The Comet Assay as a Tool in Human Biomonitoring Studies of Environmental and Occupational Exposure to Chemicals—A Systematic Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
- Search string for air pollution: Human Biomonitoring OR monitoring AND comet assay AND (air pollution OR diesel exhaust OR dust OR ozone OR particulate matter OR ultrafine particles OR formaldehyde OR hydrocarbon).
- Search string for anaesthetics: Human Biomonitoring OR monitoring AND comet assay AND (anaesthetic OR anaesthesia OR N2O OR nitrous oxide OR isoflurane OR halothane).
- Search string for antineoplastic drugs: Human Biomonitoring OR monitoring AND Comet assay AND (antineoplastic drugs OR cytostatic OR cytotoxic OR cyclophosphamide OR paclitaxel OR 5-Fluororacil).
- Search string for heavy metals: Human Biomonitoring OR monitoring AND Comet assay AND (lead OR mercury OR Cadmium OR arsenic OR heavy metals).
- Search string for pesticides: Human biomonitoring OR monitoring AND comet assay AND pesticides.
- Search string for solvents: Human Biomonitoring OR monitoring AND Comet assay AND (styrene OR benzene OR toluene OR xylene OR chloroform OR tetrachloro- or trichloroethylene OR perchloroethylene OR halogenated solvents OR solvents).
- Population: studies evaluating human subjects with environmental or occupational exposure to chemical substances;
- Exposure: studies assessing the environmental or occupational effects of exposure to the chemical substances of interest (i.e., air pollution, anaesthetics gases, antineoplastic drugs, heavy metals, pesticides, or solvents) by means of the comet assay in biological samples;
- Comparator: non-exposed human subjects or pre-post comparative data on exposure (in case of a single-arm study);
- Outcomes: comet assay measurements such as the tail moment, tail length (μm), % tail intensity, olive tail moment, visual scoring/DNA damage index parameters, and other parameters considered;
- Study design: interventional studies (controlled trials, experimental studies) or observational comparative studies, including case-control, cohort, cross-sectional studies, and quasi-experimental studies (pre–post-test).
- Studies without data for extraction (unavailable information or an unpublished paper), conference abstracts, other study designs (reviews, case reports, letters, commentaries, and protocols), non-human studies (in vitro and in vivo), in vitro studies on primary human cells or cell lines, and those in non-English languages were excluded.
2.2. Data Extraction and Synthesis
3. Results
3.1. Air Pollution
3.2. Anaesthetics
3.3. Antineoplastic Drugs
3.4. Heavy Metals
3.5. Pesticides
3.6. Solvents
4. Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO/HEP/ECH/EHD/22.01). Compendium of WHO and Other UN Guidance on Health and Environment Geneva: World Health Organization: Licence: CC BY-NC-SA 3.0 IGO. 2022. Available online: https://iris.who.int/bitstream/handle/10665/352844/WHO-HEP-ECH-EHD-22.01-eng.pdf?sequence=1 (accessed on 10 February 2024).
- Bocato, M.Z.; Bianchi Ximenez, J.P.; Hoffmann, C.; Barbosa, F. An overview of the current progress, challenges, and prospects of human biomonitoring and exposome studies. J. Toxicol. Environ. Health Part B Crit. Rev. 2019, 22, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carrillo, A.; Mustieles, V.; Salamanca-Fernandez, E.; Olivas-Martinez, A.; Suarez, B.; Bajard, L.; Baken, K.; Blaha, L.; Bonefeld-Jorgensen, E.C.; Couderq, S.; et al. Implementation of effect biomarkers in human biomonitoring studies: A systematic approach synergizing toxicological and epidemiological knowledge. Int. J. Hyg. Environ. Health 2023, 249, 114140. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, A. Biomarkers: Coming of age for environmental health and risk assessment. Environ. Sci. Technol. 1997, 31, 1837–1848. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten public health impacts of cancer—An overview. Arh. Hig. Rada Toksikol. 2017, 68, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Smajdova, L. The use of genotoxicity biomarkers in molecular epidemiology: Applications in environmental, occupational and dietary studies. AIMS Genet. 2017, 4, 166–191. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. Investigating oxidative DNA damage and its repair using the comet assay. Mutat. Res. 2009, 681, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Dusinska, M.; Collins, A.R. The comet assay in human biomonitoring: Gene-environment interactions. Mutagenesis 2008, 23, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Laffon, B.; Teixeira, J.P.; Silva, S.; Loureiro, J.; Torres, J.; Pasaro, E.; Mendez, J.; Mayan, O. Genotoxic effects in a population of nurses handling antineoplastic drugs, and relationship with genetic polymorphisms in DNA repair enzymes. Am. J. Ind. Med. 2005, 48, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Naz, S.; Ma, Y.; Ullah, Q.; Khan, M.Z.; Wang, J.; Lu, X.; Luosand, D.-Z.; Tabassum, S.; Chatha, A.M.M.; et al. An Overview of Comet Assay Application for Detecting DNA Damage in Aquatic Animals. Agriculture 2023, 13, 623. [Google Scholar] [CrossRef]
- Collins, A.; Moller, P.; Gajski, G.; Vodenkova, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA modifications with the comet assay: A compendium of protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Knudsen, L.E.; Loft, S.; Wallin, H. The comet assay as a rapid test in biomonitoring occupational exposure to DNA-damaging agents and effect of confounding factors. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1005–1015. [Google Scholar]
- Cavallo, D.; Ursini, C.L.; Rondinone, B.; Iavicoli, S. Evaluation of a suitable DNA damage biomarker for human biomonitoring of exposed workers. Environ. Mol. Mutagen. 2009, 50, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Koppen, G.; Scavone, F.; Giovannelli, L. The comet assay for human biomonitoring: Effect of cryopreservation on DNA damage in different blood cell preparations. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 843, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.; Rojas, E. Environmental and occupational biomonitoring using the Comet assay. Mutat. Res. 2009, 681, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Acito, M.; Fatigoni, C.; Villarini, M.; Moretti, M. B-Comet Assay (Comet Assay on Buccal Cells) for the Evaluation of Primary DNA Damage in Human Biomonitoring Studies. Int. J. Environ. Res. Public Health 2020, 17, 9234. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Zegura, B.; Ladeira, C.; Novak, M.; Sramkova, M.; Pourrut, B.; Del Bo, C.; Milic, M.; Gutzkow, K.B.; Costa, S.; et al. The comet assay in animal models: From bugs to whales—(Part 2 Vertebrates). Mutation research. Rev. Mutat. Res. 2019, 781, 130–164. [Google Scholar] [CrossRef]
- Gajski, G.; Zegura, B.; Ladeira, C.; Pourrut, B.; Del Bo, C.; Novak, M.; Sramkova, M.; Milic, M.; Gutzkow, K.B.; Costa, S.; et al. The comet assay in animal models: From bugs to whales—(Part 1 Invertebrates). Mutation research. Rev. Mutat. Res. 2019, 779, 82–113. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Azqueta, A.; Boutet-Robinet, E.; Koppen, G.; Bonassi, S.; Milic, M.; Gajski, G.; Costa, S.; Teixeira, J.P.; Costa Pereira, C.; et al. Minimum Information for Reporting on the Comet Assay (MIRCA): Recommendations for describing comet assay procedures and results. Nat. Protoc. 2020, 15, 3817–3826. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Fatigoni, C.; Salvatori, T.; Villarini, M.; Moretti, M. Extent of Primary DNA Damage Measured by the Comet Assay in Health Professionals Exposed to Antineoplastic Drugs: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 523. [Google Scholar] [CrossRef] [PubMed]
- Zare Sakhvidi, M.J.; Hajaghazadeh, M.; Mostaghaci, M.; Mehrparvar, A.H.; Zare Sakhvidi, F.; Naghshineh, E. Applicability of the comet assay in evaluation of DNA damage in healthcare providers’ working with antineoplastic drugs: A systematic review and meta-analysis. Int. J. Occup. Environ. Health 2016, 22, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Hemmingsen, J.G.; Jensen, D.M.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Cao, Y.; Kermanizadeh, A.; Klingberg, H.; et al. Applications of the comet assay in particle toxicology: Air pollution and engineered nanomaterials exposure. Mutagenesis 2015, 30, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.F.; Steinert, S. Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals. Mutat. Res. 2003, 544, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Banath, J.P.; Durand, R.E. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat. Res. 1990, 122, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, T.S.; Vilhar, B.; Faux, S.P.; Jha, A.N. Comet Assay measurements: A perspective. Cell Biol. Toxicol. 2009, 25, 53–64. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Munn, Z. Jonna Briggs Institute (JBI) Manual for Evidence Synthesis. 2020. Available online: https://synthesismanual.jbi.global (accessed on 12 April 2023).
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; (Updated 2020); Cochrane: Hoboken, NJ, USA, 2020. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Boogaard, H.; Walker, K.; Cohen, A.J. Air pollution: The emergence of a major global health risk factor. Int. Health 2019, 11, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Dandotiya, B. Health Effects of Air Pollution in Urban Environment. In Climate Change and Its Impact on Ecosystem Services and Biodiversity in Arid and Semi-Arid Zones; IGI Global: Hershey, PA, USA, 2019. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Urban Ambient Air Pollution Database. 2018. Available online: https://www.who.int/data/gho/data/themes/air-pollution/who-air-quality-database (accessed on 17 December 2023).
- Smith, K.R. National Burden of Disease in India from Indoor Air Pollution. Proc. Natl. Acad. Sci. USA 2000, 97, 13286–13293. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Saber, A.T.; Pedersen, J.E.; Pedersen, P.B.; Clausen, P.A.; Lohr, M.; Kermanizadeh, A.; Loft, S.; Ebbehoj, N.E.; Hansen, A.M.; et al. Assessment of polycyclic aromatic hydrocarbon exposure, lung function, systemic inflammation, and genotoxicity in peripheral blood mononuclear cells from firefighters before and after a work shift. Environ. Mol. Mutagen. 2018, 59, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Saber, A.T.; Frederiksen, M.; Clausen, P.A.; Sejbaek, C.S.; Hemmingsen, C.H.; Ebbehoj, N.E.; Catalan, J.; Aimonen, K.; Koivisto, J.; et al. Occupational exposure and markers of genetic damage, systemic inflammation and lung function: A Danish cross-sectional study among air force personnel. Sci. Rep. 2021, 11, 17998. [Google Scholar] [CrossRef] [PubMed]
- Al Zabadi, H.; Ferrari, L.; Sari-Minodier, I.; Kerautret, M.A.; Tiberguent, A.; Paris, C.; Zmirou-Navier, D. Integrated exposure assessment of sewage workers to genotoxicants: An urinary biomarker approach and oxidative stress evaluation. Environ. Health 2011, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Canpinar, H.; Undeger, U.; Guc, D.; Colakoglu, M.; Kars, A.; Basaran, N. Assessment of immunotoxicity and genotoxicity in workers exposed to low concentrations of formaldehyde. Arch. Toxicol. 2013, 87, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bacaksiz, A.; Kayaalti, Z.; Soylemez, E.; Tutkun, E.; Soylemezoglu, T. Lymphocyte DNA damage in Turkish asphalt workers detected by the comet assay. Int. J. Environ. Health Res. 2014, 24, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bagryantseva, Y.; Novotna, B.; Rossner, P., Jr.; Chvatalova, I.; Milcova, A.; Svecova, V.; Lnenickova, Z.; Solansky, I.; Sram, R.J. Oxidative damage to biological macromolecules in Prague bus drivers and garagemen: Impact of air pollution and genetic polymorphisms. Toxicol. Lett. 2010, 199, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Becit, M.; Çilekar, Ş.; Başaran, M.M.; Koca, H.B.; Çelik, S.; Dilsiz, S.A. Changes in genotoxicity, inflammatory and oxidative stress parameters of workers in marble processing plants. Environ. Res. 2021, 197, 111209. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.; Brucker, N.; Moro, A.M.; Nascimento, S.; Goethel, G.; Souto, C.; Fracasso, R.; Sauer, E.; Altknecht, L.; da Costa, B.; et al. Association between inflammation processes, DNA damage, and exposure to environmental pollutants. Environ. Sci. Pollut. Res. Int. 2017, 24, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Balamuralikrishnan, B.; Balachandar, V.; Subramaniam, M.D.; Alagumuthu, K.K.; Sureshkumar, S.; Arun, M.; Arun, S.; Padmavathi, K.; Razeena, A.H.; Gomathi, M.; et al. Assessment of genotoxic and humoral immune system alterations in silica exposed workers from pottery industries in South India. Stoch. Environ. Res. Risk Assess. 2014, 28, 1801–1814. [Google Scholar] [CrossRef]
- Bruschweiler, E.D.; Wild, P.; Huynh, C.K.; Savova-Bianchi, D.; Danuser, B.; Hopf, N.B. DNA Damage among Wood Workers Assessed with the Comet Assay. Environ. Health Insights 2016, 10, 105–112. [Google Scholar] [CrossRef]
- Carere, A.; Andreoli, C.; Galati, R.; Leopardi, P.; Marcon, F.; Rosati, M.V.; Rossi, S.; Tomei, F.; Verdina, A.; Zijno, A.; et al. Biomonitoring of exposure to urban air pollutants: Analysis of sister chromatid exchanges and DNA lesions in peripheral lymphocytes of traffic policemen. Mutat. Res. 2002, 518, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Ursini, C.L.; Bavazzano, P.; Cassinelli, C.; Frattini, A.; Perniconi, B.; Di Francesco, A.; Ciervo, A.; Rondinone, B.; Iavicoli, S. Sister chromatid exchange and oxidative DNA damage in paving workers exposed to PAHs. Ann. Occup. Hyg. 2006, 50, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Ursini, C.L.; Carelli, G.; Iavicoli, I.; Ciervo, A.; Perniconi, B.; Rondinone, B.; Gismondi, M.; Iavicoli, S. Occupational exposure in airport personnel: Characterization and evaluation of genotoxic and oxidative effects. Toxicology 2006, 223, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Boccuni, F.; Ferrante, R.; Tombolini, F.; Maiello, R.; Chiarella, P.; Buresti, G.; et al. A follow-up study on workers involved in the graphene production process after the introduction of exposure mitigation measures: Evaluation of genotoxic and oxidative effects. Nanotoxicology 2022, 16, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Cebulska-Wasilewska, A.; Wiechec, A.; Panek, A.; Binkova, B.; Sram, R.J.; Farmer, P.B. Influence of environmental exposure to PAHs on the susceptibility of lymphocytes to DNA-damage induction and on their repair capacity. Mutat. Res. 2005, 588, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Cebulska-Wasilewska, A.; Pawlyk, I.; Panek, A.; Wiechec, A.; Kalina, I.; Popov, T.; Georgieva, T.; Farmer, P.B. Exposure to environmental polycyclic aromatic hydrocarbons: Influences on cellular susceptibility to DNA damage (sampling Kosice and Sofia). Mutat. Res. 2007, 620, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Cebulska-Wasilewska, A.; Binkova, B.; Sram, R.J.; Kalina, I.; Popov, T.; Farmer, P.B. Repair competence assay in studies of the influence of environmental exposure to c-PAHs on individual susceptibility to induction of DNA damage. Mutat. Res. 2007, 620, 155–164. [Google Scholar] [CrossRef]
- Ceppi, M.; Smolkova, B.; Staruchova, M.; Kazimirova, A.; Barancokova, M.; Volkovova, K.; Collins, A.; Kocan, A.; Dzupinkova, Z.; Horska, A.; et al. Genotoxic effects of occupational exposure to glass fibres—A human biomonitoring study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2023, 885, 503572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, Y.; Yuan, J.; Chen, W.; Sun, J.; Wang, H.; Liang, H.; Guo, L.; Yang, X.; Tan, H.; et al. Association of polymorphisms in AhR, CYP1A1, GSTM1, and GSTT1 genes with levels of DNA damage in peripheral blood lymphocytes among coke-oven workers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1703–1707. [Google Scholar] [CrossRef]
- Chen, H.L.; Chen, I.J.; Chia, T.P. Occupational exposure and DNA strand breakage of workers in bottom ash recovery and fly ash treatment plants. J. Hazard. Mater. 2010, 174, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Leng, S.; Li, H.; Huang, C.; Niu, Y.; Zhang, L.; Liang, X.; Lin, H.; Zheng, Y. Suboptimal DNA repair capacity predisposes coke-oven workers to accumulate more chromosomal damages in peripheral lymphocytes. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 987–993. [Google Scholar] [CrossRef][Green Version]
- Chia, T.; Hsu, C.Y.; Chen, H.L. Oxidative damage of workers in secondary metal recovery plants affected by smoking status and joining the smelting work. Ind. Health 2008, 46, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Coelho, P.; Costa, C.; Silva, S.; Mayan, O.; Santos, L.S.; Gaspar, J.; Teixeira, J.P. Genotoxic damage in pathology anatomy laboratory workers exposed to formaldehyde. Toxicology 2008, 252, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Pina, C.; Coelho, P.; Costa, C.; Silva, S.; Porto, B.; Laffon, B.; Teixeira, J.P. Occupational exposure to formaldehyde: Genotoxic risk evaluation by comet assay and micronucleus test using human peripheral lymphocytes. J. Toxicol. Environ. Health Part A 2011, 74, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Carvalho, S.; Costa, C.; Coelho, P.; Silva, S.; Santos, L.S.; Gaspar, J.F.; Porto, B.; Laffon, B.; Teixeira, J.P. Increased levels of chromosomal aberrations and DNA damage in a group of workers exposed to formaldehyde. Mutagenesis 2015, 30, 463–473. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, M.; Lardau, S.; Buchet, J.P.; Kirsch-Volders, M.; Lison, D. Absence of significant genotoxicity in lymphocytes and urine from workers exposed to moderate levels of cobalt-containing dust: A cross-sectional study. Environ. Mol. Mutagen. 2000, 36, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Jia, X.; Zhai, Q.; Ma, L.; Wang, S.; Huang, C.; Wang, H.; Niu, Y.; Li, X.; Dai, Y.; et al. Long-term exposure to diesel engine exhaust induces primary DNA damage: A population-based study. Occup. Environ. Med. 2016, 73, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Everatt, R.; Slapsyte, G.; Mierauskiene, J.; Dedonyte, V.; Bakiene, L. Biomonitoring study of dry-cleaning workers using cytogenetic tests and the comet assay. J. Occup. Environ. Hyg. 2013, 10, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Galiotte, M.P.; Kohler, P.; Mussi, G.; Gattas, G.J. Assessment of occupational genotoxic risk among Brazilian hairdressers. Ann. Occup. Hyg. 2008, 52, 645–651. [Google Scholar] [CrossRef]
- Giri, S.K.; Yadav, A.; Kumar, A.; Dev, K.; Gupta, R.; Aggarwal, N.; Seth, N.; Gautam, S.K. Association of GSTM1 and GSTT1 polymorphisms with DNA damage in coal-tar workers. Sci. Total Environ. 2011, 409, 4465–4469. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.S.; Elmesallamy, G.E.; Sameer, M.M. Evaluation of Genotoxic Effects of Formaldehyde in Adult Albino Rats and Its Implication In Case of Human Exposure. Life Sci. J. 2012, 9, 3085–3093. [Google Scholar]
- Goethel, G.; Brucker, N.; Moro, A.M.; Charao, M.F.; Fracasso, R.; Barth, A.; Bubols, G.; Durgante, J.; Nascimento, S.; Baierle, M.; et al. Evaluation of genotoxicity in workers exposed to benzene and atmospheric pollutants. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 770, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Hachesu, V.; Naderyan Fe’li, S.; Kargar Shouroki, F.; Mehrparvar, A.H.; Zavar Reza, J.; Azimi, M.; Zare Sakhvidi, M.J. Carbon load in airway macrophages, DNA damage and lung function in taxi drivers exposed to traffic-related air pollution. Environ. Sci. Pollut. Res. Int. 2019, 26, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Guo, H.; Wu, T. Genetic variations of CYP2B6 gene were associated with plasma BPDE-Alb adducts and DNA damage levels in coke oven workers. Toxicol. Lett. 2012, 211, 232–238. [Google Scholar] [CrossRef]
- Jasso-Pineda, Y.; Díaz-Barriga, F.; Yáñez-Estrada, L.; Pérez-Vázquez, F.J.; Pérez-Maldonado, I.N. DNA damage in Mexican children living in high-risk contaminated scenarios. Sci. Total Environ. 2015, 518–519, 38–48. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, L.; Cheng, J.; Leng, S.; Dai, Y.; Zhang, Y.; Niu, Y.; Yan, H.; Qu, W.; Zhang, C.; et al. Genomic damages in peripheral blood lymphocytes and association with polymorphisms of three glutathione S-transferases in workers exposed to formaldehyde. Mutat. Res. 2010, 695, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Gautam, D.S.; Gokhale, M.; Jain, S.K. Tobacco dust induced genotoxicity as an occupational hazard in workers of bidi making cottage industry of central India. Toxicol. Int. 2014, 21, 18–23. [Google Scholar] [CrossRef][Green Version]
- Khisroon, M.; Khan, A.; Ayub, A.; Ullah, I.; Farooqi, J.; Ullah, A. DNA damage analysis concerning GSTM1 and GSTT1 gene polymorphism in gold jewellery workers from Peshawar Pakistan. Biomarkers 2020, 25, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Kianmehr, M.; Hajavi, J.; Gazeri, J. Assessment of DNA damage in blood lymphocytes of bakery workers by comet assay. Toxicol. Ind. Health 2017, 33, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.E.; Gaskell, M.; Martin, E.A.; Poole, J.; Scheepers, P.T.; Jensen, A.; Autrup, H.; Farmer, P.B. Genotoxic damage in mine workers exposed to diesel exhaust, and the effects of glutathione transferase genotypes. Mutat. Res. 2005, 583, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.F., Jr.; Mathias, P.I.; Toennis, C.A.; Clark, J.C.; Marlow, K.L.; B’Hymer, C.; Singh, N.P.; Gibson, R.L.; Butler, M.A. Detection of DNA damage in workers exposed to JP-8 jet fuel. Mutat. Res. 2012, 747, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Kvitko, K.; Bandinelli, E.; Henriques, J.A.; Heuser, V.D.; Rohr, P.; da Silva, F.R.; Schneider, N.B.; Fernandes, S.; Ancines, C.; da Silva, J. Susceptibility to DNA damage in workers occupationally exposed to pesticides, to tannery chemicals and to coal dust during mining. Genet. Mol. Biol. 2012, 35, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Cheng, J.; Pan, Z.; Huang, C.; Niu, Y.; Dai, Y.; Li, B.; He, F.; Zheng, Y. Associations between XRCC1 and ERCC2 polymorphisms and DNA damage in peripheral blood lymphocyte among coke oven workers. Biomarkers 2004, 9, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Leon-Mejia, G.; Espitia-Perez, L.; Hoyos-Giraldo, L.S.; Da Silva, J.; Hartmann, A.; Henriques, J.A.; Quintana, M. Assessment of DNA damage in coal open-cast mining workers using the cytokinesis-blocked micronucleus test and the comet assay. Sci. Total Environ. 2011, 409, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Leon-Mejia, G.; Luna-Rodriguez, I.; Trindade, C.; Oliveros-Ortiz, L.; Anaya-Romero, M.; Luna-Carrascal, J.; Navarro-Ojeda, N.; Ruiz-Benitez, M.; Franco-Valencia, K.; Da Silva, J.; et al. Cytotoxic and genotoxic effects in mechanics occupationally exposed to diesel engine exhaust. Ecotoxicol. Environ. Saf. 2019, 171, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Guo, Y.; Yi, J.; Kuang, D.; Li, X.; Deng, H.; Huang, K.; Guan, L.; He, Y.; Zhang, X.; et al. Occupational exposure to formaldehyde and genetic damage in the peripheral blood lymphocytes of plywood workers. J. Occup. Health 2013, 55, 284–291. [Google Scholar] [CrossRef]
- Marczynski, B.; Rihs, H.P.; Rossbach, B.; Holzer, J.; Angerer, J.; Scherenberg, M.; Hoffmann, G.; Bruning, T.; Wilhelm, M. Analysis of 8-oxo-7,8-dihydro-2’-deoxyguanosine and DNA strand breaks in white blood cells of occupationally exposed workers: Comparison with ambient monitoring, urinary metabolites and enzyme polymorphisms. Carcinogenesis 2002, 23, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Marczynski, B.; Raulf-Heimsoth, M.; Pesch, B.; Kendzia, B.; Kafferlein, H.U.; Vosshans, B.; Borowitzki, G.; Lee, E.H.; Bramer, R.; Bruning, T. Detection of DNA strand breaks by comet assay in sputum leucocytes of bitumen-exposed workers: A pilot study. Hum. Exp. Toxicol. 2010, 29, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Marczynski, B.; Raulf-Heimsoth, M.; Spickenheuer, A.; Pesch, B.; Kendzia, B.; Mensing, T.; Engelhardt, B.; Lee, E.H.; Schindler, B.K.; Heinze, E.; et al. DNA adducts and strand breaks in workers exposed to vapours and aerosols of bitumen: Associations between exposure and effect. Arch. Toxicol. 2011, 85 (Suppl. 1), S53–S64. [Google Scholar] [CrossRef]
- Moretti, M.; Dell’Omo, M.; Villarini, M.; Pastorelli, R.; Muzi, G.; Airoldi, L.; Pasquini, R. Primary DNA damage and genetic polymorphisms for CYP1A1, EPHX and GSTM1 in workers at a graphite electrode manufacturing plant. BMC Public Health 2007, 7, 270. [Google Scholar] [CrossRef] [PubMed]
- Novotna, B.; Topinka, J.; Solansky, I.; Chvatalova, I.; Lnenickova, Z.; Sram, R.J. Impact of air pollution and genotype variability on DNA damage in Prague policemen. Toxicol. Lett. 2007, 172, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Im, H.; Kang, H.S.; Jung, W.; Won, N.H.; Lee, E.; Sul, D. Comparison of immunnological and genotoxicological parameters in automobile emission inspectors exposed to polycyclic aromatic hydrocarbons. Environ. Toxicol. Pharmacol. 2006, 21, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Peteffi, G.P.; da Silva, L.B.; Antunes, M.V.; Wilhelm, C.; Valandro, E.T.; Glaeser, J.; Kaefer, D.; Linden, R. Evaluation of genotoxicity in workers exposed to low levels of formaldehyde in a furniture manufacturing facility. Toxicol. Ind. Health 2016, 32, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Peteffi, G.P.; Antunes, M.V.; Carrer, C.; Valandro, E.T.; Santos, S.; Glaeser, J.; Mattos, L.; da Silva, L.B.; Linden, R. Environmental and biological monitoring of occupational formaldehyde exposure resulting from the use of products for hair straightening. Environ. Sci. Pollut. Res. Int. 2016, 23, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Recio-Vega, R.; Olivas-Calderon, E.; Michel-Ramirez, G.; Martinez-Salinas, R.I.; Gallegos-Arreola, M.P.; Ocampo-Gomez, G.L.; Perez-Morales, R. Associations between sperm quality, DNA damage, and CYP1A1, GSTT1 and GSTM1 polymorphisms with 1-hydroxypyrene urinary levels in men occupationally exposed to polycyclic aromatic hydrocarbons. Int. Arch. Occup. Environ. Health 2018, 91, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Rekhadevi, P.V.; Mahboob, M.; Rahman, M.F.; Grover, P. Genetic damage in wood dust-exposed workers. Mutagenesis 2009, 24, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Rohr, P.; Kvitko, K.; da Silva, F.R.; Menezes, A.P.; Porto, C.; Sarmento, M.; Decker, N.; Reyes, J.M.; Allgayer Mda, C.; Furtado, T.C.; et al. Genetic and oxidative damage of peripheral blood lymphocytes in workers with occupational exposure to coal. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2013, 758, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sardas, S.; Omurtag, G.Z.; Tozan, A.; Gul, H.; Beyoglu, D. Evaluation of DNA damage in construction-site workers occupationally exposed to welding fumes and solvent-based paints in Turkey. Toxicol. Ind. Health 2010, 26, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Scheepers, P.T.; Coggon, D.; Knudsen, L.E.; Anzion, R.; Autrup, H.; Bogovski, S.; Bos, R.P.; Dahmann, D.; Farmer, P.; Martin, E.A.; et al. BIOMarkers for occupational diesel exhaust exposure monitoring (BIOMODEM)—A study in underground mining. Toxicol. Lett. 2002, 134, 305–317. [Google Scholar] [CrossRef]
