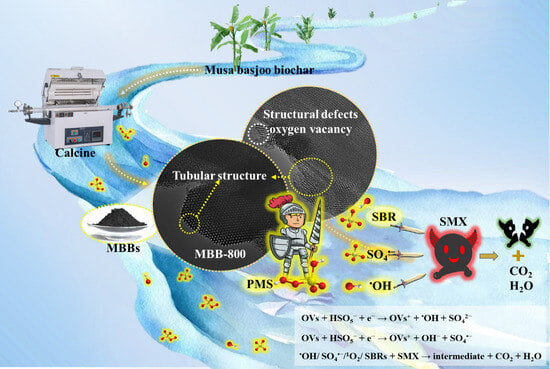
Effective Activation of Peroxymonosulfate by Oxygen Vacancy Induced Musa Basjoo Biochar to Degrade Sulfamethoxazole: Efficiency and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis
2.2.1. Synthesis of MBB
2.2.2. Synthesis of MBB-x
2.3. Characterization
2.4. Experimental Procedure and Analyses
2.5. Cell Viability Assay
3. Results and Discussion
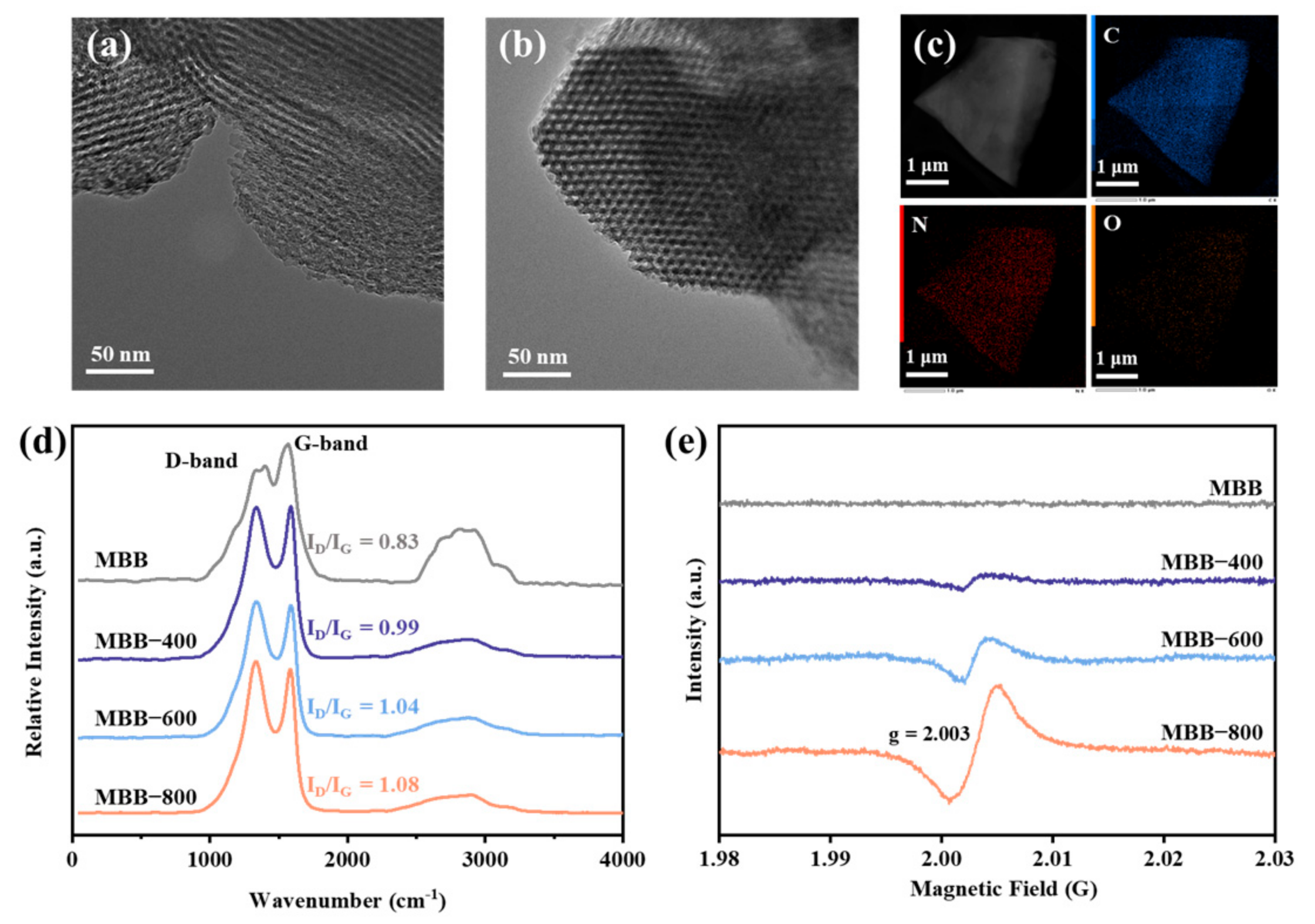
3.1. Morphology and Structure Characterization of MBBs
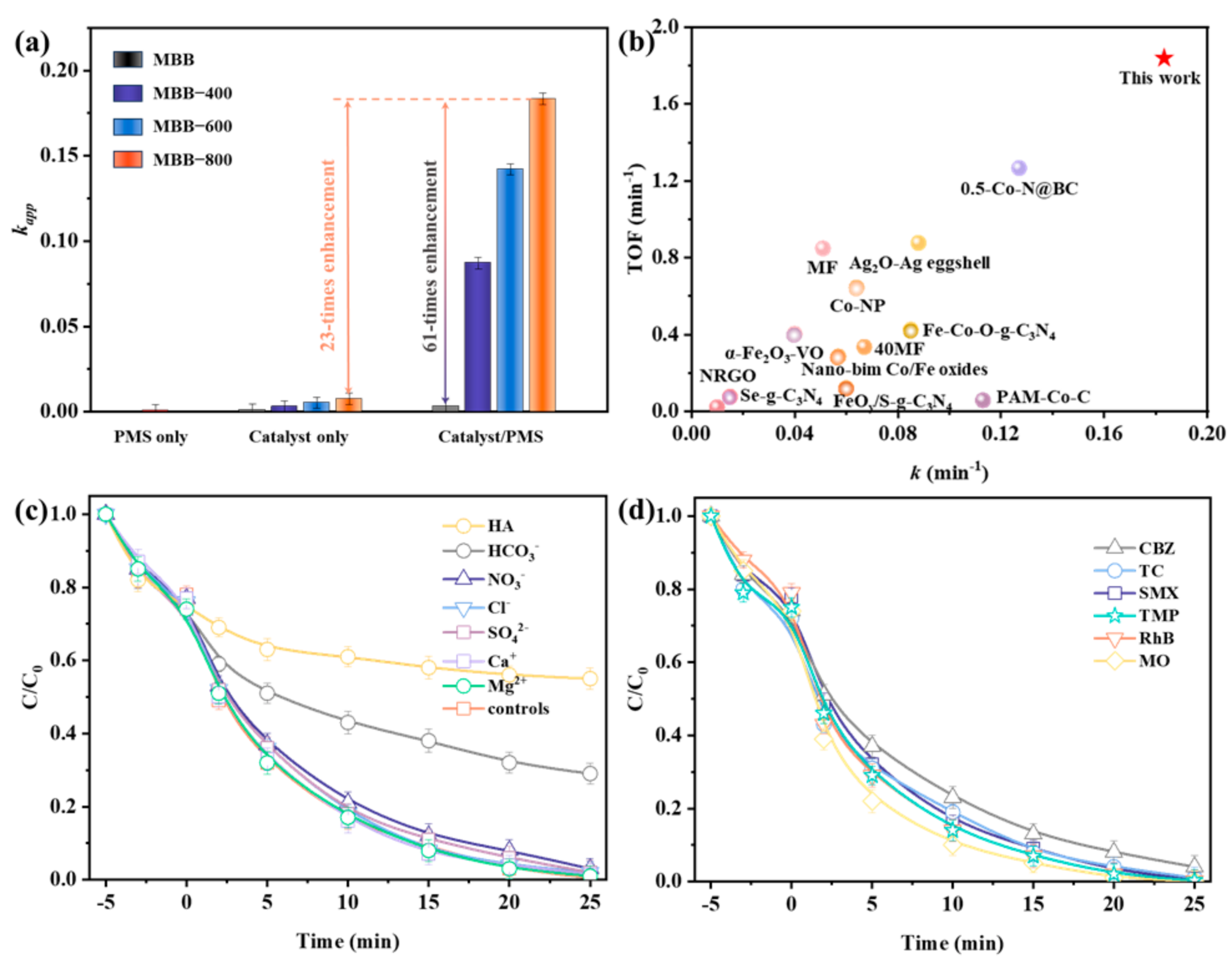
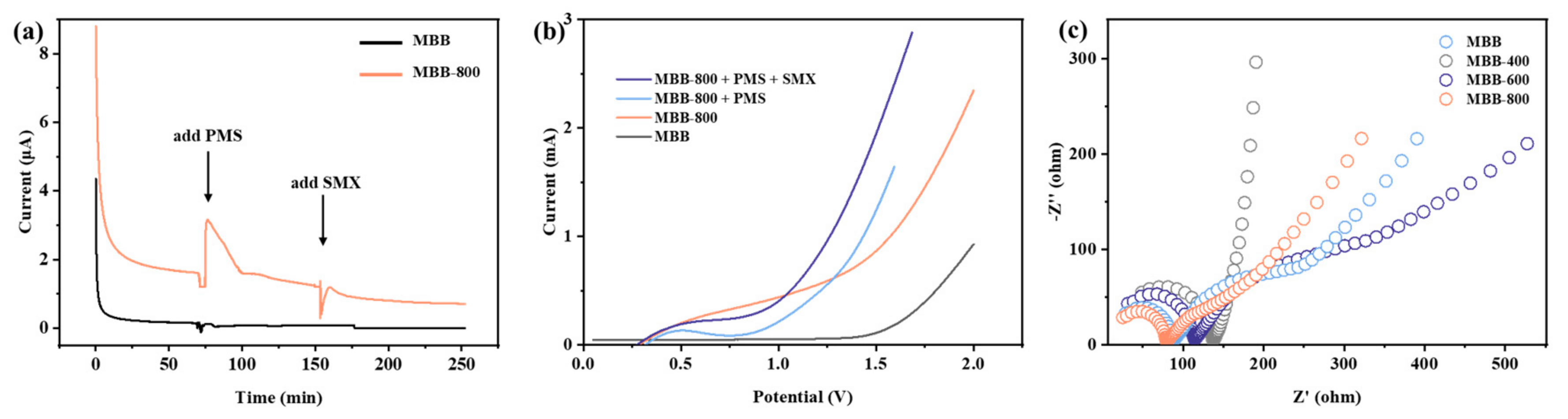
3.2. Catalytic Performance of MBBs for PMS Activation
3.3. Possible Reaction Mechanisms
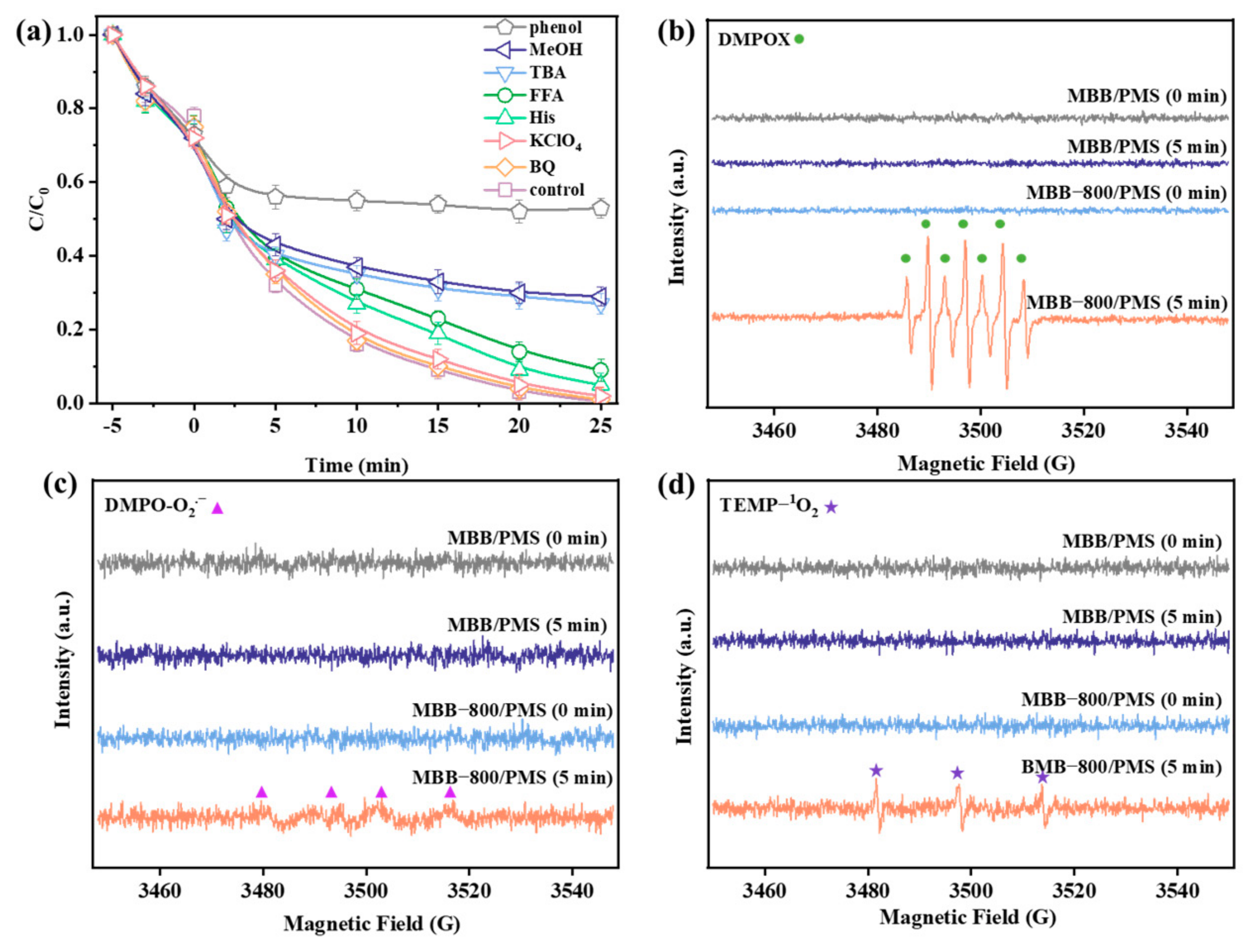
3.3.1. Identification of Key Reactive Oxygen Species
3.3.2. Electron Transfer Performance
3.3.3. Possible Degradation Pathways of SMX in PMS/MBB-800 System
3.3.4. Cytotoxicity of MBB-800
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, B.; Jin, L.; Zhao, Y.; Huang, H.; Wang, Y.; Ren, H. Adaptive Evolution Laws of Biofilm under Emerging Pollutant-Induced Stress: Community Assembly-Driven Structure Response. Environ. Sci. Technol. 2023, 57, 10721–10732. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wan, J.; Wang, Y.; Ye, G.; Yan, Z.; Ma, Y.; Sun, J. Overlooked Role of Void-Nanoconfined Effect in Emerging Pollutant Degradation: Modulating the Electronic Structure of Active Sites to Accelerate Catalytic Oxidation. Water Res. 2024, 249, 120950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Louise Leth, M.; Abou Hachem, M.; Aziz, I.; Jančič, N.; Luxbacher, T.; Hélix-Nielsen, C.; Zhang, W. Facile Fabrication of Flexible Ceramic Nanofibrous Membranes for Enzyme Immobilization and Transformation of Emerging Pollutants. Chem. Eng. J. 2023, 451, 138902. [Google Scholar] [CrossRef]
- Willach, S.; Lutze, H.V.; Eckey, K.; Löppenberg, K.; Lüling, M.; Terhalle, J.; Wolbert, J.B.; Jochmann, M.A.; Karst, U.; Schmidt, T.C. Degradation of Sulfamethoxazole Using Ozone and Chlorine Dioxide–Compound-Specific Stable Isotope Analysis, Transformation Product Analysis and Mechanistic Aspects. Water Res. 2017, 122, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Milh, H.; Cabooter, D.; Dewil, R. Degradation of Sulfamethoxazole by Ferrous Iron Activated Peroxymonosulfate: Elucidation of the Degradation Mechanism and Influence of Process Parameters. Chem. Eng. J. 2022, 430, 132875. [Google Scholar] [CrossRef]
- Sang, W.; Li, Z.; Huang, M.; Wu, X.; Li, D.; Mei, L.; Cui, J. Enhanced Transition Metal Oxide Based Peroxymonosulfate Activation by Hydroxylamine for the Degradation of Sulfamethoxazole. Chem. Eng. J. 2020, 383, 123057. [Google Scholar] [CrossRef]
- Du, J.; Guo, W.; Wang, H.; Yin, R.; Zheng, H.; Feng, X.; Che, D.; Ren, N. Hydroxyl Radical Dominated Degradation of Aquatic Sulfamethoxazole by Fe0/Bisulfite/O2: Kinetics, Mechanisms, and Pathways. Water Res. 2018, 138, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Quan, H.; Song, S.; Sun, L.; Lu, H. Comprehensive Assessment of Toxicity and Environmental Risk Associated with Sulfamethoxazole Biodegradation in Sulfur-Mediated Biological Wastewater Treatment. Water Res. 2023, 246, 120753. