Fe-CGS Effectively Inhibits the Dynamic Migration and Transformation of Cadmium and Arsenic in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
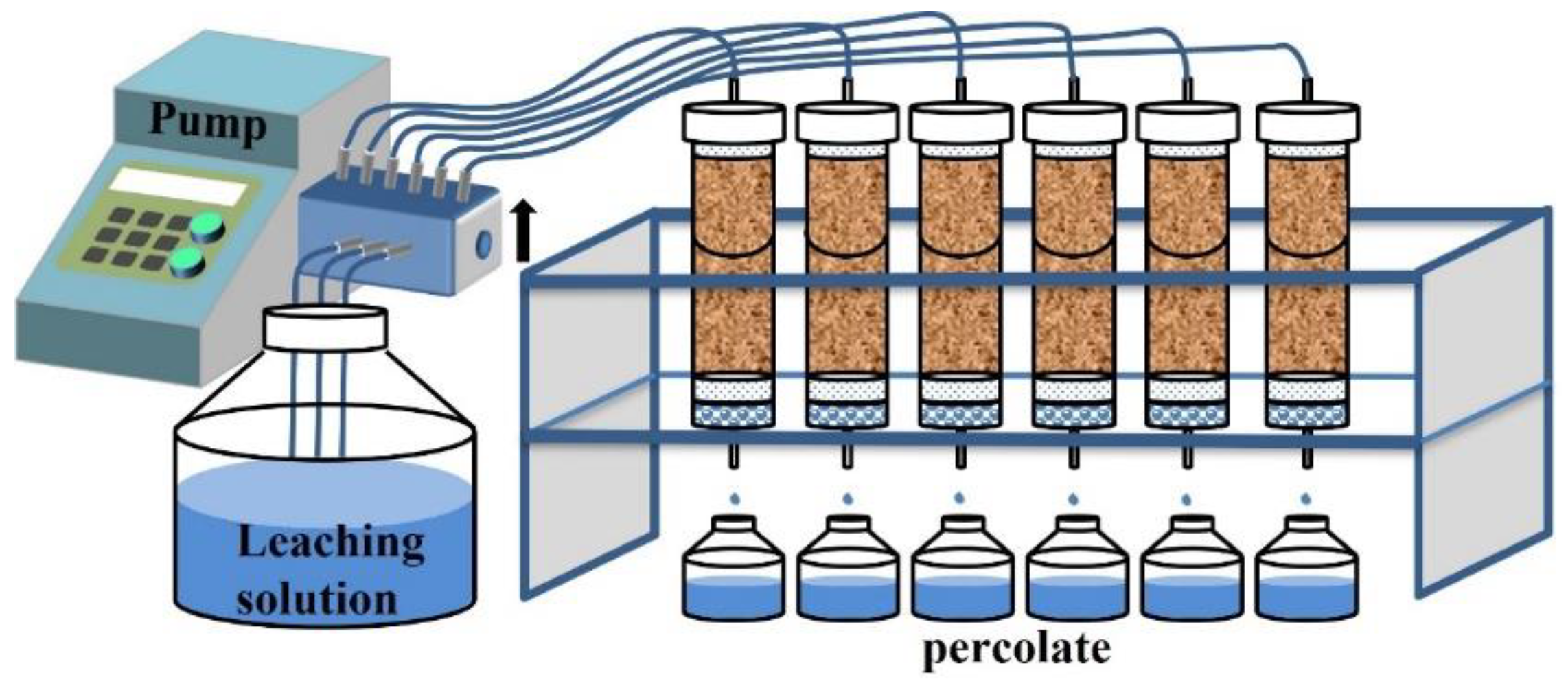
2.2.1. Soil Column Packing
2.2.2. Leaching
2.3. Determination of Effective Forms of Cd and As
2.4. Analysis of Cd and As Fractions
3. Results
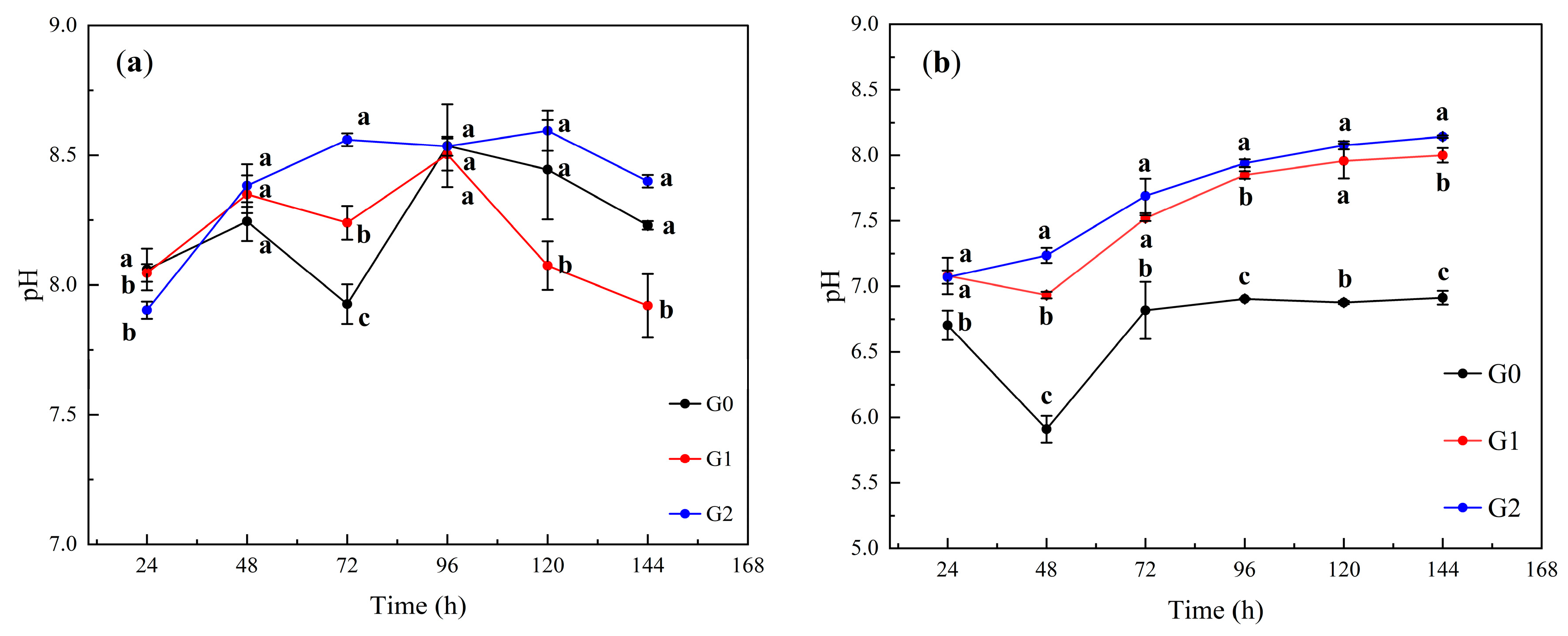
3.1. Changes in Soil Leachate pH
3.2. The Stabilizing Effect of Fe-CGS on Cd
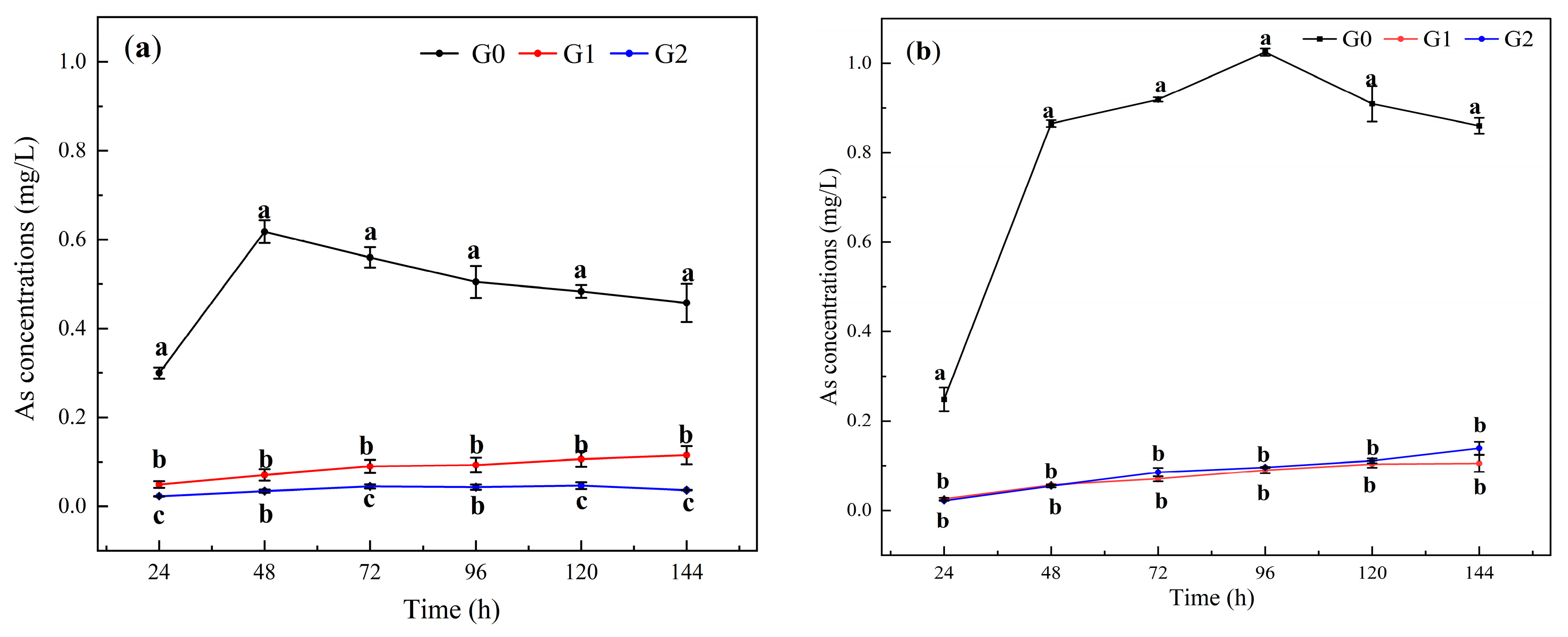
3.3. The Stabilizing Effect of Fe-CGS on As
3.4. Changes in the Available of Cd and As
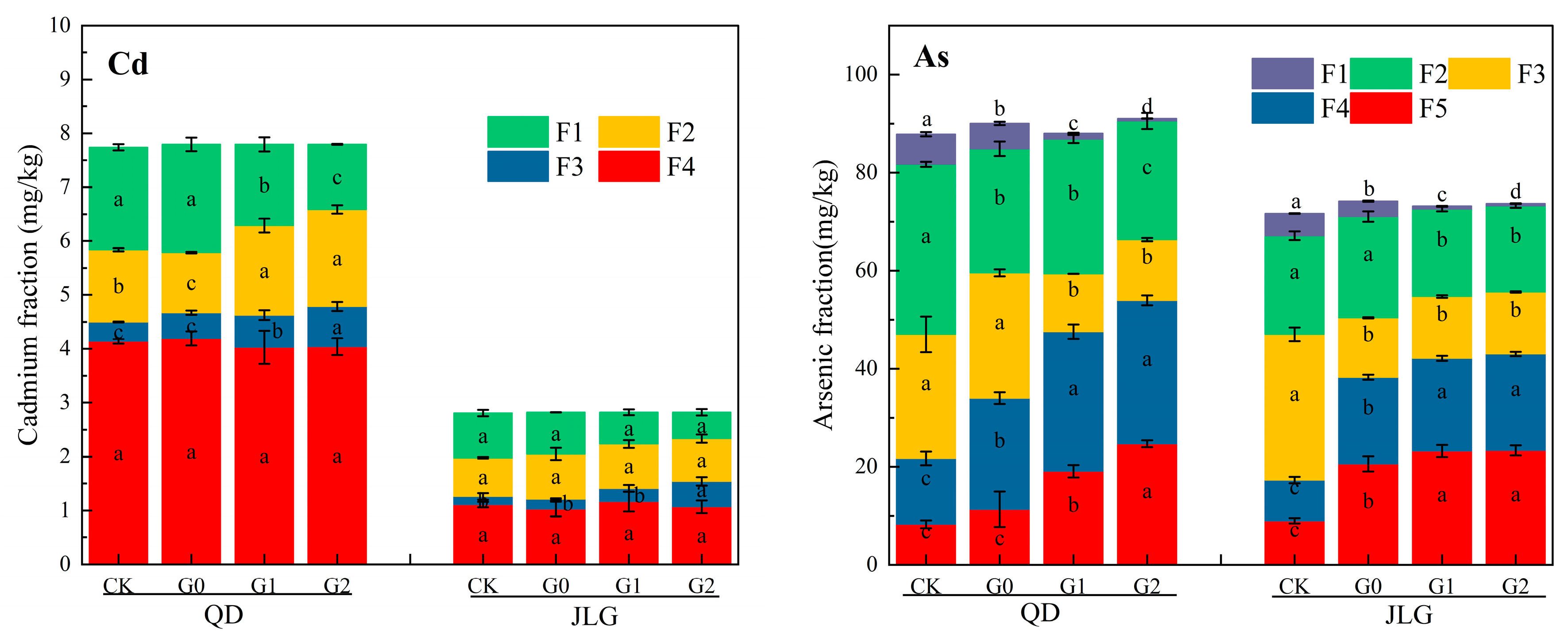
3.5. Changes in Cd and As Fractions
4. Discussion
4.1. Changes in Soil pH
4.2. Leaching Behavior and Availability of Cd and As
4.3. Cd and As Fractions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Fang, T.; Wang, J.; Liu, X.; Yan, Z.; Lin, H.; Li, F.; Guo, G. Insight into the stabilization mechanism and long-term effect on As, Cd, and Pb in soil using zeolite-supported nanoscale zero-valent iron. J. Clean. Prod. 2022, 355, 131634. [Google Scholar] [CrossRef]
- Ren, S.; Song, C.; Ye, S.; Cheng, C.; Gao, P. The spatiotemporal variation in heavy metals in China’s farmland soil over the past 20 years: A meta-analysis. Sci. Total Environ. 2022, 806, 150322. [Google Scholar] [CrossRef]
- Awual, M.R.; Khraisheh, M.; Alharthi, N.H.; Luqman, M.; Islam, A.; Rezaul Karim, M.; Rahman, M.M.; Khaleque, M.A. Efficient detection and adsorption of cadmium(II) ions using innovative nano-composite materials. Chem. Eng. J. 2018, 343, 118–127. [Google Scholar] [CrossRef]
- Jeon, E.-K.; Ryu, S.; Park, S.-W.; Wang, L.; Tsang, D.C.W.; Baek, K. Enhanced adsorption of arsenic onto alum sludge modified by calcination. J. Clean. Prod. 2018, 176, 54–62. [Google Scholar] [CrossRef]
