Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Test Fish
2.2. Adult Zebrafish Culture
2.3. Histopathological Analysis
2.4. Swimming Behavior Detection
2.5. AChE Activity Assay
2.6. Determination of Neurotransmitter Levels
2.7. HPI Axis-Related Hormone Content Determination
2.8. Quantitative Real-Time PCR (qPCR) Assay
2.9. Statistical Analysis
3. Results
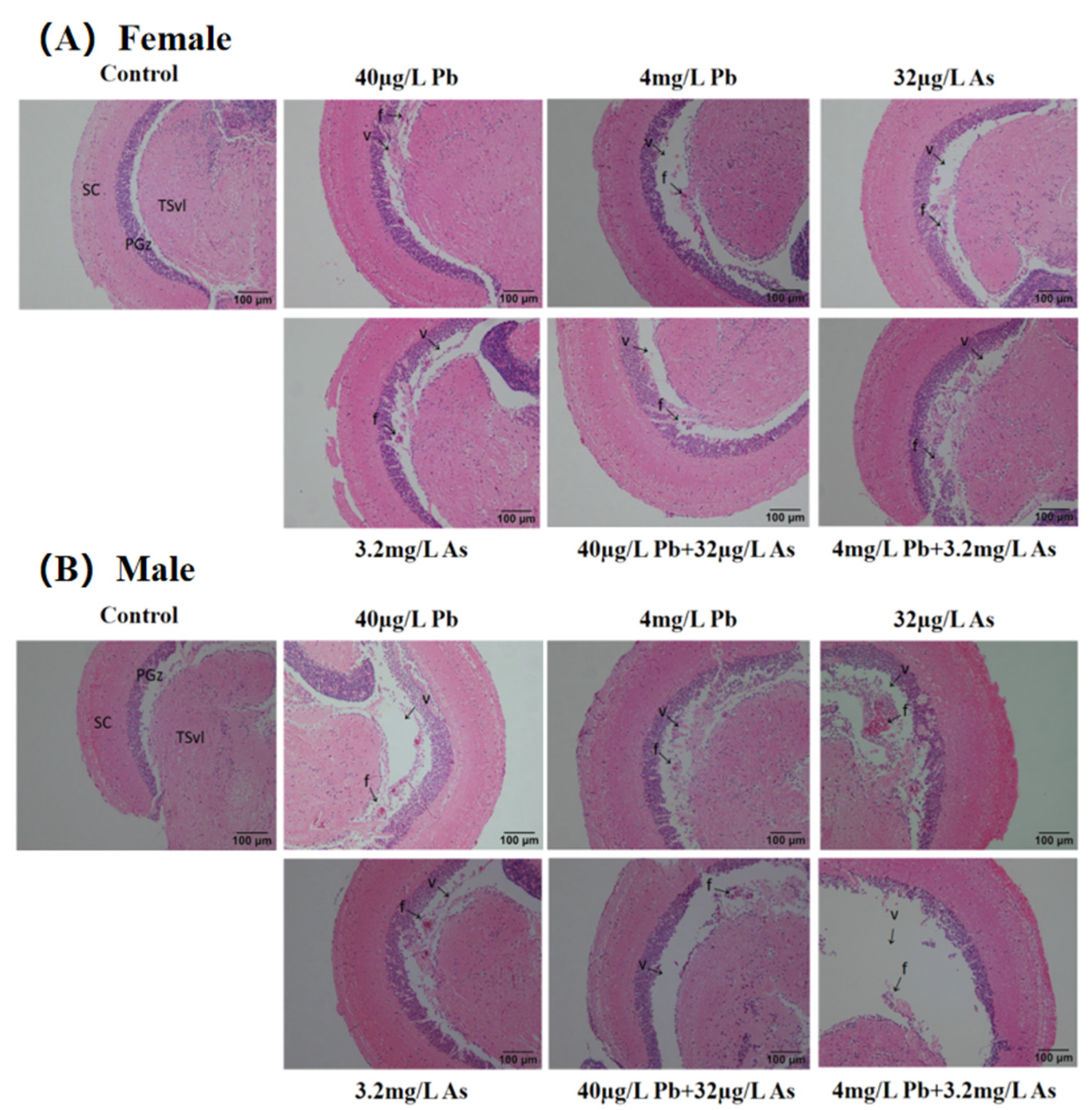
3.1. Histopathology Analysis
3.2. Swimming Performance Analysis
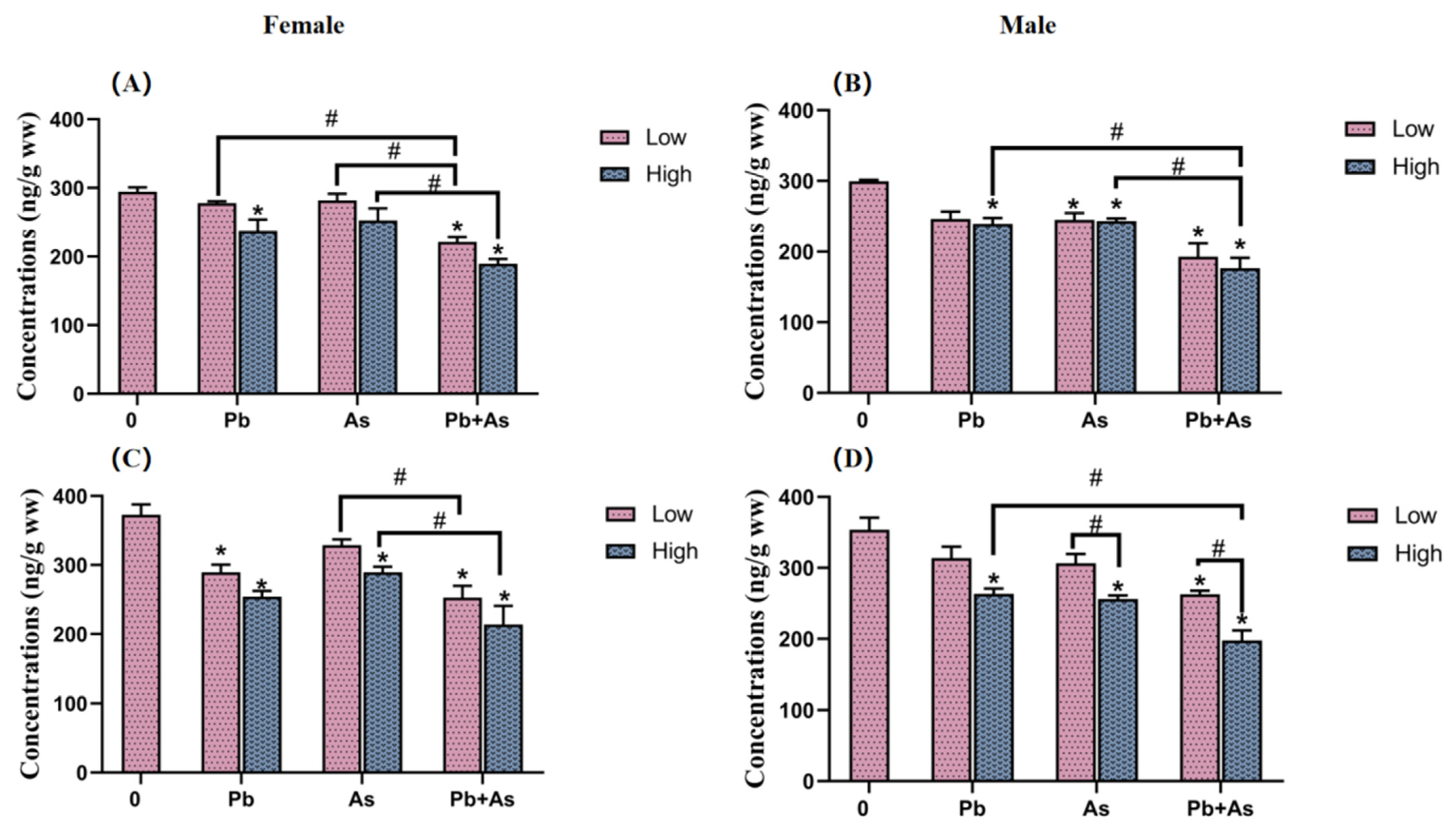
3.3. AChE Activity of Brains
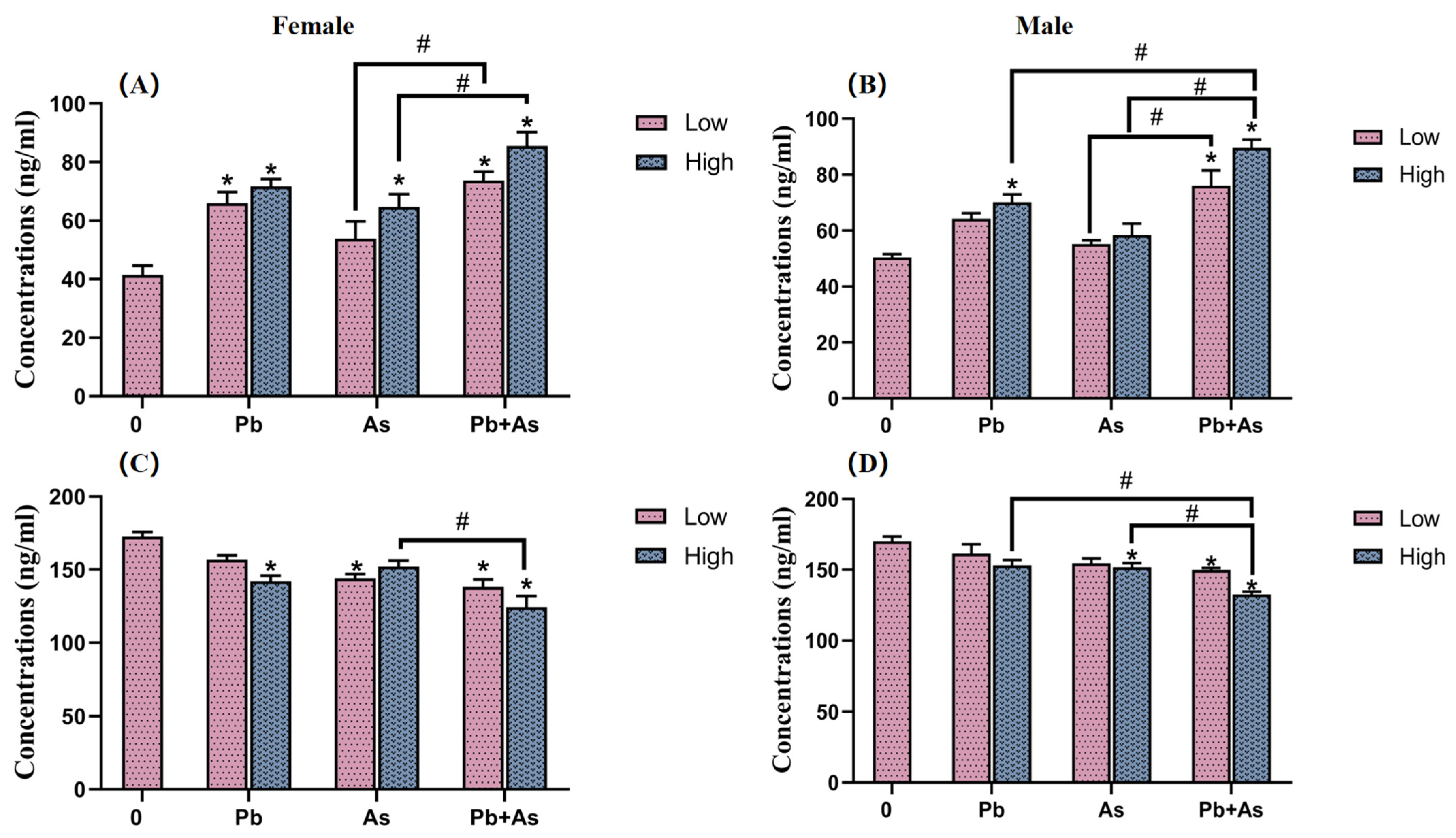
3.4. DA and 5-HT Contents in Brains
3.5. HPI Axis Hormone Content
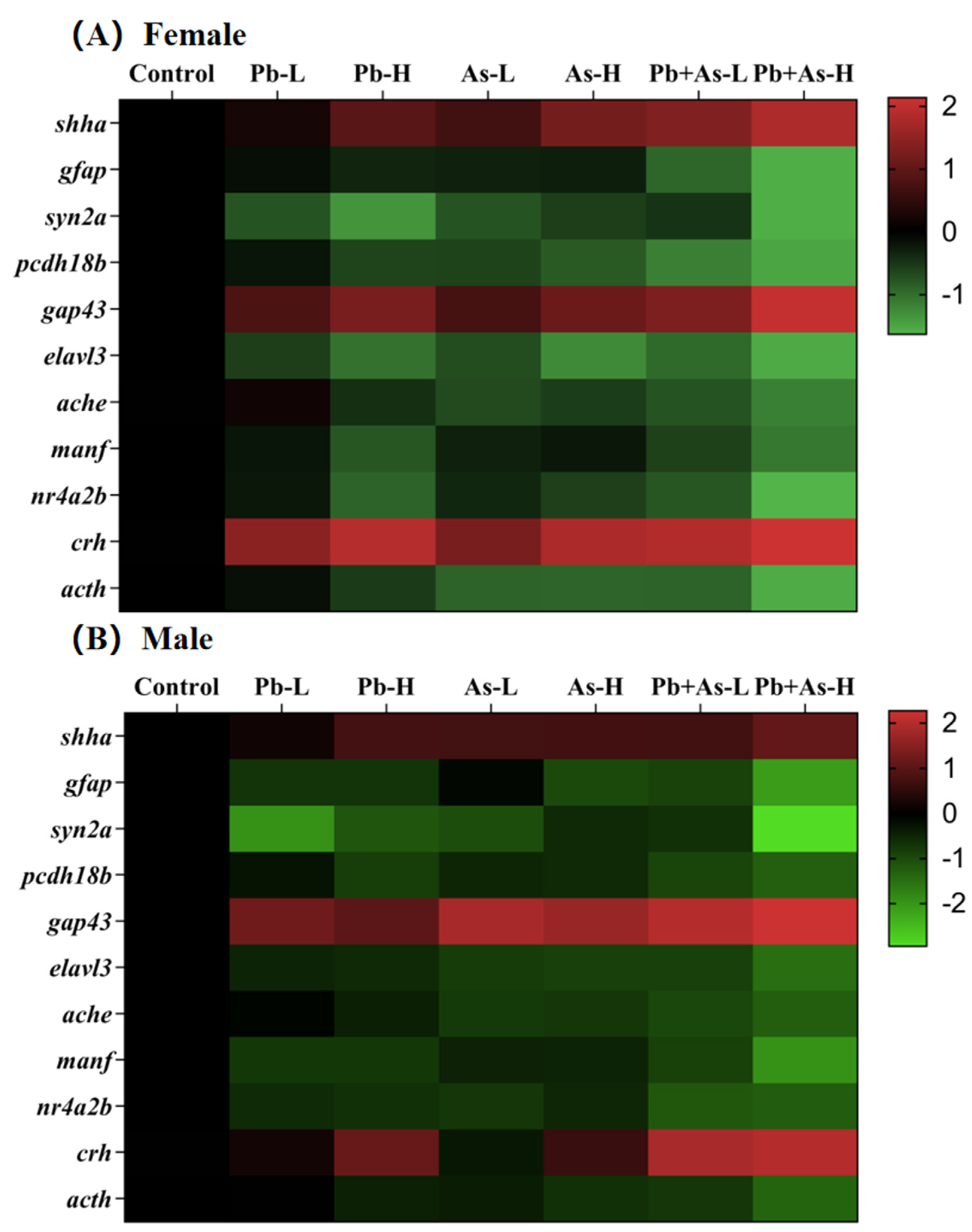
3.6. Alterations in Gene Transcript Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liao, G.; Tu, H.; Huang, Y.; Peng, T.; Xu, Y.; Chen, X.; Huang, Z.; Zhang, Y.; Meng, X.; et al. A protective role of autophagy in Pb-induced developmental neurotoxicity in zebrafish. Chemosphere 2019, 235, 1050–1058. [Google Scholar] [CrossRef]
- Xiao, C. Analysis of present situation about lead pollution in China. Environ. Sustain. Dev. 2017, 42, 91–92. [Google Scholar]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Andrade, V.; Mateus, M.L.; Santos, D.; Aschner, M.; Batoreu, M.C.; dos Santos, A.P.M. Arsenic and Manganese Alter Lead Deposition in the Rat. Biol. Trace Element Res. 2014, 158, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, S.; Hu, C.; Tang, L.; Lam, J.C.; Lam, P.K.; Chen, L. Dietary administration of probiotic Lactobacillus rhamnosus modulates the neurological toxicities of perfluorobutanesulfonate in zebrafish. Environ. Pollut. 2020, 265, 114832. [Google Scholar] [CrossRef]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb Neurotoxicity: Neuropsychological Effects of Lead Toxicity. BioMed Res. Int. 2014, 2014, 840547. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, H.; Hwang, U.-K.; Kang, J.-C.; Kang, Y.J.; Kim, K.I.; Kim, J.-H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Stansfield, K.H.; Worley, P.F.; Thompson, R.E.; Guilarte, T.R. Lead Exposure during Synaptogenesis Alters Vesicular Proteins and Impairs Vesicular Release: Potential Role of NMDA Receptor–Dependent BDNF Signaling. Toxicol. Sci. 2010, 116, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Maurya, S.K.; Khare, P.; Srivastava, A.; Bandyopadhyay, S. Characterization of Developmental Neurotoxicity of As, Cd, and Pb Mixture: Synergistic Action of Metal Mixture in Glial and Neuronal Functions. Toxicol. Sci. 2010, 118, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Bhatnagar, P.; Flora, S. Changes in tissue oxidative stress, brain biogenic amines and acetylcholinesterase following co-exposure to lead, arsenic and mercury in rats. Food Chem. Toxicol. 2015, 86, 208–216. [Google Scholar] [CrossRef]