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Xue, Y.; Zeng, W.; Bai, L.; Li, G.; Liang, H.; Ng, H.Y. Simulated-Sunlight Enhances Membrane Aerated Biofilm Reactor Performance in Sulfamethoxazole Removal and Antibiotic Resistance Genes Reduction. Water Res. 2023, 247, 120747. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Zang, J.; Zhao, X.; Jiang, F.; Jiang, L.; Xiong, C.; Wang, N.; Fu, C. Antibiotic Residues of Drinking-Water and Its Human Exposure Risk Assessment in Rural Eastern China. Water Res. 2023, 236, 119940. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Wang, X.; Zhang, Y.; Xia, S. MgFe2O4/MgO Modified Biochar with Oxygen Vacancy and Surface Hydroxyl Groups for Enhanced Peroxymonosulfate Activation to Remove Sulfamethoxazole through Singlet Oxygen-Dominated Nonradical Oxidation Process. Chem. Eng. J. 2023, 477, 146960. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Qin, J.; Wu, G.; Lian, C.; Zhang, J.; Wang, S. Iron Encapsulated in Boron and Nitrogen Codoped Carbon Nanotubes as Synergistic Catalysts for Fenton-like Reaction. Water Res. 2016, 101, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, C.; Chen, L.; Duan, J.; Li, F.; Liu, W. Different Reaction Mechanisms of SO4•− and •OH with Organic Compound Interpreted at Molecular Orbital Level in Co(II)/Peroxymonosulfate Catalytic Activation System. Water Res. 2023, 229, 119392. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Gao, P.; Zhu, J.; Zhang, Y.; Chen, Y.; Huang, S.; Wang, G.; Yu, Z.; Zhao, S.; Zhou, S. Insights into the Pollutant Electron Property Inducing the Transformation of Peroxymonosulfate Activation Mechanisms on Manganese Dioxide. Appl. Catal. B Environ. 2022, 317, 121753. [Google Scholar] [CrossRef]
- Mao, Y.; Dong, H.; Liu, S.; Zhang, L.; Qiang, Z. Accelerated Oxidation of Iopamidol by Ozone/Peroxymonosulfate (O3/PMS) Process: Kinetics, Mechanism, and Simultaneous Reduction of Iodinated Disinfection by-Product Formation Potential. Water Res. 2020, 173, 115615. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, J.; Lu, X.; Ma, J.; Liu, Y. Production of Sulfate Radical and Hydroxyl Radical by Reaction of Ozone with Peroxymonosulfate: A Novel Advanced Oxidation Process. Environ. Sci. Technol. 2015, 49, 7330–7339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, B.; Liu, Y.; Wang, Z.; Hussain, J.; Ge, R. Modulation of Reaction Pathway of Prussian Blue Analogues Derived Zn-Fe Double Oxides towards Organic Pollutants Oxidation. Chem. Eng. J. 2023, 454, 140103. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, P.; Ding, Y.; Xi, J.; Liu, L.; Wang, W.; Xu, J. Hierarchically Porous Structure of Two-Dimensional Nano-Flakes Assembled Flower-like NiO Promotes the Formation of Surface-Activated Complex during Persulfate Activation. Chem. Eng. J. 2022, 430, 133134. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of Peroxymonosulfate by Biochar-Based Catalysts and Applications in the Degradation of Organic Contaminants: A Review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, C.; Shen, Z.; Lu, Q.; He, C.; Zhang, T.C.; Liu, J. Polyethyleneimine and Carbon Disulfide Co-Modified Alkaline Lignin for Removal of Pb2+ Ions from Water. Chem. Eng. J. 2019, 359, 265–274. [Google Scholar] [CrossRef]
