The Male Reproductive Toxicity Caused by 2-Naphthylamine Was Related to Testicular Immunity Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Animals, and Study Design
2.2. H&E Staining
2.3. Periodic Acid–Schiff Stain (PAS) Staining
2.4. Immunofluorescence
2.5. Sperm Motility Assessment by Computer-Aided Sperm Analysis (Casa)
2.6. Acrosome Reaction
2.7. Malformation Detection
2.8. RNA Extraction, RNA-Sequencing, and qRT-PCR
2.9. The Detection of Total Cholesterol in Testis
2.10. Statistical Analysis
3. Results
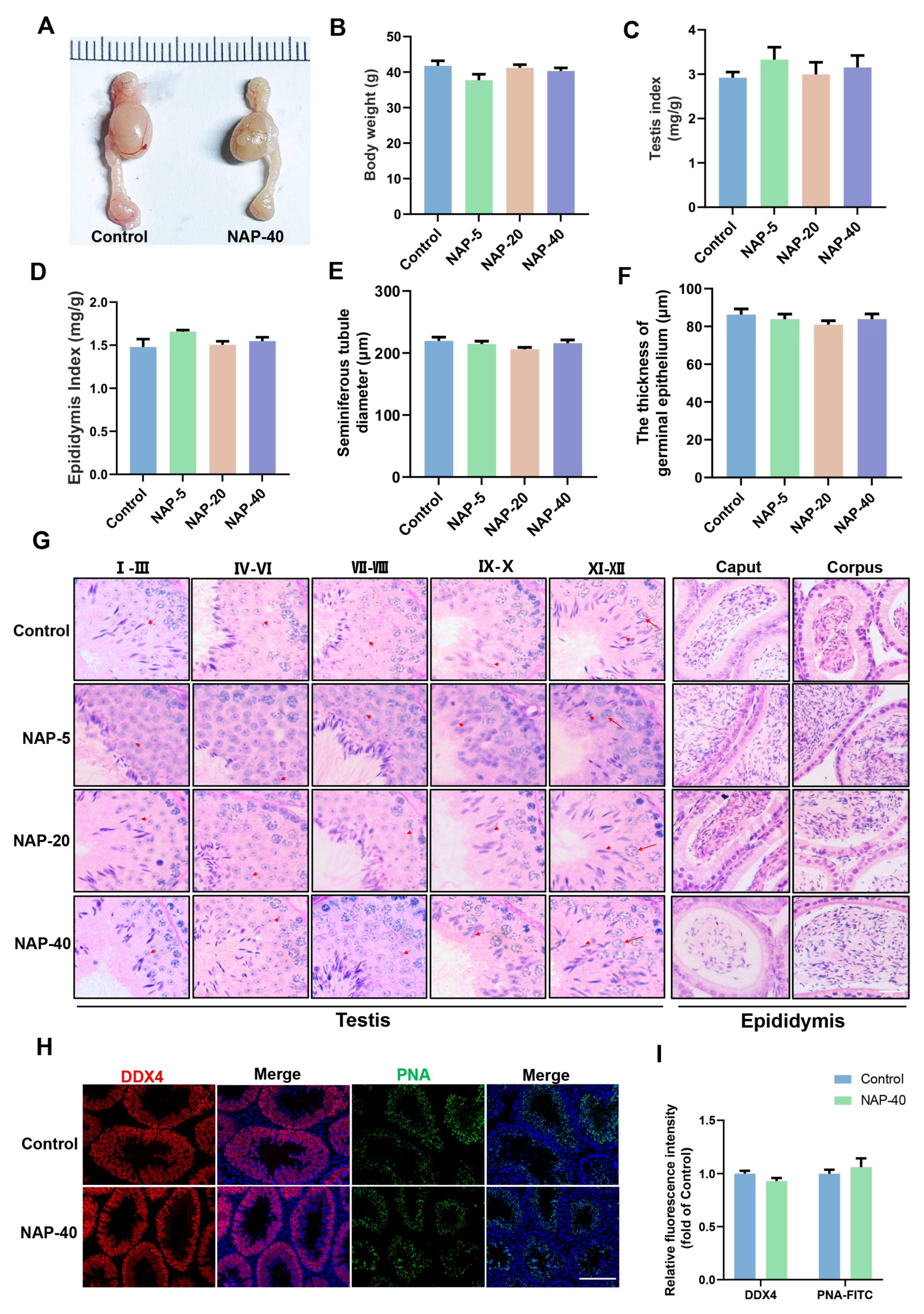
3.1. The Histological Patterns of the Testis and Epididymis Were Not Remarkably Injured after NAP Treatment
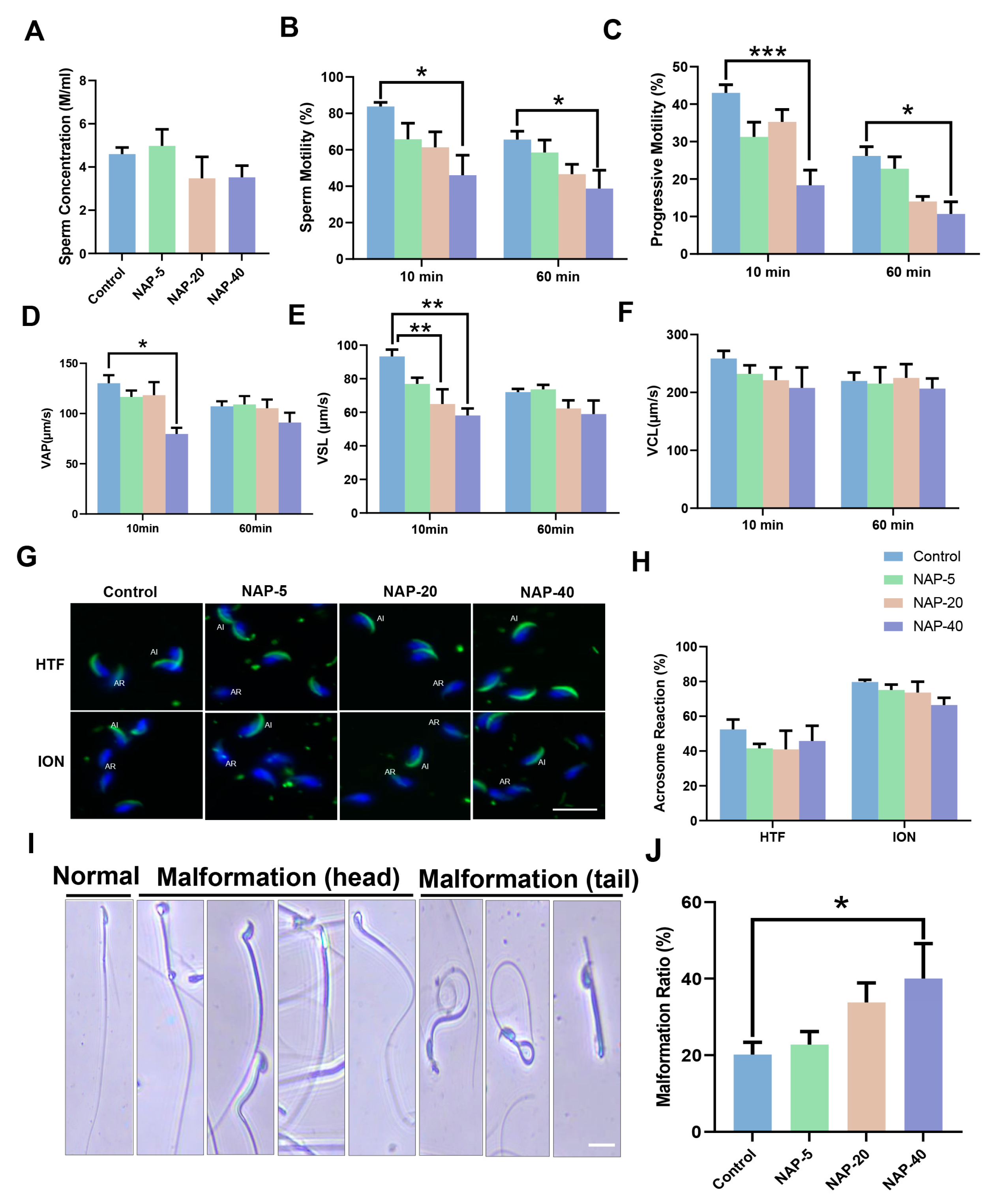
3.2. The Sperm Motility and Morphology Deteriorated following NAP Exposure
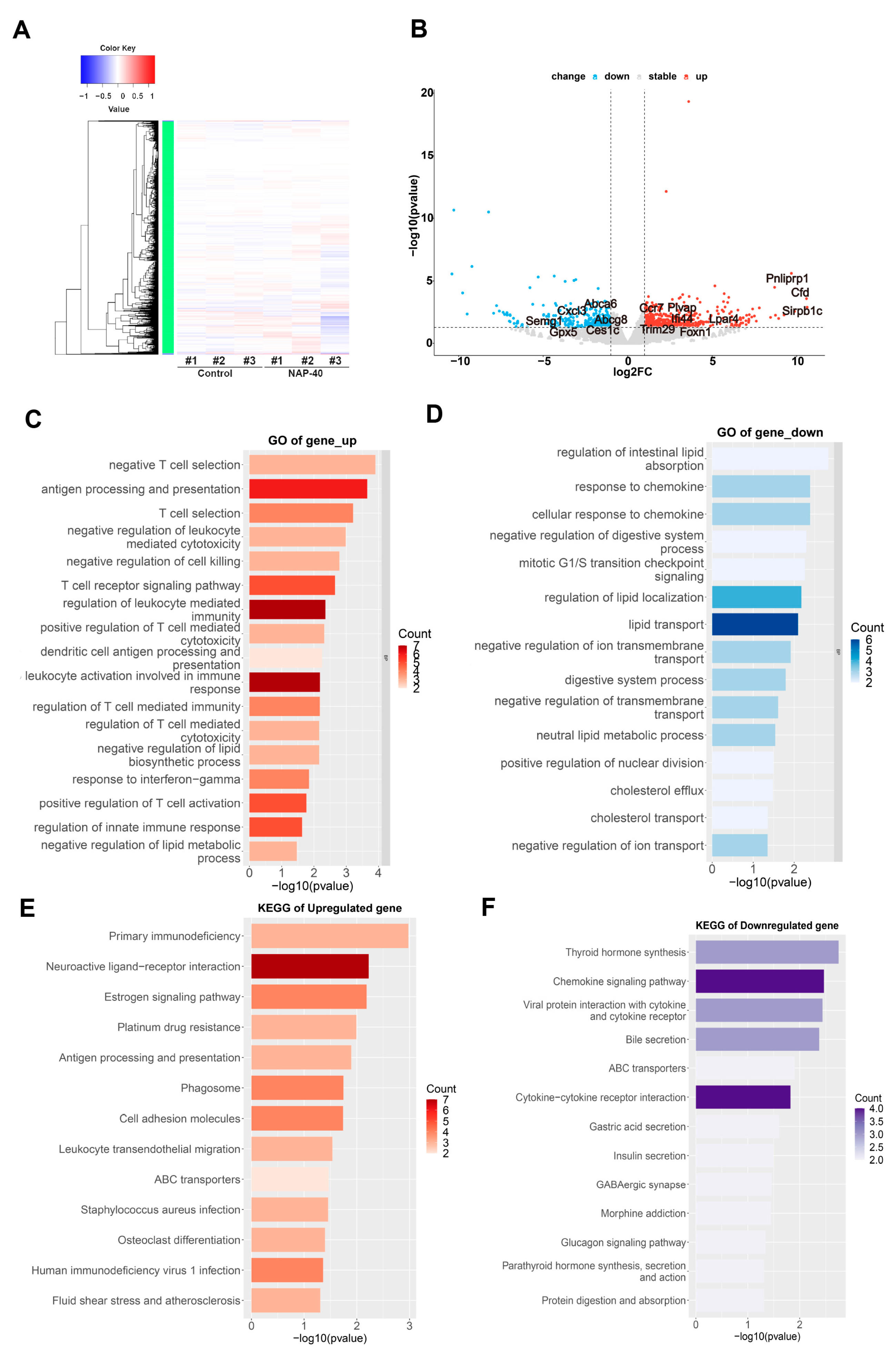
3.3. NAP Disturbed Gene Expression Profiles in the Testis
3.4. Testicular Immunity, Lipid Metabolism, and Sperm Motility Regulation Were Disturbed after NAP Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jeng, H.A.; Pan, C.H.; Lin, W.Y.; Wu, M.T.; Taylor, S.; Chang-Chien, G.P.; Zhou, G.D.; Diawara, N. Biomonitoring of polycyclic aromatic hydrocarbons from coke oven emissions and reproductive toxicity in nonsmoking workers. J. Hazard. Mater. 2013, 244, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Czubacka, E.; Czerczak, S. 2-naphthylamine toxicity. Med. Pr. 2020, 71, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.W.; Acharya, A.P.; Meyyappan, T.; Yu, M.; Bhaskar, G.; Little, S.R.; Tarin, T.V. Comparison of Bladder Carcinogens in the Urine of E-cigarette Users Versus Non E-cigarette Using Controls. Sci. Rep. 2018, 8, 507. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Human. Tobacco Smoke and Involuntary Smoking; IARC: Lyon, France, 2004. [Google Scholar]
- Patnaik, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Sung, H.L.; Araki, P.S.; Tanigawa, T.; Sakurai, S. Selective decrease of the suppressor-inducer (CD4+ CD45RA+) T lymphocytes in workers exposed to benzidine and beta-naphthylamine. Arch. Environ. Health Int. J. 1995, 50, 196–199. [Google Scholar] [CrossRef] [PubMed]
