Dimethylcyclosiloxanes in Mobile Smart Terminal Devices: Concentrations, Distributions, Profiles, and Environmental Emissions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection and Preparation
2.3. Instrumental Analysis
2.4. Quality Assurance and Quality Control
2.5. Environmental Emissions of DMCs from Silicone Rubber
3. Results and Discussion
3.1. Detection Rates and Concentrations of DMCs
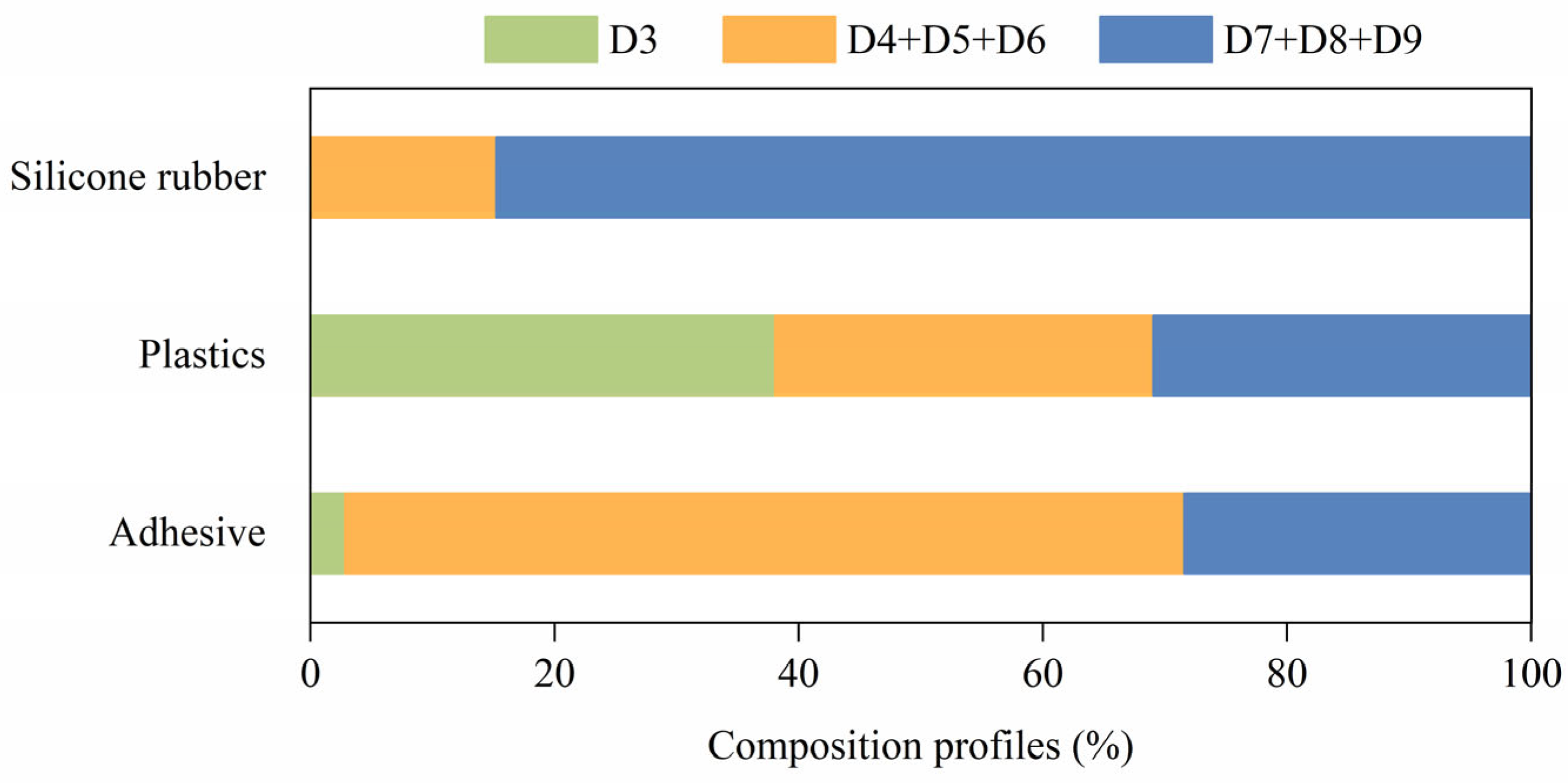
3.2. Composition Profiles of DMCs
3.3. Environmental Emissions of DMCs from Silicone Rubber
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horii, Y.; Minomo, K.; Lam, J.C.W.; Yamashita, N. Spatial Distribution and Accumulation Profiles of Volatile Methylsiloxanes in Tokyo Bay, Japan: Mass Loadings and Historical Trends. Sci. Total Environ. 2022, 806, 150821. [Google Scholar] [CrossRef] [PubMed]
- Molinier, B.; Arata, C.; Katz, E.; Lunderberg, D.; Liu, Y.; Misztal, P.; Nazaroff, W.; Goldstein, A. Volatile Methyl Siloxanes and Other Organosilicon Compounds in Residential Air. Environ. Sci. Technol. 2022, 56, 15427–15436. [Google Scholar] [CrossRef] [PubMed]
- Gerhards, R.; Seston, R.M.; Kozerski, G.E.; McNett, D.A.; Boehmer, T.; Durham, J.A.; Xu, S. Basic Considerations to Minimize Bias in Collection and Analysis of Volatile Methyl Siloxanes in Environmental Samples. Sci. Total Environ. 2022, 851, 158275. [Google Scholar] [CrossRef] [PubMed]
- Dudzina, T.; von Goetz, N.; Bogdal, C.; Biesterbos, J.; Hungerbühler, K. Concentrations of Cyclic Volatile Methylsiloxanes in European Cosmetics and Personal Care Products: Prerequisite for Human and Environmental Exposure Assessment. Environ. Int. 2014, 62, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Witte, M.; Fembacher, L.; Gruber, L.; Hagl, T.; Smolic, S.; Fiedler, D.; Sysoltseva, M.; Schober, W. Siloxane in Baking Moulds, Emission to Indoor Air and Migration to Food during Baking with an Electric Oven. Environ. Int. 2019, 126, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Varaprath, S.; Stutts, D.H.; Kozerski, G.E. A Primer on the Analytical Aspects of Silicones at Trace Levels-Challenges and Artifacts—A Review. Silicon Chem. 2006, 3, 79–102. [Google Scholar] [CrossRef]
- Wang, R.; Moody, R.; Koniecki, D.; Zhu, J. Low Molecular Weight Cyclic Volatile Methylsiloxanes in Cosmetic Products Sold in Canada: Implication for Dermal Exposure. Environ. Int. 2009, 35, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Zammarano, M.; Cazzetta, V.; Nazaré, S.; Shields, J.R.; Kim, Y.S.; Hoffman, K.M.; Maffezzoli, A.; Davis, R. Smoldering and Flame Resistant Textiles via Conformal Barrier Formation. Adv. Mater. Interfaces 2016, 3, 1600617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wong, J.W.; Begley, T.H.; Hayward, D.G.; Limm, W. Determination of Siloxanes in Silicone Products and Potential Migration to Milk, Formula and Liquid Simulants. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1311–1321. [Google Scholar] [CrossRef]