- Dong, J.; Xu, W.; Liu, S.; Gong, Y.; Yang, T.; Du, L.; Chen, Q.; Tan, X.; Liu, Y. Lignin-Derived Biochar to Support CoFe2O4: Effective Activation of Peracetic Acid for Sulfamethoxazole Degradation. Chem. Eng. J. 2022, 430, 132868. [Google Scholar] [CrossRef]
- Shen, X.; Zeng, J.; Zhang, D.; Wang, F.; Li, Y.; Yi, W. Effect of Pyrolysis Temperature on Characteristics, Chemical Speciation and Environmental Risk of Cr, Mn, Cu, and Zn in Biochars Derived from Pig Manure. Sci. Total Environ. 2020, 704, 135283. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Feng, Y.; Yang, B.; Xiong, Q.; Ying, G. Accelerated Degradation of Sulfadiazine by Nitrogen-Doped Magnetic Biochar-Activated Persulfate: Role of Oxygen Vacancy. Sep. Purif. Technol. 2022, 289, 120735. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Wang, J. Nitrogen-Doped Graphene as Peroxymonosulfate Activator and Electron Transfer Mediator for the Enhanced Degradation of Sulfamethoxazole. Chem. Eng. J. 2019, 375, 122041. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Yue, C.; Li, T.; Li, M.; Li, C. Nitrogen Vacancies Induce Sustainable Redox of Iron-Cobalt Bimetals for Efficient Peroxymonosulfate Activation: Dual-Path Electron Transfer. Chem. Eng. J. 2022, 427, 131702. [Google Scholar] [CrossRef]
- Tan, J.; Li, Z.; Li, J.; Meng, Y.; Yao, X.; Wang, Y.; Lu, Y.; Zhang, T. Visible-Light-Assisted Peroxymonosulfate Activation by Metal-Free Bifunctional Oxygen-Doped Graphitic Carbon Nitride for Enhanced Degradation of Imidacloprid: Role of Non-Photochemical and Photocatalytic Activation Pathway. J. Hazard. Mater. 2022, 423, 127048. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Li, Z.; Tan, J.; Li, J.; Wu, J.; Zhang, T.; Wang, X. Oxygen-Doped Porous Graphitic Carbon Nitride in Photocatalytic Peroxymonosulfate Activation for Enhanced Carbamazepine Removal: Performance, Influence Factors and Mechanisms. Chem. Eng. J. 2022, 429, 130860. [Google Scholar] [CrossRef]
- Begashaw, T.; Dagne, A.; Yibeltal, D. Review on Phytochemistry, Medicinal Properties, and Toxicities of the Genus Musa. Tradit. Med. 2023, 4, 17. [Google Scholar] [CrossRef]
- Zeng, T.; Tang, X.; Huang, Z.; Chen, H.; Jin, S.; Dong, F.; He, J.; Song, S.; Zhang, H. Atomically Dispersed Fe-N4 Site as a Conductive Bridge Enables Efficient and Stable Activation of Peroxymonosulfate: Active Site Renewal, Anti-Oxidative Capacity, and Pathway Alternation Mechanism. Environ. Sci. Technol. 2023, 57, 20929–20940. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.; Wang, Z.; Song, S.; Cai, Z.; Leung, K.C.F.; Zeng, T. Free-Standing Janus Nanosheets: Ultrafast Self-Assembly and Versatile Biphase-Application. Adv. Funct. Mater. 2024, 2309966. [Google Scholar] [CrossRef]
- Maziarka, P.; Wurzer, C.; Arauzo, P.J.; Dieguez-Alonso, A.; Mašek, O.; Ronsse, F. Do You BET on Routine? The Reliability of N2 Physisorption for the Quantitative Assessment of Biochar’s Surface Area. Chem. Eng. J. 2021, 418, 129234. [Google Scholar] [CrossRef]
- Taslim, R.; Refanza, R.; Hamdy, M.I.; Apriwandi, A.; Taer, E. One-Step Strategy of 3D Hierarchical Porous Carbon with Self-Heteroatom-Doped Derived Bread Waste for High-Performance Supercapacitor. J. Anal. Appl. Pyrolysis 2023, 171, 105956. [Google Scholar] [CrossRef]