- McElvenny, D.M.; Mueller, W.; Ritchie, P.; Cherrie, J.W.; Hidajat, M.; Darnton, A.J.; Agius, R.M.; De Vocht, F. British rubber and cable industry cohort: 49-year mortality follow-up. Occup. Environ. Med. 2018, 75, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, K.; Obayashi, K.; Saeki, K.; Okamoto, N.; Kurumatani, N. Increased risk of lung cancer associated with occupational exposure to benzidine and/or beta-naphthylamine. Int. Arch. Occup. Environ. Health 2015, 88, 455–465. [Google Scholar] [CrossRef]
- Wilczyńska, U.; Szadkowska-Stańczyk, I.; Szeszenia-Dabrowska, N.; Sobala, W.; Strzelecka, A. Cancer mortality in rubber tire workers in Poland. Int. J. Occup. Med. Environ. Health 2001, 14, 115–125. [Google Scholar] [PubMed]
- Delzell, E.; Macaluso, M.; Cole, P. A follow-up study of workers at a dye and resin manufacturing plant. J. Occup. Med. 1989, 31, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Hadidian, Z.; Fredrickson, T.; Weisburger, E.; Weisburger, J.; Glass, R.; Mantel, N. Tests for chemical carcinogens. Report on the activity of derivatives of aromatic amines, nitrosamines, quinolines, nitroalkanes, amides, epoxides, aziridines, and purine antimetabolites. J. Natl. Cancer Inst. 1968, 41, 985–1036. [Google Scholar]
- Bonser, G.M.; Clayson, D.; Jull, J.; Pyrah, L. The carcinogenic properties of 2-amino-1-naphthol hydrochloride and its parent amine 2-naphthylamine. Br. J. Cancer 1952, 6, 412. [Google Scholar] [CrossRef]
- Stoner, G.D.; Conran, P.B.; Greisiger, E.A.; Stober, J.; Morgan, M.; Pereira, M.A. Comparison of two routes of chemical administration on the lung adenoma response in strain AJ mice. Toxicol. Appl. Pharmacol. 1986, 82, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.W.; Fok, K.L.; Dai, P.Y.; Qiao, F.; Zhang, M.Y.; Liu, H.G.; Sang, M.M.; Ye, M.; Liu, Y.; Zhou, Y.W.; et al. Spatio-temporal landscape of mouse epididymal cells and specific mitochondria-rich segments defined by large-scale single-cell RNA-seq. Cell Discovery 2021, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Muciaccia, B.; Boitani, C.; Berloco, B.P.; Nudo, F.; Spadetta, G.; Stefanini, M.; de Rooij, D.G.; Vicini, E. Novel stage classification of human spermatogenesis based on acrosome development. Biol. Reprod. 