- Sellappa, S.; Prathyumnan, S.; Balachandar, V. DNA damage induction and repair inhibition among building construction workers in South India. Asian Pac. J. Cancer Prev. 2010, 11, 875–880. [Google Scholar] [PubMed]
- Sellappa, S.; Mani, B.; Keyan, K.S. Cytogenetic biomonitoring of road paving workers occupationally exposed to polycyclic aromatic hydrocarbons. Asian Pac. J. Cancer Prev. 2011, 12, 713–717. [Google Scholar]
- Shen, M.; Bin, P.; Li, H.; Zhang, X.; Sun, X.; Duan, H.; Niu, Y.; Meng, T.; Dai, Y.; Gao, W.; et al. Increased levels of etheno-DNA adducts and genotoxicity biomarkers of long-term exposure to pure diesel engine exhaust. Sci. Total Environ. 2016, 543, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Siwinska, E.; Mielzynska, D.; Kapka, L. Association between urinary 1-hydroxypyrene and genotoxic effects in coke oven workers. Occup. Environ. Med. 2004, 61, e10. [Google Scholar] [CrossRef]
- Sul, D.; Oh, E.; Im, H.; Yang, M.; Kim, C.W.; Lee, E. DNA damage in T- and B-lymphocytes and granulocytes in emission inspection and incineration workers exposed to polycyclic aromatic hydrocarbons. Mutat. Res. 2003, 538, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Toraason, M.; Lynch, D.W.; DeBord, D.G.; Singh, N.; Krieg, E.; Butler, M.A.; Toennis, C.A.; Nemhauser, J.B. DNA damage in leukocytes of workers occupationally exposed to 1-bromopropane. Mutat. Res. 2006, 603, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tovalin, H.; Valverde, M.; Morandi, M.T.; Blanco, S.; Whitehead, L.; Rojas, E. DNA damage in outdoor workers occupationally exposed to environmental air pollutants. Occup. Environ. Med. 2006, 63, 230–236. [Google Scholar] [CrossRef]
- Ullah, I.; Zahid, M.; Jawad, M.; Arsh, A. Assessment of DNA damage and oxidative stress among traffic conductors and coal miners. Pak. J. Med. Sci. 2021, 37, 499–502. [Google Scholar] [CrossRef]
- van Delft, J.H.M.; Steenwinkel, M.J.S.; van Asten, J.G.; de Vogel, N.; Bruijntjes-Rozier, T.C.; Schouten, T.; Cramers, P.; Maas, L.; van Herwijnen, M.H.; van Schooten, F.J.; et al. Biological monitoring the exposure to polycyclic aromatic hydrocarbons of coke oven workers in relation to smoking and genetic polymorphisms for GSTM1 and GSTT1. Ann. Occup. Hyg. 2001, 45, 395–408. [Google Scholar] [CrossRef]
- Villarini, M.; Moretti, M.; Fatigoni, C.; Agea, E.; Dominici, L.; Mattioli, A.; Volpi, R.; Pasquini, R. Evaluation of primary DNA damage, cytogenetic biomarkers and genetic polymorphisms for CYP1A1 and GSTM1 in road tunnel construction workers. J. Toxicol. Environ. Health Part A 2008, 71, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Vital, N.; Antunes, S.; Louro, H.; Vaz, F.; Simoes, T.; Penque, D.; Silva, M.J. Environmental Tobacco Smoke in Occupational Settings: Effect and Susceptibility Biomarkers in Workers From Lisbon Restaurants and Bars. Front. Public Health 2021, 9, 674142. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Zheng, H.; Guo, L.; Liang, H.; Yang, X.; Bai, Y.; Sun, J.; Su, Y.; Chen, Y.; et al. Association between plasma BPDE-Alb adduct concentrations and DNA damage of peripheral blood lymphocytes among coke oven workers. Occup. Environ. Med. 2007, 64, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, Y.; Guo, H.; Li, J.; Yang, Y.; Wu, Z.; Zheng, H.; Wu, T. Genetic variants of nucleotide excision repair genes are associated with DNA damage in coke oven workers. Cancer Epidemiol. Biomark. Prev. 2010, 19, 211–218. [Google Scholar] [CrossRef]
- Wang, J.; Luo, X.; Xu, B.; Wei, J.; Zhang, Z.; Zhu, H. Elevated oxidative damage in kitchen workers in Chinese restaurants. J. Occup. Health 2011, 53, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Wultsch, G.; Mišík, M.; Nersesyan, A.; Knasmueller, S. Genotoxic effects of occupational exposure measured in lymphocytes of waste-incinerator workers. Mutat. Res. 2011, 720, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, J.; Bai, Y.; Tian, F.; Yuan, J.; Sun, J.; Liang, H.; Guo, L.; Tan, H.; Chen, W.; et al. Using lymphocyte and plasma Hsp70 as biomarkers for assessing coke oven exposure among steel workers. Environ. Health Perspect. 2007, 115, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, M.; Fang, Q.; Zhang, X. Polycyclic aromatic hydrocarbons, long non-coding RNA expression, and DNA damage in coke oven workers. Environ. Sci. Pollut. Res. Int. 2022, 29, 57277–57286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xing, X.; Jiang, S.; Qiu, C.; Mo, Z.; Chen, S.; Chen, L.; Wang, Q.; Xiao, Y.; Dong, G.; et al. Global H3K79 di-methylation mediates DNA damage response to PAH exposure in Chinese coke oven workers. Environ. Pollut. 2021, 268, 115956. [Google Scholar] [CrossRef]
- Zendehdel, R.; Jouni, F.J.; Hajipour, B.; Panjali, Z.; Kheiri, H.; Vahabi, M. DNA damage in workers exposed to formaldehyde at concentrations below occupational exposure limits. Toxicol. Environ. Chem. 2017, 99, 1409–1417. [Google Scholar] [CrossRef]
- Zendehdel, R.; Vahabi, M.; Sedghi, R. Estimation of formaldehyde occupational exposure limit based on genetic damage in some Iranian exposed workers using benchmark dose method. Environ. Sci. Pollut. Res. Int. 2018, 25, 31183–31189. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, R.; Abdolmaleki, P.; Jouni, F.J.; Mazinani, M. Genetic variation and risk of DNA damage in peripheral blood lymphocytes of Iranian formaldehyde-exposed workers. Hum. Exp. Toxicol. 2018, 37, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Cruz, I.; Sanchez-Guerra, M.; Hernandez-Cadena, L.; De Vizcaya-Ruiz, A.; Mugica, V.; Pelallo-Martinez, N.A.; Solis-Heredia, M.J.; Byun, H.M.; Baccarelli, A.; Quintanilla-Vega, B. Increased methylation of repetitive elements and DNA repair genes is associated with higher DNA oxidation in children in an urbanized, industrial environment. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 813, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.G.; Frederiksen, M.; Saber, A.T.; Wils, R.S.; Fonseca, A.S.; Koponen, I.K.; Johannesson, S.; Roursgaard, M.; Loft, S.; Moller, P.; et al. Health effects of exposure to diesel exhaust in diesel-powered trains. Part. Fibre Toxicol. 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Avogbe, P.H.; Ayi-Fanou, L.; Autrup, H.; Loft, S.; Fayomi, B.; Sanni, A.; Vinzents, P.; Moller, P. Ultrafine particulate matter and high-level benzene urban air pollution in relation to oxidative DNA damage. Carcinogenesis 2005, 26, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Beyoglu, D.; Ozkozaci, T.; Akici, N.; Omurtag, G.Z.; Akici, A.; Ceran, O.; Sardas, S. Assessment of DNA damage in children exposed to indoor tobacco smoke. Int. J. Hyg. Environ. Health 2010, 213, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Cetkovic, T.; Haveric, A.; Behmen, S.; Hadzic Omanovic, M.; Caluk Klacar, L.; Dzaferspahic, A.; Durmisevic, I.; Mehanovic, M.; Haveric, S. A pilot biomonitoring study of air pollution in the urban area of Sarajevo, Bosnia and Herzegovina: Genotoxicity assessment in buccal cells. Mutagenesis 2023, 38, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.A.; Oh, E.; Lee, E.; Sul, D. Effects of hair dyeing on DNA damage in human lymphocytes. J. Occup. Health 2003, 45, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Sun, C.; Chen, W.; Jin, G.; Gong, J.; Zhu, M.; Yuan, J.; Dai, J.; Wang, M.; Pan, Y.; et al. Personal exposure to PM2.5, genetic variants and DNA damage: A multi-center population-based study in Chinese. Toxicol. Lett. 2015, 235, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Coronas, M.V.; Pereira, T.S.; Rocha, J.A.; Lemos, A.T.; Fachel, J.M.; Salvadori, D.M.; Vargas, V.M. Genetic biomonitoring of an urban population exposed to mutagenic airborne pollutants. Environ. Int. 2009, 35, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Coronas, M.V.; Rocha, J.A.; Salvadori, D.M.; Vargas, V.M. Evaluation of area contaminated by wood treatment activities: Genetic markers in the environment and in the child population. Chemosphere 2016, 144, 1207–1215. [Google Scholar] [CrossRef]
- Danielsen, P.H.; Brauner, E.V.; Barregard, L.; Sallsten, G.; Wallin, M.; Olinski, R.; Rozalski, R.; Moller, P.; Loft, S. Oxidatively damaged DNA and its repair after experimental exposure to wood smoke in healthy humans. Mutat. Res. 2008, 642, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Silva da Silva, C.; Rossato, J.M.; Vaz Rocha, J.A.; Vargas, V.M. Characterization of an area of reference for inhalable particulate matter (PM2.5) associated with genetic biomonitoring in children. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 778, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, L.; Moller, P.; Riddervold, I.S.; Bonlokke, J.; Massling, A.; Sigsgaard, T.; Loft, S. Controlled human wood smoke exposure: Oxidative stress, inflammation and microvascular function. Part. Fibre Toxicol. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, R.T.; Gamboa, A.R.; Bravo, A.H.; Ostrosky, W.P. Genotoxicity in child populations exposed to polycyclic aromatic hydrocarbons (PAHs) in the air from Tabasco, Mexico. Int. J. Environ. Res. Public Health 2008, 5, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Zhu, M.; Chu, M.; Sun, C.; Chen, W.; Jin, G.; Yuan, J.; Dai, J.; Wang, M.; Pan, Y.; et al. Genetic variants in SMARC genes are associated with DNA damage levels in Chinese population. Toxicol. Lett. 2014, 229, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, N.; Cui, Z.; Ma, M.; Li, L.; Cai, M.; Li, Y.; Lin, H.; Li, Y.; Ao, L.; et al. Association between urinary polycyclic aromatic hydrocarbon metabolites and sperm DNA damage: A population study in Chongqing, China. Environ. Health Perspect. 2011, 119, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, J.G.; Moller, P.; Jantzen, K.; Jonsson, B.A.; Albin, M.; Wierzbicka, A.; Gudmundsson, A.; Loft, S.; Rissler, J. Controlled exposure to diesel exhaust and traffic noise—Effects on oxidative stress and activation in mononuclear blood cells. Mutat. Res. 2015, 775, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hisamuddin, N.H.; Jalaludin, J.; Abu Bakar, S.; Latif, M.T. The Influence of Environmental Polycyclic Aromatic Hydrocarbons (PAHs) Exposure on DNA Damage among School Children in Urban Traffic Area, Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 2193. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.N.; Alaludin, J.; Bakar, S.A.; Hisamuddin, N.H.; Suhaimi, N.F. Association of Traffic-Related Air Pollution (TRAP) with DNA Damage and Respiratory Health Symptoms among Primary School Children in Selangor. Asian J. Atmos. Environ. 2019, 13, 106–116. [Google Scholar] [CrossRef]
- Jensen, A.; Karottki, D.G.; Christensen, J.M.; Bonlokke, J.H.; Sigsgaard, T.; Glasius, M.; Loft, S.; Moller, P. Biomarkers of oxidative stress and inflammation after wood smoke exposure in a reconstructed Viking Age house. Environ. Mol. Mutagen. 2014, 55, 652–661. [Google Scholar] [CrossRef]
- Koppen, G.; Verheyen, G.; Maes, A.; Van Gorp, U.; Schoeters, G.; Hond, E.D.; Staessen, J.; Nawrot, T.; Roels, H.A.; Vlietinck, R.; et al. A battery of DNA effect biomarkers to evaluate environmental exposure of Flemish adolescents. J. Appl. Toxicol. JAT 2007, 27, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Koppen, G.; Franken, C.; Den Hond, E.; Plusquin, M.; Reimann, B.; Leermakers, M.; Covaci, A.; Nawrot, T.; Van Larebeke, N.; Schoeters, G.; et al. Pooled analysis of genotoxicity markers in relation to exposure in the Flemish Environment and Health Studies (FLEHS) between 1999 and 2018. Environ. Res. 2020, 190, 110002. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.T.; Lemos, C.T.; Coronas, M.V.; Rocha, J.R.D.; Vargas, V.M.F. Integrated study of genotoxicity biomarkers in schoolchildren and inhalable particles in areas under petrochemical influence. Environ. Res. 2020, 188, 109443. [Google Scholar] [CrossRef] [PubMed]
- Leon-Mejia, G.; Vargas, J.E.; Quintana-Sosa, M.; Rueda, R.A.; Perez, J.P.; Miranda-Guevara, A.; Moreno, O.F.; Trindade, C.; Acosta-Hoyos, A.; Dias, J.; et al. Exposure to coal mining can lead to imbalanced levels of inorganic elements and DNA damage in individuals living near open-pit mining sites. Environ. Res. 2023, 227, 115773. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.K.; Mukherjee, B.; Das, D.; Ray, M.R. Micronucleus formation, DNA damage and repair in premenopausal women chronically exposed to high level of indoor air pollution from biomass fuel use in rural India. Mutat. Res. 2010, 697, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Mondal, N.K.; Bhattacharya, P.; Ray, M.R. Assessment of DNA damage by comet assay and fast halo assay in buccal epithelial cells of Indian women chronically exposed to biomass smoke. Int. J. Hyg. Environ. Health 2011, 214, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Dutta, A.; Roychoudhury, S.; Ray, M.R. Chronic inhalation of biomass smoke is associated with DNA damage in airway cells: Involvement of particulate pollutants and benzene. J. Appl. Toxicol. JAT 2013, 33, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Bindhani, B.; Saha, H.; Ray, M.R. Increased oxidative DNA damage and decreased expression of base excision repair proteins in airway epithelial cells of women who cook with biomass fuels. Environ. Toxicol. Pharmacol. 2014, 38, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Nagiah, S.; Phulukdaree, A.; Naidoo, D.; Ramcharan, K.; Naidoo, R.N.; Moodley, D.; Chuturgoon, A. Oxidative stress and air pollution exposure during pregnancy: A molecular assessment. Hum. Exp. Toxicol. 2015, 34, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Pacini, S.; Giovannelli, L.; Gulisano, M.; Peruzzi, B.; Polli, G.; Boddi, V.; Ruggiero, M.; Bozzo, C.; Stomeo, F.; Fenu, G.; et al. Association between atmospheric ozone levels and damage to human nasal mucosa in Florence, Italy. Environ. Mol. Mutagen. 2003, 42, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Bajpayee, M.; Parmar, D.; Rastogi, S.K.; Mathur, N.; Seth, P.K.; Dhawan, A. DNA damage in lymphocytes of rural Indian women exposed to biomass fuel smoke as assessed by the Comet assay. Environ. Mol. Mutagen. 2005, 45, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Pelallo-Martínez, N.A.; Batres-Esquivel, L.; Carrizales-Yáñez, L.; Díaz-Barriga, F.M. Genotoxic and hematological effects in children exposed to a chemical mixture in a petrochemical area in Mexico. Arch. Environ. Contam. Toxicol. 2014, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.S.; Beltrami, L.S.; Rocha, J.A.; Broto, F.P.; Comellas, L.R.; Salvadori, D.M.; Vargas, V.M. Toxicogenetic monitoring in urban cities exposed to different airborne contaminants. Ecotoxicol. Environ. Saf. 2013, 90, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cadahia, B.; Laffon, B.; Pasaro, E.; Mendez, J. Genetic damage induced by accidental environmental pollutants. Sci. World J. 2006, 6, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Piperakis, S.M.; Petrakou, E.; Tsilimigaki, S. Effects of air pollution and smoking on DNA damage of human lymphocytes. Environ. Mol. Mutagen. 2000, 36, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Rojas, E.; Valverde, M.; Lopez, M.C.; Naufal, I.; Sanchez, I.; Bizarro, P.; Lopez, I.; Fortoul, T.I.; Ostrosky-Wegman, P. Evaluation of DNA damage in exfoliated tear duct epithelial cells from individuals exposed to air pollution assessed by single cell gel electrophoresis assay. Mutat. Res. 2000, 468, 11–17. [Google Scholar] [CrossRef]
- Sanchez-Guerra, M.; Pelallo-Martinez, N.; Diaz-Barriga, F.; Rothenberg, S.J.; Hernandez-Cadena, L.; Faugeron, S.; Oropeza-Hernandez, L.F.; Guaderrama-Diaz, M.; Quintanilla-Vega, B. Environmental polycyclic aromatic hydrocarbon (PAH) exposure and DNA damage in Mexican children. Mutat. Res. 2012, 742, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Shermatov, K.; Zeyrek, D.; Yildirim, F.; Kilic, M.; Cebi, N.; Kocyigit, A. DNA damage in children exposed to secondhand cigarette smoke and its association with oxidative stress. Indian Pediatr. 2012, 49, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Sopian, N.A.; Jalaludin, J.; Abu Bakar, S.; Hamedon, T.R.; Latif, M.T. Exposure to Particulate PAHs on Potential Genotoxicity and Cancer Risk among School Children Living Near the Petrochemical Industry. Int. J. Environ. Res. Public Health 2021, 18, 2575. [Google Scholar] [CrossRef] [PubMed]
- Torres-Dosal, A.; Perez-Maldonado, I.N.; Jasso-Pineda, Y.; Martinez Salinas, R.I.; Alegria-Torres, J.A.; Diaz-Barriga, F. Indoor air pollution in a Mexican indigenous community: Evaluation of risk reduction program using biomarkers of exposure and effect. Sci. Total Environ. 2008, 390, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Verschaeve, L.; Koppen, G.; Gorp, U.V.; Schoeters, G.; Jacobs, G.; Zwijzen, C. Seasonal variations in spontaneous levels of DNA damage; implication in the risk assessment of environmental chemicals. J. Appl. Toxicol. JAT 2007, 27, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Vinzents, P.S.; Moller, P.; Sorensen, M.; Knudsen, L.E.; Hertel, O.; Jensen, F.P.; Schibye, B.; Loft, S. Personal exposure to ultrafine particles and oxidative DNA damage. Environ. Health Perspect. 2005, 113, 1485–1490. [Google Scholar] [CrossRef]
- Wilhelm, M.; Eberwein, G.; Holzer, J.; Gladtke, D.; Angerer, J.; Marczynski, B.; Behrendt, H.; Ring, J.; Sugiri, D.; Ranft, U. Influence of industrial sources on children’s health—Hot spot studies in North Rhine Westphalia, Germany. Int. J. Hyg. Environ. Health 2007, 210, 591–599. [Google Scholar] [CrossRef]
- Wu, F.Y.; Wu, H.D.; Yang, H.L.; Kuo, H.W.; Ying, J.C.; Lin, C.J.; Yang, C.C.; Lin, L.Y.; Chiu, T.H.; Lai, J.S. Associations among genetic susceptibility, DNA damage, and pregnancy outcomes of expectant mothers exposed to environmental tobacco smoke. Sci. Total Environ. 2007, 386, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zani, C.; Ceretti, E.; Zerbini, I.; Viola, G.C.V.; Donato, F.; Gelatti, U.; Feretti, D. Comet Test in Saliva Leukocytes of Pre-School Children Exposed to Air Pollution in North Italy: The Respira Study. Int. J. Environ. Res. Public Health 2020, 17, 3276. [Google Scholar] [CrossRef] [PubMed]
- Zani Zani, C.; Ceretti, E.; Feretti, D.; Villarini, M.; Moretti, M.; Verani, M.; De Donno, A.; Bonetta, S.; Buschini, A.; Bonetti, A.; et al. Winter Air Pollution and Genotoxic Effects in Children Living in a Highly Polluted Urban Area. Atmosphere 2021, 12, 1191. [Google Scholar] [CrossRef]
- Zeller, J.; Neuss, S.; Mueller, J.U.; Kuhner, S.; Holzmann, K.; Hogel, J.; Klingmann, C.; Bruckner, T.; Triebig, G.; Speit, G. Assessment of genotoxic effects and changes in gene expression in humans exposed to formaldehyde by inhalation under controlled conditions. Mutagenesis 2011, 26, 555–561. [Google Scholar] [CrossRef]
- Eftimova, B.; Sholjakova, M.; Mirakovski, D.; Hadzi-Nikolova, M. Health Effects Associated With Exposure to Anesthetic Gas Nitrous Oxide-N(2)O in Clinical Hospital—Shtip Personel. Open Access Maced. J. Med. Sci. 2017, 5, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Fodale, V.; Mondello, S.; Aloisi, C.; Schifilliti, D.; Santamaria, L. Genotoxic effects of anesthetic agents. Expert. Opin. Drug Saf. 2008, 7, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Schifilliti, D.; Mondello, S.; D’Arrigo, M.G.; Chille, G.; Fodale, V. Genotoxic effects of anesthetic agents: An update. Expert. Opin. Drug Saf. 2011, 10, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Kiani, F.; Jorfi, S.; Soltani, F.; Ghanbari, S.; Rezaee, R.; Mohammadi, M.J. Exposure to anesthetic gases in the operating rooms and assessment of non-carcinogenic risk among health care workers. Toxicol. Rep. 2023, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Calbayram, N.C. Exposure to anesthetic gases among operating room personnel and risk of genotoxicity: A systematic review of the human biomonitoring studies. J. Clin. Anesth. 2016, 35, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, J.M.; Escribano-Sanchez, G.; Osuna, E.; Molina-Rodriguez, A.; Diaz-Agea, J.L.; Garcia-Sanchez, A. Occupational Exposure to Inhalational Anesthetics and Teratogenic Effects: A Systematic Review. Healthcare 2023, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Nagella, A.B.; Ravishankar, M.; Hemanth Kumar, V.R. Anaesthesia practice and reproductive outcomes: Facts unveiled. Indian J. Anaesth. 2016, 60, 225. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, R.; Bjordal, C.; Andersson, M.; Bjordal, J.; Nyberg, A.; Welin, B.; Willman, A. Health risks and occupational exposure to volatile anaesthetics—A review with a systematic approach. J. Clin. Nurs. 2005, 14, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Aun, A.G.; Golim, M.A.; Nogueira, F.R.; Souza, K.M.; Arruda, N.M.; Braz, J.R.C.; Braz, L.G.; Braz, M.G. Monitoring early cell damage in physicians who are occupationally exposed to inhalational anesthetics. Mutat. Res. 2018, 812, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Baysal, Z.; Cengiz, M.; Ozgonul, A.; Cakir, M.; Celik, H.; Kocyigit, A. Oxidative status and DNA damage in operating room personnel. Clin. Biochem. 2009, 42, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, M.; Rekhadevi, P.V.; Sailaja, N.; Rahman, M.F.; Reddy, J.P.; Mahboob, M.; Grover, P. Evaluation of genetic damage in operating room personnel exposed to anaesthetic gases. Mutagenesis 2006, 21, 249–254. [Google Scholar] [CrossRef]
- El-Ebiary, A.A.; Abuelfadl, A.A.; Sarhan, N.I.; Othman, M.M. Assessment of genotoxicity risk in operation room personnel by the alkaline comet assay. Hum. Exp. Toxicol. 2013, 32, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.B.S.; Aun, A.G.; Souza, K.M.; Nishimoto, I.H.; Silva, M.A.P.; de Carvalho, L.R.; Braz, L.G.; Braz, M.G. High anesthetic (isoflurane) indoor pollution is associated with genetic instability, cytotoxicity, and proliferative alterations in professionals working in a veterinary hospital. Environ. Sci. Pollut. Res. Int. 2022, 29, 71774–71784. [Google Scholar] [CrossRef] [PubMed]
- Izdes, S.; Sardas, S.; Kadioglu, E.; Kaymak, C.; Ozcagli, E. Assessment of genotoxic damage in nurses occupationally exposed to anaesthetic gases or antineoplastic drugs by the comet assay. J. Occup. Health 2009, 51, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Izdes, S.; Sardas, S.; Kadioglu, E.; Karakaya, A.E. DNA damage, glutathione, and total antioxidant capacity in anesthesia nurses. Arch. Environ. Occup. Health 2010, 65, 211–217. [Google Scholar] [CrossRef]
- Khisroon, M.; Humayun, M.; Khan, A.; Farooqi, J.; Humayun; Khan, J. Polymorphism in GSTM1 and GSTT1 genes influence DNA damage in personnel occupationally exposed to volatile anaesthetics (VA), from Peshawar, Pakistan. Occup. Environ. Med. 2020, 77, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Rozgaj, R.; Kasuba, V.; Brozovic, G.; Jazbec, A. Genotoxic effects of anaesthetics in operating theatre personnel evaluated by the comet assay and micronucleus test. Int. J. Hyg. Environ. Health 2009, 212, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sardas, S.; Izdes, S.; Ozcagli, E.; Kanbak, O.; Kadioglu, E. The role of antioxidant supplementation in occupational exposure to waste anaesthetic gases. Int. Arch. Occup. Environ. Health 2006, 80, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Souza, K.M.; Braz, L.G.; Nogueira, F.R.; Souza, M.B.; Bincoleto, L.F.; Aun, A.G.; Corrente, J.E.; Carvalho, L.R.; Braz, J.R.C.; Braz, M.G. Occupational exposure to anesthetics leads to genomic instability, cytotoxicity and proliferative changes. Mutat. Res. 2016, 791–792, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Szyfter, K.; Szulc, R.; Mikstacki, A.; Stachecki, I.; Rydzanicz, M.; Jaloszynski, P. Genotoxicity of inhalation anaesthetics: DNA lesions generated by sevoflurane in vitro and in vivo. J. Appl. Genet. 2004, 45, 369–374. [Google Scholar] [PubMed]
- Szyfter, K.; Stachecki, I.; Kostrzewska-Poczekaj, M.; Szaumkessel, M.; Szyfter-Harris, J.; Sobczynski, P. Exposure to volatile anaesthetics is not followed by a massive induction of single-strand DNA breaks in operation theatre personnel. J. Appl. Genet. 2016, 57, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Wronska-Nofer, T.; Palus, J.; Krajewski, W.; Jajte, J.; Kucharska, M.; Stetkiewicz, J.; Wasowicz, W.; Rydzynski, K. DNA damage induced by nitrous oxide: Study in medical personnel of operating rooms. Mutat. Res. 2009, 666, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Wronska-Nofer, T.; Nofer, J.R.; Jajte, J.; Dziubaltowska, E.; Szymczak, W.; Krajewski, W.; Wasowicz, W.; Rydzynski, K. Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N(2)O). Mutat Res. 2012, 731, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.H.; McDiarmid, M.A. Preventing occupational exposures to antineoplastic drugs in health care settings. CA Cancer J. Clin. 2006, 56, 354–365. [Google Scholar] [CrossRef]
- Grosse, Y.; Baan, R.; Straif, K.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Galichet, L.; Cogliano, V.; et al. A review of human carcinogens—Part A: Pharmaceuticals. Lancet Oncol. 2009, 10, 13–14. [Google Scholar] [CrossRef] [PubMed]
- CDC. The National Institute for Occupational Safety and Health (NIOSH). Available online: http://www.cdc.gov/niosh/2004 (accessed on 8 May 2023).