- De León, J.; Cotto, M.D.C.; Olivo, C.J.; Márquez, F.M. Effects of chronic environmental exposure to waterborne lead and copper on the dopaminergic and serotonergic systems of zebrafish. Toxicol. Environ. Health Sci. 2020, 12, 265–272. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A.; Virgolini, M.B.; Rossi-George, A.; Thiruchelvam, M.; Lisek, R.; Weston, D. Lifetime Consequences of Combined Maternal Lead and Stress. Basic Clin. Pharmacol. Toxicol. 2008, 102, 218–227. [Google Scholar] [CrossRef]
- Dou, C.; Zhang, J. Effects of lead on neurogenesis during zebrafish embryonic brain development. J. Hazard. Mater. 2011, 194, 277–282. [Google Scholar] [CrossRef]
- Roy, N.M.; DeWolf, S.; Schutt, A.; Wright, A.; Steele, L. Neural alterations from lead exposure in zebrafish. Neurotoxicol. Teratol. 2014, 46, 40–48. [Google Scholar] [CrossRef]
- Kaur, S.; Kamli, M.R.; Ali, A. Role of arsenic and its resistance in nature. Can. J. Microbiol. 2011, 57, 769–774. [Google Scholar] [CrossRef]
- Sun, H.; Xiang, P.; Luo, J.; Hong, H.; Lin, H.; Li, H.-B.; Ma, L.Q. Mechanisms of arsenic disruption on gonadal, adrenal and thyroid endocrine systems in humans: A review. Environ. Int. 2016, 95, 61–68. [Google Scholar] [CrossRef]
- Sarkar, A. Ecosystem Perspective of Groundwater Arsenic Contamination in India and Relevance in Policy. Ecohealth 2010, 7, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Herrera, M.T.; Bundschuh, J.; Nath, B.; Nicolli, H.B.; Gutierrez, M.; Reyes-Gomez, V.M.; Nuñez, D.; Martín-Dominguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mater. 2013, 262, 960–969. [Google Scholar] [CrossRef] [PubMed]
- González-Horta, C.; Ballinas-Casarrubias, L.; Sánchez-Ramírez, B.; Ishida, M.C.; Barrera-Hernández, A.; Gutiérrez-Torres, D.; Zacarias, O.L.; Saunders, R.J.; Drobná, Z.; Mendez, M.A.; et al. A Concurrent Exposure to Arsenic and Fluoride from Drinking Water in Chihuahua, Mexico. Int. J. Environ. Res. Public Health 2015, 12, 4587–4601. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.M.; Singh, K.; Batal, M.; Marushka, L.; Tikhonov, C.; Sadik, T.; Schwartz, H.; Ing, A.; Fediuk, K. Levels of metals and persistent organic pollutants in traditional foods consumed by First Nations living on-reserve in Canada. Can. J. Public Health 2021, 112, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Pichhode, M.; Gaherwal, S. Toxicological Effects of Arsenic Trioxide Exposure on Haematolical Profile in Catfish, Clarias batrachus. Int. J. Curr. Res. Rev. 2019, 11, 9–12. [Google Scholar] [CrossRef]