- Horii, Y.; Ohtsuka, N.; Minomo, K.; Takemine, S.; Motegi, M.; Hara, M. Distribution Characteristics of Methylsiloxanes in Atmospheric Environment of Saitama, Japan: Diurnal and Seasonal Variations and Emission Source Apportionment. Sci. Total Environ. 2021, 754, 142399. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, Y.; Zhang, B.; Zhang, J. Distribution and Evaluation of the Fate of Cyclic Volatile Methyl Siloxanes in the Largest Lake of Southwest China. Sci. Total Environ. 2019, 657, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Nu Nguyen, H.M.; Khieu, H.T.; Ta, N.A.; Le, H.Q.; Nguyen, T.Q.; Do, T.Q.; Hoang, A.Q.; Kannan, K.; Tran, T.M. Distribution of Cyclic Volatile Methylsiloxanes in Drinking Water, Tap Water, Surface Water, and Wastewater in Hanoi, Vietnam. Environ. Pollut. 2021, 285, 117260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, M.; Tian, Y.; Zeng, G. Cyclic Volatile Methylsiloxanes in Sediment, Soil, and Surface Water from Dongting Lake, China. J. Soil. Sediment. 2018, 18, 2063–2071. [Google Scholar] [CrossRef]
- Wang, D.-G.; Alaee, M.; Steer, H.; Tait, T.; Williams, Z.; Brimble, S.; Svoboda, L.; Barresi, E.; DeJong, M.; Schachtschneider, J.; et al. Determination of Cyclic Volatile Methylsiloxanes in Water, Sediment, Soil, Biota, and Biosolid Using Large-Volume Injection-Gas Chromatography-Mass Spectrometry. Chemosphere 2013, 93, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Abualnaja, K.; Asimakopoulos, A.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.; Malarvannan, G.; Minh, T.; Moon, H.; et al. A Survey of Cyclic and Linear Siloxanes in Indoor Dust and Their Implications for Human Exposures in Twelve Countries. Environ. Int. 2015, 78, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tang, Z.; He, Y.; Wang, F.; Lyu, Y. Occurrence of Methylsiloxanes in Indoor Store Dust in China and Potential Human Exposure. Environ. Res. 2023, 218, 114969. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; de Solla, S.; Lebeuf, M.; Bisbicos, T.; Barrett, G.; Alaee, M. Determination of Linear and Cyclic Volatile Methylsiloxanes in Blood of Turtles, Cormorants, and Seals from Canada. Sci. Total Environ. 2017, 574, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Xu, L.; He, X.; Zhang, C.; Cai, Y. Distribution of Methylsiloxanes in Benthic Mollusks from the Chinese Bohai Sea. J. Environ. Sci. 2019, 76, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, Y.; Wang, Y.; Zhang, B.; Zhang, J. Assessment of Internal Exposure to Methylsiloxanes in Children and Associated Non-Dietary Exposure Risk. Environ. Int. 2021, 154, 106672. [Google Scholar] [CrossRef] [PubMed]
- Krenczkowska, D.; Mojsiewicz-Pienkowska, K.; Wielgomas, B.; Bazar, D.; Jankowski, Z. Ex Vivo Human Skin Is Not a Barrier for Cyclic Siloxanes (Cyclic Silicones): Evidence of Diffusion, Bioaccumulation, and Risk of Dermal Absorption Using a New Validated GC-FID Procedure. Pharmaceutics 2020, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Su, X.; Li, Q.; Zhao, J.; Hou, M.; Dong, S.; Yan, X.; Sun, J.; Feng, J. Dimethylsiloxanes in Dust from Nine Indoor Microenvironments of Henan Province: Occurrence and Human Exposure Assessment. Sci. Total Environ. 2023, 903, 166546. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, D.; Xu, L.; Qiu, C.; Wang, S.; Liu, N.; Sun, L. Research Progress on the Distribution, Behavior and Effects of Cyclic Volatile Methylsiloxanes in Organisms. J. Soil. Sediment. 2022, 41, 193–204. [Google Scholar] [CrossRef]