- Kopjar, N.; Garaj-Vrhovac, V.; Kasuba, V.; Rozgaj, R.; Ramic, S.; Pavlica, V.; Zeljezic, D. Assessment of genotoxic risks in Croatian health care workers occupationally exposed to cytotoxic drugs: A multi-biomarker approach. Int. J. Hyg. Environ. Health 2009, 212, 414–431. [Google Scholar] [CrossRef]
- Mahboob, M.; Rahman, M.F.; Rekhadevi, P.V.; Sailaja, N.; Balasubramanyam, A.; Prabhakar, P.V.; Singh, S.P.; Reddy, U.A.; Rao, G.S.; Grover, P. Monitoring of oxidative stress in nurses occupationally exposed to antineoplastic drugs. Toxicol. Int. 2012, 19, 20–24. [Google Scholar] [CrossRef]
- Ziegler, E.; Mason, H.J.; Baxter, P.J. Occupational exposure to cytotoxic drugs in two UK oncology wards. Occup. Environ. Med. 2002, 59, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Valanis, B.G.; Vollmer, W.M.; Labuhn, K.T.; Glass, A.G. Association of antineoplastic drug handling with acute adverse effects in pharmacy personnel. Am. J. Hosp. Pharm. 1993, 50, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Fransman, W.; Kager, H.; Meijster, T.; Heederik, D.; Kromhout, H.; Portengen, L.; Blaauboer, B.J. Leukemia from dermal exposure to cyclophosphamide among nurses in The Netherlands: Quantitative assessment of the risk. Ann. Occup. Hyg. 2014, 58, 271–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kopjar, N.; Garaj-Vrhovac, V. Application of the alkaline comet assay in human biomonitoring for genotoxicity: A study on Croatian medical personnel handling antineoplastic drugs. Mutagenesis 2001, 16, 71–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skov, T.; Maarup, B.; Olsen, J.; Rorth, M.; Winthereik, H.; Lynge, E. Leukaemia and reproductive outcome among nurses handling antineoplastic drugs. Br. J. Ind. Med. 1992, 49, 855–861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunnarsdottir, H.K.; Aspelund, T.; Karlsson, T.; Rafnsson, V.V. Occupational Risk Factors for Breast Cancer among Nurses. Int. J. Occup. Environ. Health 1997, 3, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ratner, P.A.; Spinelli, J.J.; Beking, K.; Lorenzi, M.; Chow, Y.; Teschke, K.; Le, N.D.; Gallagher, R.P.; Dimich-Ward, H. Cancer incidence and adverse pregnancy outcome in registered nurses potentially exposed to antineoplastic drugs. BMC Nurs. 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Maluf, S.W.; Erdtmann, B. Follow-up study of the genetic damage in lymphocytes of pharmacists and nurses handling antineoplastic drugs evaluated by cytokinesis-block micronuclei analysis and single cell gel electrophoresis assay. Mutat. Res. 2000, 471, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ursini, C.L.; Cavallo, D.; Colombi, A.; Giglio, M.; Marinaccio, A.; Iavicoli, S. Evaluation of early DNA damage in healthcare workers handling antineoplastic drugs. Int. Arch. Occup. Environ. Health 2006, 80, 134–140. [Google Scholar] [CrossRef]
- Yoshida, J.; Kosaka, H.; Tomioka, K.; Kumagai, S. Genotoxic risks to nurses from contamination of the work environment with antineoplastic drugs in Japan. J. Occup. Health 2006, 48, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Rekhadevi, P.V.; Sailaja, N.; Chandrasekhar, M.; Mahboob, M.; Rahman, M.F.; Grover, P. Genotoxicity assessment in oncology nurses handling anti-neoplastic drugs. Mutagenesis 2007, 22, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Cornetta, T.; Padua, L.; Testa, A.; Ievoli, E.; Festa, F.; Tranfo, G.; Baccelliere, L.; Cozzi, R. Molecular biomonitoring of a population of nurses handling antineoplastic drugs. Mutat. Res. 2008, 638, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Rombaldi, F.; Cassini, C.; Salvador, M.; Saffi, J.; Erdtmann, B. Occupational risk assessment of genotoxicity and oxidative stress in workers handling anti-neoplastic drugs during a working week. Mutagenesis 2009, 24, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Dakeishi, M.; Hoshi, S.; Ishii, N.; Murata, K. Assessment of DNA damage in Japanese nurses handling antineoplastic drugs by the comet assay. J. Occup. Health 2008, 50, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.H.; DeBord, D.G.; Pretty, J.R.; Oliver, M.S.; Roth, T.S.; Lees, P.S.; Krieg, E.F., Jr.; Rogers, B.; Escalante, C.P.; Toennis, C.A.; et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. J. Occup. Environ. Med. 2010, 52, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Dominici, L.; Piccinini, R.; Fatigoni, C.; Ambrogi, M.; Curti, G.; Morucci, P.; Muzi, G.; Monarca, S.; Moretti, M. Assessment of primary, oxidative and excision repaired DNA damage in hospital personnel handling antineoplastic drugs. Mutagenesis 2011, 26, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Buschini, A.; Villarini, M.; Feretti, D.; Mussi, F.; Dominici, L.; Zerbini, I.; Moretti, M.; Ceretti, E.; Bonfiglioli, R.; Carrieri, M.; et al. Multicentre study for the evaluation of mutagenic/carcinogenic risk in nurses exposed to antineoplastic drugs: Assessment of DNA damage. Occup. Environ. Med. 2013, 70, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Viegas, V.; Pádua, M.; Carolino, E.; Gomes, M.C.; Brito, M. Relation between DNA damage measured by comet assay and OGG1 Ser326Cys polymorphism in antineoplastic drugs biomonitoring. AIMS Genet. 2015, 2, 204–2018. [Google Scholar] [CrossRef][Green Version]
- Oltulu, C.; Yesil Devecioglu, T.; Akinci, M.; Akgun Olmez, S.G.; Obeidin, S.V.; Beceren, A. Evaluation of Genotoxicity Risk in Health Care Workers Exposed to Antineoplastic Drugs. Clin. Exp. Health Sci. 2019, 9, 166–170. [Google Scholar] [CrossRef]
- Aristizabal-Pachon, A.F.; Castillo, W.O. Genotoxic evaluation of occupational exposure to antineoplastic drugs. Toxicol. Res. 2020, 36, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, C.; Cai, W.; Tao, Y.; Zhong, X.; Liu, H.; Hong, X.; Ding, X.; Lu, H.; Lai, W.; et al. Effect of occupational exposure to antineoplastic drugs on DNA damage in nurses: A cross-sectional study. Occup. Environ. Med. 2022, 79, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hongping, D.; Jianlin, L.; Meibian, Z.; Wei, W.; Lifen, J.; Shijie, C.; Wei, Z.; Baohong, W.; Jiliang, H. Detecting the cytogenetic effects in workers occupationally exposed to vincristine with four genetic tests. Mutat. Res. 2006, 599, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Omrane, F.; Gargouri, I.; Khadhraoui, M.; Elleuch, B.; Zmirou-Navier, D. Risk assessment of occupational exposure to heavy metal mixtures: A study protocol. BMC Public Health 2018, 18, 314. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Minor heavy metal: A review on occupational and environmental intoxication. Indian. J. Occup. Environ. Med. 2008, 12, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Aksu, İ.; Anlar, H.G.; Taner, G.; Bacanlı, M.; İritaş, S.; Tutkun, E.; Basaran, N. Assessment of DNA damage in welders using comet and micronucleus assays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 843, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Balachandar, V.; Arun, M.; Mohana Devi, S.; Velmurugan, P.; Manikantan, P.; Karthick Kumar, A.; Sasikala, K.; Venkatesan, C. Evaluation of the genetic alterations in direct and indirect exposures of hexavalent chromium [Cr(VI)] in leather tanning industry workers North Arcot District, South India. Int. Arch. Occup. Environ. Health 2010, 83, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Batra, J.; Thakur, A.; Deepak, J.; Shrawan, K. Lead Induced Oxidative DNA Damage among the Occupationally Exposed Workers: A Case-Control Study. J. Clin. Diagn. Res. 2020, 14, 12–16. [Google Scholar] [CrossRef]
- Cavallo, D.; Iavicoli, I.; Setini, A.; Marinaccio, A.; Perniconi, B.; Carelli, G.; Iavicoli, S. Genotoxic risk and oxidative DNA damage in workers exposed to antimony trioxide. Environ. Mol. Mutagen. 2002, 40, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Chinde, S.; Kumari, M.; Devi, K.R.; Murty, U.S.; Rahman, M.F.; Kumari, S.I.; Mahboob, M.; Grover, P. Assessment of genotoxic effects of lead in occupationally exposed workers. Environ. Sci. Pollut. Res. Int. 2014, 21, 11469–11480. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; García-Lestón, J.; Costa, S.; Costa, C.; Silva, S.; Dall’Armi, V.; Zoffoli, R.; Bonassi, S.; de Lima, J.P.; Gaspar, J.F.; et al. Genotoxic effect of exposure to metal(loid)s. A molecular epidemiology survey of populations living and working in Panasqueira mine area, Portugal. Environ. Int. 2013, 60, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Danadevi, K.; Rozati, R.; Saleha Banu, B.; Hanumanth Rao, P.; Grover, P. DNA damage in workers exposed to lead using comet assay. Toxicology 2003, 187, 183–193. [Google Scholar] [CrossRef]
- Danadevi, K.; Rozati, R.; Banu, B.S.; Grover, P. Genotoxic evaluation of welders occupationally exposed to chromium and nickel using the Comet and micronucleus assays. Mutagenesis 2004, 19, 35–41. [Google Scholar] [CrossRef] [PubMed]
- De Olivera, J.V.; Boufleur, L.A.; Dos Santos, C.E.; Dias, J.F.; Squeff, C.H.; Silva, G.R.; Ianistcki, M.; Benvegnu, V.C.; Da Silva, J. Occupational genotoxicity among copper smelters. Toxicol. Ind. Health 2012, 28, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, H.G.; Sicard, D.; Torres, M.M. DNA damage and repair in cells of lead exposed people. Am. J. Ind. Med. 2000, 38, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, M.E.; Perbellini, L.; Soldà, S.; Talamini, G.; Franceschetti, P. Lead induced DNA strand breaks in lymphocytes of exposed workers: Role of reactive oxygen species and protein kinase C. Mutat. Res. 2002, 515, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, A.; Piccinini, R.; Ambrogi, M.; Villarini, M.; Moretti, M.; Marchetti, C.; Abbritti, G.; Muzi, G. Primary DNA damage in chrome-plating workers. Toxicology 2003, 188, 187–195. [Google Scholar] [CrossRef] [PubMed]
- García-Lestón, J.; Roma-Torres, J.; Vilares, M.; Pinto, R.; Cunha, L.M.; Prista, J.; Teixeira, J.P.; Mayan, O.; Pásaro, E.; Méndez, J.; et al. Biomonitoring of a population of Portuguese workers exposed to lead. Mutat. Res. 2011, 721, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Rekhadevi, P.V.; Danadevi, K.; Vuyyuri, S.B.; Mahboob, M.; Rahman, M.F. Genotoxicity evaluation in workers occupationally exposed to lead. Int. J. Hyg. Environ. Health 2010, 213, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Franco, P.; Maldonado-Vega, M.; Calderón-Salinas, J.V.; Rojas, E.; Valverde, M. Role of Ape1 in Impaired DNA Repair Capacity in Battery Recycling Plant Workers Exposed to Lead. Int. J. Environ. Res. Public Health 2022, 19, 7961. [Google Scholar] [CrossRef] [PubMed]
- Iarmarcovai, G.; Sari-Minodier, I.; Chaspoul, F.; Botta, C.; De Méo, M.; Orsière, T.; Bergé-Lefranc, J.L.; Gallice, P.; Botta, A. Risk assessment of welders using analysis of eight metals by ICP-MS in blood and urine and DNA damage evaluation by the comet and micronucleus assays; influence of XRCC1 and XRCC3 polymorphisms. Mutagenesis 2005, 20, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kašuba, V.; Rozgaj, R.; Milić, M.; Zelježić, D.; Kopjar, N.; Pizent, A.; Kljaković-Gašpić, Z.; Jazbec, A. Evaluation of genotoxic effects of lead in pottery-glaze workers using micronucleus assay, alkaline comet assay and DNA diffusion assay. Int. Arch. Occup. Environ. Health 2012, 85, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kašuba, V.; Milić, M.; Želježić, D.; Mladinić, M.; Pizent, A.; Kljaković-Gašpić, Z.; Balija, M.; Jukić, I. Biomonitoring findings for occupational lead exposure in battery and ceramic tile workers using biochemical markers, alkaline comet assay, and micronucleus test coupled with fluorescence in situ hybridisation. Arh. Hig. Rada Toksikol. 2020, 71, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Kayaaltı, Z.; Yavuz, İ.; Söylemez, E.; Bacaksız, A.; Tutkun, E.; Sayal, A.; Söylemezoğlu, T. Evaluation of DNA damage using 3 comet assay parameters in workers occupationally exposed to lead. Arch. Environ. Occup. Health 2015, 70, 120–125. [Google Scholar] [CrossRef]
- Khisroon, M.; Khan, A.; Shah, A.A.; Ullah, I.; Farooqi, J.; Ullah, A. Scalp Hair Metal Analysis Concerning DNA Damage in Welders of Peshawar Khyber Pakhtunkhwa Pakistan. Biol. Trace Elem. Res. 2021, 199, 1649–1656. [Google Scholar] [CrossRef]
- Liu, N.; Guan, Y.; Xue, L.; Yu, Y.; Xiao, J.; Chang, Z.; Li, Q.; Bai, Y.; Li, B.; Guan, W. Assessment of DNA/Chromosome Damage in the Peripheral Blood Lymphocytes of Workers Exposed to Indium Compounds. Toxicol. Sci. 2017, 157, 41–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, M.; Chen, Z.; Chen, Q.; Zou, H.; Lou, J.; He, J. Investigating DNA damage in tannery workers occupationally exposed to trivalent chromium using comet assay. Mutat. Res. 2008, 654, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Minozzo, R.; Deimling, L.I.; Santos-Mello, R. Cytokinesis-blocked micronucleus cytome and comet assays in peripheral blood lymphocytes of workers exposed to lead considering folate and vitamin B12 status. Mutat. Res. 2010, 697, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.D.; Garcia, S.C.; Brucker, N.; Goethel, G.; Sauer, E.; Lacerda, L.M.; Oliveira, E.; Trombini, T.L.; Machado, A.B.; Pressotto, A.; et al. Occupational risk assessment of exposure to metals in chrome plating workers. Drug Chem. Toxicol. 2022, 45, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Olewińska, E.; Kasperczyk, A.; Kapka, L.; Kozłowska, A.; Pawlas, N.; Dobrakowski, M.; Birkner, E.; Kasperczyk, S. Level of DNA damage in lead-exposed workers. Ann. Agric. Environ. Med. 2010, 17, 231–236. [Google Scholar]
- Palus, J.; Rydzynski, K.; Dziubaltowska, E.; Wyszynska, K.; Natarajan, A.T.; Nilsson, R. Genotoxic effects of occupational exposure to lead and cadmium. Mutat. Res. 2003, 540, 19–28. [Google Scholar] [CrossRef]
- Palus, J.; Lewinska, D.; Dziubaltowska, E.; Stepnik, M.; Beck, J.; Rydzynski, K.; Nilsson, R. DNA damage in leukocytes of workers occupationally exposed to arsenic in copper smelters. Environ. Mol. Mutagen. 2005, 46, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Pandeh, M.; Fathi, S.; Zare Sakhvidi, M.J.; Zavar Reza, J.; Sedghian, L. Oxidative stress and early DNA damage in workers exposed to iron-rich metal fumes. Environ. Sci. Pollut. Res. Int. 2017, 24, 9645–9650. [Google Scholar] [CrossRef] [PubMed]
- Pawlas, N.; Olewińska, E.; Markiewicz-Górka, I.; Kozłowska, A.; Januszewska, L.; Lundh, T.; Januszewska, E.; Pawlas, K. Oxidative damage of DNA in subjects occupationally exposed to lead. Adv. Clin. Exp. Med. 2017, 26, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cadahía, B.; Méndez, J.; Pásaro, E.; Lafuente, A.; Cabaleiro, T.; Laffon, B. Biomonitoring of human exposure to prestige oil: Effects on DNA and endocrine parameters. Environ. Health Insights 2008, 2, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Arshad, M.; Siddiqa, M.; Ahmad, R. Evaluation of DNA damage in traffic police wardens of Pakistan due to cadmium and zinc. Sci. Total Environ. 2018, 630, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Singh, Z.; Chadha, P. Assessment of DNA damage as an index of genetic toxicity in welding microenvironments among iron-based industries. Toxicol. Ind. Health 2016, 32, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, X.; Fang, L.; Li, K.; Yang, P.; Du, L.; Ji, K.; Wang, J.; Liu, Q.; Xu, C.; et al. Genomic instability in adult men involved in processing electronic waste in Northern China. Environ. Int. 2018, 117, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L.; Ahmad, A.; Shadab, G.G.; Usmani, J.A. Possible role of zinc in diminishing lead-related occupational stress-a zinc nutrition concern. Environ. Sci. Pollut. Res. Int. 2017, 24, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Vuyyuri, S.B.; Ishaq, M.; Kuppala, D.; Grover, P.; Ahuja, Y.R. Evaluation of micronucleus frequencies and DNA damage in glass workers exposed to arsenic. Environ. Mol. Mutagen. 2006, 47, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Zhang, X.; Wang, X.C.; Jin, L.F.; Yang, Z.P.; Jiang, C.X.; Chen, Q.; Ren, X.B.; Cao, J.Z.; Wang, Q.; et al. Chronic occupational exposure to hexavalent chromium causes DNA damage in electroplating workers. BMC Public Health 2011, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lou, J.; Chen, S.; Zheng, W.; Wu, W.; Jin, L.; Deng, H.; He, J. Evaluating the genotoxic effects of workers exposed to lead using micronucleus assay, comet assay and TCR gene mutation test. Toxicology 2006, 223, 219–226. [Google Scholar] [CrossRef]
- Andrew, A.S.; Burgess, J.L.; Meza, M.M.; Demidenko, E.; Waugh, M.G.; Hamilton, J.W.; Karagas, M.R. Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic. Environ. Health Perspect. 2006, 114, 1193–1198. [Google Scholar] [CrossRef]
- Banerjee, M.; Sarma, N.; Biswas, R.; Roy, J.; Mukherjee, A.; Giri, A.K. DNA repair deficiency leads to susceptibility to develop arsenic-induced premalignant skin lesions. Int. J. Cancer 2008, 123, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Som, A.; Ghoshal, S.; Mondal, L.; Chaubey, R.C.; Bhilwade, H.N.; Rahman, M.M.; Giri, A.K. Assessment of DNA damage in peripheral blood lymphocytes of individuals susceptible to arsenic induced toxicity in West Bengal, India. Toxicol. Lett. 2005, 159, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Esquivel, Á.; Marrugo-Negrete, J.; Calao-Ramos, C. Genetic damage in human populations at mining sites in the upper basin of the San Jorge River, Colombia. Environ. Sci. Pollut. Res. Int. 2019, 26, 10961–10971. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Turi, N.; Ain, Q.U.; Rahman, H.; Jahan, S. Evaluation of environmental effects of heavy metals on biochemical profile and oxidative stress among children at brick kiln sites. Arch. Environ. Occup. Health 2021, 76, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Franken, C.; Koppen, G.; Lambrechts, N.; Govarts, E.; Bruckers, L.; Den Hond, E.; Loots, I.; Nelen, V.; Sioen, I.; Nawrot, T.S.; et al. Environmental exposure to human carcinogens in teenagers and the association with DNA damage. Environ. Res. 2017, 152, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Pineda, Y.; Díaz-Barriga, F.; Calderón, J.; Yáñez, L.; Carrizales, L.; Pérez-Maldonado, I.N. DNA damage and decreased DNA repair in peripheral blood mononuclear cells in individuals exposed to arsenic and lead in a mining site. Biol. Trace Elem. Res. 2012, 146, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Pineda, Y.; Espinosa-Reyes, G.; Gonzalez-Mille, D.; Razo-Soto, I.; Carrizales, L.; Torres-Dosal, A.; Mejia-Saavedra, J.; Monroy, M.; Ize, A.I.; Yarto, M.; et al. An integrated health risk assessment approach to the study of mining sites contaminated with arsenic and lead. Integr. Environ. Assess. Manag. 2007, 3, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.H.; Ambreen, K.; Fatima, G.; Kumar, S. Assessment of health risks with reference to oxidative stress and DNA damage in chromium exposed population. Sci. Total Environ. 2012, 430, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, J.; Pereira, R.; Pinto, F.; Caetano, T.; Silva, A.; Carvalheiro, T.; Guimarães, A.; Gonçalves, F.; Paiva, A.; Mendo, S. Biomonitoring a human population inhabiting nearby a deactivated uranium mine. Toxicology 2013, 305, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Gomez, J.; Garcia-Vargas, G.G.; Lopez-Carrillo, L.; Calderon-Aranda, E.S.; Gomez, A.; Vera, E.; Valverde, M.; Cebrian, M.E.; Rojas, E. Genotoxic effects of environmental exposure to arsenic and lead on children in region Lagunera, Mexico. Ann. N. Y. Acad. Sci. 2008, 1140, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Sampayo-Reyes, A.; Hernández, A.; El-Yamani, N.; López-Campos, C.; Mayet-Machado, E.; Rincón-Castañeda, C.B.; Limones-Aguilar Mde, L.; López-Campos, J.E.; de León, M.B.; González-Hernández, S.; et al. Arsenic induces DNA damage in environmentally exposed Mexican children and adults. Influence of GSTO1 and AS3MT polymorphisms. Toxicol. Sci. 2010, 117, 63–71. [Google Scholar] [CrossRef]
- Staessen, J.A.; Nawrot, T.; Hond, E.D.; Thijs, L.; Fagard, R.; Hoppenbrouwers, K.; Koppen, G.; Nelen, V.; Schoeters, G.; Vanderschueren, D.; et al. Renal function, cytogenetic measurements, and sexual development in adolescents in relation to environmental pollutants: A feasibility study of biomarkers. Lancet 2001, 357, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Liou, S.H.; Lin, K.J.; Liu, T.E.; Liu, S.H.; Chen, C.Y.; Sung, F.C.; Wu, T.N. Changing blood lead levels and DNA damage (comet assay) among immigrant women in Taiwan. Sci. Total Environ. 2009, 407, 5931–5936. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, L.; García-Nieto, E.; Rojas, E.; Carrizales, L.; Mejía, J.; Calderón, J.; Razo, I.; Díaz-Barriga, F. DNA damage in blood cells from children exposed to arsenic and lead in a mining area. Environ. Res. 2003, 93, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Kanno, T.; Nakamura, K.; Ikai, H.; Kikuchi, K.; Sasaki, K.; Niwano, Y. Literature review of the role of hydroxyl radicals in chemically-induced mutagenicity and carcinogenicity for the risk assessment of a disinfection system utilizing photolysis of hydrogen peroxide. J. Clin. Biochem. Nutr. 2012, 51, 9–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- United Nations Environment Programme. Synthesis Report on the Environmental and Health Impacts of Pesticides and Fertilizers and Ways to Minimize Them. 2022. Available online: https://wedocs.unep.org/xmlui/bitstream/handle/20.500.11822/38409/pesticides.pdf (accessed on 10 June 2023).
- El-Nahhal, Y.; El-Nahhal, I. Cardiotoxicity of some pesticides and their amelioration. Environ. Sci. Pollut. Res. Int. 2021, 28, 44726–44754. [Google Scholar] [CrossRef] [PubMed]
- Gilden, R.C.; Huffling, K.; Sattler, B. Pesticides and health risks. J. Obstet. Gynecol. Neonatal Nurs. JOGNN 2010, 39, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAO Stat [PESTICIDES]. License: CC BY-NC-SA 3.0 IGO. 2023. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 14 August 2023).