- Englyst, V.; Lundström, N.-G.; Gerhardsson, L.; Rylander, L.; Nordberg, G. Lung cancer risks among lead smelter workers also exposed to arsenic. Sci. Total Environ. 2001, 273, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Ozone, K.; Ueno, S.; Ishizaki, M.; Hayashi, O. Toxicity and Oxidative Stress Induced by Organic Arsenical Diphenylarsinic Acid and Inorganic Arsenicals and Their Effects on Spatial Learning Ability in Mice. J. Health Sci. 2010, 56, 517–526. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Labonté, B.; Wen, X.L.; Turecki, G.; Meaney, M.J. Epigenetic Mechanisms for the Early Environmental Regulation of Hippocampal Glucocorticoid Receptor Gene Expression in Rodents and Humans. Neuropsychopharmacology 2012, 38, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.E.; Labrecque, M.T.; Solomon, B.R.; Ali, A.; Allan, A.M. Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicol. Teratol. 2015, 47, 66–79. [Google Scholar] [CrossRef]
- Tadanobu, I.; Zhang, Y.F.; Shigeo, M.; Hiroko, S.; Hiromichi, N.; Hiroki, M.; Yuichi, S.; Eiichi, A. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol. Lett. 1990, 54, 345–353. [Google Scholar] [CrossRef]
- Dipp, V.R.; Valles, S.; Ortiz-Kerbertt, H.; Suarez, J.V.; Bardullas, U. Neurobehavioral Alterations in Zebrafish Due to Long-Term Exposure to Low Doses of Inorganic Arsenic. Zebrafish 2018, 15, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Jay, M.; De Faveri, F.; McDearmid, J.R. Firing Dynamics and Modulatory Actions of Supraspinal Dopaminergic Neurons during Zebrafish Locomotor Behavior. Curr. Biol. 2015, 25, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Mankad, A.U.; Reddy, M.N. Lead Accumulation and its Effects on Growth and Biochemical Parameters in Tagetes erecta L. Int. J. Life Sci. Sci. Res. 2017, 3, 1142–1147. [Google Scholar] [CrossRef]
- Scherer, G. Biomonitoring of inhaled complex mixtures—Ambient air, diesel exhaust and cigarette smoke. Exp. Toxicol. Pathol. 2005, 57, 75–110. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.H.; Sarkar, S.N.; Patil, R.D.; Tripathi, H.C. Effects of Subchronic Exposure via Drinking Water to a Mixture of Eight Water-Contaminating Metals: A Biochemical and Histopathological Study in Male Rats. Arch. Environ. Contam. Toxicol. 2007, 53, 667–677. [Google Scholar] [CrossRef]
