Distribution and Risk Assessment of Organophosphate Esters in Agricultural Soils and Plants in the Coastal Areas of South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Chemical Material and Method
2.3. Sample Extraction and Purification
2.4. Instrumental Analysis
2.5. Ecological Risk and Human Health Risk Assessment
2.5.1. Ecological Risk Assessment
2.5.2. Human Health Risk Assessment
2.6. Quality Assurance and Quality Control
2.7. Data Processing and Analysis
3. Results and Discussion
3.1. General Characteristics of OPE Concentrations in Soil
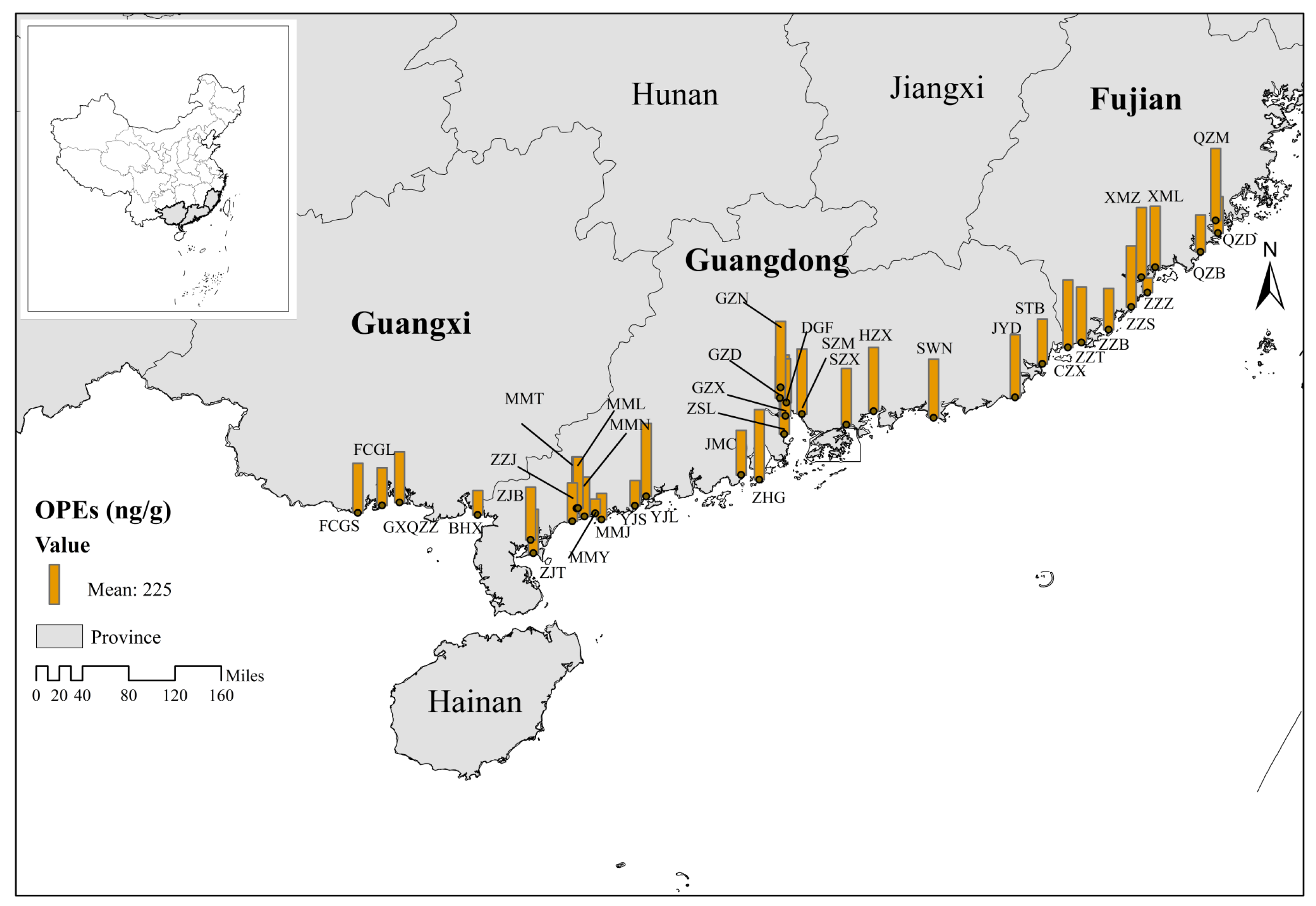
3.2. Regional Differences in OPE Contamination
3.3. Overall Characteristics of OPE Contamination in Plants
3.4. Ecological Risk and Human Risk Assessments of OPEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, G.L.; Li, D.Q.; Zhuo, M.N.; Liao, Y.S.; Xie, Z.Y.; Guo, T.L.; Li, J.J.; Zhang, S.Y.; Liang, Z.Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.M.; Shi, Y.L.; Na, G.S.; Cai, Y.Q. A review of organophosphate esters in indoor dust, air, hand wipes and silicone wristbands: Implications for human exposure. Environ. Int. 2021, 146, 106261. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate ester flame retardants: Are they a regrettable substitution for polybrominated diphenyl ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y.; Bao, L.; Wu, C.C.; Liu, L.Y.; Wong, C.S.; Zeng, E.Y. Organophosphate flame retardants emitted from thermal treatment and open burning of e-waste. J. Hazard. Mater. 2019, 367, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.J.; Yu, K.F.; Li, A.; Zeng, W.B.; Lin, T.; Wang, Y.H. Occurrence, phase distribution, and bioaccumulation of organophosphate esters (OPEs) in mariculture farms of the Beibu Gulf, China: A health risk assessment through seafood consumption. Environ. Pollut. 2020, 263, 114426. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L. Data on fate and distribution of organophosphate esters in the soil-sediments from Kathmandu Valley, Nepal. Data Brief 2020, 28, 104822. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Gu, L.Y.; Wu, Z.P.; Shan, Y.; Wang, H.; Sun, L.N. Distribution, source apportionment and ecological risks of organophosphate esters in surface sediments from the Liao River, Northeast China. Chemosphere 2020, 250, 126297. [Google Scholar] [CrossRef]
- Guo, J.; Li, Z.; Ranasinghe, P.; Rockne, K.J.; Sturchio, N.C.; Giesy, J.P.; Li, A. Halogenated flame retardants in sediments from the Upper Laurentian Great Lakes: Implications to long-range transport and evidence of long-term transformation. J. Hazard. Mater. 2020, 384, 121346. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.M.; Zhang, Y.Y.; Deng, Y.R.; Jian, K.; Li, J.H.; Ya, M.L.; Su, G.Y. Traditional and emerging organophosphate esters (OPEs) in indoor dust of Nanjing, eastern China: Occurrence, human exposure, and risk assessment. Sci. Total Environ. 2020, 712, 136494. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Tan, F.; Bao, M.; Zhang, L.; Rodgers, T.F.; Chen, J. Characteristics and risk assessment of organophosphorus flame retardants in urban road dust of Dalian, Northeast China. Sci. Total Environ. 2020, 705, 135995. [Google Scholar] [CrossRef]
- Tao, F.; Sellström, U.; De Wit, C.A. Organohalogenated Flame Retardants and Organophosphate Esters in Office Air and Dust from Sweden. Environ. Sci. Technol. 2019, 53, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Shi, Y.L.; Gao, L.H.; Wu, C.D.; Liu, J.M.; Cai, Y.Q. Occurrence, distribution and risk of organophosphate esters in urban road dust in Beijing, China. Environ. Pollut. 2018, 241, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.M.; Shi, Y.L.; Na, G.S.; Zhao, Z.S.; Cai, Y.Q. Increased Human Exposure to Organophosphate Esters via Ingestion of Drinking Water from Water Dispensers: Sources, Influencing Factors, and Exposure Assessment. Environ. Sci. Technol. Lett. 2021, 8, 884–889. [Google Scholar] [CrossRef]