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Carvalho, F. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Alavanja, M.C.; Ross, M.K.; Bonner, M.R. Increased cancer burden among pesticide applicators and others due to pesticide exposure. CA Cancer J. Clin. 2013, 63, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Laborde, A. Impact of pesticide exposure in childhood. Rev. Environ. Health 2020, 35, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.R.; Freeman, L.E.; Hoppin, J.A.; Koutros, S.; Sandler, D.P.; Lynch, C.F.; Hines, C.J.; Thomas, K.; Blair, A.; Alavanja, M.C. Occupational Exposure to Pesticides and the Incidence of Lung Cancer in the Agricultural Health Study. Environ. Health Perspect. 2017, 125, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.N.; Beane Freeman, L.E.; Murata, K.; Andreotti, G.; Shearer, J.J.; Thoren, K.; Ramanathan, L.; Parks, C.G.; Koutros, S.; Lerro, C.C.; et al. Lifetime Pesticide Use and Monoclonal Gammopathy of Undetermined Significance in a Prospective Cohort of Male Farmers. Environ. Health Perspect. 2021, 129, 17003. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Choi, K.C. Adverse effects of pesticides on the functions of immune system. Comparative biochemistry and physiology. Toxicol. Pharmacol. CBP 2020, 235, 108789. [Google Scholar] [CrossRef]
- Lerro, C.C.; Beane Freeman, L.E.; DellaValle, C.T.; Andreotti, G.; Hofmann, J.N.; Koutros, S.; Parks, C.G.; Shrestha, S.; Alavanja, M.C.R.; Blair, A.; et al. Pesticide exposure and incident thyroid cancer among male pesticide applicators in agricultural health study. Environ. Int. 2021, 146, 106187. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.O.; Condon, L.; Gomes, A.V. A Common Feature of Pesticides: Oxidative Stress-The Role of Oxidative Stress in Pesticide-Induced Toxicity. Oxidative Med. Cell. Longev. 2022, 2022, 5563759. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.J.; Juberg, D.R. Cancer and occupational exposure to pesticides: An umbrella review. Int. Arch. Occup. Environ. Health 2021, 94, 945–957. [Google Scholar] [CrossRef]
- Abhishek, S.; Kaur, N.; Kaur, S.; Lata, M.; Sharma, J.K.; Sharma, A. Association of GSTM1 and GSTT1 gene deletions with susceptibility to DNA damage in the pesticide-exposed workers of Punjab. Rejuvenation Res. 2010, 13, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Aiassa, D.E.; Manas, F.J.; Gentile, N.E.; Bosch, B.; Salinero, M.C.; Gorla, N.B.M. Evaluation of genetic damage in pesticides applicators from the province of Cordoba, Argentina. Environ. Sci. Pollut. Res. Int. 2019, 26, 20981–20988. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Ismail, M.; Asad, F.; Ashraf, A.; Waheed, U.; Khan, Q.M. Pesticide genotoxicity in cotton picking women in Pakistan evaluated using comet assay. Drug Chem. Toxicol. 2018, 41, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.S.; da Silva, F.R.; da Silva, G.F.; Salvador, M.; Kvitko, K.; Rohr, P.; dos Santos, C.E.; Dias, J.F.; Henriques, J.A.; da Silva, J. Investigation of potential biomarkers for the early diagnosis of cellular stability after the exposure of agricultural workers to pesticides. An. Da Acad. Bras. De. Cienc. 2016, 88, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Siddiqa, M.; Rashid, S.; Hashmi, I.; Awan, M.A.; Ali, M.A. Biomonitoring of Toxic Effects of Pesticides in Occupationally Exposed Individuals. Saf. Health Work. 2016, 7, 156–160. [Google Scholar] [CrossRef]
- Benedetti, D.; Nunes, E.; Sarmento, M.; Porto, C.; Dos Santos, C.E.; Dias, J.F.; da Silva, J. Genetic damage in soybean workers exposed to pesticides: Evaluation with the comet and buccal micronucleus cytome assays. Mutat. Res. 2013, 752, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Bhalli, J.A.; Khan, Q.M.; Nasim, A. DNA damage in Pakistani pesticide-manufacturing workers assayed using the Comet assay. Environ. Mol. Mutagen. 2006, 47, 587–593. [Google Scholar] [CrossRef]
- Bhalli, J.A.; Ali, T.; Asi, M.R.; Khalid, Z.M.; Ceppi, M.; Khan, Q.M. DNA damage in Pakistani agricultural workers exposed to mixture of pesticides. Environ. Mol. Mutagen. 2009, 50, 37–45. [Google Scholar] [CrossRef]
- Bian, Q.; Xu, L.C.; Wang, S.L.; Xia, Y.K.; Tan, L.F.; Chen, J.F.; Song, L.; Chang, H.C.; Wang, X.R. Study on the relation between occupational fenvalerate exposure and spermatozoa DNA damage of pesticide factory workers. Occup. Environ. Med. 2004, 61, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Lopez, Y.; Gomez-Arroyo, S.; Villalobos-Pietrini, R.; Calderon-Segura, M.E.; Martinez-Arroyo, A. Biomonitoring of agricultural workers exposed to pesticide mixtures in Guerrero state, Mexico, with comet assay and micronucleus test. Environ. Sci. Pollut. Res. Int. 2016, 23, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Cayir, A.; Coskun, M.; Coskun, M.; Cobanoglu, H. Comet assay for assessment of DNA damage in greenhouse workers exposed to pesticides. Biomarkers 2019, 24, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Huang, C.H.; Wu, M.T.; Chou, C.H.; Huang, C.C.; Tseng, T.Y.; Chang, F.Y.; Li, Y.T.; Tsai, C.C.; Wang, T.S.; et al. Multidrug resistance 1 gene variants, pesticide exposure, and increased risk of DNA damage. Biomed. Res. Int. 2014, 2014, 965729. [Google Scholar] [CrossRef]
- Costa, C.; Garcia-Leston, J.; Costa, S.; Coelho, P.; Silva, S.; Pingarilho, M.; Valdiglesias, V.; Mattei, F.; Dall’Armi, V.; Bonassi, S.; et al. Is organic farming safer to farmers’ health? A comparison between organic and traditional farming. Toxicol. Lett. 2014, 230, 166–176. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.; Moraes, C.R.; Heuser, V.D.; Andrade, V.M.; Silva, F.R.; Kvitko, K.; Emmel, V.; Rohr, P.; Bordin, D.L.; Andreazza, A.C.; et al. Evaluation of genetic damage in a Brazilian population occupationally exposed to pesticides and its correlation with polymorphisms in metabolizing genes. Mutagenesis 2008, 23, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.R.; Da Silva, J.; Allgayer Mda, C.; Simon, C.F.; Dias, J.F.; dos Santos, C.E.; Salvador, M.; Branco, C.; Schneider, N.B.; Kahl, V.; et al. Genotoxic biomonitoring of tobacco farmers: Biomarkers of exposure, of early biological effects and of susceptibility. J. Hazard. Mater. 2012, 225–226, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.R.; Kvitko, K.; Rohr, P.; Abreu, M.B.; Thiesen, F.V.; Da Silva, J. Genotoxic assessment in tobacco farmers at different crop times. Sci. Total Environ. 2014, 490, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Dalberto, D.; Alves, J.; Garcia, A.L.H.; de Souza, M.R.; Abella, A.P.; Thiesen, F.V.; Salvador, M.; Santos Branco, C.D.; Marroni, N.; Bona, S.; et al. Exposure in the tobacco fields: Genetic damage and oxidative stress in tobacco farmers occupationally exposed during harvest and grading seasons. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 878, 503485. [Google Scholar] [CrossRef] [PubMed]
- Dhananjayan, V.; Ravichandran, B.; Panjakumar, K.; Kalaiselvi, K.; Rajasekar, K.; Mala, A.; Avinash, G.; Shridhar, K.; Manju, A.; Wilson, R. Assessment of genotoxicity and cholinesterase activity among women workers occupationally exposed to pesticides in tea garden. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 841, 1–7. [Google Scholar] [CrossRef]
- Dutta, S.; Bahadur, M. Comet assay genotoxicity evaluation of occupationally exposed tea-garden workers in northern West Bengal, India. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 844, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.C.; Alves, A.A.; Godoy, F.R.; Avelar, J.B.; Rodrigues, D.D.; Pedroso, T.M.; da Cruz, A.D.; Nomura, F.; de Melo, E.S.D. Evaluating genotoxic risks in Brazilian public health agents occupationally exposed to pesticides: A multi-biomarker approach. Environ. Sci. Pollut. Res. Int. 2016, 23, 19723–19734. [Google Scholar] [CrossRef] [PubMed]
- Garaj-Vrhovac, V.; Zeljezic, D. Evaluation of DNA damage in workers occupationally exposed to pesticides using single-cell gel electrophoresis (SCGE) assay. Pestic. Genotoxicity Reveal. By Comet. Assay. Mutat. Res. 2000, 469, 279–285. [Google Scholar] [CrossRef]
- Garaj-Vrhovac, V.; Zeljezic, D. Cytogenetic monitoring of croatian population occupationally exposed to a complex mixture of pesticides. Toxicology 2001, 165, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Garaj-Vrhovac, V.; Zeljezic, D. Assessment of genome damage in a population of Croatian workers employed in pesticide production by chromosomal aberration analysis, micronucleus assay and Comet assay. J. Appl. Toxicol. JAT 2002, 22, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Godoy, F.R.; Nunes, H.F.; Alves, A.A.; Carvalho, W.F.; Franco, F.C.; Pereira, R.R.; da Cruz, A.S.; da Silva, C.C.; Bastos, R.P.; de Melo, E.S.D. Increased DNA damage is not associated to polymorphisms in OGGI DNA repair gene, CYP2E1 detoxification gene, and biochemical and hematological findings in soybeans farmers from Central Brazil. Environ. Sci. Pollut. Res. Int. 2019, 26, 26553–26562. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Danadevi, K.; Mahboob, M.; Rozati, R.; Banu, B.S.; Rahman, M.F. Evaluation of genetic damage in workers employed in pesticide production utilizing the Comet assay. Mutagenesis 2003, 18, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Kahl, V.F.S.; da Silva, F.R.; Alves, J.D.S.; da Silva, G.F.; Picinini, J.; Dhillon, V.S.; Fenech, M.; de Souza, M.R.; Dias, J.F.; de Souza, C.T.; et al. Role of PON1, SOD2, OGG1, XRCC1, and XRCC4 polymorphisms on modulation of DNA damage in workers occupationally exposed to pesticides. Ecotoxicol. Environ. Saf. 2018, 159, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Kyriakopoulou, K.; Emmanouil, C.; Tsantila, N.; Liesivuori, J.; Souki, H.; Manakis, S.; Machera, K. Monitoring of systemic exposure to plant protection products and DNA damage in orchard workers. Toxicol. Lett. 2012, 210, 182–188. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, S.; Lata, M. Evaluation of DNA damage in agricultural workers exposed to pesticides using single cell gel electrophoresis (comet) assay. Indian. J. Hum. Genet. 2011, 17, 179–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaur, K.; Kaur, R. Polymorphisms in XPC and XPD genes modulate DNA damage in pesticide-exposed agricultural workers of Punjab, North-West India. Mol. Biol. Rep. 2020, 47, 5253–5262. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kaur, R. Impact of single nucleotide polymorphisms in the OGG1 and XRCC1 genes on modulation of DNA damage in pesticide-exposed agricultural workers in Punjab, North-West India. Biomarkers 2020, 25, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kaur, R. Modulation of DNA damage by XPF, XPG and ERCC1 gene polymorphisms in pesticide-exposed agricultural workers of Punjab, North-West India. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 861–862, 503302. [Google Scholar] [CrossRef]
- Khayat, C.B.; Costa, E.O.; Goncalves, M.W.; da Cruz e Cunha, D.M.; da Cruz, A.S.; de Araujo Melo, C.O.; Bastos, R.P.; da Cruz, A.D.; de Melo e Silva, D. Assessment of DNA damage in Brazilian workers occupationally exposed to pesticides: A study from Central Brazil. Environ. Sci. Pollut. Res. Int. 2013, 20, 7334–7340. [Google Scholar] [CrossRef] [PubMed]
- Lebailly, P.; Devaux, A.; Pottier, D.; De Meo, M.; Andre, V.; Baldi, I.; Severin, F.; Bernaud, J.; Durand, B.; Henry-Amar, M.; et al. Urine mutagenicity and lymphocyte DNA damage in fruit growers occupationally exposed to the fungicide captan. Occup. Environ. Med. 2003, 60, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Huang, P.L.; Chang, Y.F.; Chen, Y.H.; Chiou, Y.H.; Xu, Z.L.; Wong, R.H. GSTP1 genetic polymorphism is associated with a higher risk of DNA damage in pesticide-exposed fruit growers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Muniz, J.F.; McCauley, L.; Scherer, J.; Lasarev, M.; Koshy, M.; Kow, Y.W.; Nazar-Stewart, V.; Kisby, G.E. Biomarkers of oxidative stress and DNA damage in agricultural workers: A pilot study. Toxicol. Appl. Pharmacol. 2008, 227, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Naravaneni, R.; Jamil, K. Determination of AChE levels and genotoxic effects in farmers occupationally exposed to pesticides. Hum. Exp. Toxicol. 2007, 26, 723–731. [Google Scholar] [CrossRef]
- Paiva, J.C.; Cabral, I.O.; Soares, B.M.; Sombra, C.M.; Ferreira, J.R.; Moraes, M.O.; Cavalcanti, B.C.; Pessoa, C. Biomonitoring of rural workers exposed to a complex mixture of pesticides in the municipalities of Tiangua and Ubajara (Ceara state, Brazil): Genotoxic and cytogenetic studies. Environ. Mol. Mutagen. 2011, 52, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Paz-y-Mino, C.; Arevalo, M.; Sanchez, M.E.; Leone, P.E. Chromosome and DNA damage analysis in individuals occupationally exposed to pesticides with relation to genetic polymorphism for CYP 1A1 gene in Ecuador. Mutat. Res. 2004, 562, 77–89. [Google Scholar] [CrossRef]
- Prabha & Pooja Chadha. Risk Assessment of Occupational Exposure to Pesticides among Pesticide Distributors of Punjab (India) Using Single Cell Gel Electrophoresis. Int. J. Hum. Genet. 2015, 15, 149–155. [Google Scholar] [CrossRef]
- Ramos, J.S.A.; Pedroso, T.M.A.; Godoy, F.R.; Batista, R.E.; de Almeida, F.B.; Francelin, C.; Ribeiro, F.L.; Parise, M.R.; de Melo, E.S.D. Multi-biomarker responses to pesticides in an agricultural population from Central Brazil. Sci. Total Environ. 2021, 754, 141893. [Google Scholar] [CrossRef] [PubMed]
- Remor, A.P.; Totti, C.C.; Moreira, D.A.; Dutra, G.P.; Heuser, V.D.; Boeira, J.M. Occupational exposure of farm workers to pesticides: Biochemical parameters and evaluation of genotoxicity. Environ. Int. 2009, 35, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rohr, P.; da Silva, J.; Erdtmann, B.; Saffi, J.; Guecheva, T.N.; Antonio Pegas Henriques, J.; Kvitko, K. BER gene polymorphisms (OGG1 Ser326Cys and XRCC1 Arg194Trp) and modulation of DNA damage due to pesticides exposure. Environ. Mol. Mutagen. 2011, 52, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Saad-Hussein, A.; Noshy, M.; Taha, M.; El-Shorbagy, H.; Shahy, E.; Abdel-Shafy, E.A. GSTP1 and XRCC1 polymorphisms and DNA damage in agricultural workers exposed to pesticides. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2017, 819, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Saad-Hussein, A.; Beshir, S.; Taha, M.M.; Shahy, E.M.; Shaheen, W.; Abdel-Shafy, E.A.; Thabet, E. Early prediction of liver carcinogenicity due to occupational exposure to pesticides. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 838, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sapbamrer, R.; Khacha-Ananda, S.; Sittitoon, N.; Wunnapuk, K.; Seesen, M.; Sidthilaw, S.; Chittrakul, J.; Suwannakul, B. A longitudinal follow-up study of oxidative stress and DNA damage among farmers exposed to pesticide mixtures. Environ. Sci. Pollut. Res. Int. 2019, 26, 13185–13194. [Google Scholar] [CrossRef]
- Simoniello, M.F.; Kleinsorge, E.C.; Scagnetti, J.A.; Grigolato, R.A.; Poletta, G.L.; Carballo, M.A. DNA damage in workers occupationally exposed to pesticide mixtures. J. Appl. Toxicol. JAT 2008, 28, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Simoniello, M.F.; Kleinsorge, E.C.; Scagnetti, J.A.; Mastandrea, C.; Grigolato, R.A.; Paonessa, A.M.; Carballo, M.A. Biomarkers of cellular reaction to pesticide exposure in a rural population. Biomarkers 2010, 15, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Thakur, S.; Banerjee, B.D.; Chandna, S.; Rautela, R.S.; Grover, S.S.; Rawat, D.S.; Pasha, S.T.; Jain, S.K.; et al. DNA damage and cholinesterase activity in occupational workers exposed to pesticides. Environ. Toxicol. Pharmacol. 2011, 31, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Singh, P.; Thakur, S.; Banerjee, B.D.; Rautela, R.S.; Grover, S.S.; Rawat, D.S.; Pasha, S.T.; Jain, S.K.; et al. Genetic polymorphisms of GSTM1, GSTT1 and GSTP1 and susceptibility to DNA damage in workers occupationally exposed to organophosphate pesticides. Mutat. Res. 2011, 725, 36–42. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singh, P.; Banerjee, B.D.; Rautela, R.S.; Grover, S.S.; Rawat, D.S.; Pasha, S.T.; Jain, S.K.; Rai, A. Influence of CYP2C9, GSTM1, GSTT1 and NAT2 genetic polymorphisms on DNA damage in workers occupationally exposed to organophosphate pesticides. Mutat. Res. 2012, 741, 101–108. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Vashisht, K.; Singh, P.; Banerjee, B.D.; Rautela, R.S.; Grover, S.S.; Rawat, D.S.; Pasha, S.T.; Jain, S.K.; et al. Role of genetic polymorphisms of CYP1A1, CYP3A5, CYP2C9, CYP2D6, and PON1 in the modulation of DNA damage in workers occupationally exposed to organophosphate pesticides. Toxicol. Appl. Pharmacol. 2011, 257, 84–92. [Google Scholar] [CrossRef]
- Valencia-Quintana, R.; Lopez-Duran, R.M.; Milic, M.; Bonassi, S.; Ochoa-Ocana, M.A.; Uriostegui-Acosta, M.O.; Perez-Flores, G.A.; Gomez-Olivares, J.L.; Sanchez-Alarcon, J. Assessment of Cytogenetic Damage and Cholinesterases’ Activity in Workers Occupationally Exposed to Pesticides in Zamora-Jacona, Michoacan, Mexico. Int. J. Environ. Res. Public Health 2021, 18, 6269. [Google Scholar] [CrossRef]
- Varona-Uribe, M.E.; Torres-Rey, C.H.; Diaz-Criollo, S.; Palma-Parra, R.M.; Narvaez, D.M.; Carmona, S.P.; Briceno, L.; Idrovo, A.J. Exposure to pesticide mixtures and DNA damage among rice field workers. Arch. Environ. Occup. Health 2016, 71, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Perumalla Venkata, R.; Rahman, M.F.; Mahboob, M.; Indu Kumari, S.; Chinde, S.; M, B.; Dumala, N.; Grover, P. Assessment of genotoxicity in female agricultural workers exposed to pesticides. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2017, 22, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, C.M.; Calsing, A.K.; da Silva, L.B. Assessment of DNA damage in floriculturists in southern Brazil. Environ. Sci. Pollut. Res. Int. 2015, 22, 8182–8189. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Chang, S.Y.; Ho, S.W.; Huang, P.L.; Liu, Y.J.; Chen, Y.C.; Yeh, Y.H.; Lee, H.S. Polymorphisms in metabolic GSTP1 and DNA-repair XRCC1 genes with an increased risk of DNA damage in pesticide-exposed fruit growers. Mutat. Res. 2008, 654, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Sehrawat, G. Evaluation of Genetic Damage in Farmers Exposed to Pesticide Mixtures. Int. J. Hum. Genet. 2011, 11, 105–109. [Google Scholar] [CrossRef]
- Zepeda-Arce, R.; Rojas-Garcia, A.E.; Benitez-Trinidad, A.; Herrera-Moreno, J.F.; Medina-Diaz, I.M.; Barron-Vivanco, B.S.; Villegas, G.P.; Hernandez-Ochoa, I.; Solis Heredia, M.J.; Bernal-Hernandez, Y.Y. Oxidative stress and genetic damage among workers exposed primarily to organophosphate and pyrethroid pesticides. Environ. Toxicol. 2017, 32, 1754–1764. [Google Scholar] [CrossRef]
- Zeljezic, D.; Garaj-Vrhovac, V. Chromosomal aberration and single cell gel electrophoresis (Comet) assay in the longitudinal risk assessment of occupational exposure to pesticides. Mutagenesis 2001, 16, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Hernandez, D.L.; Montero-Montoya, R.; Serrano-Garcia, L.; Arellano-Aguilar, O.; Jasso-Pineda, Y.; Yanez-Estrada, L. Assessment of exposure to organochlorine pesticides and levels of DNA damage in mother-infant pairs of an agrarian community. Environ. Mol. Mutagen. 2013, 54, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, N.; Mahdi, A.A.; Deo, S.; Ahmad, M.K.; Kumar, D. Assessment of genotoxicity and oxidative stress in pregnant women contaminated to organochlorine pesticides and its correlation with pregnancy outcome. Environ. Res. 2022, 204, 112010. [Google Scholar] [CrossRef] [PubMed]
- How, V.; Hashim, Z.; Ismail, P.; Md Said, S.; Omar, D.; Bahri Mohd Tamrin, S. Exploring cancer development in adulthood: Cholinesterase depression and genotoxic effect from chronic exposure to organophosphate pesticides among rural farm children. J. Agromed. 2014, 19, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kapka-Skrzypczak, L.; Czajka, M.; Sawicki, K.; Matysiak-Kucharek, M.; Gabelova, A.; Sramkova, M.; Bartyzel-Lechforowicz, H.; Kruszewski, M. Assessment of DNA damage in Polish children environmentally exposed to pesticides. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 843, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.B.; Franco de Diana, D.M.; Segovia Abreu, J.A.; Avalos, D.S.; Denis, M.A.; Ovelar, C.C.; Samaniego Royg, M.J.; Thielmann Arbo, B.A.; Corvalan, R. DNA damage induced by exposure to pesticides in children of rural areas in Paraguay. Indian. J. Med. Res. 2019, 150, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Sutris, J.M.; How, V.; Sumeri, S.A.; Muhammad, M.; Sardi, D.; Mohd Mokhtar, M.T.; Muhammad, H.; Ghazi, H.F.; Isa, Z.M. Genotoxicity following Organophosphate Pesticides Exposure among Orang Asli Children Living in an Agricultural Island in Kuala Langat, Selangor, Malaysia. Int. J. Occup. Environ. Med. 2016, 7, 42–51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Azqueta, A.; Ladeira, C.; Giovannelli, L.; Boutet-Robinet, E.; Bonassi, S.; Neri, M.; Gajski, G.; Duthie, S.; Del Bo, C.; Riso, P.; et al. Application of the comet assay in human biomonitoring: An hCOMET perspective. Mutation research. Rev. Mutat. Res. 2020, 783, 108288. [Google Scholar] [CrossRef] [PubMed]
- Durrani, T.; Clapp, R.; Harrison, R.; Shusterman, D. Solvent-based paint and varnish removers: A focused toxicologic review of existing and alternative constituents. J. Appl. Toxicol. JAT 2020, 40, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Brauner, C.; Joveleviths, D.; Alvares-da-Silva, M.R.; Marroni, N.; Bona, S.; Schemitt, E.; Nardi, R. Exposure to organic solvents and hepatotoxicity. J. Environ. Sci. Health Part A 2020, 55, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Sainio, M.A., Sr. Neurotoxicity of solvents. Handb. Clin. Neurol. 2015, 131, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Sit, G.; Letellier, N.; Iwatsubo, Y.; Goldberg, M.; Leynaert, B.; Nadif, R.; Ribet, C.; Roche, N.; Roquelaure, Y.; Varraso, R.; et al. Occupational Exposures to Organic Solvents and Asthma Symptoms in the CONSTANCES Cohort. Int. J. Environ. Res. Public Health 2021, 18, 9258. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J. DNA damage induced by occupational and environmental exposure to miscellaneous chemicals. Mutat. Res. Rev. Mutat. Res. 2016, 770, 170–182. [Google Scholar] [CrossRef] [PubMed]