- Gunadasa, S.G.; Tighe, M.K.; Wilson, S.C. Arsenic and cadmium leaching in co-contaminated agronomic soil and the influence of high rainfall and amendments. Environ. Pollut. 2023, 316, 120591. [Google Scholar] [CrossRef]
- He, Y.M.; Yang, R.; Lei, G.; Li, B.; Jiang, M.; Yan, K.; Zu, Y.Q.; Zhan, F.D.; Li, Y. Arbuscular mycorrhizal fungi reduce cadmium leaching from polluted soils under simulated heavy rainfall. Environ. Pollut. 2020, 263, 114406. [Google Scholar] [CrossRef]
- Ren, W.; Ran, Y.; Mou, Y.; Cui, Y.; Sun, B.; Yu, L.; Wan, D.; Hu, D.; Zhao, P. Pollution characteristics and risk assessment of antimony and arsenic in a typical abandoned antimony smelter. Environ. Geochem. Health 2023, 45, 5467–5480. [Google Scholar] [CrossRef]
- Tica, D.; Udovic, M.; Lestan, D. Immobilization of potentially toxic metals using different soil amendments. Chemosphere 2011, 85, 577–583. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Du, H.; Nie, N.; Rao, W.; Lu, L.; Lei, M.; Tie, B. Ferrihydrite-organo composites are a suitable analog for predicting Cd(II)-As(V) coexistence behaviors at the soil solid-liquid interfaces. Environ. Pollut. 2021, 290, 118040. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Wang, Q.; Leng, Z.; Geng, Y.; Sun, S.; Hou, H. Simultaneous adsorption of Cd and As by a novel coal gasification slag based composite: Characterization and application in soil remediation. Sci. Total. Environ. 2023, 882, 163374. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Chen, L.; Yue, S.; Yang, W. Effect of biochar on immobilization remediation of Cd rectanglecontaminated soil and environmental quality. Environ. Res. 2022, 204, 111840. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Yao, A.; Ju, L.; Ling, X.; Liu, C.; Wei, X.; Qiu, H.; Tang, Y.; Morel, J.L.; Qiu, R.; Li, C.; et al. Simultaneous attenuation of phytoaccumulation of Cd and As in soil treated with inorganic and organic amendments. Environ. Pollut. 2019, 250, 464–474. [Google Scholar] [CrossRef]