- Regnery, J.; Püttmann, W. Seasonal fluctuations of organophosphate concentrations in precipitation and storm water runoff. Chemosphere 2010, 78, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hao, Y.F.; Li, Y.M.; Yang, R.Q.; Wang, P.; Zhang, G.X.; Zhang, Q.H.; Jiang, G.B. Occurrence and distribution of organophosphate esters in the air and soils of Ny-Alesund and London Island, Svalbard, Arctic. Environ. Pollut. 2020, 263, 114495. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Li, J.; Zhang, G.; Covaci, A. Concentration and spatial distribution of organophosphate esters in the soil-sediment profile of Kathmandu Valley, Nepal: Implication for risk assessment. Sci. Total Environ. 2018, 613–614, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, J.H.; Mi, W.Y.; Tian, C.G.; Emeis, K.C.; Ebinghaus, R.; Xie, Z.Y. Spatial Distribution and Seasonal Variation of Organophosphate Esters in Air above the Bohai and Yellow Seas, China. Environ. Sci. Technol. 2018, 52, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Marklund, A.; Andersson, B.; Haglund, P. Screening of organophosphorus compounds and their distribution in various indoor environments. Chemosphere 2003, 53, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.C.; Xie, Z.Y.; Song, T.L.; Tang, J.H.; Zhang, Y.Y.; Mi, W.Y.; Peng, J.H.; Zhao, Y.; Zou, S.C.; Ebinghaus, R. Occurrence and dry deposition of organophosphate esters in atmospheric particles over the northern South China Sea. Chemosphere 2015, 127, 195–200. [Google Scholar] [CrossRef]
- Li, J.; Xie, Z.Y.; Mi, E.Y.; Lai, S.C.; Tian, C.G.; Emeis, K.C.; Ebinghaus, R. Organophosphate Esters in Air, Snow, and Seawater in the North Atlantic and the Arctic. Environ. Sci. Technol. 2017, 51, 6887–6896. [Google Scholar] [CrossRef]
- Huang, Y.C.; Tan, H.L.; Li, L.Z.; Yang, L.; Sun, F.J.; Li, J.; Gong, X.; Chen, D. A broad range of organophosphate tri- and di-esters in house dust from Adelaide, South Australia: Concentrations, compositions, and human exposure risks. Environ. Int. 2020, 142, 105872. [Google Scholar] [CrossRef]
- Wang, G.G.; Liu, Y.; Zhao, X.D.; Tao, W.; Wang, H.X. Geographical distributions and human exposure of organophosphate esters in college library dust from Chinese cities. Environ. Pollut. 2019, 255, 113332. [Google Scholar] [CrossRef] [PubMed]
- Smythe, T.A.; Mattioli, L.C.; Letcher, R.J. Distribution behaviour in body compartments and in ovo transfer of flame retardants in North American Great Lakes herring gulls. Environ. Pollut. 2020, 262, 114306. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.M.; Shi, Y.L.; Jin, Q.; Cai, Y.Q. Organophosphate esters and their metabolites in paired human whole blood, serum, and urine as biomarkers of exposure. Environ. Int. 2020, 139, 105698. [Google Scholar] [CrossRef]
- Wang, G.W.; Shi, H.H.; Du, Z.K.; Chen, H.Y.; Peng, J.B.; Gao, S.X. Bioaccumulation mechanism of organophosphate esters in adult zebrafish (Danio rerio). Environ. Pollut. 2017, 229, 177–187. [Google Scholar] [CrossRef]
- Long, S.X.; Hamilton, P.B.; Fu, B.; Xu, J.; Han, L.C.; Suo, X.H.; Lai, Y.Q.; Shen, G.F.; Xu, F.L.; Li, B.G. Bioaccumulation and emission of organophosphate esters in plants affecting the atmosphere’s phosphorus cycle. Environ. Int. 2023, 318, 120803. [Google Scholar] [CrossRef]
- Deng, X.; Yin, H.L.; HE, W.L.; Luo, Y.; Wu, D.; Luo, L.; Chen, J. Distribution and migration of OPEs in soil profile and crops in urban and suburban areas of Chengdu. Environ. Chem. 2019, 38, 679–685. [Google Scholar]