- Jin, Z.; Jin, S.; Tang, X.; Tan, W.; Wang, D.; Song, S.; Zhang, H.; Zeng, T. Rational Design of Conjugated Acetylenic Polymers Enables a Two-Electron Water Oxidation Pathway for Enhanced Photosynthetic Hydrogen Peroxide Generation. Small 2024, 20, 2305004. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Shao, G.; Wang, S. Porous Carbons: Structure-Oriented Design and Versatile Applications. Adv. Funct. Mater. 2020, 30, 1909265. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and Graphitized Porous Biochar Composites for Activating Peroxymonosulfate to Degrade P-Hydroxybenzoic Acid: Insights on the Mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Hu, P.; Su, H.; Chen, Z.; Yu, C.; Li, Q.; Zhou, B.; Alvarez, P.J.J.; Long, M. Selective Degradation of Organic Pollutants Using an Efficient Metal-Free Catalyst Derived from Carbonized Polypyrrole via Peroxymonosulfate Activation. Environ. Sci. Technol. 2017, 51, 11288–11296. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Niu, Q.; Zeng, G.; Zhang, C.; Huang, D.; Shao, B.; Zhou, C.; Yang, Y.; Liu, Y.; Guo, H.; et al. 1D Porous Tubular G-C3N4 Capture Black Phosphorus Quantum Dots as 1D/0D Metal-Free Photocatalysts for Oxytetracycline Hydrochloride Degradation and Hexavalent Chromium Reduction. Appl. Catal. B Environ. 2020, 273, 119051. [Google Scholar] [CrossRef]
- Ai, M.; Zhang, J.W.; Gao, R.; Pan, L.; Zhang, X.; Zou, J.J. MnOx-Decorated 3D Porous C3N4 with Internal Donor–Acceptor Motifs for Efficient Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2019, 256, 117805. [Google Scholar] [CrossRef]
- Lan, X.; Li, Q.; Zhang, Y.; Li, Q.; Ricardez-Sandoval, L.; Bai, G. Engineering Donor-Acceptor Conjugated Organic Polymers with Boron Nitride to Enhance Photocatalytic Performance towards Visible-Light-Driven Metal-Free Selective Oxidation of Sulfides. Appl. Catal. B Environ. 2020, 277, 119274. [Google Scholar] [CrossRef]
- Shao, P.; Tian, J.; Yang, F.; Duan, X.; Gao, S.; Shi, W.; Luo, X.; Cui, F.; Luo, S.; Wang, S. Identification and Regulation of Active Sites on Nanodiamonds: Establishing a Highly Efficient Catalytic System for Oxidation of Organic Contaminants. Adv. Funct. Mater. 2018, 28, 1705295. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.D.; Lim, T.T. Graphene- and CNTs-Based Carbocatalysts in Persulfates Activation: Material Design and Catalytic Mechanisms. Chem. Eng. J. 2018, 354, 941–976. [Google Scholar] [CrossRef]
- Bayoka, H.; Snoussi, Y.; Bhakta, A.K.; El Garah, M.; Khalil, A.M.; Jouini, M.; Ammar, S.; Chehimi, M.M. Evidencing the Synergistic Effects of Carbonization Temperature, Surface Composition and Structural Properties on the Catalytic Activity of Biochar/Bimetallic Composite. J. Anal. Appl. Pyrolysis 2023, 173, 106069. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Wei, T.; Ren, Y.; Ma, J. Oxygen Vacancies Induced Heterogeneous Catalysis of Peroxymonosulfate by Ni-Doped AgFeO2 Materials: Evolution of Reactive Oxygen Species and Mechanism. Chem. Eng. J. 2020, 388, 124371. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, S.; Li, N.; Chen, G.; Hou, L. Applications of Vacancy Defect Engineering in Persulfate Activation: Performance and Internal Mechanism. J. Hazard. Mater. 2023, 449, 130971. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, Y.; Hong, P.; Li, Y.; Xie, C.; Wu, Z.; Li, M.; Kong, L. Journal of Environmental Chemical Engineering Oxygen Vacancies of Mn/CeOx-H Induced Non-Radical Activation of Peroxymonosulfate through Electron Mediation for Bisphenol A Degradation. J. Environ. Chem. Eng. 2023, 11, 111078. [Google Scholar] [CrossRef]