- International International Agency for Research on Cancer (IARC). Occupational Exposure as a Painter—IARC Monographs on the Evaluation of the Carcinogenic Risks of Chemicals to Humans, Chemical Agents and Related Occupations. 2018, pp. 509–539. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-35.pdf (accessed on 10 June 2023).
- International Agency for Research on Cancer (IARC). Boot and Shoe Manufacture and Repair—IARC Monographs on the Evaluation of the Carcinogenic Risks of Chemicals to Humans, Chemical Agents and Related Occupations. 1987, pp. 232–235. Available online: http://monographs.iarc.fr/ENG/Monographs/suppl7/Suppl7-96.pdf (accessed on 10 June 2023).
- Onyije, F.M.; Hosseini, B.; Togawa, K.; Schuz, J.; Olsson, A. Cancer Incidence and Mortality among Petroleum Industry Workers and Residents Living in Oil Producing Communities: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4343. [Google Scholar] [CrossRef] [PubMed]
- Azimi, M.; Bahrami, M.R.; Rezaei Hachesu, V.; Zavar Reza, J.; Mihanpour, H.; Zare Sakhvidi, M.J.; Mostaghaci, M. Primary DNA Damage in Dry Cleaners with Perchlorethylene Exposure. Int. J. Occup. Environ. Med. 2017, 8, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Buschini, A.; De Palma, G.; Poli, P.; Martino, A.; Rossi, C.; Mozzoni, P.; Scotti, E.; Buzio, L.; Bergamaschi, E.; Mutti, A. Genetic polymorphism of drug-metabolizing enzymes and styrene-induced DNA damage. Environ. Mol. Mutagen. 2003, 41, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Cassini, C.; Calloni, C.; Bortolini, G.; Garcia, S.C.; Dornelles, M.A.; Henriques, J.A.; Erdtmann, B.; Salvador, M. Occupational risk assessment of oxidative stress and genotoxicity in workers exposed to paints during a working week. Int. J. Occup. Med. Environ. Health 2011, 24, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Tranfo, G.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Paci, E.; Pigini, D.; Gherardi, M.; Gatto, M.P.; et al. Biomarkers of early genotoxicity and oxidative stress for occupational risk assessment of exposure to styrene in the fibreglass reinforced plastic industry. Toxicol. Lett. 2018, 298, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Buresti, G.; Paci, E.; Pigini, D.; Gherardi, M.; Carbonari, D.; et al. Occupational Exposure in Industrial Painters: Sensitive and Noninvasive Biomarkers to Evaluate Early Cytotoxicity, Genotoxicity and Oxidative Stress. Int. J. Environ. Res. Public Health 2021, 18, 4645. [Google Scholar] [CrossRef] [PubMed]
- Cok, I.; Sardas, S.; Kadioglu, E.; Ozcagli, E. Assessment of DNA damage in glue sniffers by use of the alkaline comet assay. Mutat. Res. 2004, 557, 131–136. [Google Scholar] [CrossRef]
- Costa, C.; Costa, S.; Silva, S.; Coelho, P.; Botelho, M.; Gaspar, J.; Rueff, J.; Laffon, B.; Teixeira, J.P. DNA damage and susceptibility assessment in industrial workers exposed to styrene. J. Toxicol. Environ. Health Part A 2012, 75, 735–746. [Google Scholar] [CrossRef]
- Costa-Amaral, I.C.; Carvalho, L.V.B.; Santos, M.V.C.; Valente, D.; Pereira, A.C.; Figueiredo, V.O.; Souza, J.M.; Castro, V.S.; Trancoso, M.F.; Fonseca, A.S.A.; et al. Environmental Assessment and Evaluation of Oxidative Stress and Genotoxicity Biomarkers Related to Chronic Occupational Exposure to Benzene. Int. J. Environ. Res. Public Health 2019, 16, 2240. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, T.; Zenkner, F.F.; Ellwanger, J.H.; Pra, D.; Rieger, A. DNA damage and cytotoxicity in pathology laboratory technicians exposed to organic solvents. An. Da Acad. Bras. De Cienc. 2016, 88, 227–236. [Google Scholar] [CrossRef]
- Fracasso, M.E.; Doria, D.; Bartolucci, G.B.; Carrieri, M.; Lovreglio, P.; Ballini, A.; Soleo, L.; Tranfo, G.; Manno, M. Low air levels of benzene: Correlation between biomarkers of exposure and genotoxic effects. Toxicol. Lett. 2010, 192, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, M.E.; Doria, D.; Carrieri, M.; Bartolucci, G.B.; Quintavalle, S.; De Rosa, E. DNA single- and double-strand breaks by alkaline- and immuno-comet assay in lymphocytes of workers exposed to styrene. Toxicol. Lett. 2009, 185, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Godderis, L.; De Boeck, M.; Haufroid, V.; Emmery, M.; Mateuca, R.; Gardinal, S.; Kirsch-Volders, M.; Veulemans, H.; Lison, D. Influence of genetic polymorphisms on biomarkers of exposure and genotoxic effects in styrene-exposed workers. Environ. Mol. Mutagen. 2004, 44, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Hanova, M.; Stetina, R.; Vodickova, L.; Vaclavikova, R.; Hlavac, P.; Smerhovsky, Z.; Naccarati, A.; Polakova, V.; Soucek, P.; Kuricova, M.; et al. Modulation of DNA repair capacity and mRNA expression levels of XRCC1, hOGG1 and XPC genes in styrene-exposed workers. Toxicol. Appl. Pharmacol. 2010, 248, 194–200. [Google Scholar] [CrossRef]
- Heuser, V.D.; de Andrade, V.M.; da Silva, J.; Erdtmann, B. Comparison of genetic damage in Brazilian footwear-workers exposed to solvent-based or water-based adhesive. Mutat. Res. 2005, 583, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Heuser, V.D.; Erdtmann, B.; Kvitko, K.; Rohr, P.; da Silva, J. Evaluation of genetic damage in Brazilian footwear-workers: Biomarkers of exposure, effect, and susceptibility. Toxicology 2007, 232, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Keretetse, G.S.; Laubscher, P.J.; Du Plessis, J.L.; Pretorius, P.J.; Van Der Westhuizen, F.H.; Van Deventer, E.; Van Dyk, E.; Eloff, F.C.; Van Aarde, M.N.; Du Plessis, L.H. DNA damage and repair detected by the comet assay in lymphocytes of african petrol attendants: A pilot study. Ann. Occup. Hyg. 2008, 52, 653–662. [Google Scholar] [CrossRef]
- Ladeira, C.; Gajski, G.; Meneses, M.; Geric, M.; Viegas, S. The genotoxicity of an organic solvent mixture: A human biomonitoring study and translation of a real-scenario exposure to in vitro. Regul. Toxicol. Pharmacol. RTP 2020, 116, 104726. [Google Scholar] [CrossRef] [PubMed]
- Laffon, B.; Pasaro, E.; Mendez, J. Evaluation of genotoxic effects in a group of workers exposed to low levels of styrene. Toxicology 2002, 171, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.H.; Zhu, C.Q.; Jiang, C.Q. Lymphocyte DNA damage in elevator manufacturing workers in Guangzhou, China. Mutat. Res. 2002, 515, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; He, Z.; Sun, Q.; Qin, F.; Huang, Z.; Zhang, X.; Sun, X.; Liu, L.; Chen, L.; et al. MGMT hypomethylation is associated with DNA damage in workers exposed to low-dose benzene. Biomarkers 2017, 22, 470–475. [Google Scholar] [CrossRef]
- Londono-Velasco, E.; Martinez-Perafan, F.; Carvajal-Varona, S.; Garcia-Vallejo, F.; Hoyos-Giraldo, L.S. Assessment of DNA damage in car spray painters exposed to organic solvents by the high-throughput comet assay. Toxicol. Mech. Methods 2016, 26, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Martino-Roth, M.G.; Viegas, J.; Roth, D.M. Occupational genotoxicity risk evaluation through the comet assay and the micronucleus test. Genet. Mol. Res. GMR 2003, 2, 410–417. [Google Scholar] [PubMed]
- Migliore, L.; Colognato, R.; Naccarati, A.; Bergamaschi, E. Relationship between genotoxicity biomarkers in somatic and germ cells: Findings from a biomonitoring study. Mutagenesis 2006, 21, 149–152. [Google Scholar] [CrossRef]
- Migliore, L.; Naccarati, A.; Zanello, A.; Scarpato, R.; Bramanti, L.; Mariani, M. Assessment of sperm DNA integrity in workers exposed to styrene. Hum. Reprod. 2002, 17, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.M.; Brucker, N.; Charao, M.; Bulcao, R.; Freitas, F.; Baierle, M.; Nascimento, S.; Valentini, J.; Cassini, C.; Salvador, M.; et al. Evaluation of genotoxicity and oxidative damage in painters exposed to low levels of toluene. Mutat. Res. 2012, 746, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Navasumrit, P.; Chanvaivit, S.; Intarasunanont, P.; Arayasiri, M.; Lauhareungpanya, N.; Parnlob, V.; Settachan, D.; Ruchirawat, M. Environmental and occupational exposure to benzene in Thailand. Chemico-Biol. Interact. 2005, 153–154, 75–83. [Google Scholar] [CrossRef]
- Pandey, A.K.; Bajpayee, M.; Parmar, D.; Kumar, R.; Rastogi, S.K.; Mathur, N.; Thorning, P.; de Matas, M.; Shao, Q.; Anderson, D.; et al. Multipronged evaluation of genotoxicity in Indian petrol-pump workers. Environ. Mol. Mutagen. 2008, 49, 695–707. [Google Scholar] [CrossRef]
- Poca, K.S.D.; Giardini, I.; Silva, P.V.B.; Geraldino, B.R.; Bellomo, A.; Alves, J.A.; Conde, T.R.; Zamith, H.; Otero, U.B.; Ferraris, F.K.; et al. Gasoline-station workers in Brazil: Benzene exposure; Genotoxic and immunotoxic effects. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 865, 503322. [Google Scholar] [CrossRef] [PubMed]
- Rekhadevi, P.V.; Rahman, M.F.; Mahboob, M.; Grover, P. Genotoxicity in filling station attendants exposed to petroleum hydrocarbons. Ann. Occup. Hyg. 2010, 54, 944–954. [Google Scholar] [CrossRef]
- Roma-Torres, J.; Teixeira, J.P.; Silva, S.; Laffon, B.; Cunha, L.M.; Mendez, J.; Mayan, O. Evaluation of genotoxicity in a group of workers from a petroleum refinery aromatics plant. Mutat. Res. 2006, 604, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Sakhvidi, M.J.Z.; Zarei, A.; Hachesu, V.R.; Zolfaghari, A. Evaluating the relationship between the respiratory exposure to the benzene with the primary damages of deoxyribonucleic acid and total antioxidant capacity in one of the oil companies in Iran. Environ. Sci. Pollut. Res. Int. 2022, 29, 48340–48346. [Google Scholar] [CrossRef] [PubMed]
- Sul, D.; Lee, D.; Im, H.; Oh, E.; Kim, J.; Lee, E. Single strand DNA breaks in T- and B-lymphocytes and granulocytes in workers exposed to benzene. Toxicol. Lett. 2002, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sul, D.; Lee, E.; Lee, M.Y.; Oh, E.; Im, H.; Lee, J.; Jung, W.W.; Won, N.; Kang, H.S.; Kim, E.M.; et al. DNA damage in lymphocytes of benzene exposed workers correlates with trans,trans-muconic acids and breath benzene levels. Mutat. Res. 2005, 582, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.; Gaspar, J.; Coelho, P.; Costa, C.; Pinho-Silva, S.; Costa, S.; Da Silva, S.; Laffon, B.; Pasaro, E.; Rueff, J.; et al. Cytogenetic and DNA damage on workers exposed to styrene. Mutagenesis 2010, 25, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Li, Q.; Zhou, B.; Huang, J.; Liang, G.; Zhang, L.; Ma, S.; Qing, L.; Liang, L.; Su, J.; et al. Oxidative Stress and Genotoxicity of Long-Term Occupational Exposure to Low Levels of BTEX in Gas Station Workers. Int. J. Environ. Res. Public Health 2016, 13, 1212. [Google Scholar] [CrossRef]
- Zhao, Z.; Xing, X.; Ou, X.; Liu, X.; Zhou, R.; Zhang, H.; Yang, L.; Zhuang, Z.; Su, X.; Lu, Y.; et al. DNA damage levels in electronics workers in Southern China: A micro-whole blood comet assay. Mutat. Res. 2017, 803–805, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, M.; Skov, H.; Autrup, H.; Hertel, O.; Loft, S. Urban benzene exposure and oxidative DNA damage: Influence of genetic polymorphisms in metabolism genes. Sci. Total Environ. 2003, 309, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Serrana, M.; Au, W.W. Biomonitoring using accessible human cells for exposure and health risk assessment. Mutat. Res. 1999, 436, 99–112. [Google Scholar] [CrossRef]
- Collins, A.; Dusinska, M.; Franklin, M.; Somorovska, M.; Petrovska, H.; Duthie, S.; Fillion, L.; Panayiotidis, M.; Raslova, K.; Vaughan, N. Comet assay in human biomonitoring studies: Reliability, validation, and applications. Environ. Mol. Mutagen. 1997, 30, 139–146. [Google Scholar] [CrossRef]
- Villarini, M.; Dominici, L.; Fatigoni, C.; Muzi, G.; Monarca, S.; Moretti, M. Biological effect monitoring in peripheral blood lymphocytes from subjects occupationally exposed to antineoplastic drugs: Assessment of micronuclei frequency. J. Occup. Health 2012, 54, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Viegas, S.; Padua, M.; Gomes, M.; Carolino, E.; Gomes, M.C.; Brito, M. Assessment of genotoxic effects in nurses handling cytostatic drugs. J. Toxicol. Environ. Health Part A 2014, 77, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Zare Jeddi, M.B.; Hopf, N.; Bessems, J.; Palmen, N.S.; Galea, K.; Jones, K.; Kujath, P.; Duca, R.C.; Verhagen, H.; et al. Biomonitoring as an Underused Exposure Assessment Tool in Occupational Safety and Health Context-Challenges and Way Forward. Int. J. Environ. Res. Public Health 2020, 17, 5884. [Google Scholar] [CrossRef] [PubMed]
- Manno, M.; Viau, C.; in collaboration, w.; Cocker, J.; Colosio, C.; Lowry, L.; Mutti, A.; Nordberg, M.; Wang, S. Biomonitoring for occupational health risk assessment (BOHRA). Toxicol. Lett. 2010, 192, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Viegas, S. Human biomonitoring: An overview on biomarkers and their application in occupational and environmental health. Biomonitoring 2016, 3, 15–24. [Google Scholar] [CrossRef]
- Decker, J.A.; DeBord, D.G.; Bernard, B.; Dotson, G.S.; Halpin, J.; Hines, C.J.; Kiefer, M.; Myers, K.; Page, E.; Schulte, P.; et al. Recommendations for biomonitoring of emergency responders: Focus on occupational health investigations and occupational health research. Mil. Med. 2013, 178, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Au, W.W. Biomarkers in molecular epidemiology studies for health risk prediction. Mutat. Res. 2002, 511, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.J.; Gargon, E.; Clarke, M.; Williamson, P.R. Can a core outcome set improve the quality of systematic reviews?—A survey of the Co-ordinating Editors of Cochrane Review Groups. Trials 2013, 14, 21. [Google Scholar] [CrossRef]
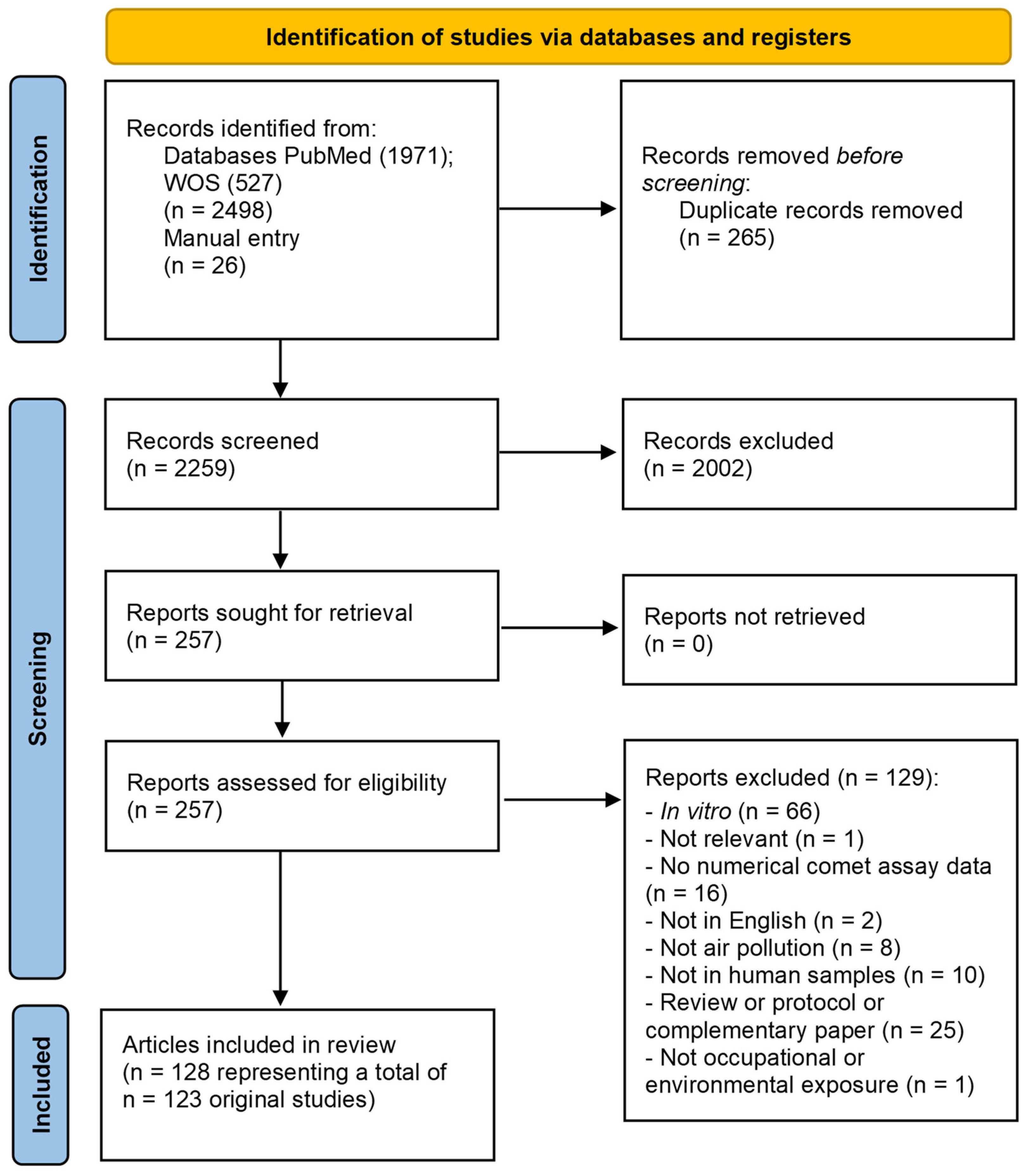
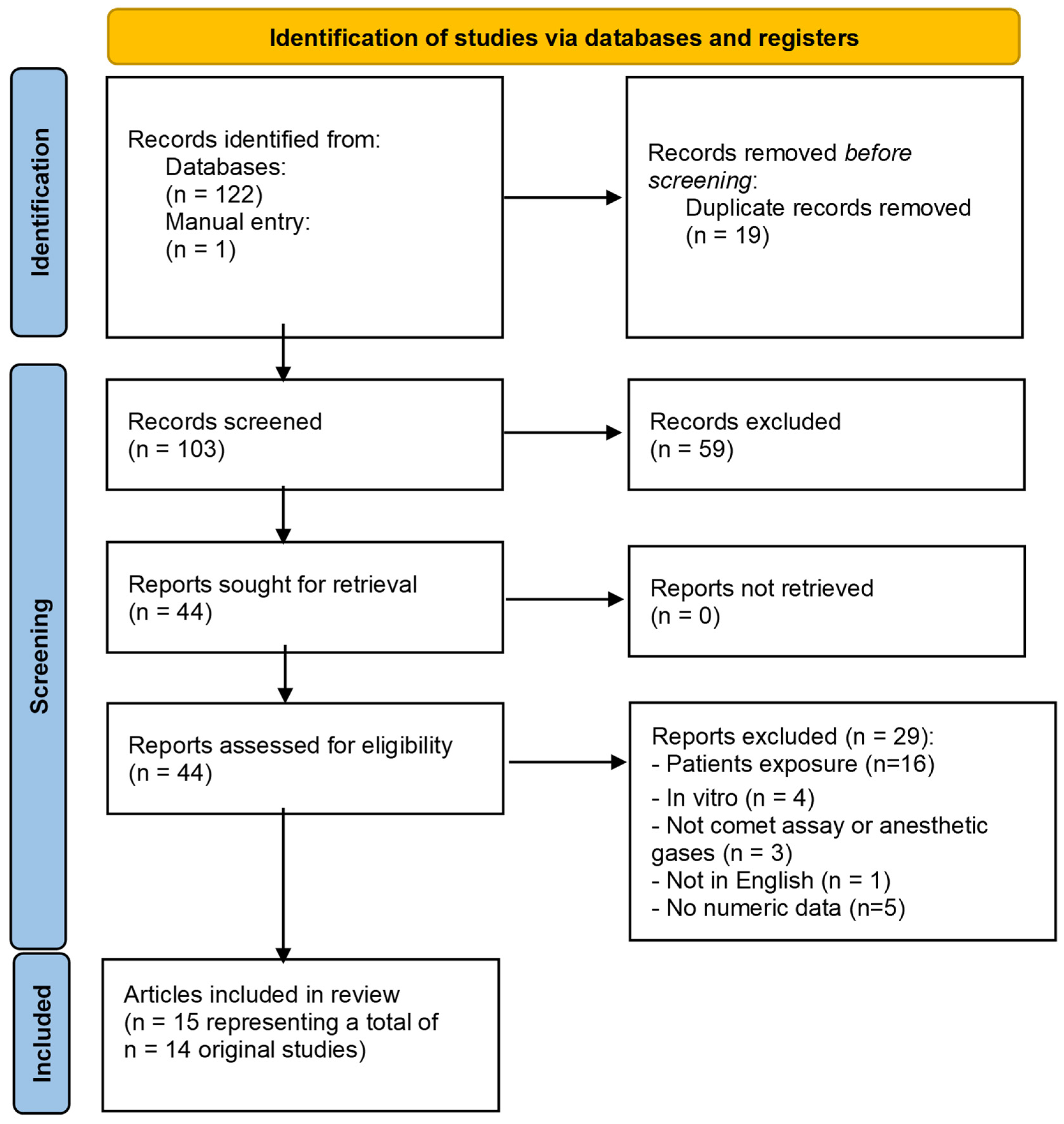
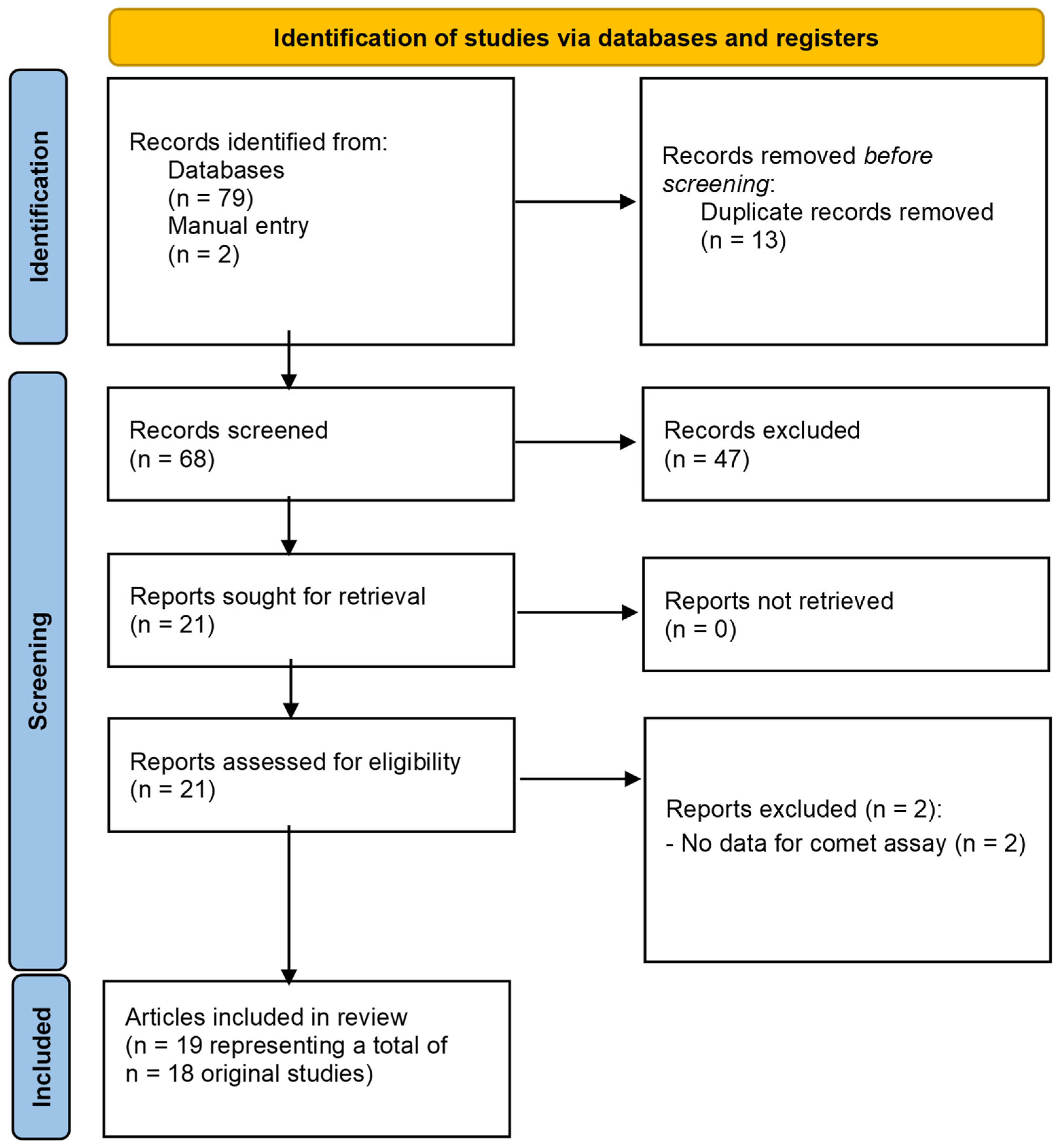

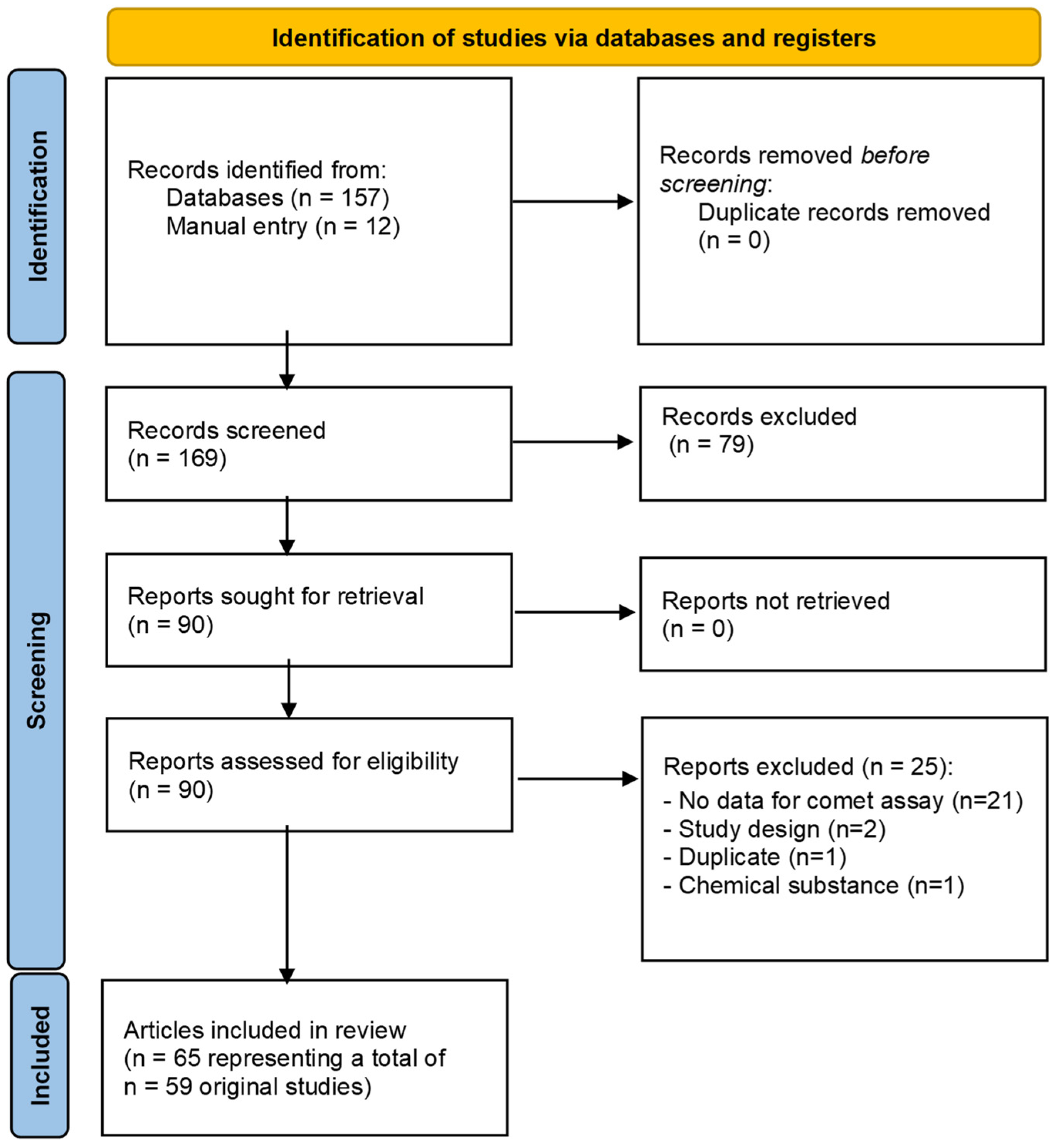

| Author | Year | Main Chemical Exposure | Country | Exposure Assessment or Biomarkers of Exposure | Population Characteristics | DNA Damage | Reference/DOI |
|---|---|---|---|---|---|---|---|
| Occupational exposure | |||||||
| Andersen | 2018 | PAH | Denmark | Urinary 1-OHP | 22 professional firefighters |
| [39] 10.1002/em.22193 |
| Andersen | 2021 | PAH fluorene | Denmark | Exposure levels to PAH (silicone bands, skin wipes) Exposure levels to PAHs and organophosphate esters (OPEs) Urinary excretion of PAH metabolites (OH-PAHs). | 116 air force personnel (79 exposed, 37 controls) |
| [40] 10.1038/s41598-021-97382-5 |
| Al Zabadi ** | 2011 | PAH, VOC | France | Air concentration PAH and benzene | 64 sewage workers (34 exposed, 30 unexposed) |
| [41] 10.1186/1476-069X-10-23 |
| Aydin | 2013 | Formaldehyde | Turkey | Passive air samplers (TWA8h) | 92 medium-density fibreboard plants (46 exposed, 46 unexposed) |
| [42] 10.1007/s00204-012-0961-9 |
| Bacaksiz | 2013 | PAH and heterocyclic compounds | Turkey | -- | 60 (30 exposed asphalt workers, 30 controls) |
| [43] 10.1080/09603123.2013.773586 |
| Bagryants | 2010 | PAH, VOC | Czech Republic | Personal samplers, quantitative analysis of PAHs, radial diffusive samplers for VOC exposure, cotinine | 120 (50 bus drivers, 20 garagemen, 50 controls) |
| [44] 10.1016/j.toxlet.2010.08.007 |
| Becit | 2021 | Marble dust | Turkey | Air samples and particle analysis | 89 (48 exposed workers in marble processing plants, 41 controls) |
| [45] 10.1016/j.envres.2021.111209 |
| Barth | 2016 | Air pollution (outdoor) | Brazil | Urinary 1-hydroxy-pyrene (1-OHP) | 82 (45 taxi drivers, 37 controls) |
| [46] 10.1007/s11356-016-7772-0 |
| Balamur likrishnan | 2014 | Silica dust exposure | India | -- | 85 (50 exposed subjects: Group I ≤ 40 years and ≤13 years working duration (23 individuals) Group II above 40 years and above 13 years (27 individuals) working duration, 35 controls; Group I (17), Group II (18)) |
| [47] 10.1007/s00477-013-0843-6 |
| Bruschweiler | 2016 | Wood dust | Switzerland | Wood dust, PAH, and B(a)P exposure | nonsmoking wood workers (n = 31, furniture and construction workers, natural wood, 12; wooden board, 19) and controls (n = 19) |
| [48] 10.4137/EHI.S38344 |
| Carere ** | 2002 | Air pollution | Italy | Benzene exposure | 190 (133 traffic policemen, 57 office workers as controls) |
| [49] 10.1016/s1383-5718(02)00108-0 |
| Cavallo | 2005 | PAH | Italy | Personal air sampling, urinary OH-pyrene | 41 (19 paving workers, 22 controls) |
| [50] 10.1093/annhyg/mei072 |
| Cavallo | 2006 | PAH | Italy | Urinary 1-hydroxy-pyrene (1-OHP) | 71 (41 exposed airport personnel (group A, 24 persons, group B, 17 persons; 31 controls)) |
| [51] 10.1016/j.tox.2006.03.003 |
| Cavallo | 2009 | PAHs, antineoplastic drugs | Italy | Exposure assessment studies cited (reported in previous papers) | 163 (30 workers exposed to antineoplastic drugs, 57 workers exposed to PAHs, 76 controls) |
| [16] 10.1002/em.20501 |
| Cavallo | 2022 | Graphene | Italy | Particle number concentration (PNC, particles/ cm3) from 10 nm to 1000 nm; airborne particle matter from 250 nm to 10 mm | 6 graphene workers and 11 controls |
| [52] 10.1080/17435390.2022.2149359 |
| Cebulska-Wasilewska * | 2005 | PAH | Czech Republic | PM2.5 and PAH analyses | 78 (40 policemen, 38 controls) |
| [53] 10.1016/j.mrgentox.2005.08.013 |
| Cebulska-Wasilewska * | 2007 | PAH | Slovakia/Bulgaria | PM2.5 and PAH analyses | 174 policemen (99 exposed, 75 controls) |
| [54] 10.1016/j.mrfmmm.2007.03.004 |
| Cebulska-Wasilewska * | 2007* | PAH | Slovakia/Bulgaria | Environmental PAHs | 259 (144 exposed, who were municipal policemen or bus drivers; 115 controls) |
| [55] 10.1016/j.mrfmmm.2007.03.005 |
| Ceppi | 2023 | PAH and glass fibres | Slovakia | Air sampling for the PAH analysis, air fibre sampling, personal exposure monitoring for PAH, cotinine | 116 (76 exposed shop floor workers, 34 controls) |
| [56] 10.1016/j.mrgentox.2022.503572 |
| Chen | 2006 | PAH (coke-oven exposure) | China | PAH analysis | 363 (240 coke-oven workers and 123 controls, all males) |
| [57] 10.1158/1055-9965.EPI-06-0291 |
| Chen | 2010 | PCDD, metals, and silica particles, | Taiwan | Air samples analysis, metal analysis | 78 (37 workers were recruited from a bottom ash recovery plant and 41 workers from fly ash treatment plants) |
| [58] 10.1016/j.jhazmat.2009.09.010 |
| Cheng | 2009 | PAH (coke-oven exposure) | China | Urinary 1-hydroxypyrene (1-OHP) | 158 (94 coke-oven workers and 64 controls) |
| [59] 10.1158/1055-9965.EPI-08-0763 |
| Chia | 2008 | Zinc and copper smelting work | Taiwan | 8-hydroxydeoxyguanosine (8-OH-dG) in urine (ELISA), lipid peroxidation (MDA in plasma) | 67 (39 smelting workers, 28 non-exposed) |
| [60] 10.2486/indhealth.46.174 |
| Costa § | 2008 | Formaldehyde | Portugal | Air samplers (TWA8h): ranging from 1.50 and 4.43 ppm | 60 (30 pathology anatomy workers, 30 controls) |
| [61] 10.1016/j.tox.2008.07.056 |
| Costa § | 2011 | Formaldehyde | Portugal | Air sampling and FA analysis | 98 (48 pathology anatomy workers, 50 non-exposed) |
| [62] 10.1080/15287394.2011.582293 |
| Costa | 2015 | Formaldehyde | Portugal | Air sampling (TWA8h) level of exposure | 171 (84 pathology anatomy workers, 87 controls) |
| [63] 10.1093/mutage/gev002 |
| De Boeck | 2000 | Cobalt dust, hard metal dust | Belgium | Urinary 8-OH-dG | 99 (24 workers exposed to cobalt dust, 27 workers exposed to hard metal dust, and 27 controls) |
| [64] 10.1002/1098-2280(2000)36:2<151::aid-em10>3.3.co;2-m |
| Duan | 2016 | Diesel engine exhaust | China | Air sampling: PM2.5, elemental carbon, NO2, SO2, and airborne PAHs urinary 1-OHP | 207 (101 DEE-exposed workers and 106 controls) |
| [65] 10.1136/oemed-2015-102919 |
| Everatt ** | 2013 | Perchloroethylene | Lithuania | PCE concentration in air: 31.40 ± 23.51 | 59 (30 dry cleaner workers, 29 control) |
| [66] 10.1080/15459624.2013.818238 |
| Galiotte | 2008 | Hair dyes, waving, and straightening preparations | Brazil | -- | 124 hairdressers (69 exposed females, 55 unexposed) |
| [67] 10.1093/annhyg/men037 |
| Giri | 2011 | PAH | India | Air sampling, [B(a)P] analysis | 220 (115 coal-tar workers, 105 controls) |
| [68] 10.1016/j.scitotenv.2011.07.009 |
| Gomaa | 2012 | Formaldehyde | Egypt | -- | 45 (30 lab technicians, 15 unexposed) |
| [69] |
| Göethel ** | 2014 | Air pollution, benzene, and CO | Brazil | Urinary t,t-muconic acid (t,t-MA) and 8OHdG carboxyhaemoglobin (COHb) in whole blood | 99 (43 gas station staff, 34 drivers, 22 unexposed) |
| [70] 10.1016/j.mrgentox.2014.05.008 |
| Hachesu | 2019 | Air pollution (traffic) | Iran | -- | 104 taxi drivers (11 smokers, 93 non-smokers) |
| [71] 10.1007/s11356-019-04179-1 |
| Huang | 2012 | PAH (coke-oven exposure) | China | Airborne samples analysis | 298 (202 exposed coke-oven workers: bottom 67, side 57, top 78 of the coke-oven; 96 controls) |
| [72] 10.1016/j.toxlet.2012.04.004 |
| Jasso-Pineda **,ɣ | 2015 | Arsenic, lead, PAH, DDT/DDE | Mexico | As and 1-OHP in urine Lead and total DDT/DDE in blood | 276 children total; 191 for air pollution (65 low PAH exposure; 50 biomass combustion; 76 high PAH exposure) |
| [73] 10.1016/j.scitotenv.2015.02.073 |
| Jiang | 2010 | Formaldehyde | China | Air samplers (TWA8h): 0.83 ppm, ranging 0.08–6.30 ppm | 263 (151 plywood industry workers, 112 controls) |
| [74] 10.1016/j.mrgentox.2009.09.011 |
| Khanna | 2014 | Tobacco dust | India | -- | 61 (31 female bidi rollers, 30 controls) |
| [75] 10.4103/0971-6580.128785 |
| Khisroon | 2020 | Gold jewellery fumes | Pakistan | -- | 94 (54 gold jewellery workers, 40 controls) |
| [76] 10.1080/1354750X.2020.1791253 |
| Kianmehr | 2017 | Fuel smoke | Iran | -- | 55 (11 exposed to natural gas, 11 exposed to diesel, 11 exposed to kerosene, 11 exposed to firewood, 11 unexposed) |
| [77] 10.1177/0748233717712408 |
| Knudsen | 2005 | Diesel-powered truck exhausts | Estonia | Cited in a previous paper | 92 (50 underground mine workers, 42 surface workers) |