- Yang, D.; Yang, S.; Yuan, H.; Wang, F.; Wang, H.; Xu, J.; Liu, X. Co-benefits of biochar-supported nanoscale zero-valent iron in simultaneously stabilizing soil heavy metals and reducing their bioaccessibility. J. Hazard. Mater. 2021, 418, 126292. [Google Scholar] [CrossRef]
- Hu, Y.B.; Zhang, M.; Li, X.Y. Improved longevity of nanoscale zero-valent iron with a magnesium hydroxide coating shell for the removal of Cr(VI) in sand columns. Environ. Int. 2019, 133, 105249. [Google Scholar] [CrossRef]
- Yuan, N.; Zhao, A.; Hu, Z.; Tan, K.; Zhang, J. Preparation and application of porous materials from coal gasification slag for wastewater treatment: A review. Chemosphere 2022, 287, 132227. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Ai, W.; Wei, C. A new method to prepare mesoporous silica from coal gasification fine slag and its application in methylene blue adsorption. J. Clean. Prod. 2019, 212, 1062–1071. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhang, J.; Zuo, J.; Zhang, J.; Qiu, F.; Wei, C.; Miao, S. Preparation of mesoporous coal gasification slag and applications in polypropylene resin reinforcement and deodorization. Powder Technol. 2021, 386, 437–448. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Guo, F.; Shao, Z.; Wu, J. Zeolite Synthesized from Coal Fly Ash Produced by a Gasification Process for Ni2+ Removal from Water. Minerals 2018, 8, 116. [Google Scholar] [CrossRef]
- Xu, Y.; Chai, X. Characterization of coal gasification slag-based activated carbon and its potential application in lead removal. Environ. Technol. 2018, 39, 382–391. [Google Scholar] [CrossRef]