2013, 89, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.I.; Mruk, D.D.; Cheng, C.Y. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 59, pp. 35–45. [Google Scholar]
- Xiong, W.; Shen, C.; Wang, Z. The molecular mechanisms underlying acrosome biogenesis elucidated by gene-manipulated mice. Biol. Reprod. 2021, 105, 789–807. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. The mammalian blood-testis barrier: Its biology and regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Rantakari, P.; Auvinen, K.; Jäppinen, N.; Kapraali, M.; Valtonen, J.; Karikoski, M.; Gerke, H.; Iftakhar-E-Khuda, I.; Keuschnigg, J.; Umemoto, E. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 2015, 16, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Weng, L.; Yuan, B.; Wang, Z.; Jia, L.; Jin, R.; Lu, H.; Li, X.C.; Liu, Y.-J.; Zhang, Z. Identification of a role for TRIM29 in the control of innate immunity in the respiratory tract. Nat. Immunol. 2016, 17, 1373–1380. [Google Scholar] [CrossRef]
- Igarashi, H.; Akahoshi, N.; Ohto-Nakanishi, T.; Yasuda, D.; Ishii, S. The lysophosphatidic acid receptor LPA4 regulates hematopoiesis-supporting activity of bone marrow stromal cells. Sci. Rep. 2015, 5, 11410. [Google Scholar] [CrossRef]
- Yang, L.; Kraemer, M.; Fang, X.F.; Angel, P.M.; Drake, R.R.; Morris, A.J.; Smyth, S.S. LPA receptor 4 deficiency attenuates experimental atherosclerosis. J. Lipid Res. 2019, 60, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Maibaum, J.; Liao, S.-M.; Vulpetti, A.; Ostermann, N.; Randl, S.; Rüdisser, S.; Lorthiois, E.; Erbel, P.; Kinzel, B.; Kolb, F.A. Small-molecule factor D inhibitors targeting the alternative complement pathway. Nat. Chem. Biol. 2016, 12, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Kwan W-h van der Touw, W.; Heeger, P.S. Complement regulation of T cell immunity. Immunol. Res. 2012, 54, 247–253. [Google Scholar] [CrossRef]