- Xie, L.; Hao, J.; Wu, Y.; Xing, S. Non-Radical Activation of Peroxymonosulfate with Oxygen Vacancy-Rich Amorphous MnO X for Removing Sulfamethoxazole in Water. Chem. Eng. J. 2022, 436, 135260. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The Potential Role of Biochar in the Removal of Organic and Microbial Contaminants from Potable and Reuse Water: A Review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Tang, X.; Cai, X.; Jin, S.; Zhu, Y.; Xu, W.; Song, S.; Zhang, H. Fluorinated Defect-Engineered Acetylenic Polymers with Separated Active Centers for Switching the Photosensitized Activation Pathway of Peroxymonosulfate. ACS Catal. 2024, 14, 1405–1418. [Google Scholar] [CrossRef]
- Bao, Y.; Shan, Y.; Lim, T.; Wang, R.; David, R. Polyacrylonitrile (PAN) -Induced Carbon Membrane with in-Situ Encapsulated Cobalt Crystal for Hybrid Peroxymonosulfate Oxidation- Fi Ltration Process: Preparation, Characterization and Performance Evaluation. Chem. Eng. J. 2019, 373, 425–436. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, X.; Zeng, W.; Nie, Y.; Yang, C.; Dai, C.; Li, Y.; Lu, L. Enhanced Peroxymonosulfate Decomposition into •OH and 1O2 for Sulfamethoxazole Degradation over Se Doped g-C3N4 Due to Induced Exfoliation and N Vacancies Formation. Sep. Purif. Technol. 2021, 267, 118664. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Iron and Sulfur Co-Doped Graphite Carbon Nitride (FeOy/S-g-C3N4) for Activating Peroxymonosulfate to Enhance Sulfamethoxazole Degradation. Chem. Eng. J. 2020, 382, 122836. [Google Scholar] [CrossRef]
- Bao, Y.; Oh, W.; Lim, T.; Wang, R.; David, R.; Hu, X. Elucidation of Stoichiometric Efficiency, Radical Generation and Transformation Pathway during Catalytic Oxidation of Sulfamethoxazole via Peroxymonosulfate Activation. Water Res. 2019, 151, 64–74. [Google Scholar] [CrossRef]
- Xu, X.; Lin, R.; Deng, X.; Liu, J. In Situ Synthesis of FeOOH-Coated Trimanganese Tetroxide Composites Catalyst for Enhanced Degradation of Sulfamethoxazole by Peroxymonosulfate Activation. Sep. Purif. Technol. 2021, 275, 119184. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, T.; Zhang, J.; Wei, R.; You, S.; Xu, Y. Facile Synthesis of Oxygen Vacancies Enriched α-Fe2O3 for Peroxymonosulfate Activation: A Non-Radical Process for Sulfamethoxazole Degradation. J. Hazard. Mater. 2021, 419, 126447. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Peroxymonosulfate Activation by Fe−Co−O−Codoped Graphite Carbon Nitride for Degradation of Sulfamethoxazole. Environ. Sci. Technol. 2020, 54, 10361–10369. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, H.; Pan, Z.; Liu, Y.; Yao, G.; Guo, Y.; Lai, B. Degradation of Sulfamethoxazole by Cobalt-Nickel Powder Composite Catalyst Coupled with Peroxymonosulfate: Performance, Degradation Pathways and Mechanistic Consideration. J. Hazard. Mater. 2020, 400, 123322. [Google Scholar] [CrossRef]