- Qiao, J.T.; Liu, T.X.; Wang, X.Q.; Li, F.B.; Lv, Y.H.; Cui, J.H.; Zeng, X.D.; Yuan, Y.Z.; Liu, C.P. Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils. Chemosphere 2018, 195, 260–271. [Google Scholar] [CrossRef]
- Moon, D.H.; Dermatas, D. Arsenic and lead release from fly ash stabilized/solidified soils under modified semi-dynamic leaching conditions. J. Hazard. Mater. 2007, 141, 388–394. [Google Scholar] [CrossRef]
- Voglar, G.E.; Lestan, D. Solidification/stabilisation of metals contaminated industrial soil from former Zn smelter in Celje, Slovenia, using cement as a hydraulic binder. J. Hazard. Mater. 2010, 178, 926–933. [Google Scholar] [CrossRef]
- Zhang, H.-q.; Yang, Y.-Y.; Yi, Y.-C. Effect of sulfate erosion on strength and leaching characteristic of stabilized heavy metal contaminated red clay. Trans. Nonferrous Met. Soc. China 2017, 27, 666–675. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Xu, Y.; Wang, L.; Liang, X.; Li, Y. Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials. Environ. Pollut. 2016, 208, 739–746. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef]
- Wang, P.; Xue, Q.; Li, J.-S.; Zhang, T.-T. Effects of pH on leaching behavior of compacted cement solidified/stabilized lead contaminated soil. Environ. Prog. Sustain. Energy 2016, 35, 149–155. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J.; Zeng, X.; Zhang, N.; Wen, J.; Liu, J.; Jiku, M.A.S.; Wu, C.; Su, S. The performance and mechanism of cadmium availability mitigation by biochars differ among soils with different pH: Hints for the reasonable choice of passivators. J. Environ. Manag. 2022, 312, 114903. [Google Scholar] [CrossRef]