| [78] 10.1016/j.mrgentox.2005.03.004 |
| Krieg | 2012 | JP-8 jet fuel | USA | Urinary (2-methoxy ethoxy) acetic acid (MEAA) and creatinine, benzene, and naphthalene in exhaled breath | 310 (Before: low 152, moderate 42, and high exposure 116; After a 4 h work shift exposure: low 151, moderate 43, high 116) |
| [79] 10.1016/j.mrgentox.2012.05.005 |
| Kvitko | 2012 | PAH, PM, pesticides, solvents | Brazil | -- | For PAH and PM exposure 109 (44 coal miners, 65 controls) |
| [80] 10.1590/S1415-47572012000600022 |
| Leng | 2004 | PAH (coke-oven exposure) | China | Urinary 1-hydroxypyrene (1-OHP) | 193 (143 Coke-oven workers, 50 controls) |
| [81] 10.1080/13547500400015618 |
| León-Mejía | 2011 | Dust particles | Colombia | -- | 200 (100 exposed open-cast coal mine workers, 100 controls) |
| [82] 10.1016/j.scitotenv.2010.10.049 |
| León-Mejía | 2019 | Diesel exhaust (gases, PAH, PM) | Colombia | -- | 220 (120 exposed mechanics and 100 controls) |
| [83] 10.1016/j.ecoenv.2018.12.067 |
| Lin | 2013 | Formaldehyde | China | Air-monitoring badges | 178 (96 plywood industry, 82 controls) |
| [84] 10.1539/joh.12-0288-oa |
| Marczynski | 2002 | PAH (coke-oven exposure) | Germany | 1-Hydroxypyrene (1-OHP) and sum of five hydroxyphenanthrenes (OHPHs), creatinine, and cotinine | 95 19 coke-oven workers, 29 graphite-electrode-producing workers), 32 controls |
| [85] 10.1093/carcin/23.2.273 |
| Marczynski | 2010 | Bitumen | Germany | -- | 42 bitumen-exposed workers |
| [86] 10.1177/0960327109359635 |
| Marczynski | 2011 | Vapours and aerosols of bitumen | Germany | Urinary hydroxylated metabolites of naphthalene, phenanthrene, pyrene | 438 (320 exposed construction workers, 118 unexposed) |
| [87] 10.1007/s00204-011-0682-5 |
| Moretti | 2007 | PAH | Italy | Urinary 1-OHP | 191 (109 graphite-electrode-producing workers, 82 controls) |
| [88] 10.1186/1471-2458-7-270 |
| Novotna | 2007 | Air pollution | Czech Republic | Air samples analysis; personal air sampler. Quantitative analysis of cPAHs | 65 non-smoking city policemen (54 outdoor policemen, 11 indoor policemen) |
| [89] 10.1016/j.toxlet.2007.05.013 |
| Oh | 2006 | PAH | South Korea | Urinary 1-OHP,2-naphthol, and creatinine in urine | 138 (54 automobile emission inspectors, 84 controls) |
| [90] 10.1016/j.etap.2005.08.004 |
| Peteffi | 2016 | Formaldehyde | Brazil | Urinary formic acid concentrations | 91 (46 exposed furniture manufacturing workers, 45 controls) |
| [91] 10.1177/0748233715584250 |
| Peteffi | 2016 | Formaldehyde | Brazil | Environmental FA concentrations; urinary formic acid | 50 hairdresser workers |
| [92] 10.1007/s11356-015-5343-4 |
| Recio-Vega | 2018 | PAH | Mexico | Urinary 1-OHP | 70 brick factory workers (35 exposed; 35 controls) |
| [93] 10.1007/s00420-018-1320-9 |
| Rekhadevi | 2009 | wood dust | India | Wood dust levels | 120 (60 carpentry workers, 60 controls) |
| [94] 10.1093/mutage/gen053 |
| Rohr | 2013 | Coal dust | Brazil | -- | 128 (71 coal-exposed workers and 57 controls) |
| [95] 10.1016/j.mrgentox.2013.08.006 |
| Sardas | 2010 | Welding fumes and solvent-based paints | Turkey | -- | 78 (52 workers in construction, 26 controls) |
| [96] 10.1177/0748233710374463 |
| Scheepers ** | 2002 | Diesel exhaust (benzene, PAHs) | Estonia, Czech Republic | Analysis of air samples, urinary metabolites of PAH and benzene | 92 underground miners (drivers of diesel-powered excavators) (46 underground workers, 46 surface workers) |
| [97] 10.1016/s0378-4274(02)00195-9 |
| Sellappa | 2010 | Cement dust exposure | India | -- | 164 (96 building construction workers and 68 controls) |
Workers: Age ≤ 40 (16.85 ± 2.08); sig.; ≥41 (14.12 ± 2.33); sig.; Smoking Yes (15.97 ± 2.61); sig.; No (13.71 ± 2.89); sig.; Tobacco chewing Yes (15.71 ± 2.34); sig.; No (15.71 ± 2.34); sig.; Alcohol Consumption Yes (14.05 ± 2.59); sig.; No (12.90 ± 2.98); sig. | [98] |
| Sellappa | 2011 | PAH | India | Urinary 1-OHP | 73 (36 road pavers; 37 control) |
| [99] |
| Shen | 2016 | Diesel | China | Urinary OH-PAHs, urinary εdA levels | 185 (86 exposed diesel engine testing workers, 99 unexposed) |
| [100] 10.1016/j.scitotenv.2015.10.165 |
| Siwińska | 2004 | PAH | Poland | Urinary 1-hydroxypyrene (HpU) | 98 coke-oven workers (49 exposed; 49 controls) |
| [101] 10.1136/oem.2002.006643 |
| Sul | 2003 | PAH | South Korea | Urinary 1-OH-pyrene and creatinine, 2-naphthol | 95 (24 workers from automobile emission companies, 28 workers from waste incinerating company, 43 unexposed) |
| [102] 10.1016/s1383-5718(03)00095-0 |
| Toraason | 2006 | 1-Bromopropane | USA | Personal-breathing zone samples collected for 1–3 days up to 8 h per (TWA8h). Bromide (Br) in blood and urine. | 64 workers (42 facility A (non-sprayer—low exposure 29; sprayer—high exposure 13) and 22 workers facility B (non-sprayer—low exposure 16; sprayer—high exposure 6)) |
| [103] 10.1016/j.mrgentox.2005.08.015 |
| Tovalin ** | 2006 | Air pollution (traffic), VOCs, PM2.5, ozone | Mexico | Personal occupational and non-occupational monitoring for VOCs, PM2.5, O3 | 55 City traffic exposure (28 outdoor workers, 27 indoor workers) |
| [104] 10.1136/oem.2005.019802 |
| Ullah | 2021 | Air pollution (traffic), coal mining dust | Pakistan | -- | 240 (60 participants exposed to traffic pollution, 60 controls, 60 mine workers, 60 controls) |
| [105] 10.12669/pjms.37.2.2848 |
| van Delft | 2001 | PAH (coke-oven exposure) | Netherlands | Urinary 1-hydroxypyrene | 72 (28 coke-oven workers, 37 controls) |
| [106] 10.1016/S0003-4878(00)00065-X |
| Villarini | 2008 | Dust (a-quartz and other particles from blasting), gases (nitrogen dioxide, NO2), diesel exhausts, oil mist | Italy | -- | 73 (39 underground workers and 34 unexposed subjects) |
| [107] 10.1080/15287390802328580 |
| Vital | 2021 | Environmental tobacco smoke (occupational settings) | Portugal | Monitoring the level of indoor air contaminants, namely, particulate matter (PM2.5), CO, and CO2 | 76 (17 smoker workers (SW), 32 non-exposed non-smoker workers (NE NSW), 32 exposed non-smoker workers E NSW) |
| [108] 10.3389/fpubh.2021.674142 |
| Wang | 2007 | PAH (coke-oven exposure) | China | Benzo[a]pyrene-r-7, t-8, t-9, c-10-tetrahydotetrol-albumin (BPDE-Alb) adducts | 309 (207 coke-oven workers exposed, 102 controls) |
| [109] 10.1136/oem.2006.030445 |
| Wang | 2010 | PAH (coke-oven exposure) | China | Airborne PAH monitoring and urinary 1-Hydroxypyrene | 475 workers (157 low, 160 intermediates, 158 high exposure) |
| [110] 10.1158/1055-9965.EPI-09-0270 |
| Wang | 2011 | PAH (cooking oil fumes) | China | Urinary 1-OHP | 110 (67 kitchen workers, 43 controls) |
| [111] 10.1539/joh.11-0074-oa |
| Wultsch | 2011 | PAH | Austria | Cr, Mn, Ni, As, in urine, creatinine | 42 waste incinerator workers (23 exposed, 19 unexposed) |
| [112] 10.1016/j.mrgentox.2010.08.002 |
| Yang | 2007 | PAH (coke-oven exposure) | China | PAH and urinary 1-OHP monitoring | 101 coke-oven workers (Low (n = 33) Intermediate (n = 35) High (n = 33) exposure) |
| [113] 10.1289/ehp.10104 |
| Yu | 2022 | PAH (coke-oven exposure) | China | Urinary monohydroxy PAHs (OH-PAHs) | 332 coke-oven workers |
| [114] 10.1007/s11356-022-19828-1 |
| Zhang | 2021 | PAHs (coke-oven exposure) | China | Urinary 1-hydroxypyrene (1-OHP) analysis | 256 (173 male coke-oven workers, 83 male hot-rolling workers not exposed as a control group) |
| [115] 10.1016/j.envpol.2020.115956 |
| Zendehdel Ø | 2017 | Formaldehyde | Iran | Monitoring FA exposure | 83 (49 melamine tableware workshop workers, 34 controls) |
| [116] 10.1080/02772248.2017.1343335 |
| Zendehdel Ø | 2018 | Formaldehyde | Iran | Air sampling | 87 (53 melamine tableware workshop workers, 34 unexposed) |
| [117] 10.1007/s11356-018-3077-9 |
| Zendehdel Ø | 2018 | Formaldehyde | Iran | Air sampling | 88 (54 melamine tableware workshop workers, 34 controls) |
| [118] 10.1177/0960327117728385 |
| Environmental exposure | |||||||
| Alvarado-Cruz | 2017 | Air pollution | Mexico | PM10 characterization, urinary levels of 1-OHP (PAHs exposure) and t,t-MA (benzene exposure) | 141 children |
| [119] 10.1016/j.mrgentox.2016.11.007 |
| Andersen | 2019 | Diesel-powered trains particles | Denmark | Levels of 1-OHP, 2-OHF, 1-NAPH, and 2-NAPH in urine | 83 healthy volunteers 54 exposed to diesel, 29 exposed in electric train) |
| [120] 10.1186/s12989-019-0306-4 |
| Avogbe ** | 2005 | PM (UFPs), benzene | Benin | Ambient UFP, urinary excretion of S-PMA | 135 city traffic exposure (29 drivers, 37 roadside residents, 42 suburban, 27 rural) |
| [121] 10.1093/carcin/bgh353 |
| Beyoglu | 2010 | Indoor tobacco smoke | Turkey | -- | 60 children from paediatric unit (30 exposed, 30 controls) |
| [122] 10.1016/j.ijheh.2009.10.001 |
| Cetkovic | 2023 | Air pollution | Bosnia and Herzegov | -- | 33 volunteers (Summer and winter sampling) |
| [123] 10.1093/mutage/geac016 |
| Cho | 2003 | Hair dye fumes | Korea | -- | 20 volunteers (before and after hair-dyeing) |
| [124] 10.1539/joh.45.376 |
| Chu | 2015 | Air pollution | China | Personal 24 h PM2.5 exposure | 301 (108 from Zhuhai, 114 from Wuhan, 79 from Tianjin) |
| [125] 10.1016/j.toxlet.2015.04.007 |
| Coronas | 2009 | PM | Brazil | Weekly airborne particulate matter (PM10) samples | 74 healthy men recruits, 18–40 years old, living or working at the target site (37 exposed, 37 unexposed) |
| [126] 10.1016/j.envint.2009.05.001 |
| Coronas | 2016 | PAHs (in PM) | Brazil | Air sampling Quantification of 16 PAHs from organic extract of PM 2.5: Acenaphthene, Acenaphthlene, Anthracene, Benzo(a)anthracene, Benzo(a)pyrene, Benzo(a)fluoranthene, Benzo(g,h,i)perylene, Indeno(1,2,3-cd)pyrene, Benzo(k)fluoranthene, Chrysene, Dibenzo(a,h) Anthracene, Phenanthrene, Fluoranthene, Fluorene, Naphthalene, and Pyrene. | 62 children aged 5–12 years (42 exposed, 20 controls) |
| [127] 10.1016/j.chemosphere.2015.09.084 |
| Danielsen | 2008 | Wood smoke | Sweden | Urinary 8-oxoGua, 8-oxodG | 13 never-smoking subjects |
| [128] 10.1016/j.mrfmmm.2008.04.001 |
| da Silva | 2015 | PAH | Brazil | -- | 45 children of Santo Antônio da Patrulha, Rio Grande do Sul |
| [129] 10.1016/j.mrgentox.2014.11.006 |
| Forchhammer | 2012 | Wood smoke (controlled exposure) | Denmark | 14, 220, or 354 μg/m3 of particles from a well-burning modern wood stove for 3 h in a climate-controlled chamber with 2-week intervals | 20 healthy non-smoking subjects (controlled exposure) |
| [130] 10.1186/1743-8977-9-7 |
| Gamboa | 2008 | PAH | Mexico | Air sampling | 6–15 years old children (37) (12 from oil extraction activity; 10 from no extraction activity regions, 15 controls) |
| [131] 10.3390/ijerph5050349 |
| Gong | 2014 | Air pollution | China | PM2.5 (mg/m3): Zhuhai 68.35 (37.17–116.79); Wuhan 114.96 (86.55–153.20); Tianjin 146.60 (88.63–261.41) | 307 (110 from Zhuhai, 118 from Wuhan, 79 from Tianjin) |
| [132] 10.1016/j.toxlet.2014.06.034 |
| Han | 2010 | PAH | China | PAH metabolites (2-OHNa, 9-OHPh, 2-OHFlu, and 1-OHP) in urine | 232 men from Chongqing, China. |
| [133] 10.1289/ehp.1002340 |
| Hemmingsen | 2015 | Diesel exhaust | Sweden | 3 h to diesel exhaust (276 μg/m3) from a passenger car or filtered air, with co-exposure to traffic noise at 48 or 75 dB(A) | 18 individuals with controlled exposure (3 h) |
| [134] 10.1016/j.mrfmmm.2015.03.009 |
| Hisamuddin | 2022 | PAHs (in PM) | Malaysia | Gravimetric sampling of PM2.5 PAHs Extraction: Acenaphthene, Acenaphthlene, Anthracene, Benzo(a)anthracene, Benzo(a)pyrene, Benzo(a)fluoranthene, Benzo(g,h,i)perylene, Indeno(1,2,3-cd)pyrene, Benzo(k)fluoranthene, Chrysene, Dibenzo(a,h) Anthracene, Phenanthrene, Fluoranthene, Fluorene, Naphthalene, and Pyrene. | 228 school children |
| [135] 10.3390/ijerph19042193 |
| Ismail | 2019 | Traffic-related air pollution | Malaysia | Air samples analysis | 104 (52 exposed group, 52 controls) |
| [136] 10.5572/ajae.2019.13.2.106 |
| Jasso-Pineda ** | 2015 | Arsenic, lead, PAH, DDT/DDE | Mexico | Arsenic and 1-OHP in urine Lead and total DDT/DDE in blood | 276 children (40/25 with high/low arsenic, 55/10 with high/low lead) |
| [73] 10.1016/j.scitotenv.2015.02.073 |
| Jensen | 2014 | wood smoke exposure | Denmark | Exposure to high indoor concentrations of PM2.5 (700–3,600 μg/m3), CO (10.7–15.3 ppm), and NO2 (140–154 μg/m(3)) during 1 week. | 11 university students |
| [137] 10.1002/em.21877 |
| Koppen ** | 2007 | Air pollution, PAHs, VOCs (benzene and toluene) | Belgium | Outdoor ozone concentrations, urinary concentrations of PAH, t,t′-muconic acid, o-cresol, VOCs metabolites | 200 adolescents |
| [138] 10.1002/jat.1174 |
| Koppen **,§ | 2020 | PAH, metals, benzene, POPs, phthalates, PM | Belgium | Ar, Cd, Cu, Ni, Pb, Tl, Cr in blood, outdoor air analysis | 2283 adolescents (14–18 years old) |
| [139] 10.1016/j.envres.2020.110002 |
| Lemos | 2020 | PAHs (in PM) | Brazil | Air sampling Quantification of 16 PAHs from organic extract of PM 2.5: Acenaphthene, Acenaphthlene, Anthracene, Benzo(a)anthracene, Benzo(a)pyrene, Benzo(a)fluoranthene, Benzo(g,h,i)perylene, Indeno(1,2,3-cd)pyrene, Benzo(k)fluoranthene, Chrysene, Dibenzo(a,h) Anthracene, Phenanthrene, Fluoranthene, Fluorene, Naphthalene, and Pyrene. | 54 children living in industrial areas |
| [140] 10.1016/j.envres.2020.109443 |
| León-Mejía | 2023 | Coal mining | Colombia | -- | 270 150 individuals exposed to coal mining residues from the locality of Loma-Cesar, 120 nonexposed individuals from the City of Barranquilla |
| [141] 10.1016/j.envres.2023.115773 |
| Mondal | 2010 | Fuel smoke (biomass and liquefied petroleum) | India | PM2.5 and PM10 (stationary sampling) | 217 (132 biomass users, 85 liquefied petroleum gas users) |
| [142] 10.1016/j.mrgentox.2010.02.006 |
| Mondal | 2011 | Fuel smoke (biomass and liquefied petroleum) | India | PM2.5 and PM10 (stationary sampling) | 161 premenopausal women (85 cooking with biomass; 76 control women cooking with liquid petroleum gas) |
| [143] 10.1016/j.ijheh.2011.04.003 |
| Mukherjee ƍ | 2013 | Fuel smoke (biomass and liquefied petroleum) | India | Urinary trans, trans-muconic acid | 105 (56 biomass users, 49 cleaner liquefied petroleum gas users) |
| [144] 10.1002/jat.1748 |
| Mukherjee ƍ | 2014 | Fuel smoke (biomass and liquefied petroleum) | India | PM2.5 and PM10 (stationary sampling) | 150 (80 biomass users, 70 liquefied petroleum gas (LPG) users) |
| [145] 10.1016/j.etap.2014.06.010 |
| Nagiah | 2015 | Air pollution | South Africa | -- | 100 pregnant women (50 from a highly industrialised south Durban and 50 from the less industrialised north Durban) |
| [146] 10.1177/0960327114559992 |
| Pacini | 2003 | Ozone | Italy | Air quality monitoring | 119 (102 subjects from Florence, 17 controls from Sardinia) |
| [147] 10.1002/em.10188 |
| Pandey | 2005 | Fuel smoke (biomass fuel liquefied petroleum gas) | India | -- | 144 volunteers (70 biomass fuel users, 74 liquefied petroleum gas (LPG) users) |
| [148] 10.1002/em.20106 |
| Pelallo-Martínez **,ɣ | 2014 | PAH, lead, benzene, toluene | Mexico | Urinary and blood Pb, benzene, toluene, PAHs | 97 children, air pollution (44 Allende, 37 Nuevo Mundo, 16 Lopez Mateos) |
| [149] 10.1007/s00244-014-9999-4 |
| Pereira | 2013 | PAH | Brazil | PAH analysis | 59 subjects from two towns of Rio Grande do Sul State (24, site 1 (exposed)—high quantity of nitro and amino derivatives of PAHs; 35 from site 2 (controls)—lesser anthropogenic influence) |
| [150] 10.1016/j.ecoenv.2012.12.029 |
| Pérez-Cadahia | 2006 | Air pollution | Spain | VOCs determination by dosimeters | 110 (25 volunteers cleaning beaches, 20 manual workers beach, 23 high-pressure cleaners, 42 controls) |
| [151] 10.1100/tsw.2006.206 |
| Piperakis | 2000 | Air pollution | Greece | -- | 80 healthy individuals living in urban and rural areas with different smoking habits |
| [152] 10.1002/1098-2280(2000)36:3<243::aid-em8 > 3.0.co;2- |
| Rojas | 2000 | Ozone | Mexico | Ozone values | 38 (27 exposed to hydrocarbons northward and 11 southward, exposed to ozone) |
| [153] 10.1016/s1383-5718(00)00035-8 |
| Sánchez-Guerra | 2012 | PAH | Mexico | Urinary 1-OHP | 82 children |
| [154] 10.1016/j.mrgentox.2011.12.006 |
| Shermatov | 2012 | Second hand cigarette smoking | Turkey | Urinary cotinine and creatinine | 57 children (27 exposed, 27 controls) |
| [155] 10.1007/s13312-012-0250-y |
| Sopian | 2021 | PAHs (PM) | Malaysia | 60 indoor and outdoor PM2.5 samples PAHs analysis: naphthalene (NAP), acenaphthene (ACP), acenaphthylene (ACY), anthracene (ANT), fluorene (FLU), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLA), pyrene (PYR), benzo(a)anthracene (BaA), chrysene (CYR), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), indeno(1,2,3-cd)pyrene (IcP), dibenzo(a,h)anthracene (DbA), and benzo(ghi)perylene (BgP) | 234 children (near petrochemical industry) |
| [156] 10.3390/ijerph18052575 |
| Torres-Dosal | 2008 | Wood smoke | Mexico | Urinary 1-OHP Carboxyhemoglobin determination | 20 healthy volunteers (pre- and post-intervention) |
| [157] 10.1016/j.scitotenv.2007.10.039 |
| Verschaeve | 2007 | PAH | Belgium | 1-Hydroxypyrene | 45 healthy subjects in different seasons |
| [158] 10.1002/jat.1244 |
| Vinzents | 2005 | PM (UFPs) | Denmark | Personal exposure in terms of number of concentrations of UFPs in the breathing zone, using portable instruments in six 18 h periods | 15 subjects bicycling in traffic or indoors on six occasions (controlled exposure) |
| [159] 10.1289/ehp.7562 |
| Wilhelm **,ɣ | 2007 | PAH, benzene, heavy metals | Germany | Monitored ambient air quality data, urinary (PAH) metabolites, benzene metabolites | 935 air pollution close to industrial settings (620 exposed children, 315 unexposed) |
| [160] 10.1016/j.ijheh.2007.02.007 |
| Wu | 2007 | Environmental tobacco smoke | Taiwan | -- | 291 (18 smokers, 143 environmental tobacco exposure, 130 non-smokers) |
| [161] |
| Zani ** | 2020 | PM10, PM2.5, NO2, CO, SO2, benzene, and O3 | Italy | Air sampling | 152 pre-school children (3–6 years old) |
| [162] 10.3390/ijerph17093276 |
| Zani | 2021 | Air pollution | Italy | Air pollutant levels | 142 children 6–8 years old (71 first winter, 71 second winter) |
| [163] 10.3390/atmos12091191 |
| Zeller | 2011 | Controlled exposure to formaldehyde | Germany | FA vapours (0 to 0.8 ppm) for 4 h/day over a period of five working days under strictly controlled conditions and bicycling (∼80 W) four times for 15 min. | 37 volunteers |
| [164] 10.1093/mutage/ger016 |
| Author | Year | Main Chemical Exposure | Country | Exposure Assessment or Biomarkers of Exposure | Population Characteristics | DNA Damage | Reference/DOI |
|---|---|---|---|---|---|---|---|
| Occupational exposure | |||||||
| Aun | 2018 | Isoflurane, sevoflurane, desflurane, and N2O | Brazil | -- | 26 medical residents |
| [173] 10.1016/j.mrfmmm.2018.10.002 |
| Baysal | 2009 | Halothane, isoflurane, sevoflurane, N2O, and desflurane | Turkey | -- | 60 (30 anaesthesiologist, certified registered nurse anaesthetist, surgeons, 30 controls) |
| [174] 10.1016/j.clinbiochem.2008.09.103 |
| Chandrasekhar | 2006 | Halothane, isoflurane, sevoflurane, sodium pentothal, N2O, Desflurane, and enflurane | India | -- | 99 (45 exposed operating room staff, 54 controls) |
| [175] 10.1093/mutage/gel029 |
| El-Ebiary | 2013 | Halothane, Isoflurane, (sevoflurane), and N2O (as pure, liquefied compressed, medical grade nitrous oxide gas) | Egypt | -- | 60 [40 operating room staff (anaesthetists, nurses, technicians), 20 controls] |
| [176] 10.1177/0960327111426584 |
| Figueiredo | 2022 | Inhalational of aesthetic isoflurane | Brazil | Workplace exposure assessment: waste anaesthetic gases (WAG), isoflurane, monitoring | 76 (39 professionals working in a veterinary hospital, 37 matched controls) |
| [177] 10.1007/s11356-022-20444-2 |
| Izdes * | 2009 | N2O, isoflurane, sevoflurane, and desfluran | Turkey | -- | 74 [19 office workers, 17 anaesthesia nurses, 19 nurses—antineoplastic drugs; 19 controls (unexposed office workers)] |
| [178] 10.1539/joh.m8012 |
| Izdes | 2010 | Waste anaesthetic gases (N2O, isoflurane, sevoflurane, and desflurane) | Turkey | -- | 80 [40 nurses, 40 controls (unexposed health care workers)] |
| [179] 10.1080/19338244.2010.486421 |
| Khisroon | 2020 | Mixture not specified | Pakistan | -- | 99 (50 exposed, 49 unexposed) |
| [180] 10.1136/oemed-2020-106561 |
| Rozgaj | 2009 | Sevoflurane, isoflurane, and N2O | Croatia | -- | 100 (50 room staff [anaesthetists, nurses, technicians], 50 controls) |
| [181] 10.1016/j.ijheh.2007.09.001 |
| Sardas * | 2006 | N2O, isoflurane, sevoflurane, and desflurane | Turkey | -- | 34 [17 exposed anaesthesiology staff, 17 controls (unexposed office workers)] |
| [182] 10.1007/s00420-006-0115-6 |
| Souza | 2016 | Waste anaesthetic gases (isoflurane, sevoflurane, desflurane, and N2O) | Brazil | Concentrations of halogenated anaesthetics (isoflurane, sevoflurane, and desflurane) and N2O using a sample flow rate of 10 L/min | 60 (30 anaesthesiologists, 27 internal medicine physicians) |
| [183] 10.1016/j.mrfmmm.2016.09.002 |
| Szyfter § | 2004 | Sevoflurane, halothane, and isoflurane | Poland | Analysis of N2O, volatile anaesthetics and organic solvents in the ambient air of operating rooms | 49 [29 operating room staff (anaesthetists, nurses, technicians), 20 controls] |
| [184] |
| Szyfter § | 2016 | N2O, halothane, isoflurane, and sevoflurane | Poland | Concentration of waste anaesthetic gases (N2O, halothane, isoflurane, and sevoflurane) | 200 (100 anaesthetists, 100 controls) |
| [185] 10.1007/s13353-015-0329-y |
| Wrońska-Nofer | 2009 | N2O, sevoflurane or isoflurane and halogenated hydrocarbons | Poland | Air N2O (breathing zone sampling) and volatile anaesthetics (individual dosimeters) | 167 medical staff members (84 exposed male anaesthetists and 55 nurses, and 83 unexposed controls without a history of working in operating rooms) |
| [186] 10.1016/j.mrfmmm.2009.03.012 |
| Wrońska-Nofer | 2012 | N2O | Poland | Air N2O (stationary monitoring sampling) halogenated anaesthetics and toxic solvents, 8 individual dosimeters) | 72 (36 exposed nurses in operating rooms, 36 unexposed nurses) |
| [187] 10.1016/j.mrfmmm.2011.10.010 |