- Kumari, K.; Singh, A.; Marathe, D. Cyclic Volatile Methyl Siloxanes (D4, D5, and D6) as the Emerging Pollutants in Environment: Environmental Distribution, Fate, and Toxicological Assessments. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.; Greene, T.; Van Landingham, C.; Gentry, R. Toxicology of Octamethylcyclotetrasiloxane (D4). Toxicol. Lett. 2017, 279, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Falany, J.; Xie, X.; Falany, C. Induction of Rat Hepatic Drug Metabolizing Enzymes by Dimethylcyclosiloxanes. Chem. Biol. Interact. 2000, 124, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.; Park, S.-M.; Kwon, J.-S.; Jeung, E.-B. Effects of Decamethylcyclopentasiloxane on Reproductive Systems in Female Rats. Toxics 2023, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.H.; Stump, D.G.; Plotzke, K.P.; Holson, J.F.; Meeks, R.G. A Two-Generation Reproductive Toxicity Study of Octamethylcyclotetrasiloxane (D4) in Rats Exposed by Whole-Body Vapor Inhalation. Reprod. Toxicol. 2007, 23, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Park, S.-M.; Jung, E.-M.; Jeung, E.-B. Prenatal Octamethylcyclotetrasiloxane Exposure Impaired Proliferation of Neuronal Progenitor, Leading to Motor, Cognition, Social and Behavioral Functions. Int. J. Mol. Sci. 2021, 22, 12949. [Google Scholar] [CrossRef]
- Hossain, M.M.; Yuan, Y.; Huang, H.; Wang, Z.; Baki, M.A.; Dai, Z.; Rizwan, M.; Xiong, S.; Cao, M.; Tu, S. Exposure to Dodecamethylcyclohexasiloxane (D6) Affects the Antioxidant Response and Gene Expression of Procambarus clarkii. Sustainability 2021, 13, 3495. [Google Scholar] [CrossRef]
- Jean, P.A.; Plotzke, K.P. Chronic Toxicity and Oncogenicity of Octamethylcyclotetrasiloxane (D4) in the Fischer 344 Rat. Toxicol. Lett. 2017, 279, 75–97. [Google Scholar] [CrossRef]
- Young, L.J.; Morfeld, P. Statistical Considerations for a Chronic Bioassay Study: Exposure to Decamethylcyclopentasiloxane (D5) and Incidence of Uterine Endometrial Adenocarcinomas in a 2-Year Inhalation Study with Fischer Rats. Regul. Toxicol. Pharmacol. 2016, 74, S14–S24. [Google Scholar] [CrossRef] [PubMed]
- Farasani, A.; Darbre, P.D. Exposure to Cyclic Volatile Methylsiloxanes (cVMS) Causes Anchorage-Independent Growth and Reduction of BRCA1 in Non-Transformed Human Breast Epithelial Cells. J. Appl. Toxicol. 2017, 37, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, Y.; Guo, J. Association of Volatile Methylsiloxanes Exposure with Non-Alcoholic Fatty Liver Disease among Chinese Adults. Environ. Pollut. 2023, 334, 122128. [Google Scholar] [CrossRef]