- Xue, W.; Peng, Z.; Huang, D.; Zeng, G.; Wan, J.; Xu, R.; Cheng, M.; Zhang, C.; Jiang, D.; Hu, Z. Nanoremediation of cadmium contaminated river sediments: Microbial response and organic carbon changes. J. Hazard. Mater. 2018, 359, 290–299. [Google Scholar] [CrossRef]
- Harikishore Kumar Reddy, D.; Lee, S.-M. Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Liang, L.; Lin, Z.; Su, X.; Zhang, W. Immobilization of cadmium in contaminated soils using sulfidated nanoscale zero-valent iron: Effectiveness and remediation mechanism. J. Hazard. Mater. 2021, 420, 126605. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, S.E.; Chapin, F.S. Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 1996, 35, 327–338. [Google Scholar] [CrossRef]
- Huang, D.; Xue, W.; Zeng, G.; Wan, J.; Chen, G.; Huang, C.; Zhang, C.; Cheng, M.; Xu, P. Immobilization of Cd in river sediments by sodium alginate modified nanoscale zero-valent iron: Impact on enzyme activities and microbial community diversity. Water Res. 2016, 106, 15–25. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Li, Z.; Tang, X.; He, M.; Yang, S.; Liu, X.; Xu, J. Simultaneous adsorption of Cd(II)andAs(III)by a novel biochar-supported nanoscale zero-valent iron in aqueous systems. Sci. Total. Environ. 2020, 708, 134823. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Deng, Y.; Shu, Z.; Zhang, C.; Ye, S.; Chen, Q.; Yang, H.; Yang, L. Phytoremediation plants (ramie) and steel smelting wastes for calcium silicate coated-nZVI/biochar production: Environmental risk assessment and efficient As(V) removal mechanisms. Sci. Total. Environ. 2022, 844, 156924. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Deng, W.; Tan, A.; Chu, Z.; Wei, W.; Zheng, R.; Shangguan, Y.; Sasaki, A.; Endo, M.; Chen, H. Protonation stabilized high As/F mobility red mud for Pb/As polluted soil remediation. J. Hazard. Mater. 2021, 404, 124143. [Google Scholar] [CrossRef]