- Bohlson, S.S.; O’Conner, S.D.; Hulsebus, H.J.; Ho, M.-M.; Fraser, D.A. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front. Immunol. 2014, 5, 402. [Google Scholar] [CrossRef]
- Bhushan, S.; Meinhardt, A. The macrophages in testis function. J. Reprod. Immunol. 2017, 119, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hasan, H.; Bhushan, S.; Fijak, M.; Meinhardt, A. Mechanism of inflammatory associated impairment of sperm function, spermatogenesis and steroidogenesis. Front. Endocrinol. 2022, 13, 897029. [Google Scholar] [CrossRef]
- Abd-Allah, A.R.; Helal, G.K.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Bakheet, S.A. Pro-inflammatory and oxidative stress pathways which compromise sperm motility and survival may be altered by L-carnitine. Oxidative Med. Cell. Longev. 2009, 2, 73–81. [Google Scholar] [CrossRef]
- Meng, Y.; Lin, R.; Wu, F.; Sun, Q.; Jia, L. Decreased capacity for sperm production induced by perinatal bisphenol a exposure is associated with an increased inflammatory response in the offspring of C57BL/6 male mice. Int. J. Environ. Res. Public Health 2018, 15, 2158. [Google Scholar] [CrossRef]
- Bill, C.A.; Allen, C.M.; Vines, C.M. CC chemokine receptor 7 in cancer. Cells 2022, 11, 656. [Google Scholar] [CrossRef]
- Brandum, E.P.; Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Dendritic cells and CCR7 expression: An important factor for autoimmune diseases, chronic inflammation, and cancer. Int. J. Mol. Sci. 2021, 22, 8340. [Google Scholar] [CrossRef]
- Vigliano, I.; Gorrese, M.; Fusco, A.; Vitiello, L.; Amorosi, S.; Panico, L.; Ursini, M.; Calcagno, G.; Racioppi, L.; Del Vecchio, L. FOXN1 mutation abrogates prenatal T-cell development in humans. J. Med. Genet. 2011, 48, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Rota, I.A.; Dhalla, F. FOXN1 deficient nude severe combined immunodeficiency. Orphanet J. Rare Dis. 2017, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Schrama, D.; thor Straten, P.; Becker, J.C. Cytotoxic T cells. J. Investig. Dermatol. 2006, 126, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, C.; Letvin, N.L.; Distaso, J.A.; Aldrich, W.R.; Schlossman, S.F. The isolation and characterization of the human suppressor inducer T cell subset. J. Immunol. 1985, 134, 1508–1515. [Google Scholar] [CrossRef]