- Picó, Y.; Campo, J.; Alfarhan, A.H.; El-Sheikh, M.A.; Barceló, D. A reconnaissance study of pharmaceuticals, pesticides, perfluoroalkyl substances and organophosphorus flame retardants in the aquatic environment, wild plants and vegetables of two Saudi Arabia urban areas: Environmental and human health risk assessment. Sci. Total Environ. 2021, 776, 145843. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, M.S.E.; Mäkinen, M.R.A.; Koistinen, J.T.B.; Pasanen, A.L.; Pasanen, P.O.; Kalliokoski, P.J.; Korpi, A.M. Respiratory and dermal exposure to organophosphorus flame retardants and tetrabromobisphenol A at five work environments. Environ. Sci. Technol. 2009, 43, 941–947. [Google Scholar] [CrossRef]
- Meeker, J.D.; Cooper, E.M.; Stapleton, H.M.; Hauser, R. Urinary metabolites of organophosphate flame retardants: Temporal variability and correlations with house dust concentrations. Environ. Health Perspect. 2013, 121, 580–585. [Google Scholar] [CrossRef]
- Zheng, G.M.; Schreder, E.; Dempsey, J.C.; Uding, N.; Chu, V.; Andres, G.; Sathyanarayana, S.; Salamova, A. Organophosphate Esters and Their Metabolites in Breast Milk from the United States: Breastfeeding Is an Important Exposure Pathway for Infants. Environ. Sci. Technol. Lett. 2021, 8, 224–230. [Google Scholar] [CrossRef]
- Su, G.Y.; Crump, D.; Letcher, R.J.; Kennedy, S.W. Rapid in vitro metabolism of the flame retardant triphenyl phosphate and effects on cytotoxicity and mRNA expression in chicken embryonic hepatocytes. Environ. Sci. Technol. 2014, 48, 13511–13519. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.M.; Lee, H.J.; Lee, B.C.; Ra, J.S.; Kim, K.T. Effects of the chorion on the developmental toxicity of organophosphate esters in zebrafish embryos. J. Hazard. Mater. 2021, 401, 123389. [Google Scholar] [CrossRef] [PubMed]
- Sutha, J.; Anila, P.A.; Umamaheswari, S.; Ramesh, M.; Narayanasamy, A.; Poopal, R.K.; Ren, Z.M. Biochemical responses of a freshwater fish Cirrhinus mrigala exposed to tris(2-chloroethyl) phosphate (TCEP). Environ. Sci. Pollut. Res. 2020, 27, 34369–34387. [Google Scholar] [CrossRef]
- Hales, B.F.; Robaire, B. Effects of brominated and organophosphate ester flame retardants on male reproduction. Andrology 2020, 8, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xu, Y.P.; Wang, Z.J. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, A.; Mubeen, S.; Liu, M.Y.; Bekele, T.G.; Ohoro, C.R.; Adeniji, A.O.; Alraih, A.M.; Ajmal, Z.; Alshammari, A.S.; Al-Hadeethi, Y.; et al. Global environmental and toxicological impacts of polybrominated diphenyl ethers versus organophosphate esters: A comparative analysis and regrettable substitution dilemma. J. Hazard. Mater. 2024, 466, 133543. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.W.; Tan, H.N.; Peng, T.; Wang, S.S.; Xu, W.B.; Qian, H.F.; Jin, Y.X.; Fu, Z.W. Developmental neurotoxicity of organophosphate flame retardants in early life stages of Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2016, 35, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Hammel, S.C.; Nordone, S.; Zhang, S.; Lorenzo, A.M.; Eichner, B.; Moody, M.A.; Harrington, L.; Gandee, J.; Schmidt, L.; Smith, S.; et al. Infants’ diminished response to DTaP vaccine is associated with exposure to organophosphate esters. Sci. Total Environ. 2022, 837, 155782. [Google Scholar] [CrossRef]
- Lee, G.; Kim, S.; Bastiaensen, M.; Malarvannan, G.; Poma, G.; Casero, N.C.; Gys, C.; Covaci, A.; Le, S.; Lim, J.E.; et al. Exposure to organophosphate esters, phthalates, and alternative plasticizers in association with uterine fibroids. Environ. Res. 2020, 189, 109874. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, Y.; Dong, S.S.; Han, L.; Guo, R.X.; Fu, Y.R.; Zhang, S.H.; Chen, J.Q. The risk and impact of organophosphate esters on the development of female-specific cancers: Comparative analysis of patients with benign and malignant tumors. J. Hazard. Mater. 2021, 404, 124020. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Li, J.; Zhang, G. Organophosphate ester flame retardants in Nepalese soil: Spatial distribution, source apportionment and air-soil exchange assessment. Chemosphere 2018, 190, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Liu, J.F.; Yin, Y.G.; Shen, M.H. Evaluating the sorption of organophosphate esters to different sourced humic acids and its effects on the toxicity to Daphnia magna. Environ. Toxicol. Chem. 2013, 32, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Z.; Zhao, H.X.; Bekele, T.G.; Qu, B.C.; Chen, J.W. Organophosphate esters (OPEs) in wetland soil and Suaeda salsa from intertidal Laizhou Bay, North China: Levels, distribution, and soil-plant transfer model. Sci. Total Environ. 2021, 764, 142891. [Google Scholar] [CrossRef]
- You, J.; Chen, Z.M.; Hou, X.Y.; Guo, J.S.; Wang, C.C.; Gao, J.M. Occurrence, potential sources and risks of organophosphate esters in the high-elevation region, Tibet, China. Sci. Total Environ. 2022, 806, 151348. [Google Scholar] [CrossRef]
- Liu, M.Y.; Guo, C.S.; Zhu, C.F.; Lv, J.F.; Yang, W.L.; Wu, L.L.; Xu, J. Vertical profile and assessment of soil pollution from a typical coking plant by suspect screening and non-target screening using GC/QTOF-MS. Sci. Total Environ. 2022, 810, 151278. [Google Scholar] [CrossRef]
- Ge, X.; Ma, S.T.; Zhang, X.L.; Yang, Y.; Li, G.Y.; Yu, Y.X. Halogenated and organophosphorus flame retardants in surface soils from an e-waste dismantling park and its surrounding area: Distributions, sources, and human health risks. Environ. Int. 2020, 139, 105741. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Yu, X.L.; Zhang, D.Q.; Jin, L.; Huang, J.H.; Zhu, X.F.; Sun, J.T.; Yu, M.; Zhu, L.Z. Biotransformation of Organophosphate Esters by Rice and Rhizosphere Microbiome: Multiple Metabolic Pathways, Mechanism, and Toxicity Assessment. Environ. Sci. Technol. 2023, 57, 1776–1787. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Huang, J.H.; Jin, L.; Yu, M.; Yu, X.L.; Zhu, X.F.; Sun, J.T.; Zhu, L.Z. Translocation and metabolism of tricresyl phosphate in rice and microbiome system: Isomer-specific processes and overlooked metabolites. Environ. Int. 2023, 172, 107793. [Google Scholar] [CrossRef]
- Yu, X.L.; Li, M.; Tang, S.Y.; Wei, Z.; Yu, Y.Y.; Sun, J.T.; Lu, G.N.; Yin, H. Photocatalysis of Tris-(2-chloroethyl) phosphate by ultraviolet driven peroxymonosulfate oxidation process: Removal performance, energy evaluation and toxicity on bacterial metabolism network. Chem. Eng. J. 2021, 423, 130261. [Google Scholar] [CrossRef]
- Yu, X.L.; Yin, H.; Peng, H.; Lu, G.N.; Liu, Z.H.; Li, H.Y.; Dang, Z. Degradation mechanism, intermediates and toxicology assessment of tris-(2-chloroisopropyl) phosphate using ultraviolet activated hydrogen peroxide. Chemosphere 2020, 241, 124991. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.K.G.; Garcia, L.F.; Lobón, G.S.; Brito, L.B.; Oliveira, G.A.R.; Luque, R.; Gil, E.D.S. Ecotoxicological assessment and electrochemical remediation of doxorubicin. Ecotox. Environ. Safe. 2019, 179, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Pan, L.L.; Tsang, D.C.W.; Li, Z.H.; Zhu, L.Z.; Li, X.D. Phthalate esters and organochlorine pesticides in agricultural soils and vegetables from fast-growing regions: A case study from eastern China. Environ. Sci. Pollut. Res. 2018, 25, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Pan, L.L.; Zhan, Y.; Lu, H.N.; Tsang, D.C.W.; Liu, W.X.; Wang, X.L.; Li, X.D.; Zhu, L.Z. Contamination of phthalate esters, organochlorine pesticides and polybrominated diphenyl ethers in agricultural soils from the Yangtze River Delta of China. Sci. Total Environ. 2016, 544, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.W.; Mei, X.X.; Sun, J.X.; Hu, Y. Sources, Distribution and Major Transformation Process of Typical Endocrine Disruptors in the Environment. Asian J. Ecotoxicol. 2018, 13, 42–55. [Google Scholar]