- Cantu, M.; Gobas, F. Bioaccumulation of Dodecamethylcyclohexasiloxane (D6) in Fish. Chemosphere 2021, 281, 130948. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Norwood, W.; Alaee, M.; Byer, J.D.; Brimble, S. Review of Recent Advances in Research on the Toxicity, Detection, Occurrence and Fate of Cyclic Volatile Methyl Siloxanes in the Environment. Chemosphere 2013, 93, 711–725. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Candidate List of Substances of Very High Concern for Authorisation. Available online: https://echa.europa.eu/candidate-list-table (accessed on 22 January 2024).
- ECHA. List of Substances Proposed as POPs. Available online: https://echa.europa.eu/list-of-substances-proposed-as-pops/-/dislist/details/0b0236e184f17c3e (accessed on 12 April 2024).
- ACMI. 2023 China Silicone Series Product Market Report Was Released Grandly; Advanced Chemical Materials Institution: Beijing, China, 2023; Available online: https://acmi.org.cn/marketresearch/info.aspx?itemid=508&eqid=8dd6ecea000024680000000464537057 (accessed on 17 December 2023).
- Arshad; Nekahi, A.; McMeekin, S.G.; Farzaneh, M. Measurement of Surface Resistance of Silicone Rubber Sheets under Polluted and Dry Band Conditions. Electr. Eng. 2018, 100, 1729–1738. [Google Scholar] [CrossRef]
- Li, G.; Gong, J.M.; Tan, J.Z.; Zhu, D.S.; Jia, W.H.; Lu, X.J. Acidic-Thermal Ageing Effect on Compression Stress Relaxation of Silicone Rubber. Strength. Mater. 2019, 51, 660–666. [Google Scholar] [CrossRef]
- Mollah, M.S.I.; Kwon, Y.-D.; Islam, M.M.; Seo, D.-W.; Jang, H.-H.; Lim, Y.-D.; Lee, D.-K.; Kim, W.-G. Synthesis and Characterization of Polycarbonates Containing Terminal and Chain Interior Siloxane. Polym. Bull. 2012, 68, 1551–1564. [Google Scholar] [CrossRef]
- Yuan, D.; Cai, X. Synthesis of a Silicon-Containing Flame Retardant and Its Synergistic Effect with Potassium-4-(Phenylsulfonyl)Benzenesulfonate (KSS) in Polycarbonate (PC). Chin. J. Polym. Sci. 2013, 31, 1352–1358. [Google Scholar] [CrossRef]
- Antosik, A.K.; Makuch, E.; Gziut, K. Influence of Modified Attapulgite on Silicone Pressure-Sensitive Adhesives Properties. J. Polym. Res. 2022, 29, 135. [Google Scholar] [CrossRef]
- Antosik, A.K.; Weisbrodt, M.; Mozelewska, K.; Czech, Z.; Piątek-Hnat, M. Impact of Environmental Conditions on Silicone Pressure-Sensitive Adhesives. Polym. Bull. 2020, 77, 6625–6639. [Google Scholar] [CrossRef]
- Storozhenko, P.A.; Minas’yan, R.M.; Polivanov, A.N.; Nikitushkin, I.V.; Minas’yan, O.I. New Thermally Conductive Silicone Adhesive Sealants. Polym. Sci. Ser. D 2017, 10, 221–224. [Google Scholar] [CrossRef]
- He, X.; Xu, L.; Zhang, C.; Cai, Y. Pollution Characteristics of Methyl Siloxanes in Soil from an Electronic Waste(e-Waste) Dismantling Area in Taizhou, China. J. Soil. Sediment. 2016, 35, 2287–2294. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Z.; Zhang, Q.; Xiang, X.; Zhang, S.; Cai, Y. Methylsiloxanes and Their Brominated Products in One E-Waste Recycling Area in China: Emission, Environmental Distribution, and Elimination. Environ. Sci. Technol. 2020, 54, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- ACMI. Adhesives Market Report; ACMI: Beijing, China, 2022; Available online: https://acmi.org.cn/Achievement/info.aspx?itemid=482 (accessed on 23 January 2024).