- Guo, R.; Wang, Y.; Li, J.; Cheng, X.; Dionysiou, D.D. Sulfamethoxazole Degradation by Visible Light Assisted Peroxymonosulfate Process Based on Nanohybrid Manganese Dioxide Incorporating Ferric Oxide. Appl. Catal. B Environ. 2020, 278, 119297. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Q.; Li, Y.; Li, Y.; Gou, J.; Cheng, X. Degradation of Sulfamethoxazole by Peroxymonosulfate Activated by Waste Eggshell Supported Ag2O–Ag Nano–Particles. Chem. Eng. J. 2021, 405, 126719. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, Y.; He, P.; Zhu, J.; Gan, M. Insights into Biochar Supported Atomically Dispersed Cobalt as an Efficient Peroxymonosulfate Activator for Sulfamethoxazole Degradation: Robust Performance, ROS and Surface Electron-Transfer Pathways. Environ. Sci. Nano 2022, 9, 3551–3561. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Zhang, Y.; Cao, Q.; Si, Y.; Wu, Z.; Akram, M.; Xu, X. Applied Catalysis B: Environmental Novel Lignin-Based Single Atom Catalysts as Peroxymonosulfate Activator for Pollutants Degradation: Role of Single Cobalt and Electron Transfer Pathway. Appl. Catal. B Environ. 2021, 286, 119910. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, X.; Tang, M.; Du, K.; Yin, H.; Mao, X. Waste Eggshell-Derived N, P, S Tri-Doped Core-Shell Catalysts for Efficient Fenton-like Catalysis. Chem. Eng. J. 2022, 440, 135879. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Z.; Wu, P.; Li, Y.; Dong, X.; Li, C.; Zhu, N.; Duan, X.; Dionysiou, D.D. Rapid Removal of Tetrabromobisphenol A by A-Fe2O3-X@Graphene@Montmorillonite Catalyst with Oxygen Vacancies through Peroxymonosulfate Activation: Role of Halogen and A-Hydroxyalkyl Radicals. Appl. Catal. B Environ. 2020, 260, 118129. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, H.; Wang, W.; Li, W.; Ren, Y.; Li, X. Synthesis of Fe0/Fe3O4@Porous Carbon through a Facile Heat Treatment of Iron-Containing Candle Soots for Peroxymonosulfate Activation and Efficient Degradation of Sulfamethoxazole. J. Hazard. Mater. 2021, 411, 124952. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, M.; Xie, C.; Wang, H.; Wang, J.; Chen, Z.; Xiao, J.; Chen, Z. Applied Catalysis B: Environmental Tunable S Doping from Co3O4 to Co9S8 for Peroxymonosulfate Activation: Distinguished Radical / Nonradical Species and Generation Pathways. Appl. Catal. B Environ. 2021, 282, 119605. [Google Scholar] [CrossRef]
- Qin, L.; Sun, Q.; Lai, C.; Liu, S.; Qin, X.; Chen, W.; Fu, Y.; Zhou, X.; Xu, F.; Ma, D. Sulfonic Acid Metal-Organic Frameworks Derived Iron-Doped Carbon as Novel Heterogeneous Electro-Fenton Catalysts for the Degradation of Tetracycline: Performance and Mechanism Investigation. Chem. Eng. J. 2023, 474, 145722. [Google Scholar] [CrossRef]
- Pang, K.; Sun, W.; Ye, F.; Yang, L.; Pu, M.; Yang, C.; Zhang, Q.; Niu, J. Sulfur-Modified Chitosan Derived N, S-Co-Doped Carbon as a Bifunctional Material for Adsorption and Catalytic Degradation Sulfamethoxazole by Persulfate. J. Hazard. Mater. 2022, 424, 127270. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Insight into the Mechanism of Persulfate Activated by Bone Char: Unraveling the Role of Functional Structure of Biochar. Chem. Eng. J. 2020, 401, 126127. [Google Scholar] [CrossRef]