- US Environmental Protection Agency. USEPA Exposure Factors Handbook, final ed.; US Environmental Protection Agency: Washington, DC, USA, 2011; [EPA/600/R-09/052F]. [Google Scholar]
- Zhang, X.L.; Zou, W.; Mu, L.; Chen, Y.M.; Ren, C.X.; Hu, X.G.; Zhou, Q.X. Rice ingestion is a major pathway for human exposure to organophosphate flame retardants (OPFRs) in China. J. Hazard. Mater. 2016, 318, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Wang, L.M.; Gao, X.X.; Huang, Z.J.; Zhang, X.; Zhao, Z.P.; Li, C.; Zhang, M. Intake of vegetables and fruit among adults in China in 2018. Chin. J. Prev. Contr. Chron. Dis. 2022, 30, 561–566. [Google Scholar]
- Li, L.; Ouyang, Y.F.; Wang, H.J.; Huang, F.F.; Wang, Y.; Zhang, J.G.; Sui, C.; Du, W.W.; Jia, X.F.; Jiang, H.R.; et al. Status of fruit and vegetable intake among children and adolescents in 15 provinces of China. Chin. J. Health Educ. 2020, 36, 3–7. [Google Scholar]
- Kurt-Karakus, P.; Alegria, H.; Birgul, A.; Gungormus, E.; Jantunen, L. Organophosphate ester (OPEs) flame retardants and plasticizers in air and soil from a highly industrialized city in Turkey. Sci. Total Environ. 2018, 625, 555–565. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.Y.; Tan, F.; Xu, Y.; Zhao, H.X.; Chen, J.W. Occurrence and air-soil exchange of organophosphate flame retardants in the air and soil of Dalian, China. Environ. Pollut. 2020, 265 Pt A, 114850. [Google Scholar] [CrossRef]
- Tang, J.F.; Sun, J.; Ke, Z.Y.; Yin, H.L.; Yang, L.; Yen, H.; Li, X.H.; Xu, Y.Y. Organophosphate esters in surface soils from a heavily urbanized region of Eastern China: Occurrence, distribution, and ecological risk assessment. Environ. Pollut. 2021, 291, 118200. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.Y.; Wen, J.X.; Zeng, F.; Li, S.C.; Zhou, X.; Zeng, Z.X. Occurrence and distribution of organophosphate esters in urban soils of the subtropical city, Guangzhou, China. Chemosphere 2017, 175, 514–520. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Z.X.; Zhao, L.Y.; Ma, H.B.; Huang, J.; Li, H.Y.; Mao, X.X.; Huang, T.; Gao, H.; Ma, J.M. Gridded emission inventory of organophosphorus flame retardants in China and inventory validation. Environ. Pollut. 2021, 290, 118071. [Google Scholar] [CrossRef] [PubMed]
- He, M.J.; Yang, T.; Yang, Z.H.; Zhou, H.; Wei, S.Q. Current State, Distribution, and Sources of Phthalate Esters and Organophosphate Esters in Soils of the Three Gorges Reservoir Region, China. Arch. Environ. Contam. Toxicol. 2018, 74, 502–513. [Google Scholar] [CrossRef]
- Li, X.H.; Ma, J.; Fang, D.; Shi, T.R.; Gong, Y.W. Organophosphate Flame Retardants in Soils of Zhejiang Province, China: Levels, Distribution, Sources, and Exposure Risks. Arch. Environ. Contam. Toxicol. 2020, 78, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Zhu, H.K. A pilot study of organophosphate esters in surface soils collected from Jinan City, China: Implications for risk assessments. Environ. Sci. Pollut. Res. 2021, 28, 3344–3353. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wang, P.; Li, Y.M.; Wang, D.; Matsiko, J.; Yang, R.Q.; Sun, H.Z.; Hao, Y.F.; Zhang, Q.H.; Jiang, G.B. Spatial and temporal distribution of organophosphate esters in the atmosphere of the Beijing-Tianjin-Hebei region, China. Environ. Pollut. 2019, 244, 182–189. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, Y.; Yao, Y.M.; Ren, C.; Lan, Z.H.; Fang, X.G.; Zhang, K.; Sun, W.J.; Alder, A.C.; Sun, H.W. Occurrence of organophosphate flame retardants in farmland soils from northern China: Primary source analysis and risk assessment. Environ. Pollut. 2019, 247, 832–838. [Google Scholar] [CrossRef]
- Mihajlović, I.; Miloradov, M.V.; Fries, E. Application of Twisselmann Extraction, SPME, and GCMS To Assess Input Sources for Organophosphate Esters into Soil. Environ. Sci. Technol. 2011, 45, 2264–2269. [Google Scholar] [CrossRef]