- Amghar, N.; Moreno, V.; Sánchez-Jiménez, P.E.; Perejón, A.; Pérez-Maqueda, L.A. Ca-Based Materials Derived from Calcined Cigarette Butts for CO2 Capture and Thermochemical Energy Storage. J. Environ. Sci. 2024, 140, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Forti, V.; Bald, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020. E-Waste Monitor. Available online: https://ewastemonitor.info/gem-2020/ (accessed on 26 January 2024).
- Fromme, H.; Cequier, E.; Kim, J.; Hanssen, L.; Hilger, B.; Thomsen, C.; Chang, Y.; Völkel, W. Persistent and Emerging Pollutants in the Blood of German Adults: Occurrence of Dechloranes, Polychlorinated Naphthalenes, and Siloxanes. Environ. Int. 2015, 85, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, L.; Warner, N.; Braathen, T.; Odland, J.; Lund, E.; Nieboer, E.; Sandanger, T. Plasma Concentrations of Cyclic Volatile Methylsiloxanes (cVMS) in Pregnant and Postmenopausal Norwegian Women and Self-Reported Use of Personal Care Products (PCPs). Environ. Int. 2013, 51, 82–87. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Liu, N.; Cai, Y. Methyl Siloxanes in Environmental Matrices and Human Plasma/Fat from Both General Industries and Residential Areas in China. Sci. Total Environ. 2015, 505, 454–463. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Background Document to the Opinion on the Annex XV Dossier Proposing Restrictions on Octamethylcyclotetrasiloxane (D4) and Decamethylcyclopentasiloxane (D5), 2016. Available online: https://echa.europa.eu/documents/10162/fefaa3a2-ffc0-4b74-4ec8-3c869d4adae7 (accessed on 29 March 2024).
- Xiao, R.; Zammit, I.; Wei, Z.; Hu, W.-P.; MacLeod, M.; Spinney, R. Kinetics and Mechanism of the Oxidation of Cyclic Methylsiloxanes by Hydroxyl Radical in the Gas Phase: An Experimental and Theoretical Study. Environ. Sci. Technol. 2015, 49, 13322–13330. [Google Scholar] [CrossRef] [PubMed]
- IDC. IDC Media Center. IDC: The Premier Global Market Intelligence Company. Available online: https://www.idc.com/about/press (accessed on 26 January 2024).
