A Simple, Ecofriendly, and Fast Method for Nitrate Quantification in Bottled Water Using Visible Spectrophotometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Solutions
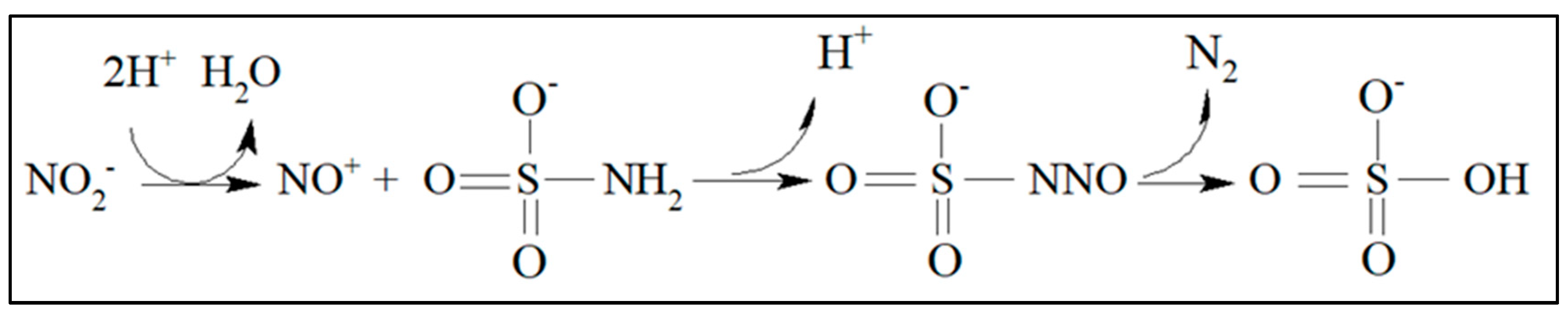
2.3. Reduction Reaction of Nitrate to Nitrite and Diazotation Reaction
2.4. Preparation of Calibration Standards
2.5. Sampling and Preparation