- Casas, G.; Martinez-Varela, A.; Vila-Costa, M.; Jiménez, B.; Dachs, J. Rain amplification of persistent organic pollutants. Environ. Sci. Technol. 2021, 55, 12961–12972. [Google Scholar] [CrossRef]
- He, J.H.; Li, J.F.; Ma, L.Y.; Wu, N.; Zhang, Y.; Niu, Z.G. Large-scale distribution of organophosphate esters (flame retardants and plasticizers) in soil from residential area across China: Implications for current level. Sci. Total Environ. 2019, 697, 133997. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hu, Q.P.; Liu, J.; Liu, S.H.; Liu, C.J.; Deng, Q.Y.; Zeng, X.Y.; Yu, Z.Q. Occurrence of organophosphate esters and their diesters degradation products in industrial wastewater treatment plants in China: Implication for the usage and potential degradation during production processing. Environ. Pollut. 2019, 250, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.H.; Shi, Y.L.; Li, W.H.; Liu, J.M.; Cai, Y.Q. Occurrence and distribution of organophosphate triesters and diesters in sludge from sewage treatment plants of Beijing, China. Sci. Total Environ. 2016, 544, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.F.; Du, B.B.; Wang, F.; Lam, J.C.W.; Zeng, L.X.; Zeng, E.Y. Organophosphate triesters and diester degradation products in municipal sludge from wastewater treatment plants in China: Spatial patterns and ecological implications. Environ. Sci. Technol. 2017, 51, 13614–13623. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Zhang, W.J.; Zhang, S.Y.; Wang, Y.; Zhang, X.Y.; Lu, Y.; Sun, H.W.; Wang, L. Organophosphite antioxidants in mulch films are important sources of organophosphate pollutants in farmlands. Environ. Sci. Technol. 2021, 55, 7398–7406. [Google Scholar] [CrossRef] [PubMed]
- Veen, I.V.D.; Boer, J.D. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.X.; Jiang, X.X.; Xu, H.Z.; Luo, Y.Q.; Long, T.T.; Li, J.; Xing, L.Q. Spatial occurrence and composition profile of organophosphate esters (OPEs) in farmland soils from different regions of China: Implications for human exposure. Environ. Pollut. 2021, 276, 116729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, Y.M.; Li, W.H.; Zhu, H.K.; Wang, L.; Sun, H.W.; Kannan, K. A nationwide survey of 19 organophosphate esters in soils from China: Spatial distribution and hazard assessment. Sci. Total Environ. 2019, 671, 528–535. [Google Scholar] [CrossRef]
- Sang, Y.Z.; Li, W.H.; Liu, J.M.; Qu, C.; Zhao, Y.J.; Cai, H.M.; Hong, S.C. Simultaneous Determination of 13 Organophosphate Esters in Greenhouse Soil by Gas Chromatography-Mass Spectrometry. J. Instrum. Anal. 2020, 39, 1538–1543. [Google Scholar]
- Fisk, P.R.; Girling, A.E.; Wildey, R.J. Prioritisation of Flame Retardants for Environmental Risk Assessment; Environment Agency: Wallingford, UK, 2003. [Google Scholar]
- Regnery, J.; Püttmann, W. Occurrence and fate of organophosphorus flame retardants and plasticizers in urban and remote surface waters in Germany. Water Res. 2010, 44, 4097–4104. [Google Scholar] [CrossRef]
- Kawagoshi, Y.; Nakamura, S.; Fukunaga, I. Degradation of organophosphoric esters in leachate from a sea-based solid waste disposal site. Chemosphere 2002, 48, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Zhang, Q.; Luo, T.W.; Xing, L.Q.; Xu, H.Z. Occurrence, distribution and health risk assessment of organophosphate esters in outdoor dust in Nanjing, China: Urban vs. rural areas. Chemosphere 2019, 231, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.J.; Kannan, K. Occurrence and distribution of organophosphate flame retardants/plasticizers in surface waters, tap water, and rainwater: Implications for human exposure. Environ. Sci. Technol. 2018, 52, 5625–5633. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Gao, L.H.; Li, W.H.; Wang, Y.; Liu, J.M.; Cai, Y.Q. Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ. Pollut. 2016, 209, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.Q.; Tao, M.; Zhang, Q.; Kong, M.; Sun, J.; Jia, S.Y.; Liu, C.H. Occurrence, spatial distribution and risk assessment of organophosphate esters in surface water from the lower Yangtze River Basin. Sci. Total Environ. 2020, 734, 139380. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Cui, K.Y.; Xie, Z.Y.; Wu, L.N.; Luo, D.L.; Chen, L.X.; Lin, Y.J.; Liu, M.; Sun, G.X. Distribution of phthalate esters in urban soils of subtropical city, Guangzhou, China. J. Hazard. Mater. 2009, 164, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Wang, X.K.; Zhang, Y.; Chen, L.; Sun, Y.F.; Li, M.; Wu, M.H. Short-and medium-chain chlorinated paraffins in urban soils of Shanghai: Spatial distribution, homologue group patterns and ecological risk assessment. Sci. Total Environ. 2014, 490, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Chen, L.G.; Feng, Y.B.; Ye, Z.X.; He, Q.S.; Feng, Q.H.; Qing, X.; Liu, M.; Gao, B. Short-chain chlorinated paraffins in the soils of two different Chinese cities: Occurrence, homologue patterns and vertical migration. Sci. Total Environ. 2016, 557–558, 644–651. [Google Scholar] [CrossRef]
- Luo, Q.; Shan, Y.; Muhammad, A.; Wang, S.y.; Sun, L.N.; Wang, H. Levels, distribution, and sources of organophosphate flame retardants and plasticizers in urban soils of Shenyang. China. Environ. Sci. Pollut. Res. 2018, 25, 31752–31761. [Google Scholar] [CrossRef]
- He, C.; Wang, X.Y.; Tang, S.Y.; Thai, P.; Li, Z.R.; Baduel, C.; Mueller, J.F. Concentrations of Organophosphate Esters and Their Specific Metabolites in Food in Southeast Queensland, Australia: Is Dietary Exposure an Important Pathway of Organophosphate Esters and Their Metabolites? Environ. Sci. Technol. 2018, 52, 12765–12773. [Google Scholar] [CrossRef]
- Poma, G.; Sales, C.; Bruyland, B.; Christia, C.; Goscinny, S.; Loco, J.V.; Covaci, A. Occurrence of Organophosphorus Flame Retardants and Plasticizers (PFRs) in Belgian Foodstuffs and Estimation of the Dietary Exposure of the Adult Population. Environ. Sci. Technol. 2018, 52, 2331–2338. [Google Scholar] [CrossRef] [PubMed]
- Poma, G.; Glynn, A.; Malarvannan, G.; Covaci, A.; Darnerud, P.O. Dietary intake of phosphorus flame retardants (PFRs) using Swedish food market basket estimations. Food Chem. Toxicol. 2017, 100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.X. Study on the Occurrence Status and Environmental Risk of Organophosphate Esters in Soils of Beijing Urban Parks; Chinese Research Academy of Environmental Sciences: Beijing, China, 2023. [Google Scholar]
- Wang, S.Y. Pollution Characteristics and Risk Assessment of Organic Phosphate Easters of the Liaohekou; Shenyang University: Shenyang, China, 2018. [Google Scholar]
- Schmidt, N.; Castro-Jiménez, J.; Oursel, B.; Sempéré, R. Phthalates and organophosphate esters in surface water, sediments and zooplankton of the NW Mediterranean Sea: Exploring links with microplastic abundance and accumulation in the marine food web. Environ. Pollut. 2021, 272, 115970. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.Q.; Zhang, Y.Y.; Chang, S.; Tao, L.Y.; Su, G.Y. Uptake, accumulation and translocation of traditional and novel organophosphate esters by rice seedlings in the presence of micro(nano)-polystyrene plastics: Effects of concentration and size of particles. Sci. Total Environ. 2023, 898, 165534. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Identification and Evaluation of Data On flame Retardants in Consumer Products. 2017. Available online: https://www.europeanfiresafetyalliance.org/publications/identification-and-evaluation-of-data-on-flame-retardants-in-consumer-products/ (accessed on 12 January 2024).