- Yang, D.; Wang, R.; Feng, X.; Chu, Z.; Li, J.; Wei, W.; Zheng, R.; Zhang, J.; Chen, H. Transferring waste red mud into ferric oxide decorated ANA-type zeolite for multiple heavy metals polluted soil remediation. J. Hazard. Mater. 2022, 424, 127244. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhou, H.; Gu, J.F.; Huang, F.; Yang, W.J.; Wang, S.L.; Yuan, T.Y.; Liao, B.H. Effects of nano-Fe(3)O(4)-modified biochar on iron plaque formation and Cd accumulation in rice (Oryza sativa L.). Environ. Pollut. 2020, 260, 113970. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Zhang, R.; Min, X.; Yang, Z.; Chai, L.; Zhao, F. Highly efficient removal of arsenic (III/V) from groundwater using nZVI functionalized cellulose nanocrystals fabricated via a bioinspired strategy. Sci. Total. Environ. 2022, 842, 156937. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, F.; Su, B.; Sun, M.; Owens, G.; Chen, Z. The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation. J. Hazard. Mater. 2019, 379, 120832. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, H.; Guo, Y.; Liu, Z.; Jiao, W. Immobilization of heavy metals in contaminated soils by modified hydrochar: Efficiency, risk assessment and potential mechanisms. Sci. Total. Environ. 2019, 685, 1201–1208. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, J.; Zhang, S.; Zhang, X.; Chen, H. Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J. Hazard. Mater. 2020, 390, 121349. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.Y.; Liu, C.; Li, F.; Xu, X.; Wang, Q. Arsenic availability in rice from a mining area: Is amorphous iron oxide-bound arsenic a source or sink? Environ. Pollut. 2015, 199, 95–101. [Google Scholar] [CrossRef]