- Hou, L.; Li, X.; Yang, Q.; Chen, F.; Wang, S.; Ma, Y.; Wu, Y.; Zhu, X.; Huang, X.; Wang, D. Heterogeneous Activation of Peroxymonosulfate Using Mn-Fe Layered Double Hydroxide: Performance and Mechanism for Organic Pollutant Degradation. Sci. Total Environ. 2019, 663, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; He, D.; Chang, Y.; Zhang, Q.; Wen, F.; Wang, X. Cobalt Oxyhydroxide as an Ef Fi Cient Heterogeneous Catalyst of Peroxymonosulfate Activation for Oil-Contaminated Soil Remediation. Sci. Total Environ. 2019, 680, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Z.; Jiang, X.; Yu, J.; Wang, Y.; Jin, S.; Zhang, H.; Song, S.; Zeng, T. Thiophene-Incorporated CTFs with Boosted Intramolecular Charge Transfer and Defect-Abundant Networks for Potent Photosensitized Oxidation of Organics. Chem. Eng. J. 2022, 431, 133900. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Wu, X.; Liu, Y. Non-Photochemical Production of Singlet Oxygen via Activation of Persulfate by Carbon Nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- You, J.; Sun, W.; Su, S.; Ao, Z.; Liu, C.; Yao, G.; Lai, B. Degradation of Bisphenol A by Peroxymonosulfate Activated with Oxygen Vacancy Modified Nano-NiO-ZnO Composite Oxides: A Typical Surface-Bound Radical System. Chem. Eng. J. 2020, 400, 125915. [Google Scholar] [CrossRef]
- Feng, Y.; Lee, P.; Wu, D.; Shih, K. Surface-Bound Sulfate Radical-Dominated Degradation of 1,4-Dioxane by Alumina-Supported Palladium (Pd/Al2O3) Catalyzed Peroxymonosulfate. Water Res. 2017, 120, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Bin, Q.; Shen, Y.; Huang, J.; He, D.; Chen, W. In-Situ Formed N-Doped Bamboo-like Carbon Nanotubes Encapsulated with Fe Nanoparticles Supported by Biochar as Highly efficient Catalyst for Activation of Persulfate (PS) toward Degradation of Organic Pollutants. Chem. Eng. J. 2020, 402, 126090. [Google Scholar] [CrossRef]
- Kong, X.; Chen, A.; Chen, L.; Feng, L.; Wang, W.; Li, J.; Du, Q.; Sun, W.; Zhang, J. Enhanced Fenton-like Catalytic Performance of Freestanding CuO Nanowires by Coating with g-C3N4 Nanosheets. Sep. Purif. Technol. 2021, 272, 118850. [Google Scholar] [CrossRef]
- Liu, H.; Dai, X.; Kong, L.; Sui, C.; Nie, Z.; Liu, Y.; Cai, B.; Ni, S.; Boczkaj, G.; Zhan, J. Ball Milling Treatment of Mn3O4 Regulates Electron Transfer Pathway for Peroxymonosulfate Activation. Chem. Eng. J. 2023, 467, 143339. [Google Scholar] [CrossRef]
- Huang, K.Z.; Zhang, H. Direct Electron-Transfer-Based Peroxymonosulfate Activation by Iron-Doped Manganese Oxide (δ-MnO2) and the Development of Galvanic Oxidation Processes (GOPs). Environ. Sci. Technol. 2019, 53, 12610–12620. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, R.; Kang, J.; Li, Z.; Pan, Y.; Bai, J.; Li, J.; Qiu, R. Atomic Cation-Vacancy Modulated Peroxymonosulfate Nonradical Oxidation of Sulfamethoxazole via High-Valent Iron-Oxo Species. Appl. Catal. B Environ. 2023, 330, 122640. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Ren, N.; Zeng, L.; Zhu, M. New Insight into the Substituents Affecting the Peroxydisulfate Nonradical Oxidation of Sulfonamides in Water. Water Res. 2020, 171, 115374. [Google Scholar] [CrossRef]