- USEPA Mid Atlantic Risk Assessment, Regional Screening Levels (RSLs)-Generic Tables. 2023. Available online: http://www.epa.gov/region9/superfund/prg (accessed on 16 January 2024).
- Ding, J.J.; Shen, X.L.; Liu, W.P.; Covaci, A.; Yang, F.X. Occurrence and risk assessment of organophosphate esters in drinking water from Eastern China. Sci. Total Environ. 2015, 538, 959–965. [Google Scholar] [CrossRef]
- Ali, N.; Dirtu, A.C.; Eede, N.V.D.; Goosey, E.; Harrad, S.; Neels, H.; Mannetje, A.; Coakley, J.; Douwes, J.; Covaci, A. Occurrence of alternative flame retardants in indoor dust from New Zealand: Indoor sources and human exposure assessment. Chemosphere 2012, 88, 1276–1282. [Google Scholar] [CrossRef]
- USDoE. The Risk Assessment Information System (RAIS); U.S. Department of Energy’s Oak Ridge Operations Office (ORO): Oak Ridge, TN, USA, 2011. [Google Scholar]
- MEPC, Ministry of Environmental Protection of the People’s Republic of China. Exposure Factors Handbook of Chinese Population; China Environmental Science Press: Beijing, China, 2013. [Google Scholar]
- USEPA (US Environmental Protection Agency). Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; OSWER9355.4-24; Office of Solid Waste and Emergency Response; US Environmental Protection Agency: Washington, DC, USA, 2001. [Google Scholar]






| Compound | Detection Rate (%) | Mean | Median | Min | Max |
|---|---|---|---|---|---|
| TCEP | 100 | 28.3 | 16.7 | 3.99 | 154 |
| TcPP | 100 | 55.5 | 62.9 | 6.17 | 106 |
| TDCIPP | 100 | 139 | 154 | 16.2 | 245 |
| TPHP | 97.3 | 7.96 | 7.57 | - | 16.5 |
| EHDPP | 78.3 | 6.30 | 4.04 | - | 71.9 |
| ToCP | 83.8 | 6.76 | 4.85 | - | 51.8 |
| TpCP | 73.0 | 5.23 | 3.44 | - | 30.3 |
| TmCP | 86.5 | 6.43 | 5.26 | - | 35.2 |
| Σ8OPEs | 100 | 255 | 264 | 74.7 | 410 |
| Compound | Detection Rate (%) | Mean | Median | Min | Max |
|---|---|---|---|---|---|
| TCEP | 100 | 30.9 | 19.1 | 5.47 | 157 |
| TcPP | 100 | 102 | 105 | 14.1 | 177 |
| TDCIPP | 97.3 | 129 | 134 | - | 226 |
| TPHP | 100 | 11.3 | 7.05 | 2.94 | 121 |
| EHDPP | 83.8 | 29.3 | 19.3 | - | 231 |
| ToCP | 64.9 | 49.5 | 18.0 | - | 182 |
| TpCP | 72.2 | 2.44 | 2.70 | - | 7.44 |
| TmCP | 64.9 | 26.1 | 5.16 | - | 278 |
| Σ8OPEs | 100 | 381 | 350 | 202 | 751 |
| Compound | Children | Adult | RfD ng/kg bw Day | ||||
|---|---|---|---|---|---|---|---|
| Max | Min | Mean | Max | Min | Mean | ||
| TCEP | 904.12 | 31.360 | 177.53 | 924.27 | 32.060 | 181.49 | 22,000 |
| TDCIPP | 1296.2 | 0.0000 | 741.75 | 1325.1 | 0.0000 | 758.28 | 15,000 |
| TPHP | 694.93 | 16.850 | 64.740 | 710.41 | 17.230 | 66.180 | 70,000 |
| Σ3OPEs | 1708.1 | 239.46 | 984.03 | 1746.1 | 244.80 | 1005.9 | na a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Yao, S.; Huang, J.; Wu, H.; Zhou, H.; Du, M.; Jin, L.; Sun, J. Distribution and Risk Assessment of Organophosphate Esters in Agricultural Soils and Plants in the Coastal Areas of South China. Toxics 2024, 12, 286. https://doi.org/10.3390/toxics12040286
Luo W, Yao S, Huang J, Wu H, Zhou H, Du M, Jin L, Sun J. Distribution and Risk Assessment of Organophosphate Esters in Agricultural Soils and Plants in the Coastal Areas of South China. Toxics. 2024; 12(4):286. https://doi.org/10.3390/toxics12040286
Chicago/Turabian StyleLuo, Wangxing, Siyu Yao, Jiahui Huang, Haochuan Wu, Haijun Zhou, Mingjiang Du, Ling Jin, and Jianteng Sun. 2024. "Distribution and Risk Assessment of Organophosphate Esters in Agricultural Soils and Plants in the Coastal Areas of South China" Toxics 12, no. 4: 286. https://doi.org/10.3390/toxics12040286