- Fowler, B.A.; Whittaker, M.H.; Lipsky, M.; Wang, G.; Chen, X.-Q. Oxidative stress induced by lead, cadmium and arsenic mixtures: 30-day, 90-day, and 180-day drinking water studies in rats: An overview. BioMetals 2004, 17, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Aktar, S.; Jahan, M.; Alam, S.; Mohanto, N.C.; Arefin, A.; Rahman, A.; Haque, A.; Himeno, S.; Hossain, K.; Alam Saud, Z. Individual and Combined Effects of Arsenic and Lead on Behavioral and Biochemical Changes in Mice. Biol. Trace Element Res. 2016, 177, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Toma, N.J.; Anwar, S.; Kabir, T.; Hosen, M.J. Lead and lead–arsenic combined exposure induces mortality and developmental impairments in zebrafish embryos: A study using wild-caught zebrafish from Bangladesh. Drug Chem. Toxicol. 2021, 45, 2833–2842. [Google Scholar] [CrossRef] [PubMed]
- Kiper, K.; Freeman, J.L. Joint Action Toxicity of Arsenic (As) and Lead (Pb) Mixtures in Developing Zebrafish. Biomolecules 2022, 12, 1833. [Google Scholar] [CrossRef] [PubMed]
- Scinicariello, F.; Buser, M.C. Blood cadmium and depressive symptoms in young adults (aged 20–39 years). Psychol. Med. 2014, 45, 807–815. [Google Scholar] [CrossRef]
- Wu, Q.; Leung, J.Y.S.; Geng, X.; Chen, S.; Huang, X.; Li, H.; Huang, Z.; Zhu, L.; Chen, J.; Lu, Y. Heavy metal contamination of soil and water in the vicinity of an abandoned e-waste recycling site: Implications for dissemination of heavy metals. Sci. Total Environ. 2015, 506–507, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-J.; Zhao, W.-J.; Teng, X.-Q.; Shu, S.-P.; Li, S.-W.; Hong, H.-C.; Guan, D.-X. Antioxidant responses and pathological changes in the gill of zebrafish (Danio rerio) after chronic exposure to arsenite at its reference dose. Ecotoxicol. Environ. Saf. 2020, 200, 110743. [Google Scholar] [CrossRef]
- Wang, G.; Wang, T.; Zhang, X.; Chen, J.; Feng, C.; Yun, S.; Cheng, Y.; Cheng, F.; Cao, J. Sex-specific effects of fluoride and lead exposures on histology, antioxidant physiology, and immune system in the liver of zebrafish (Danio rerio). Ecotoxicology 2022, 31, 396–414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yan, W.; Wu, Q.; Lu, J.; Liu, C.; Hung, T.-C.; Li, G. Adverse reproductive performance in zebrafish with increased bioconcentration of microcystin-LR in the presence of titanium dioxide nanoparticles. Environ. Sci. Nano 2018, 5, 1208–1217. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, Y.; Sun, B.; Zhou, X.; Chen, L. Exposure to methylparaben at environmentally realistic concentrations significantly impairs neuronal health in adult zebrafish. J. Environ. Sci. 2023, 132, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.V.; Begum, G.; Pallela, R.; Usman, P.K.; Rao, R.N. Changes in Behavior and Brain Acetylcholinesterase Activity in Mosquito Fish, Gambusia affinis in Response to the Sub-Lethal Exposure to Chlorpyrifos. Int. J. Environ. Res. Public Health 2005, 2, 478–483. [Google Scholar] [CrossRef]