| Author | Year | Main Chemical Exposure | Country | Exposure Assessment or Biomarkers of Exposure | Population Characteristics | DNA Damage | Reference/DOI |
|---|---|---|---|---|---|---|---|
| Aristizabal-Pachon | 2002 | Antineoplastic drugs | Colombia | -- | 80 (40 exposed, 40 unexposed) hospital workers |
| [212] 10.1007/s43188-019-00003-7 |
| Buschini | 2013 | Antineoplastic drugs | Italy | -- | 137 (63 exposed, 74 unexposed) nurses |
| [209] 10.1136/oemed-2013-101475 |
| Cavallo | 2009 | Antineoplastic drugs | Italy | -- | 106 (30 exposed, 76 unexposed) hospital workers |
| [16] 10.1002/em.20501 |
| Connor | 2010 | Antineoplastic drugs | USA | Fixed-location and personal breathing zone air samples Cyclophosphamide, ifosfamide, paclitaxel, 5-fluorouracil, and cytarabine surface contamination Urinary cyclophosphamide and paclitaxel. | 121 (68 exposed, 53 unexposed) hospital workers |
| [207] 10.1097/JOM.0b013e3181f72b63 |
| Cornetta | 2008 | Antineoplastic drugs | Italy | - | 90 (83 exposed and 73 unexposed) hospital workers |
| [204] 10.1016/j.mrfmmm.2007.08.017 |
| Hongping | 2006 | Vincristine | China | -- | 30 (15 exposed, 15 unexposed) workers from a plant production |
| [214] 10.1016/j.mrfmmm.2006.02.003 |
| Huang | 2022 | Antineoplastic drugs | China | -- | 455 (305 exposed, 150 unexposed) nurses |
| [213] 10.1136/oemed-2021-107913 |
| Kopjar * | 2009 | Antineoplastic drugs | Croatia | -- | 100 (50 exposed, 50 unexposed) healthcare workers |
| [191] 10.1016/j.ijheh.2008.10.001 |
| Kopjar * | 2001 | Antineoplastic drugs | Croatia | -- | 70 (50 exposed, 20 unexposed) hospital workers |
| [196] 10.1093/mutage/16.1.71 |
| Ladeira | 2015 | Antineoplastic drugs | Portugal | Cyclophosphamide, 5-Fluorouracil, and Paclitaxel surface contamination | 92 (46 exposed, 46 unexposed) hospital workers |
| [210] 10.3934/genet.2015.3.204 |
| Laffon | 2005 | Antineoplastic drugs (cyclophosphamide, cisplatin, doxorubicin, mitomycin C, 5-fluorouracil, methotrexate) | Portugal | -- | 52 (30 exposed, 22 unexposed) nurses |
| [12] 10.1002/ajim.20189 |
| Maluf | 2000 | Antineoplastic drugs | Brazil | -- | 24 (12 exposed, 12 unexposed, plus a historic control of 34 non-exposed workers) hospital workers |
| [200] 10.1016/S1383-5718(00)00107-8 |
| Oltulu | 2019 | Antineoplastic drugs | Turkey | -- | 59 (29 exposed, 30 unexposed) hospital workers |
| [211] 10.33808/clinexphealthsci.563988 |
| Rekhadevi | 2007 | Antineoplastic drugs | India | Urinary cyclophosphamide | 120 (60 exposed nurses and 60 unexposed subjects) |
| [203] 10.1093/mutage/gem032 |
| Rombaldi | 2008 | Antineoplastic drugs | Brazil | - | 40 (20 exposed and 20 unexposed) hospital workers |
| [205] 10.1093/mutage/gen060 |
| Sasaki | 2008 | Antineoplastic drugs | Japan | -- | 224 (121 exposed, 57 highly exposed [antineoplastic preparation], 46 unexposed) female nurses |
| [206] 10.1539/joh.50.7 |
| Ursini | 2006 | Antineoplastic drugs | Italy | 5-Fluorouracil, cytarabine, gemcitabine, cyclophosphamide, and ifosfamide surface contamination Biological monitoring of α-Xuoro-β-alanine in urine (metabolite of 5-Xuorouracile) | 65 (30 exposed, 35 unexposed) hospital workers |
| [201] 10.1007/s00420-006-0111-x |
| Villarini | 2011 | Antineoplastic drugs | Italy | 5-Fluorouracil and cytarabine surface contamination Urinary cyclophosphamide | 104 (52 exposed, 52 unexposed) healthcare workers |
| [208] 10.1093/mutage/geq102 |
| Yoshida | 2006 | Antineoplastic drugs (cyclophosphamide, dacarbazine, isophosphamide, aclarubicin, amrubicin, bleomycin, daunorubicin, doxorubicin, pirarubicin, carboplatin, cisplatin, docetaxel, etoposide, irinotecan, paclitaxel, vinblastine, vincristine, vinorelbine, rituximab) | Japan | umu assay from surface contamination | 37 (19 exposed, 18 unexposed) female nurses |
| [202] 10.1539/joh.48.517 |
| Author | Year | Main Chemical Exposure | Country | Exposure Assessment or Biomarkers of Exposure | Population Characteristics | DNA Damage | Reference/DOI |
|---|---|---|---|---|---|---|---|
| Occupational exposure | |||||||
| Aksu | 2019 | Cr, Cu, Cd, Ni, Pb | Turkey | Cr, Mn, Ni, Cu, As, Cd, Pb in blood | 96 (48 welders, 48 controls) |
| [218] 10.1016/j.mrgentox.2018.11.006 |
| Balachandar | 2010 | Chromium | India | Cr in air and urine Cr in air | 108 (36 leather tanning industry workers, 36 environmental exposure subjects, 36 controls) |
| [219] 10.1007/s00420-010-0562-y |
| Batra | 2010 | Lead | India | Pb in blood | 220 (110 workers occupationally exposed to lead, 110 controls) |
| [220] 10.7860/JCDR/2020/43682.13572 |
| Cavallo | 2002 | Antimony | Italy | Airborne Sb2O2; personal air samplers | 46 (23 workers assigned to different fire-retardant treatment tasks in the car upholstery industry, 23 controls) |
| [221] 10.1002/em.10102 |
| Chinde | 2014 | Lead | India | Pb in blood | 400 (200 lead–acid storage battery recycling and manufacturing industry workers, 200 controls) |
| [222] 10.1007/s11356-014-3128-9 |
| Coelho | 2013 | Lead, Cd, As | Portugal | Metalloids levels in blood | 122 (41 miners, 41 subjects living near a mine, 40 controls) |
| [223] 10.1016/j.envint.2013.08.014 |
| Danadevi | 2003 | Lead | India | Pb, Cd in blood | 81 (45 workers employed in a secondary Pb recovery unit, 36 controls) |
| [224] 10.1016/s0300-483x(03)00054-4 |
| Danadevi | 2004 | Cr, Ni | India | Cr, Ni in blood | 204 (102 welders, 102 controls) |
| [225] 10.1093/mutage/geh001 |
| De Boeck | 2000 | Cobalt | Belgium, Norway, Finland, Sweden, England | Co in urine | 99 (35 cobalt dust, 29 carbide-cobalt, 35 unexposed) |
| [64] 10.1002/1098-2280(2000)36:2<151::aid-em10>3.3.co;2-m |
| De Olivera | 2012 | Copper (and other metals) | Brazil | Cu in blood | 22 (11 copper-smelter, 11 controls) |
| [226] 10.1177/0748233711422735 |
| De Restrepo | 2000 | Lead | Colombia | Lead in air Pb in blood | 56 (43 workers of electric battery factories exposed to lead compounds, 13 controls) |
| [227] 10.1002/1097-0274(200009)38:3<330::aid-ajim13>3.0.co;2-z |
| Fracasso | 2002 | Lead | Italy | Pb, Cd in blood | 66 (37 battery plant workers, 29 controls) |
| [228] 10.1016/s1383-5718(02)00012-8 |
| Gambelunghe | 2003 | Chromium | Italy | Cr urine | 39 (19 chrome-plating workers, 20 controls) |
| [229] 10.1016/s0300-483x(03)00088-x |
| García-Lestón | 2011 | Lead | Portugal | Lead in blood Zn protoporphyrin, δ-aminolaevulinic acid dehydratase activity | 108 (70 workers in plants using inorganic lead, 38 controls) |
| [230] 10.1016/j.mrgentox.2011.01.001 |
| Grover | 2010 | Lead | India | 4.5 μg/m3 Pb in air Pb in blood and urine | 180 (90 workers of secondary Pb recovery unit, 90 controls) |
| [231] 10.1016/j.ijheh.2010.01.005 |
| Hernandez-Franco | 2022 | Lead | Mexico | Pb in blood | 53 (37 battery recycling workers, 16 controls) |
| [232] 10.3390/ijerph19137961 |
| Iarmarcovai | 2005 | Lead, cadmium | France | Al, Cd, Cr, Co, Pb, Mn, Ni, Zn in blood and urine | 57 (27 welders, 30 controls) |
| [233] 10.1093/mutage/gei058 |
| Kašuba | 2012 | Lead, cadmium | Croatia | Pb, Cd in blood | 60 (30 pottery-glaze workers, 30 controls) |
| [234] 10.1007/s00420-011-0726-4 |
| Kašuba | 2020 | Lead | Croatia | Pb in blood ALAD activity and EP level | 98 (50 manufacture lead workers, 48 unexposed) |
| [235] 10.2478/aiht-2020-71-3427 |
| Kayaalti | 2015 | Lead | Turkey | Pb in blood | 61 occupationally exposed to lead workers (36 low exposure, 25 high exposure) |
| [236] 10.1080/19338244.2013.787964 |
| Khisroon | 2021 | Cd, Cr, Fe, Mn, Ni, Pb | Pakistan | Cd, Cr, Fe, Mn, Ni, Pb in scalp hair | 118 (59 welders, 59 controls) |
| [237] 10.1007/s12011-020-02281-x |
| Liu | 2017 | Indium | China | In in urine In in ambient | 120 (57 indium exposed workers, 63 controls) |
| [238] 10.1093/toxsci/kfx017 |
| Meibian-Zhang | 2008 | Chromium | China | Cr in air Cr in blood and urine | 90 Exposure group I: 30 tannery workers exposed to trivalent chromium from tanning department; exposure group II: 30 tannery workers from finishing department; 30 controls. |
| [239] 10.1016/j.mrgentox.2008.04.011 |
| Minozzo | 2010 | Lead | Brazil | Lead in blood | 106 (53 workers in recycling of automotive batteries, 53 controls) |
| [240] 10.1016/j.mrgentox.2010.01.009 |
| Muller | 2022 | Chromium | Brazil | Cr, Pb, As, Ni, V in blood | 100 (50 male chrome-plating workers, 50 unexposed) |
| [241] 10.1080/01480545.2020.1731527 |
| Olewińska | 2010 | Lead | Poland | Lead (PbB) and zinc protoporphyrin (ZPP) in blood | 88 (62 metalworkers exposed to lead, 26 controls) |
| [242] |
| Palus | 2003 | Lead, cadmium | Poland | Pb, Cd in blood | 106 (44 Pb exposed, 22 Cd exposed, 40 unexposed) |
| [243] 10.1016/s1383-5718(03)00167-0 |
| Palus | 2005 | Arsenic | Poland | As concentration in dust and fumes As in urine | 155 (71 copper-smelter workers, 80 controls) |
| [244] 10.1002/em.20132 |
| Pandeh | 2017 | Fe | Iran | Iron status (including serum iron) | 56 (30 steel company workers, 26 controls) |
| [245] 10.1007/s11356-017-8657-6 |
| Pawlas | 2017 | Lead | Poland | Cd, Zn in blood | 116 (78 lead and zinc-smelter and battery recycling plan workers, 38 controls) |
| [246] 10.17219/acem/64682 |
| Pérez-Cadahía | 2008 | Lead | Spain | Al, Ni, Cd, Pb, Zn in blood | 240 (61 oil collectors, 59 hired workers, 60 high-pressure machine workers, 60 unexposed) |
| [247] 10.4137/ehi.s954 |
| Rashid | 2018 | Cd, Zn | Pakistan | Cd, Zn in blood | 60 (35 traffic police wardens, 25 controls) |
| [248] 10.1016/j.scitotenv.2018.02.254 |
| Singh | 2016 | Lead | India | Pb in blood | 70 (35 welders, 35 unexposed) |
| [249] 10.1177/0748233715590518 |
| Wang | 2018 | Pb | China | Pb in blood | 267 146 electronic waste processing workers, 121 controls) |
| [250] 10.1016/j.envint.2018.04.027 |
| Wani | 2017 | Lead, Zn | India | Pb in blood Zn in blood | 130 (92 occupationally exposed to lead or lead and zinc, 38 unexposed controls were selected from neighbouring with similar age) |
| [251] 10.1007/s11356-017-8569-5 |
| Vuyyuri | 2006 | Arsenic | India | As in blood | 365 (200 glass workers, 165 controls) |
| [252] 10.1002/em.20229 |
| Wultsch | 2011 | As, Mn, Ni, Cr | Austria | Cr, Mn, Ni, As in urine | 42 (23 waste incinerator workers, 19 controls) |
| [112] 10.1016/j.mrgentox.2010.08.002 |
| Zhang | 2011 | Chromium | China | Cr in air Cr in blood | 250 (157 electroplating workers, 93 unexposed) |
| [253] 10.1186/1471-2458-11-224 |
| Zhijian Chen | 2006 | Lead | China | Pb in air Pb in blood | 50 storage battery workers (25 exposed, 25 unexposed) |
| [254] 10.1016/j.tox.2006.03.016 |
| Environmental exposure | |||||||
| Andrew | 2006 | Arsenic | USA, Mexico | As in drinking water | 24 subjects (12 low exposure, 12 high exposure) |
| [255] 10.1289/ehp.9008 |
| Banerjee | 2008 | Arsenic | India | As in water As in urine, nail, hair | 90 (30 exposed subjects with skin lesions, 30 without skin lesions, 30 controls) |
| [256] 10.1002/ijc.23478 |
| Basu | 2005 | Arsenic | India | As in water As in urine, nails, hair | 60 volunteers (30 high-level exposure, 30 controls) |
| [257] 10.1016/j.toxlet.2005.05.001 |
| Cruz-Esquivel | 2019 | As, Hg | Colombia | As, Hg in blood | 100 volunteers (50 exposed, 50 unexposed) |
| [258] 10.1007/s11356-019-04527-1 |
| David | 2021 | Cd, Cr, Zn | Pakistan | Ni, Cd, Zn, Cr in blood | 232 children (134 living at brick kiln industries, 98 controls) |
| [259] 10.1080/19338244.2020.1854645 |
| Franken | 2017 | PAHs, metals | Belgium | Cr, Cd, Ni in urine As in blood MeHg in hair | 598 adolescents (14–15 years old) |
| [260] 10.1016/j.envres.2016.10.012 |
| Jasso-Pineda | 2012 | Lead, arsenic | Mexico | Pb in blood As in urine | 85 exposed subjects (48 high area, 12 middle area, 25 low area) |
| [261] 10.1007/s12011-011-9237-0 |
| Jasso-Pineda * | 2015 | Arsenic, lead, PAH, DDT/DDE | Mexico | As and 1-OHP in urine Lead and total DDT/DDE in blood | 276 children (40/25 with high/low arsenic, 55/10 with high/low lead) |
| [73] 10.1016/j.scitotenv.2015.02.073 |
| Jasso-Pineda | 2007 | Lead, As | Mexico | As, Pb, Cd, Cu, and Zn in soil Pb in blood, As in urine | 60 children (12 low area, 28 medium area, 20 high area exposure) |
| [262] 10.1002/ieam.5630030305 |
| Khan | 2012 | Chromium | India | Cr in blood | 200 volunteers (100 exposed, 100 unexposed) |
| [263] 10.1016/j.scitotenv.2012.04.063 |
| Koppen *,§ | 2020 | PAHs, metals, benzene, POPs, phthalates | Belgium | Ar, Cd, Cu, Ni, Pb, Tl, Cr in blood Outdoor air | 2283 adolescents (14–18 years old) |
| [139] 10.1016/j.envres.2020.110002 |
| Lourenço | 2013 | Uranium | Portugal | U, Zn, Mn in blood | 84 volunteers (54 exposed, 30 unexposed) |
| [264] 10.1016/j.tox.2013.01.011 |
| Mendez-Gomez | 2008 | As, Pb | Mexico | As, Cd, Pb in air (playground) and drinking water, As in urine, Pb in blood | 65 subjects (living near a smelter facility, 22 near, 22 intermediate, 21 distant) |
| [265] 10.1196/annals.1454.027 |
| Pelallo-Martinez *,§ | 2014 | Lead | Mexico | Pb in blood | 97 volunteers 44 Allede, 37 Mundo Nuevo, 16 Lopez Mateo) |
| [149] 10.1007/s00244-014-9999-4 |
| Sampayo-Reyes | 2010 | Arsenic | Mexico | As in water As in urine | 286 subjects (five villages) |
| [266] 10.1093/toxsci/kfq173 |
| Staessen | 2001 | Lead, cadmium | Belgium | Pb, Hg in blood Hg in urine | 200 exposed volunteers (100 in Peer, 42 in Wilrijk, 58 in Hoboken) |
| [267] 10.1016/s0140-6736(00)04822-4 |
| Wu | 2009 | Lead | Taiwan | Lead in blood | 154 volunteers (71 immigrant women from China, 83 native women from Taiwan) |
| [268] 10.1016/j.scitotenv.2009.07.025 |
| Yanez | 2003 | Lead, arsenic | Mexico | As, Pb in soil and house dust Pb in blood, As in urine | 55 children (20 exposed, 35 unexposed) |
| [269] 10.1016/j.envres.2003.07.005 |
| Author | Year | Main Chemical Exposure | Country | Exposure Assessment [Mean Concentration Pesticides (ppm)] or Biomarkers of Exposure | Population Characteristics | DNA Damage | Reference |
|---|---|---|---|---|---|---|---|
| Occupational exposure | |||||||
| Abhishek | 2010 | -- | India | -- | 67 (40 exposed, 27 unexposed agricultural workers |
| [288] 10.1089/rej.2009.0931 |
| Aiassa | 2019 | Glyphosate, cypermethrin, chlorpyrifos | Argentina | -- | 52 (30 exposed, 22 unexposed) agricultural workers |
| [289] 10.1007/s11356-019-05344-2 |
| Ali | 2018 | Cyhalothrin, endosulfan, deltamethrin | Pakistan | Serum concentrations: Deltamethrin: exposed (0.54 ± 0.22) vs. unexposed (0.28 ± 0.13); p < 0.01 Endosalfan: exposed (1.07 ± 0.52) vs. unexposed (0.36 ± 0.12); p < 0.001 Cyhalothrin: exposed (1.04 ± 0.38) vs. unexposed (0.33 ± 0.15); p < 0.01 | 138 (69 exposed, 69 unexposed) cotton-picking workers |
| [290] 10.1080/01480545.2017.1343342 |
| Alves | 2016 | Dithiocarbamate, carbamate, dicarboximide, organophosphate, neonicotinoid, pyrethoid, isoxazolidinone, dinitroaniline | Brazil | List of compounds commonly used in the area | 137 (77 exposed, 60 unexposed) tobacco farmers |
| [291] 10.1590/0001-3765201520150181 |
| Arshad | 2016 | Carbamates, organophosphates, pyrethroids | Pakistan | Blood malathion levels: detected in 72% of the exposed blood samples with na average value of 0.14 mg/L (range 0.01–0.31 mg/L) | 58 (38 exposed, 20 unexposed) pesticide-manufacturing workers |
| [292] 10.1016/j.shaw.2015.11.001 |
| Benedetti | 2013 | Organophosphorouscarbamates, pyrethroids, organochlorines | Brazil | BuChE—U/L: exposed (8231 ± 1368) vs. unexposed (8068 ± 920); p > 0.05 List of compounds used by volunteers | 127 (81 exposed, 46 unexposed) agricultural workers |
| [293] 10.1016/j.mrgentox.2013.01.001 |
| Bhalli | 2006 | Organophosphates, carbamates, pyrethroids | Pakistan | -- | 64 (29 exposed, 35 unexposed) pesticide-manufacturing workers |
| [294] 10.1002/em.20232 |
| Bhalli | 2009 | Carbamate, organophosphate, organochlorine, pyrethroids | Pakistan | Cypermethrin, cyhalothrin, deltamethrin, and endosulfan serum levels before and after spraying | 97 (47 exposed, 50 unexposed) agricultural workers |
| [295] 10.1002/em.20435 |
| Bian | 2004 | Pyrethroids (fenvalerate), organophosphorus compounds (phoxim), carbamates (carbaryl) | China | Fenvalerate concentration 21.55 × 10−4 mg/m3 (operation site) vs. 1.19 × 10−4 mg/m3 (control site), and dermal contamination 1.59 mg/m2 higher than control | 63 (21 exposed, 23 internal controls, 19 external controls) pesticide-manufacturing workers |
| [296] 10.1136/oem.2004.014597 |
| Carbajal-López | 2016 | Organochlorines, organophosphorus, carbamates, pyrethroids | Mexico | List of compounds commonly used in the area | 171 (111 exposed, 60 unexposed) agricultural workers |
| [297] 10.1007/s11356-015-5474-7 |
| Cayir | 2019 | Propineb, captan, boscalid, pyraclostrobin, cycloxydim, cypermethrin, alphacypermethri, deltamethrin, chlorpyrifos, permethrin | Turkey | Pesticides exposure assessment List of compounds used by the volunteers | 86 (41 exposed, 45 unexposed) greenhouse workers |
| [298] 10.1080/1354750X.2019.1610498 |
| Chen | 2014 | Fungicides, herbicides, inseticides | China | Pesticides exposure assessment | 337 (83 low exposure, 113 high exposure, 141 unexposed) fruit growers |
| [299] 10.1155/2014/965729. |
| Costa | 2014 | Fungicides, herbicides, inseticides | Portugal | Urinary metabolites: organic farmers PYR 0.06 ± 0.05, OP/CRB 1.86 ± 0.30, THIO 62.56 ± 5.60; pesticide workers PYR 0.08 ± 0.03, OP/CRB 2.23 ± 0.19, THIO 54.33 ± 3.16, unexposed PYR 0.13 ± 0.04, OP/CRB 1.54 ± 0.23, THIO 51.83 ± 3.28 BuChE—U/L: exposed farmers (6245.62 ± 191.41) vs. exposed pesticide workers (7063.66 ± 202.31) vs. unexposed (6425.44 ± 224.15); p = 0.943 List of compounds used by volunteers | 182 (36 organic farmers, 85 pesticide workers, 61 unexposed) agricultural workers |
| [300] 10.1016/j.toxlet.2014.02.011 |
| da Silva | 2008 | Carbamates and organophosphates | Brazil | -- | 173 (108 exposed, 65 unexposed) agricultural workers |
| [301] 10.1093/mutage/gen031 |
| da Silva | 2012 | -- | Brazil | -- | 167 (111 exposed, 56 unexposed) tobacco farmers |
| [302] 10.1016/j.jhazmat.2012.04.074 |
| da Silva | 2014 | Organophosphorate, carbamate, dithiocarbamate, pyrethroid | Brazil | BuChE activity—did not differ between exposed and unexposed | 60 (30 exposed, 30 unexposed) tobacco farmers |
| [303] 10.1016/j.scitotenv.2014.05.018 |
| Dalberto | 2022 | Neonicotinoid, pyrethroid, carbamate, organophosphate | Brazil | List of compounds used by the volunteers | 241 (84 exposed harvest, 72 exposed grading, 85 unexposed) tobacco farmers |
| [304] 10.1016/j.mrgentox.2022.503485 |
| Dhananjayan | 2019 | Organophosphorus, organochlorine, synthetic pyrethroid, benzoylurea, limonoid, benzoylphenylurea, organosulfite, quinazoline, stereoisomers, triazole, copper compounds, diphenyl ether, phosphanoglycine, chlorophenoxyacetic, ammonium salt, bipyridilium | India | AchE activity—U/mL: exposed (2.86 ± 0.75) vs. unexposed (3.93 ± 0.87); p < 0.001 BuChE activity—U/mL: exposed (2.02 ± 0.74) vs. unexposed (2.60 ± 0.74); p < 0.001 | 143 (77 exposed, 66 unexposed) tea garden workers |
| [305] 10.1016/j.mrgentox.2019.03.002 |
| Dutta and Bahadur | 2019 | Organophosphates, carbamates, pyrethroids | India | AchE activity—μmol/min/mL: exposed (6.43 ± 1.85) vs. unexposed (11.81 ± 3.40); p ≤ 0.001 BuChE activity—μmol/min/mL: exposed (3.50 ± 1.89) vs. unexposed (4.73 ± 1.84); p ≤ 0.001 | 155 (95 exposed, 60 unexposed) tea garden workers |
| [306] 10.1016/j.mrgentox.2019.06.005 |
| Franco | 2016 | Pyrethroids, carbamates, organophosphates, organochlorines, benzoylureas | Brazil | -- | 249 (161 exposed, 88 unexposed) community health agents |
| [307] 10.1007/s11356-016-7179-y |
| Garaj-Vrhovac and Želježić * | 2000 | Atrazine, alachlor, cyanazine, dichlorophenoxyacetic acid, malathion | Croatia | -- | 20 (10 exposed, 10 unexposed) pesticide-manufacturing workers |
| [308] 10.1016/s1383-5718(00)00092-9 |
| Garaj-Vrhovac and Želježić * | 2001 | Atrazine, alachlor, cyanazine, 2,4-dichlorophenoxyacetic acid, malathion | Croatia | -- | 40 (20 exposed, 20 unexposed) pesticide-manufacturing workers |
| [309] 10.1016/s0300-483x(01)00419-x |
| Garaj-Vrhovac and Želježić * | 2002 | Atrazine, alachlor, cyanazine, 2,4-dichlorophenoxyacetic acid, malathion | Croatia | -- | 30 (10 exposed, 20 unexposed) pesticide-manufacturing workers |
| [310] 10.1002/jat.855 |
| Godoy et al. | 2019 | Organochlorines, carbamates, pyrethroids | Brazil | List of compounds used by the volunteers | 163 (74 exposed, 89 unexposed) agricultural workers |
| [311] 10.1007/s11356-019-05882-9 |
| Grover | 2003 | Organophosphates, carbamates, pyrethroids | India | -- | 108 (54 exposed, 54 unexposed) pesticide-manufacturing workers |
| [312] 10.1093/mutage/18.2.201 |
| Kahl | 2018 | Glyphosate, flumetralin, clomazone, imidacloprid, sulfentrazone, dithiocarbamate, magnesium aluminium phosphide, fertilizers | Brazil | -- | 242 (121 exposed, 121 unexposed) tobacco farmers |
| [313] 10.1016/j.ecoenv.2018.04.052 |
| Kasiotis | 2012 | Chlorpyrifos, captan, myclobutanil, propargite, acetamiprid, cypermethrin, deltamethrin | Greece | Serum levels: Myclobutanil: 1.12–5.54 ppb Cypermethrin: 22.92–30.32 ppb Deltamethrin: <LOD–30.96 ppb Propargite, chlorpyrifos, captan, acetamiprid <LOD | 19 (all exposed) fruit growers |
| [314] 10.1016/j.toxlet.2011.10.020 |
| Kaur | 2011 | Carbamates, organophosphates, pyrethroids | India | List and frequency of compounds used by the volunteers | 260 (210 exposed [60 of them selected for follow-up], 50 unexposed) agricultural workers |
| [315] 10.4103/0971-6866.92100 |
| Kaur and Kaur § | 2020 | Organophosphates, carbamates, pyrethroids | India | -- | 450 (225 exposed, 225 unexposed) agricultural workers |
| [316] 10.1007/s11033-020-05600-6 |
| Kaur and Kaur § | 2020 | Organophosphates, carbamates, pyrethroids | India | -- | 450 (225 exposed, 225 unexposed) agricultural workers |
| [317] 10.1080/1354750X.2020.1794040 |
| Kaur and Kaur § | 2021 | Organophosphates, carbamates, pyrethroids | India | List of compounds used by the volunteers | 450 (225 exposed, 225 unexposed) agricultural workers |
| [318] 10.1016/j.mrgentox.2020.503302 |
| Khayat | 2013 | Glyphosate, fenpropathrin, carbofuran | Brazil | List of pesticide mixtures | 73 (41 exposed, 32 unexposed) agricultural workers |