- Horii, Y.; Kannan, K. Survey of Organosilicone Compounds, Including Cyclic and Linear Siloxanes, in Personal-Care and Household Products. Arch. Environ. Contam. Toxicol. 2008, 55, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhi, L.; Cai, Y. Methylsiloxanes in Children Silicone-Containing Products from China: Profiles, Leaching, and Children Exposure. Environ. Int. 2017, 101, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yuan, T.; Wang, W.; Kannan, K. Concentrations and Assessment of Exposure to Siloxanes and Synthetic Musks in Personal Care Products from China. Environ. Pollut. 2011, 159, 3522–3528. [Google Scholar] [CrossRef] [PubMed]
- Helling, R.; Mieth, A.; Altmann, S.; Simat, T.J. Determination of the Overall Migration from Silicone Baking Moulds into Simulants and Food Using 1H-NMR Techniques. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Xu, S. Quantitative Structure-reactivity Relationships of Hydroxyl Radical Rate Constants for Linear and Cyclic Volatile Methylsiloxanes. Environ. Toxicol. Chem. 2017, 36, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Harrison, R.M.; Song, C.; Hou, S.; Wei, L.; Fu, P.; Li, H.; Li, W.; Shi, Z. PM2.5-Bound Silicon-Containing Secondary Organic Aerosols (Si-SOA) in Beijing Ambient Air. Chemosphere 2022, 288 Pt 1, 132377. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Hoang, A.Q.; Le, S.T.; Minh, T.B.; Kannan, K. A Review of Contamination Status, Emission Sources, and Human Exposure to Volatile Methyl Siloxanes (VMSs) in Indoor Environments. Sci. Total Environ. 2019, 691, 584–594. [Google Scholar] [CrossRef]
- Cui, S.; Fu, Q.; An, L.; Yu, T.; Zhang, F.; Gao, S.; Liu, D.; Jia, H. Trophic Transfer of Cyclic Methyl Siloxanes in the Marine Food Web in the Bohai Sea, China. Ecotoxicol. Environ. Saf. 2019, 178, 86–93. [Google Scholar] [CrossRef] [PubMed]

| Name | Abbreviation | CAS-No. | Boiling Points | Molar Weight |
|---|---|---|---|---|
| Hexamethylcyclotrisiloxane | D3 | 541-05-9 | 134 °C | 222.47 |
| Octamethylcyclotetrasiloxane | D4 | 556-67-2 | 175–176 °C | 296.62 |
| Decamethylcyclopentasiloxane | D5 | 541-02-6 | 205 °C | 370.72 |
| Dodecamethylcyclohexasiloxane | D6 | 540-97-6 | 245 °C | 444.93 |
| Tetradecamethylcycloheptasiloxane | D7 | 107-50-6 | 337 °C | 519.08 |
| Hexadecamethylcyclooctasiloxane | D8 | 556-68-3 | 290 °C | 593.23 |
| Octadecamethylcyclononasiloxane | D9 | 556-71-8 | 416 °C | 667.39 |
| Samples | Statistics | D3 | D4 | D5 | D6 | D7 | D8 | D9 | D4+D5+D6 | D7+D8+D9 | ∑DMCs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Silicone rubber (n = 22) | DR (%) | 9 | 59 | 95.5 | 95.5 | 95.5 | 91 | 91 | 95.5 | 95.5 | 95.5 |
| Mean | 0.53 | 9.91 | 66.3 | 136.9 | 159.3 | 171.8 | 159.0 | 212.9 | 490.0 | 703.0 | |
| Median | 0.50 | 1.55 | 14.5 | 78.9 | 180.1 | 158.2 | 132.5 | 86.0 | 481.5 | 802.2 | |
| Range | <LOQ-1.1 | <LOQ-63 | <LOQ-335 | <LOQ-422 | <LOQ-479 | <LOQ-481 | <LOQ-438.9 | <LOQ-820 | <LOQ-1331 | <LOQ-1788 | |
| Plastics (n = 18) | DR (%) | 61.1 | 61.1 | 50 | 38.9 | 44.4 | 50 | 50 | 61.1 | 50 | 66.7 |
| Mean | 22.1 | 19.0 | 9.36 | 5.32 | 4.46 | 4.53 | 3.69 | 33.1 | 12.2 | 66.9 | |
| Median | 2.95 | 2.15 | 0.50 | 0.50 | 0.50 | 0.75 | 1.65 | 2.40 | 2.40 | 8.25 | |