- Tian, J.; Hu, J.; Liu, D.; Yin, J.; Chen, M.; Zhou, L.; Yin, H. Cadmium chloride-induced transgenerational neurotoxicity in zebrafish development. Environ. Toxicol. Pharmacol. 2021, 81, 103545. [Google Scholar] [CrossRef]
- Dwivedi, N.; Flora, S.J. Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Food Chem. Toxicol. 2011, 49, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.; Hernández-Sánchez, J.; Dipp, V.R.; Huerta-González, D.; Olivares-Bañuelos, T.N.; González-Fraga, J.; Bardullas, U. Exposure to low doses of inorganic arsenic induces transgenerational changes on behavioral and epigenetic markers in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2020, 396, 115002. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cai, F.; Yan, W.; Li, C.; Wang, J. A Proteomic Analysis of MCLR-induced Neurotoxicity: Implications for Alzheimer’s Disease. Toxicol. Sci. 2012, 127, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, E.A.; Campbell, P.D.; Nelson, J.C.; Granato, M. A single base pair substitution in zebrafish distinguishes between innate and acute startle behavior regulation. PLoS ONE 2024, 19, e0300529. [Google Scholar] [CrossRef]
- Liao, G.; Li, R.; Chen, X.; Zhang, W.; Du, S.; Yuan, Y. Sodium valproate prevents radiation-induced injury in hippocampal neurons via activation of the Nrf2/HO-1 pathway. Neuroscience 2016, 331, 40–51. [Google Scholar] [CrossRef]
- Eriksson, P.; Ankarberg, E.; Viberg, H.; Fredriksson, A. The developing cholinergic system as target for environmental toxicants, nicotine and polychlorinated biphenyls (PCBs): Implications for neurotoxicological processes in mice. Neurotox. Res. 2001, 3, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.H.B.; Thi, N.A.N.; Audira, G.; Siregar, P.; Liang, S.-T.; Huang, J.-C.; Hsiao, C.-D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. Int. J. Mol. Sci. 2020, 21, 1844. [Google Scholar] [CrossRef]
- Behra, M.; Cousin, X.; Bertrand, C.; Vonesch, J.-L.; Biellmann, D.; Chatonnet, A.; Strähle, U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat. Neurosci. 2002, 5, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, C.; Hu, C.; Yu, K.; Yang, L.; Zhou, B. Acute exposure to DE-71: Effects on locomotor behavior and developmental neurotoxicity in zebrafish larvae. Environ. Toxicol. Chem. 2012, 31, 2338–2344. [Google Scholar] [CrossRef]