| [319] 10.1007/s11356-013-1747-1 |
| Lebailly | 2003 | Fungicide captan | France | UK Predictive Operator Exposure Model suggested 14.4 mg (0.9–66.1 mg) of captan absorbed. List of other compounds used a day before | 19 (all exposed) fruit growers |
| [320] 10.1136/oem.60.12.910 |
| Liu ɣ | 2006 | Organophosphates, carbamates, pyrethroid insecticides, fungicides, growth regulator | China (Taiwan) | List of pesticides used, area of use, and frequency of use | 197 (43 low exposure, 48 high exposure, 106 unexposed) agricultural workers |
| [321] 10.1158/1055-9965.EPI-05-0617 |
| Muniz | 2008 | Organophosphonate | USA | Adjusted urinary dialkylphosphate (DAP) metabolite levels: sum methyl DAP (μmol/L): Farmworker 1.03 ± 37%, Applicator 0.774 ± 36%, Control 0.126 ± 42% | 31 (10 farmworkers, 12 applicators, 9 unexposed) agricultural workers |
| [322] 10.1016/j.taap.2007.10.027 |
| Naravaneni, Jamil | 2007 | Carbamates, organophosphates, pyrethroids | India | AchE activity- U/mL: exposed (253.5 ± 21.7) vs. unexposed (311.1 ± 7.99); p < 0.001 | 370 (210 exposed, 160 unexposed) agricultural workers |
| [323] 10.1177/0960327107083450 |
| Paiva | 2011 | Organochlorates, organophosphates, pyrethroids, carbamates | Brazil | List of compounds used by the volunteers | 63 (16 exposed region A, 16 exposed region B, 31 unexposed) agricultural workers |
| [324] 10.1002/em.20647 |
| Paz-y-Miño | 2004 | Fungicides, herbicides, inseticides | Ecuador | List of compounds used by the volunteers | 66 (45 exposed, 21 unexposed) agricultural workers |
| [325] 10.1016/j.mrgentox.2004.05.005 |
| Prabha, Chadha | 2017 | -- | India | -- | 100 (50 exposed, 50 unexposed) pesticide-manufacturing workers |
| [326] 10.1080/09723757.2015.11886263 |
| Ramos | 2021 | Glyphosate, dichlorophenoxyacetic acid, atrazine, cypermethrin, deltamethrin, | Brazil | -- | 360 (180 exposed, 180 unexposed) agricultural workers |
| [327] 10.1016/j.scitotenv.2020.141893 |
| Remor | 2009 | Fungicides, herbicides, inseticides | Brazil | ALA-D and BuChE activity—lower in exposed group | 57 (37 exposed, 20 unexposed) agricultural workers |
| [328] 10.1016/j.envint.2008.06.011 |
| Rohr | 2011 | Bipyridyl, organophosphates, copper sulfate, carbamates | Brazil | Pesticide exposure assessment List of compounds used by the volunteers | 173 (108 exposed, 65 unexposed) agricultural workers |
| [329] 10.1002/em.20562 |
| Saad-Hussein | 2017 | Malathion, chloropyrifos, dimethoate, carbofuran | Egypt | List of compounds commonly used in the area | 101 (51 exposed, 50 unexposed) agricultural workers |
| [330] 10.1016/j.mrgentox.2017.05.005 |
| Saad-Hussein | 2019 | Malathionchloropyrifos, dimethoate, carbofuran | Egypt | BuChE activity—U/L: rural exposed (2836 ± 189) vs. rural unexposed (3444.9 ± 148.4) vs. urban exposed (2653.2 ± 112.6) vs. urban unexposed (3040.8 ± 83.4) | 200 (50 rural exposed, 50 urban exposed, 50 rural unexposed, 50 urban unexposed) agricultural workers |
| [331] 10.1016/j.mrgentox.2018.12.004 |
| Sapbamrer | 2019 | Organophosphates, glyphosate, paraquat | Thailand | -- | 56 (all exposed) agricultural workers |
| [332] 10.1007/s11356-019-04650-z |
| Simoniello | 2008 | Thiophthalimide, inorganic-copper, dithiocarbamate-inorganic zinc, organophosphorus, carbamate, pyrethroid, organophosphorus, organochlorine, chloronicotinyl, phosphonoglycine | Argentina | List of compounds used by volunteers | 84 (27 farmers, 27 pesticide workers, 30 unexposed) agricultural workers |
| [333] 10.1002/jat.1361 |
| Simoniello | 2010 | Thiophthalimide, inorganic-copper, dithiocarbamate-inorganic zinc, organophosphorus, carbamate, pyrethroid, organophosphorus, organochlorine, chloronicotinyl, phosphonoglycine | Argentina | AchE activity—U/L: exposed farmers (7651.52 ± 2062.07) vs. exposed pesticide workers (6740.33 ± 1454.48) vs. unexposed (9045.54 ± 2191.56); p < 0.05 BuChE activity—U/L: exposed farmers (6313.86 ± 1268.26) vs. exposed pesticide workers (6777.77 ± 1281.84) vs. unexposed (6993.31 ± 1131.92); p > 0.05 | 123 (23 farmers, 18 pesticide workers, 82 unexposed) agricultural workers |
| [334] 10.3109/13547500903276378 |
| Singh | 2011 | Pirimiphos methyl, chlorpyrifos, temephos, malathion | India | AchE activity—KAU/L: exposed (3.45 ± 0.95) vs. unexposed (9.55 ± 0.35); p < 0.001 Pesticides exposure index | 140 (70 exposed, 70 unexposed) pesticide-manufacturing workers |
| [335] 10.1016/j.etap.2010.11.005 |
| Singh | 2011 | Organophosphate | India | Pesticides exposure index | 230 (115 exposed, 115 unexposed) pesticide-manufacturing workers |
| [336] 10.1016/j.mrgentox.2011.06.006 |
| Singh | 2012 | Organophosphate | India | AchE activity—KAU/L: exposed (3.76 ± 1.06) vs. unexposed (9.33 ± 0.52); p < 0.001 PONase activity nmol/min/mL: exposed (180.97 ± 37.59) vs. unexposed (246.70 ± 43.23) Pesticides exposure index | 268 (134 exposed, 134 unexposed), Community health agents |
| [337] 10.1016/j.mrgentox.2011.11.001 |
| Singh | 2011 | Organophosphate | India | AchE activity—KAU/L: exposed (3.71 ± 1.04) vs. unexposed (9.33 ± 0.52); p < 0.001 PONase activity nmol/min/mL: exposed (181.76 ± 37.10) vs. unexposed (246.70 ± 43.24) Pesticides exposure index | 284 (150 exposed, 134 unexposed) community health agents |
| [338] 10.1016/j.taap.2011.08.021 |
| Valencia-Quintana | 2021 | Organophosphate, carbamate, organochlorine, piretroides | Mexico | AchE activity—U/L: exposed (52.35 ± 10.04) vs. unexposed (35.32 ± 11.07); p ≤ 0.006 BuChE activity—U/L: exposed (297.73 ± 60.78) vs. unexposed (231.76 ± 81.60); p ≤ 0.047 List of compounds used by the volunteers | 80 (54 exposed, 26 unexposed) agricultural workers |
| [339] 10.3390/ijerph18126269 |
| Varona-Uribe | 2016 | Organochlorines, organophosphorus, carbamates, ethylenethiourea | Colombia | Blood/serum/urine concentrations: Organophosphorus (8 substances) range 0.56–21.05; Carbamates (2 substances) range 0.03–0.04; Dithiocarbamates (1 substance) 0.90; Organochlorines (14 substances) range 0.42–46.36 | 223 (all exposed) agricultural workers |
| [340] 10.1080/19338244.2014.910489 |
| Venkata | 2017 | Carbamates, organochlorine, organophosphorus, pyrethroid | India | AchE activity—U/L: exposed (1090.76 ± 71.28) vs. unexposed (1290.80 ± 78.68); p = 0.02 List of compounds used by the volunteers | 212 (106 exposed, 106 unexposed) tea garden workers |
| [341] 10.1080/1354750X.2016.1252954 |
| Wilhelm | 2015 | Fungicides, herbicides, inseticides | Brazil | List of compounds commonly used in the area | 74 (37 exposed, 37 unexposed) floriculturists |
| [342] 10.1007/s11356-014-3959-4 |
| Wong ɣ | 2008 | Organophosphates, carbamates, pyrethroid insecticides, fungicides, growth regulator | China (Taiwan) | List of pesticides used, area of use, and frequency of use | 241 (62 low exposure, 73 high exposure, 106 unexposed) fruit growers |
| [343] 10.1016/j.mrgentox.2008.06.005 |
| Yadav | 2011 | Organophosphates | India | List of compounds used by the volunteers | 62 (33 exposed, 29 unexposed) agricultural workers |
| [344] 10.1080/09723757.2011.11886131 |
| Zepeda-Arce | 2017 | Organochlorines, carbamates, pyrethroids | Mexico | AchE—U/g Hb: moderate exposed (19.4) vs. high exposed (20.5) vs. unexposed (18.8); p > 0.05 BuChE—U/L: moderate exposed (5943.97) vs. high exposed (4333.2) vs. unexposed (6673.27); p > 0.05 MDA concentration (nmol/mL): moderate exposed (0.98) vs. high exposed (1.0) vs. unexposed (0.97); p = 0.79. Pesticides exposure assessment List of compounds used by the volunteers | 208 (186 moderate exposure, 60 high exposure, 22 unexposed) agricultural workers |
| [345] 10.1002/tox.22398 |
| Želježić, Garaj-Vrhovac * | 2001 | Atrazine, alachlor, cyanazine, 2,4-dichlorophenoxyacetic acid, malathion | Croatia | -- | 40 (20 exposed, 20 unexposed) pesticide-manufacturing workers |
| [346] 10.1093/mutage/16.4.359 |
| Environmental exposure | |||||||
| Alvarado-Hernandez | 2013 | Organochlorine | Mexico | 17 analysed pesticides (detection range 58–100% in maternal blood, and 66–100% in umbilical cord blood) Most abundant in maternal blood: Heptachlor epoxide: 3764 ng/g lipids; Oxychlordane: 1672 ng/g lipides; Beta-HCH: 1320 ng/g lipides. Most abundant in umbilical cord blood: Heptachlor epoxide: 8707 ng/g lipides; Oxychlordane: 1411 ng/g lipides; Beta-HCH: 2815 ng/g lipides. | 50 mother–infant pairs, pregnant women and their infants from rural areas |
| [347] 10.1002/em.21753 |
| Dwivedi | 2022 | Organochlorines | India | 10 analysed pesticides: maximum concentration found for aldrin (3.26 mg/L) in maternal blood and dieldrin (2.69 mg/L) in cord blood | 221 (104 preterm delivery, 117 full-term delivery) pregnant women and their infants from rural areas |
| [348] 10.1016/j.envres.2021.112010 |
| How | 2014 | Organophosphates | Malaysia | Blood cholinesterase levels—unexposed (79.55 ± 13.48) vs. exposed (56.32 ± 12.35) | 180 (95 exposed, 85 unexposed) children exposed lived < 2 km from paddy farmland |
| [349] 10.1080/1059924X.2013.866917 |
| Kapka-Skrzypczak | 2019 | Carbetamide, carbofuran, chloridazon, dodemorph, cyclopropanecarboxamide, permethrin | Poland | Sweat pesticides (19 positive samples) for carbetamide, carbofuran, chloridazon, dodemorph, cyclopropanecarboxamide, permethrin AchE activity and BuChE activity significantly lower in exposed group | 200 children (108 exposed, 92 unexposed), lived <1 km from the nearest orchards, cultivated fields, greenhouses |
| [350] 10.1016/j.mrgentox.2018.12.012 |
| Leite | 2019 | -- | Paraguay | Plasma cholinesterase activity did not differ among groups | 84 children (43 exposed, 41 unexposed). Children exposed were born < 1 km from fumigated fields and have been living in that location for >5 years |
| [351] 10.4103/ijmr.IJMR_1497_17 |
| Sutris | 2016 | Dimethyphosphate, diethylphosphate, dimethylthiophosp, diethylthiophosph, dimethylthiophosph diethyldithiphosph | Malaysia | Urine organophosphate metabolites: 46.7% positive results: dimethyphosphate (46.7%), diethylphosphate (16.7%), dimethylthiophosphate (3.3%) | 180 children (all exposed) living on agricultural island |
| [352] 10.15171/ijoem.2016.705 |
| Author | Year | Main Chemical Exposure | Country | Exposure Assessment or Biomarkers of Exposure | Population Characteristics | DNA Damage | Reference/DOI |
|---|---|---|---|---|---|---|---|
| Occupational exposure | |||||||
| Al Zabadi ** | 2011 | PAHs, VOCs | France | Air concentration PAH and benzene | 64 sewage workers (34 exposed, 30 unexposed) |
| [41] 10.1186/1476-069X-10-23 |
| Azimi | 2017 | Perchloroethylene | Iran | -- | 59 dry cleaners (33 exposed, 26 unexposed) |
| [362] 10.15171/ijoem.2017.1089 |
| Buschini | 2003 | Styrene | Italy | Passive air samplers (TWA8h) Urinary excretion of MA and PGA | 62 workers in polyester resins and fibreglass-reinforced plastics factories (48 exposed, 14 unexposed) |
| [363] 10.1002/em.10150 |
| Careree ** | 2002 | Benzene and other aromatic hydrocarbons | Italy | Passive air samplers (TWA7h) | 190 traffic policemen (133 exposed, 57 unexposed) |
| [49] 10.1016/s1383-5718(02)00108-0 |
| Cassini | 2011 | Paint complex mixtures | Brazil | -- | 62 painters (33 exposed, 29 unexposed) |
| [364] 10.2478/s13382-011-0030-2 |
| Cavallo | 2018 | Styrene | Italy | Passive air samplers (4–7 h) Urinary excretion of MA and PGA | 39 workers in fibreglass- reinforced plastics factories (11 workers on open moulding plastic process, 16 workers on closed moulding plastic process, 12 controls) |
| [365] 10.1016/j.toxlet.2018.06.006 |
| Cavallo | 2021 | VOC | Italy | Personal VOCs exposure Urinary VOCs metabolites | 35 (17 shipyard painters, 18 unexposed) |
| [366] 10.3390/ijerph18094645 |
| Cok | 2004 | Toluene, other VOCs | Turkey | Urinary hippuric acid and o-cresol | 40 (20 male glue sniffers, 20 smoking habit matched controls) |
| [367] 10.1016/j.mrgentox.2003.10.009 |
| Costa | 2012 | Styrene | Portugal | Styrene in workplace air Urinary mandelic and phenylglyoxylic acids | 152 (75 workers from a fibreglass factory, 77 unexposed) |
| [368] 10.1080/15287394.2012.688488 |
| Costa-Amaral | 2019 | Benzene | Brazil | Benzene and toluene in air Urinary excretion of MA and S-PMA | 86 (51 employees of filling stations, 35 controls) |
| [369] 10.3390/ijerph16122240 |
| de Aquino | 2016 | Xylene, other organic solvents | Brazil | -- | 29 technicians in pathology laboratory (18 exposed, 11 unexposed) |
| [370] 10.1590/0001-3765201620150194 |
| Everatt ** | 2013 | Perchloroethylene | Lithuania | PCE concentration in air: 31.40 ± 23.51 | 59 dry cleaning workers (30 exposed, 29 unexposed) |
| [66] 10.1080/15459624.2013.818238 |
| Fracasso | 2010 | Benzene | Italy | Personal passive air samplers Urinary excretion of MA and S-PMA | 133 (33 petrochemical industry operators, 28 service station staff, 21 gasoline pump staff, 51 unexposed) |
| [371] 10.1016/j.toxlet.2009.04.028 |
| Fracasso | 2009 | Styrene | Italy | Personal passive air samplers Urinary excretion of MA and S-PMA | 63 workers in fibreglass-reinforced plastics factories (34 exposed, 29 unexposed) |
| [372] 10.1016/j.toxlet.2008.11.010 |
| Godderis | 2004 | Styrene | Belgium | Urinary mandelic acid: 201.57 mg/g creatinine ± 148.32 in exposed workers | 88 workers in fibreglass-reinforced plastics factories (44 exposed, 44 unexposed) |
| [373] 10.1002/em.20069 |
| Göethel ** | 2014 | Benzene and CO | Brazil | Urinary t,t-muconic acid (t,t-MA) and 8OhdG Carboxyhaemoglobin (COHb) in whole blood | 99 (43 gas station staff, 34 drivers, 22 unexposed) |
| [70] 10.1016/j.mrgentox.2014.05.008 |
| Hanova | 2010 | Styrene | Czechia | Styrene concentration at workplace and in blood | 122 hand lamination workers in a plastics factory (71 exposed, 51 unexposed) |
| [374] 10.1016/j.taap.2010.07.027 |
| Heuser | 2005 | Toluene, n-hexane, acetone, MEK | Brazil | Urinary hippuric acid | 70 (29 solvent-based adhesive workers, 16 water-based adhesive workers, 25 controls) |
| [375] 10.1016/j.mrgentox.2005.03.002 |
| Heuser | 2007 | Toluene, n-hexane, acetone, MEK | Brazil | Urinary hippuric acid | 94 footwear workers (39 exposed, 55 unexposed) |
| [376] 10.1016/j.tox.2007.01.011 |
| Keretetse | 2008 | BTX | South Africa | Air samplers (TWA) | 40 (20 petrol station staff, 20 controls) |
| [377] 10.1093/annhyg/men047 |
| Ladeira | 2020 | Styrene, xylene | Portugal | Styrene and xylene air-monitoring campaigns (NIOSH 1501) | 34 workers in polymer producing factory (17 exposed, 17 unexposed) |
| [378] 10.1016/j.yrtph.2020.104726 |
| Laffon | 2002 | Styrene | Spain | Urinary mandelic acid: average exposures of 16.76 ± 5.9, 17.51 ± 4.64, 19.33 ± 9.95 ppm) | 44 workers in fiberglass-reinforced plastics factory (14 exposed, 30 unexposed) |
| [379] 10.1016/s0300-483x(01)00572-8 |
| Lam | 2002 | Benzene | China | -- | 718 workers in elevator manufacturing factory (359 workers manufacturing, 205 department staff, 154 controls) |
| [380] 10.1016/s1383-5718(02)00010-4 |
| Li | 2017 | Benzene, toluene | China | Air levels of benzene and toluene Urinary S-phenylmercapturic acid (SPMA) and S-benzylmercapturic acid (SBMA) | 196 (96 petrochemical staff, 100 controls) |
| [381] 10.1080/1354750X.2016.1274335 |
| Londoño-Velasco | 2016 | Organic solvents | Spain | -- | 104 (52 painters, 52 unexposed) |
| [382] 10.3109/15376516.2016.1158892 |
| Martino-Roth | 2003 | Organic solvents, lead | Brazil | -- | 40 (10 car painters, 10 storage staff, 20 controls) |
| [383] |
| Migliore ¥ | 2006 | Styrene | Italy | Urinary excretion styrene metabolites, mandelic, and phenylglyoxylic acids (MAPGA) | 67 workers in fibreglass-reinforced plastics factory (42 exposed, 25 unexposed) |
| [384] 10.1093/mutage/gel012 |
| Migliore ¥ | 2002 | Styrene | Italy | Urinary concentration of mandelic acid (MA) | 73 workers in fibreglass-reinforced plastics factory (46 exposed, 27 unexposed) |
| [385] 10.1093/humrep/17.11.2912 |
| Moro | 2012 | Toluene | Brazil | Urinary levels of hippuric acid (HA) | 61 painters (34 exposed, 27 unexposed) |
| [386] 10.1016/j.mrgentox.2012.02.007 |
| Navasumrit | 2005 | Benzene | Thailand | Personal benzene exposure by diffusive badges Urinary metabolites, blood benzene | 148 (28 children in Chonburi, 41 children in Bangkok, 29 gasoline service staff in Bangkok, 23 factory staff, 27 controls) |
| [387] 10.1016/j.cbi.2005.03.010 |
| Pandey | 2008 | BTX | India | Benzene monitoring in air Benzene, toluene, and xylene in blood samples | 200 petrol pump workers (100 exposed, 100 unexposed) |
| [388] 10.1002/em.20419 |
| Poça | 2021 | Benzene in gasoline | Brazil | Urinary t,t-muconic acid | 349 (154 exposed filling station workers, 95 convenience store workers, 100 unexposed office workers) |
| [389] 10.1016/j.mrgentox.2021.503322 |
| Rekhadevi | 2010 | BTX | India | Monitoring of ambient and breathing zone air BTX in blood | 400 (200 fuel station staff, 200 controls) |
| [390] 10.1093/annhyg/meq065 |
| Roma-Torres | 2006 | BTX | Portugal | Urinary t,t-Muconic acid (t,t-MA), hippuric acid (HA), and methylhippuric acid (MHA) | 78 (48 petroleum unit workers, 30 controls) |
| [391] 10.1016/j.mrgentox.2005.12.005 |
| Sakhvidi | 2022 | Benzene found in petroleum compounds | Iran | Air sampling for benzene | 32 petroleum products workers exposed to benzene, 32 non-exposed administrative |
| [392] 10.1007/s11356-022-19015-2 |
| Sardas | 2010 | Welding fume, solvent base paint | Turkey | -- | 78 (26 welders, 26 painters, 26 controls) |
| [96] 10.1177/0748233710374463 |
| Scheepers ** | 2002 | Diesel exhaust (benzene, PAHs) | Estonia, Czech Republic | Analysis of air samples Urinary metabolites of PAH and benzene | 92 underground miners (drivers of diesel-powered excavators) (46 underground workers, 46 surface workers) |
| [97] 10.1016/s0378-4274(02)00195-9 |
| Sul * | 2002 | Benzene | South Korea | Urinary t,t-muconic acid (t,t-MA), and creatinine | 81 printing factory (41 exposed, 41 unexposed) |
| [393] 10.1016/s0378-4274(02)00167-4 |
| Sul * | 2005 | Benzene | South Korea | Personal sampler benzene Urinary trans, trans-muconic acid (t,t-MA), phenol, creatinine | 61 subjects (working in printing, shoemaking, production of methylene di-aniline (MDA), nitrobenzene, carbomer, and benzene) |
| [394] 10.1016/j.mrgentox.2004.12.011 |
| Teixeira | 2010 | Styrene | Portugal | Styrene in inhaled air Urinary excretion styrene metabolites, mandelic, and phenylglyoxylic acids (MAPGA) | 106 (52 fibreglass workers, 54 controls) |
| [395] 10.1093/mutage/geq049 |
| Tovalin ** | 2006 | VOCs, PM2.5, ozone | Mexico | Personal occupational and non-occupational monitoring | 55 city traffic exposure (28 outdoor workers, 27 indoor workers) |
| [104] 10.1136/oem.2005.019802 |
| Xiong | 2016 | Benzene, toluene, ethylbenzene, and xylenes (BTEX) | China | Air sampling | 252 gas station workers (200 refueling workers, 52 controls) |
| [396] 10.3390/ijerph13121212 |
| Zhao | 2017 | Benzene, acetone, xylene, toluene, lead, isopropanol, and physical factors | China | Air sampling | 722 workers in electronics factory (584 exposed, 138 controls) |
| [397] 10.1016/j.mrfmmm.2017.07.005 |
| Environmental exposure | |||||||
| Avogbe ** | 2005 | Benzene, ultrafine particles | Benin | Ambient UFP Urinary excretion of S-PMA | 135 city traffic exposure (29 drivers, 37 roadside residents, 42 suburban, 27 rural) |
| [121] 10.1093/carcin/bgh353 |
| Koppen ** | 2007 | PAHs, VOCs (benzene and toluene) | Belgium | Outdoor ozone concentrations Urinary concentrations of PAH, t,t′-muconic acid, o-cresol, VOCs metabolites | 200 adolescents air pollution |
| [138] 10.1002/jat.1174 |
| Mukherjee ** | 2013 | Particulate pollutants and benzene | India | Urinary trans, trans-muconic acid | 105 (56 biomass users, 49 cleaner liquefied petroleum gas users) |
| [144] 10.1002/jat.1748 |
| Pelallo-Martínez **,ɣ | 2014 | Lead, benzene, toluene, PAHs | Mexico | Urinary and blood Pb, benzene, toluene, PAHs | 97 children, air pollution (44 Allende, 37 Nuevo Mundo, 16 Lopez Mateos) |
| [149] 10.1007/s00244-014-9999-4 |
| Sørensen | 2003 | Benzene | Denmark | Exposure benzene, toluene, MTBE 8-oxodG in blood Urinary ttMA, S-PMA | 40 subjects, air pollution |
| [398] 10.1016/S0048-9697(03)00054-8 |
| Wilhelm **,ɣ | 2007 | PAH, benzene, heavy metals | Germany | Monitored ambient air quality data Urinary (PAH) metabolites, benzene metabolites | 935 air pollution close to industrial settings (620 exposed children, 315 unexposed) |
| [160] 10.1016/j.ijheh.2007.02.007 |
| Zani ** | 2020 | PM10, PM2.5, NO2, CO, SO2, benzene, O3 | Italy | Air monitoring by regional agency | 152 children, air pollution | Saliva leukocytes from sputum
| [162] 10.3390/ijerph17093276 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladeira, C.; Møller, P.; Giovannelli, L.; Gajski, G.; Haveric, A.; Bankoglu, E.E.; Azqueta, A.; Gerić, M.; Stopper, H.; Cabêda, J.; et al. The Comet Assay as a Tool in Human Biomonitoring Studies of Environmental and Occupational Exposure to Chemicals—A Systematic Scoping Review. Toxics 2024, 12, 270. https://doi.org/10.3390/toxics12040270
Ladeira C, Møller P, Giovannelli L, Gajski G, Haveric A, Bankoglu EE, Azqueta A, Gerić M, Stopper H, Cabêda J, et al. The Comet Assay as a Tool in Human Biomonitoring Studies of Environmental and Occupational Exposure to Chemicals—A Systematic Scoping Review. Toxics. 2024; 12(4):270. https://doi.org/10.3390/toxics12040270
Chicago/Turabian StyleLadeira, Carina, Peter Møller, Lisa Giovannelli, Goran Gajski, Anja Haveric, Ezgi Eyluel Bankoglu, Amaya Azqueta, Marko Gerić, Helga Stopper, José Cabêda, and et al. 2024. "The Comet Assay as a Tool in Human Biomonitoring Studies of Environmental and Occupational Exposure to Chemicals—A Systematic Scoping Review" Toxics 12, no. 4: 270. https://doi.org/10.3390/toxics12040270
APA StyleLadeira, C., Møller, P., Giovannelli, L., Gajski, G., Haveric, A., Bankoglu, E. E., Azqueta, A., Gerić, M., Stopper, H., Cabêda, J., Tonin, F. S., & Collins, A. (2024). The Comet Assay as a Tool in Human Biomonitoring Studies of Environmental and Occupational Exposure to Chemicals—A Systematic Scoping Review. Toxics, 12(4), 270. https://doi.org/10.3390/toxics12040270