| Range | <LOQ-279 | <LOQ-181 | <LOQ-86 | <LOQ-42.5 | <LOQ-27.5 | <LOQ-33.6 | <LOQ-26.4 | <LOQ-309.5 | <LOQ-87.5 | <LOQ-631.6 | |
| Adhesive (n = 12) | DR (%) | 41.7 | 50 | 100 | 100 | 91.7 | 75 | 50 | 100 | 91.7 | 100 |
| Mean | 2.11 | 5.33 | 28.9 | 43.8 | 38.2 | 41.9 | 48.4 | 78.5 | 129.0 | 208.6 | |
| Median | 0.50 | 0.95 | 2.35 | 7.35 | 2.10 | 1.20 | 0.90 | 12.1 | 5.0 | 21.5 | |
| Range | <LOQ-10.3 | <LOQ-27.7 | 1–119 | 1.8–160 | <LOQ-153 | <LOQ-169 | <LOQ-204 | 3.7–289.6 | <LOQ-515 | 6.3–807.9 | |
| All (n = 52) | DR (%) | 34.6 | 57.7 | 80.8 | 76.9 | 76.9 | 73.1 | 67.3 | 84.6 | 78.8 | 86.5 |
| Mean | 8.52 | 12.0 | 38.0 | 79.9 | 77.8 | 83.9 | 79.7 | 119.7 | 241.3 | 368.7 | |
| Median | 0.50 | 1.55 | 6.60 | 11.0 | 7.35 | 8.10 | 7.55 | 33.3 | 23.2 | 70.8 | |
| Range | <LOQ-279 | <LOQ-181 | <LOQ-335 | <LOQ-422 | <LOQ-479 | <LOQ-481 | <LOQ-438.9 | <LOQ-820 | <LOQ-1331 | <LOQ-1788 |
| Region | Samples | Sample Size | Statistics | D3 | D4 | D5 | D6 | D7 | D8 | D9 | Units | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA | Silicone nipples | 22 | DR (%) | - | 100 | 100 | 100 | - | - | - | Zhang et al., 2012 [9] | |
| Range | - | 0.6–49 | 0.6–269 | 0.3–108 | - | - | - | mg/kg | ||||
| Canada | Cosmetic products | 252 | DR (%) | 0.79 | 4.8 | 14 | 9.1 | - | - | - | Wang et al., 2009 [7] | |
| China | Soft rubber toys | 44 | DR (%) | - | 100 | 91 | 100 | - | - | - | Xu et al., 2017 [58] | |
| Median | - | 0.062 | 0.086 | 0.101 | - | - | - | μg/g | ||||
| Pacifiers | 74 | DR (%) | - | 100 | 100 | 100 | - | - | - | |||
| Median | - | 0.107 | 0.924 | 1.46 | - | - | - | μg/g | ||||
| Hard toys | 40 | DR (%) | - | 10 | 50 | 40 | - | - | - | |||
| Median | - | <0.6 × 10−3 | <0.45 × 10−3 | <0.7 × 10−3 | - | - | - | μg/g | ||||
| Europe | Cosmetics and PCPs | 51 | Median | - | 0.011 | 25.7 | 0.64 | - | - | - | mg/g | Dudzina et al., 2014 [4] |
| Germany | Bakeware products | 4 | Mean | - | 505 | 284 | 753 | 1265 | 1127 | 1325 | mg/kg | Fromme et al., 2019 [5] |
| Shanghai, China | PCPs | 158 | Median | - | 0.29 | 1.70 | 0.66 | 0.06 | - | - | μg/g | Lu et al., 2011 [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Ge, Y.; Lu, S.; Yang, T.; Peng, X. Dimethylcyclosiloxanes in Mobile Smart Terminal Devices: Concentrations, Distributions, Profiles, and Environmental Emissions. Toxics 2024, 12, 287. https://doi.org/10.3390/toxics12040287
Xing Y, Ge Y, Lu S, Yang T, Peng X. Dimethylcyclosiloxanes in Mobile Smart Terminal Devices: Concentrations, Distributions, Profiles, and Environmental Emissions. Toxics. 2024; 12(4):287. https://doi.org/10.3390/toxics12040287
Chicago/Turabian StyleXing, Yuanna, Yiming Ge, Shaoyou Lu, Tao Yang, and Xianzhi Peng. 2024. "Dimethylcyclosiloxanes in Mobile Smart Terminal Devices: Concentrations, Distributions, Profiles, and Environmental Emissions" Toxics 12, no. 4: 287. https://doi.org/10.3390/toxics12040287
APA StyleXing, Y., Ge, Y., Lu, S., Yang, T., & Peng, X. (2024). Dimethylcyclosiloxanes in Mobile Smart Terminal Devices: Concentrations, Distributions, Profiles, and Environmental Emissions. Toxics, 12(4), 287. https://doi.org/10.3390/toxics12040287