- Chen, L.; Yu, K.; Huang, C.; Yu, L.; Zhu, B.; Lam, P.K.S.; Lam, J.C.W.; Zhou, B. Prenatal Transfer of Polybrominated Diphenyl Ethers (PBDEs) Results in Developmental Neurotoxicity in Zebrafish Larvae. Environ. Sci. Technol. 2012, 46, 9727–9734. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicol. Teratol. 2010, 32, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, L.; Gibson, A.; Hieb, A. Chronic postnatal DE-71 exposure: Effects on learning, attention and thyroxine levels. Neurotoxicol. Teratol. 2009, 31, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Iversen, S.D.; Iversen, L.L. Dopamine: 50 years in perspective. Trends Neurosci. 2007, 30, 188–193. [Google Scholar] [CrossRef]
- Bradner, J.M.; Suragh, T.A.; Caudle, W.M. Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture, DE-71. Toxicology 2013, 312, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.; Kelly, P.; Hunter, D.; MacPhail, R.; Padilla, S. Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol. Biochem. Behav. 2013, 103, 792–813. [Google Scholar] [CrossRef]
- Vitalis, T.; Parnavelas, J.G. The Role of Serotonin in Early Cortical Development. Dev. Neurosci. 2003, 25, 245–256. [Google Scholar] [CrossRef]
- Kala, S.V.; Jadhav, A.L. Region-specific alterations in dopamine and serotonin metabolism in brains of rats exposed to low levels of lead. Neurotoxicology 1995, 16, 297–308. [Google Scholar]
- Airhart, M.J.; Lee, D.H.; Wilson, T.D.; Miller, B.E.; Miller, M.N.; Skalko, R.G.; Monaco, P.J. Adverse effects of serotonin depletion in developing zebrafish. Neurotoxicol. Teratol. 2012, 34, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, L.; Wu, J.; Hua, J.; Yang, L.; Wang, Q.; Zhang, W.; Lee, J.S.; Zhou, B. Parental co-exposure to bisphenol A and nano-TiO2 causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish offspring. Sci. Total Environ. 2019, 650, 557–565. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.-H. Environmental co-exposure to TBT and Cd caused neurotoxicity and thyroid endocrine disruption in zebrafish, a three-generation study in a simulated environment. Environ. Pollut. 2020, 259, 113868. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lam, J.C.-W.; Man, Y.-C.; Lai, N.L.-S.; Kwok, K.Y.; Guo, Y.Y.; Lam, P.K.-S.; Zhou, B. Bioconcentration, metabolism and neurotoxicity of the organophorous flame retardant 1,3-dichloro 2-propyl phosphate (TDCPP) to zebrafish. Aquat. Toxicol. 2015, 158, 108–115. [Google Scholar] [CrossRef]
- da Rocha, A.; Kist, L.; Almeida, E.; Silva, D.; Bonan, C.; Altenhofen, S.; Kaufmann, C.; Bogo, M.; Barros, D.; Oliveira, S.; et al. Neurotoxicity in zebrafish exposed to carbon nanotubes: Effects on neurotransmitters levels and antioxidant system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 30–35. [Google Scholar] [CrossRef]