- Ahn, Y.-Y.; Yun, E.-T.; Seo, J.-W.; Lee, C.; Kim, S.H.; Kim, J.-H.; Lee, J. Activation of Peroxymonosulfate by Surface-Loaded Noble Metal Nanoparticles for Oxidative Degradation of Organic Compounds. Environ. Sci. Technol. 2016, 50, 10187–10197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, X.; Wu, Z.; Wu, W.D.; Wang, X.; Chen, X.D.; Sun, S. Fe-Mn Bimetallic Oxide-Enabled Facile Cleaning of Micro Fi Ltration Ceramic Membranes for E Ffl Uent Organic Matter Fouling Mitigation via Activation of Oxone. ACS ES&T Water 2022, 2, 1234–1246. [Google Scholar] [CrossRef]
- Chu, C.; Yang, J.; Zhou, X.; Huang, D.; Qi, H.; Weon, S.; Li, J.; Elimelech, M.; Wang, A.; Kim, J. Cobalt Single Atoms on Tetrapyridomacrocyclic Support for E Ffi Cient Peroxymonosulfate Activation. Environ. Sci. Technol. 2021, 55, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Ma, S.; Xu, S.; Wang, B.; He, M.; Li, G.; Fu, H.; Zhao, P. Soybean Straw Biochar Activating Peroxydisulfate to Simultaneously Eliminate Tetracycline and Tetracycline Resistance Bacteria: Insights on the Mechanism. Water Res. 2022, 218, 118489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jin, R.; Zhang, B. A Novel Z-Scheme Er3+:Yb0.5Y2.5Al5N0.01F0.01O11.98/g-C3N4–Pt–BiPO4 Photocatalyst for Enhanced Photocatalytic Degradation of Azofuchsin and Ciprofloxacin. J. Am. Ceram. Soc. 2021, 104, 6319–6334. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Huang, Y.; Shi, X.; Zhang, Y.; Huang, T.; Cao, J.; Ho, W.; Lee, S.C. Self-Assembly Synthesis of Boron-Doped Graphitic Carbon Nitride Hollow Tubes for Enhanced Photocatalytic NOx Removal under Visible Light. Appl. Catal. B Environ. 2018, 239, 352–361. [Google Scholar] [CrossRef]
- Jin, Z.; Jin, S.; Zou, R.; Wang, D.; Dong, F.; Liu, M.; Song, S.; Zeng, T. Intramolecular Engineering of Defective Terminations within Triazine-Based Conjugated Polymers for Augmented Photocatalytic H2 Production and Cr(VI) Reduction. J. Clean. Prod. 2023, 422, 138556. [Google Scholar] [CrossRef]
- Eun, J.; Kant, S.; Jin, H.; Yoo, E.; Jin, H.; Yoon, J. Bioresource Technology Adsorptive Removal of Tetracycline from Aqueous Solution by Maple Leaf- Derived Biochar. Bioresour. Technol. 2020, 306, 123092. [Google Scholar] [CrossRef]
- Sievers, M. Advanced Oxidation Processes; Springer International Publishing: Cham, Switzerland, 2024; Volume 4, ISBN 9780444531933. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yang, J.; Zheng, K.; He, S.; Liu, Z.; Song, S.; Zeng, T. Effective Activation of Peroxymonosulfate by Oxygen Vacancy Induced Musa Basjoo Biochar to Degrade Sulfamethoxazole: Efficiency and Mechanism. Toxics 2024, 12, 283. https://doi.org/10.3390/toxics12040283
Li S, Yang J, Zheng K, He S, Liu Z, Song S, Zeng T. Effective Activation of Peroxymonosulfate by Oxygen Vacancy Induced Musa Basjoo Biochar to Degrade Sulfamethoxazole: Efficiency and Mechanism. Toxics. 2024; 12(4):283. https://doi.org/10.3390/toxics12040283
Chicago/Turabian StyleLi, Shuqi, Jian Yang, Kaiwen Zheng, Shilong He, Zhigang Liu, Shuang Song, and Tao Zeng. 2024. "Effective Activation of Peroxymonosulfate by Oxygen Vacancy Induced Musa Basjoo Biochar to Degrade Sulfamethoxazole: Efficiency and Mechanism" Toxics 12, no. 4: 283. https://doi.org/10.3390/toxics12040283
APA StyleLi, S., Yang, J., Zheng, K., He, S., Liu, Z., Song, S., & Zeng, T. (2024). Effective Activation of Peroxymonosulfate by Oxygen Vacancy Induced Musa Basjoo Biochar to Degrade Sulfamethoxazole: Efficiency and Mechanism. Toxics, 12(4), 283. https://doi.org/10.3390/toxics12040283