- Hecker, N.; Sharma, V.; Hiller, M. Convergent gene losses illuminate metabolic and physiological changes in herbivores and carnivores. Proc. Natl. Acad. Sci. USA 2019, 116, 3036–3041. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Zhang, D.; Liang, Y.; Shan, Z.; Teng, X.; Teng, W. Proteomic analysis of the intestinal resistance to thyroid hormone mouse model with thyroid hormone receptor alpha mutations. Front. Endocrinol. 2022, 13, 773516. [Google Scholar] [CrossRef] [PubMed]
- Batista-Gonzalez, A.; Vidal, R.; Criollo, A.; Carreño, L.J. New insights on the role of lipid metabolism in the metabolic reprogramming of macrophages. Front. Immunol. 2020, 10, 501852. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular changes induced by oxidative stress that impair human sperm motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.; Welch, G. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Heidary, Z.; Zaki-Dizaji, M.; Saliminejad, K.; Khorramkhorshid, H.R. Expression Analysis of the CRISP2, CATSPER1, PATE1 and SEMG1 in the Sperm of Men with Idiopathic Asthenozoospermia. J. Reprod. Infertil. 2019, 20, 70. [Google Scholar] [PubMed]
- Yu, Q.; Zhou, Q.; Wei, Q.; Li, J.; Feng, C.; Mao, X. SEMG 1 may be the candidate gene for idiopathic asthenozoospermia. Andrologia 2014, 46, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Selvam, M.K.P.; Agarwal, A.; Baskaran, S. Proteomic analysis of seminal plasma from bilateral varicocele patients indicates an oxidative state and increased inflammatory response. Asian J. Androl. 2019, 21, 544. [Google Scholar]
- Finelli, R.; Leisegang, K.; Kandil, H.; Agarwal, A. Oxidative stress: A comprehensive review of biochemical, molecular, and genetic aspects in the pathogenesis and management of varicocele. World J. Men’s Health 2022, 40, 87–103. [Google Scholar] [CrossRef]