- Mikko, A.; Shen, H.; Kuo, C.-C.; Peränen, J.; Saarma, M. Mesencephalic astrocyte-derived neurotrophic factor (ARMET; ARP; MANF). Sci. Bus. Exch. 2009, 2, 1191. [Google Scholar]
- Chen, Y.-C.; Sundvik, M.; Rozov, S.; Priyadarshini, M.; Panula, P. MANF regulates dopaminergic neuron development in larval zebrafish. Dev. Biol. 2012, 370, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.R.; Chen, Y.; Li, X.P.; Liu, T.X.; Le, W.D. Nr4a2 is essential for the differentiation of dopaminergic neurons during zebrafish embryogenesis. Mol. Cell. Neurosci. 2008, 39, 202–210. [Google Scholar] [CrossRef]
- Mukherjee, K.; Knisely, A.; Jacobson, L. Partial Glucocorticoid Agonist-Like Effects of Imipramine on Hypothalamic-Pituitary-Adrenocortical Activity, Thymus Weight, and Hippocampal Glucocorticoid Receptors in Male C57BL/6 Mice. Endocrinology 2004, 145, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Young, E.A.; Lopez, J.F.; Murphy-Weinberg, V.; Watson, S.J.; Akil, H. Mineralocorticoid Receptor Function in Major Depression. Arch. Gen. Psychiatry 2003, 60, 24–28. [Google Scholar] [CrossRef]
- A Bryce, C.; Floresco, S.B. Perturbations in Effort-Related Decision-Making Driven by Acute Stress and Corticotropin-Releasing Factor. Neuropsychopharmacology 2016, 41, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Devilbiss, D.M.; Martin, A.J.; Spencer, R.C.; Jenison, R.L. Stress degrades working memory-related frontostriatal circuit function. Cereb. Cortex 2023, 33, 7857–7869. [Google Scholar] [CrossRef]
- Gump, B.B.; Stewart, P.; Reihman, J.; Lonky, E.; Darvill, T.; Parsons, P.J.; Granger, D.A. Low-Level Prenatal and Postnatal Blood Lead Exposure and Adrenocortical Responses to Acute Stress in Children. Environ. Health Perspect. 2008, 116, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The developmental neurotoxicity of arsenic: Cognitive and behavioral consequences of early life exposure. Ann. Glob. Health 2014, 80, 303–314. [Google Scholar] [CrossRef]
- E Newell-Fugate, A.; Taibl, J.N.; Clark, S.G.; Alloosh, M.; Sturek, M.; Krisher, R.L. Effects of diet-induced obesity on metabolic parameters and reproductive function in female Ossabaw minipigs. Comp. Med. 2014, 64, 44–49. [Google Scholar]
- Young, T.L.; Whisenhunt, K.N.; LaMartina, S.M.; Hewitt, A.W.; Mackey, D.A.; Tompson, S.W. Sonic Hedgehog Intron Variant Associated with an Unusual Pediatric Cortical Cataract. Investig. Ophthalmol. Vis. Sci. 2022, 63, 25. [Google Scholar] [CrossRef] [PubMed]