- Niazi, N.K.; Singh, B.; Shah, P. Arsenic speciation and phytoavailability in contaminated soils using a sequential extraction procedure and XANES spectroscopy. Environ. Sci. Technol. 2011, 45, 7135–7142. [Google Scholar] [CrossRef]
- Tuutijarvi, T.; Lu, J.; Sillanpaa, M.; Chen, G. As(V) adsorption on maghemite nanoparticles. J. Hazard. Mater. 2009, 166, 1415–1420. [Google Scholar] [CrossRef]
- Koomson, J.A.; Koomson, B.; Owusu, C.; Agyemang, F.O. Detoxification of lead and arsenic from galamsey polluted water using nano synthesized iron oxide from cupola furnace slag. Mater. Chem. Phys. 2023, 308, 128301. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Zhou, C.; Wang, J.; Yin, M.; Wu, Z.; Song, N.; Song, X.; Shangguan, Y.; Sun, Z.; Zong, Q.; et al. Fe-CGS Effectively Inhibits the Dynamic Migration and Transformation of Cadmium and Arsenic in Soil. Toxics 2024, 12, 273. https://doi.org/10.3390/toxics12040273
Yin H, Zhou C, Wang J, Yin M, Wu Z, Song N, Song X, Shangguan Y, Sun Z, Zong Q, et al. Fe-CGS Effectively Inhibits the Dynamic Migration and Transformation of Cadmium and Arsenic in Soil. Toxics. 2024; 12(4):273. https://doi.org/10.3390/toxics12040273
Chicago/Turabian StyleYin, Hongliang, Changzhi Zhou, Junhuan Wang, Mengxue Yin, Zhihao Wu, Ningning Song, Xin Song, Yuxian Shangguan, Zaijin Sun, Quanli Zong, and et al. 2024. "Fe-CGS Effectively Inhibits the Dynamic Migration and Transformation of Cadmium and Arsenic in Soil" Toxics 12, no. 4: 273. https://doi.org/10.3390/toxics12040273
APA StyleYin, H., Zhou, C., Wang, J., Yin, M., Wu, Z., Song, N., Song, X., Shangguan, Y., Sun, Z., Zong, Q., & Hou, H. (2024). Fe-CGS Effectively Inhibits the Dynamic Migration and Transformation of Cadmium and Arsenic in Soil. Toxics, 12(4), 273. https://doi.org/10.3390/toxics12040273