| Gene | The Sequence of Primers | Gene | The Sequence of Primers |
|---|---|---|---|
| Ifi44 | F: AACTGACTGCTCGCAATAATGT R: GTAACACAGCAATGCCTCTTGT | Cfd | F: GCAAGTGAACGGCACACAC R: GAGTCGTCATCCGTCACTCC |
| Trim29 | F: AGAATGGCACTAAAGCAGACAG R: AAATAGGCCACTCTTCCCCTC | Pnliprp1 | F: CTTCTCCCTTGGAGCCCTGA R: GCCATGGATGATGAACCGAG |
| Foxn1 | F: ATGGTGTCGCTACTCCCTCC R: AGGCACAAACGACGAGCAG | Lpar4 | F: AGTGCCTCCCTGTTTGTCTTC R: GCCAGTGGCGATTAAAGTTGTAA |
| Gpx5 | F: TCTAGCCAGCTATGTGCAGAC R: TCCTTCCCATTAAGAGACAGAGC | Cxcl3 | F: CCCTACCAAGGGTTGATTTTGA R: GGCTATGACTTCTGTCTGGGTG |
| Ccr7 | F: GTGGTGGCTCTCCTTGTCAT R: CTTGAAGCACACCGACTCGT | Semg1 | F: TCTGTCTTCGTCCTTTCTCTGC R: GATCCCGAAACTGAGACGGC |
| Plvap | F: CATCGCCGCTATCATCCTGA R: AGCTTCGCAGGTCTTGTTGA | β-actin | F: GATCTTCATTGTGCTGGGTG R: GGGAAATCGTGCGTGACATT |
| Sirpb1c | F: CCATCAGAGCCTGACATGGA R: CTGCAGGACCGGAGACCATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, P.; Ding, M.; Yu, J.; Gao, Y.; Wang, M.; Ling, J.; Dong, S.; Zhang, X.; Zeng, X.; Sun, X. The Male Reproductive Toxicity Caused by 2-Naphthylamine Was Related to Testicular Immunity Disorders. Toxics 2024, 12, 342. https://doi.org/10.3390/toxics12050342
Dai P, Ding M, Yu J, Gao Y, Wang M, Ling J, Dong S, Zhang X, Zeng X, Sun X. The Male Reproductive Toxicity Caused by 2-Naphthylamine Was Related to Testicular Immunity Disorders. Toxics. 2024; 12(5):342. https://doi.org/10.3390/toxics12050342
Chicago/Turabian StyleDai, Pengyuan, Mengqian Ding, Jingyan Yu, Yuan Gao, Miaomiao Wang, Jie Ling, Shijue Dong, Xiaoning Zhang, Xuhui Zeng, and Xiaoli Sun. 2024. "The Male Reproductive Toxicity Caused by 2-Naphthylamine Was Related to Testicular Immunity Disorders" Toxics 12, no. 5: 342. https://doi.org/10.3390/toxics12050342
APA StyleDai, P., Ding, M., Yu, J., Gao, Y., Wang, M., Ling, J., Dong, S., Zhang, X., Zeng, X., & Sun, X. (2024). The Male Reproductive Toxicity Caused by 2-Naphthylamine Was Related to Testicular Immunity Disorders. Toxics, 12(5), 342. https://doi.org/10.3390/toxics12050342