- Heng, J.; Shi, B.; Zhou, J.-Y.; Zhang, Y.; Ma, D.; Yang, Y.-G.; Liu, F. Cpeb1b-mediated cytoplasmic polyadenylation of shha mRNA modulates zebrafish definitive hematopoiesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2212212120. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Pang, Y.; Ren, Z.; Fu, X.; Jia, L. Delivering synaptic protein mRNAs via extracellular vesicles ameliorates cognitive impairment in a mouse model of Alzheimer’s disease. BMC Med. 2024, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Alm, H.; Kultima, K.; Scholz, B.; Nilsson, A.; Andrén, P.E.; Fex-Svenningsen, Å.; Dencker, L.; Stigson, M. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. NeuroToxicology 2008, 29, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Jørgensen, A.L. Structural and functional characterization of the zebrafish gene for glial fibrillary acidic protein, GFAP. Gene 2003, 310, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.; Costa, S.; Pestarino, M.; Candiani, S. Differential expression of synapsin genes during early zebrafish development. Neuroscience 2014, 280, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-B.; Jiang, Q.; Hu, J.-Y.; Wang, Y.-X.; Song, H.-Y. The Effects of Protocadherin18b Down Regulation on Embryonic Neurogenesis in Zebrafish*. Prog. Biochem. Biophys. 2010, 37, 897–903. [Google Scholar] [CrossRef]
- Pascale, A.; Gusev, P.A.; Amadio, M.; Dottorini, T.; Govoni, S.; Alkon, D.L.; Quattrone, A. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proc. Natl. Acad. Sci. USA 2004, 101, 1217–1222. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Deng, P.; Li, G.; Liu, H.; Zuo, J.; Cui, W.; Zhang, H.; Chen, X.; Yao, J.; Peng, X.; et al. Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish (Danio rerio). Toxics 2024, 12, 282. https://doi.org/10.3390/toxics12040282
Liu M, Deng P, Li G, Liu H, Zuo J, Cui W, Zhang H, Chen X, Yao J, Peng X, et al. Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish (Danio rerio). Toxics. 2024; 12(4):282. https://doi.org/10.3390/toxics12040282
Chicago/Turabian StyleLiu, Ming, Ping Deng, Guangyu Li, Haoling Liu, Junli Zuo, Wenwen Cui, Huixian Zhang, Xin Chen, Jingjing Yao, Xitian Peng, and et al. 2024. "Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish (Danio rerio)" Toxics 12, no. 4: 282. https://doi.org/10.3390/toxics12040282
APA StyleLiu, M., Deng, P., Li, G., Liu, H., Zuo, J., Cui, W., Zhang, H., Chen, X., Yao, J., Peng, X., Peng, L., Liu, J., Zheng, W., Yan, W., & Luan, N. (2024). Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish (Danio rerio). Toxics, 12(4), 282. https://doi.org/10.3390/toxics12040282