2.6. Analytical Parameters and Statistics
3. Results and Discussion
3.1. Nitrate and Nitrite Reduction Reactions
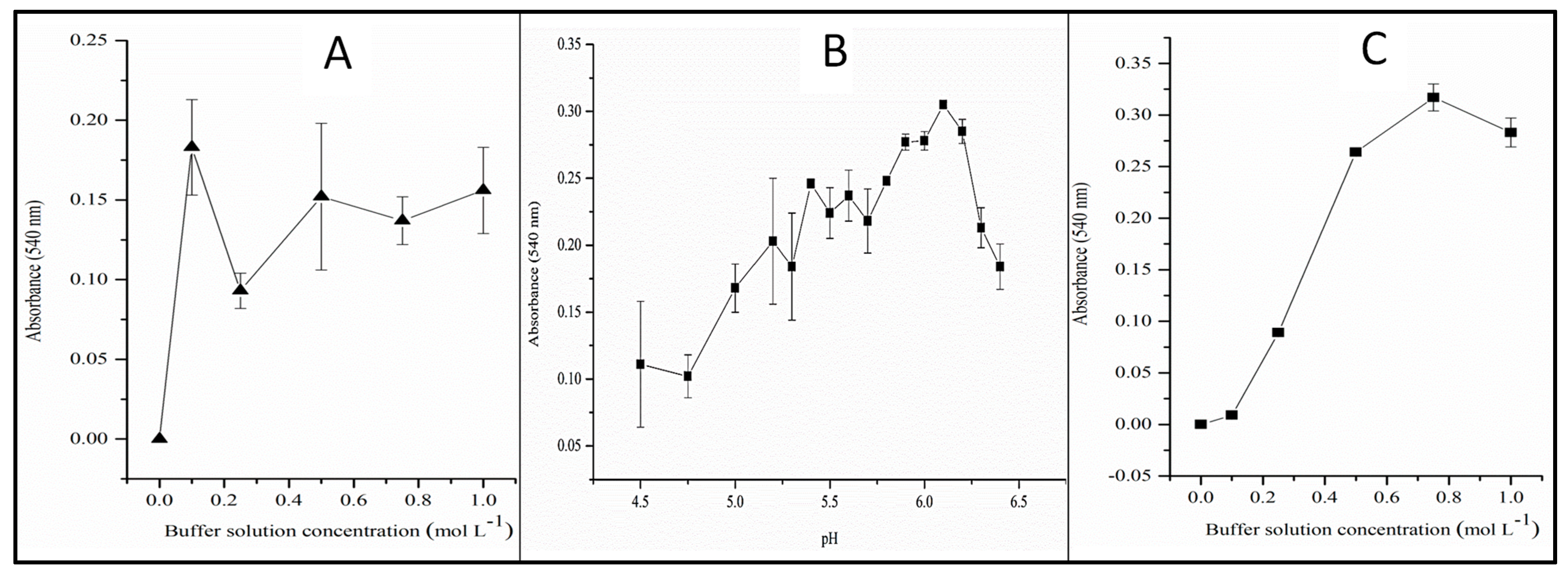
3.2. Influence of Buffer Solution Concentration and pH
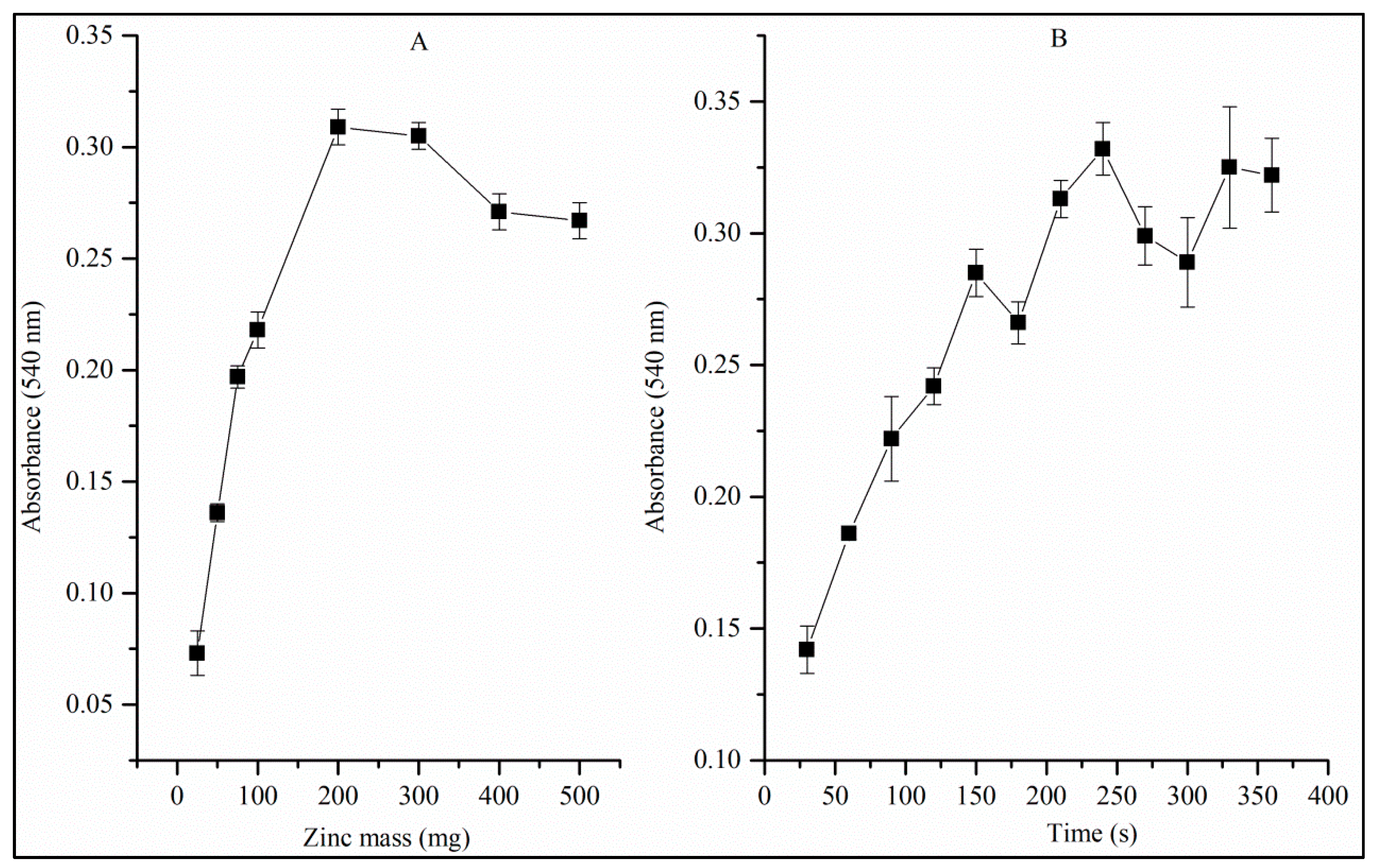
3.3. Univariate Studies of the Reduction Reaction of Nitrate to Nitrite

4. Method Validation
4.1. Selectivity
4.2. Working Range
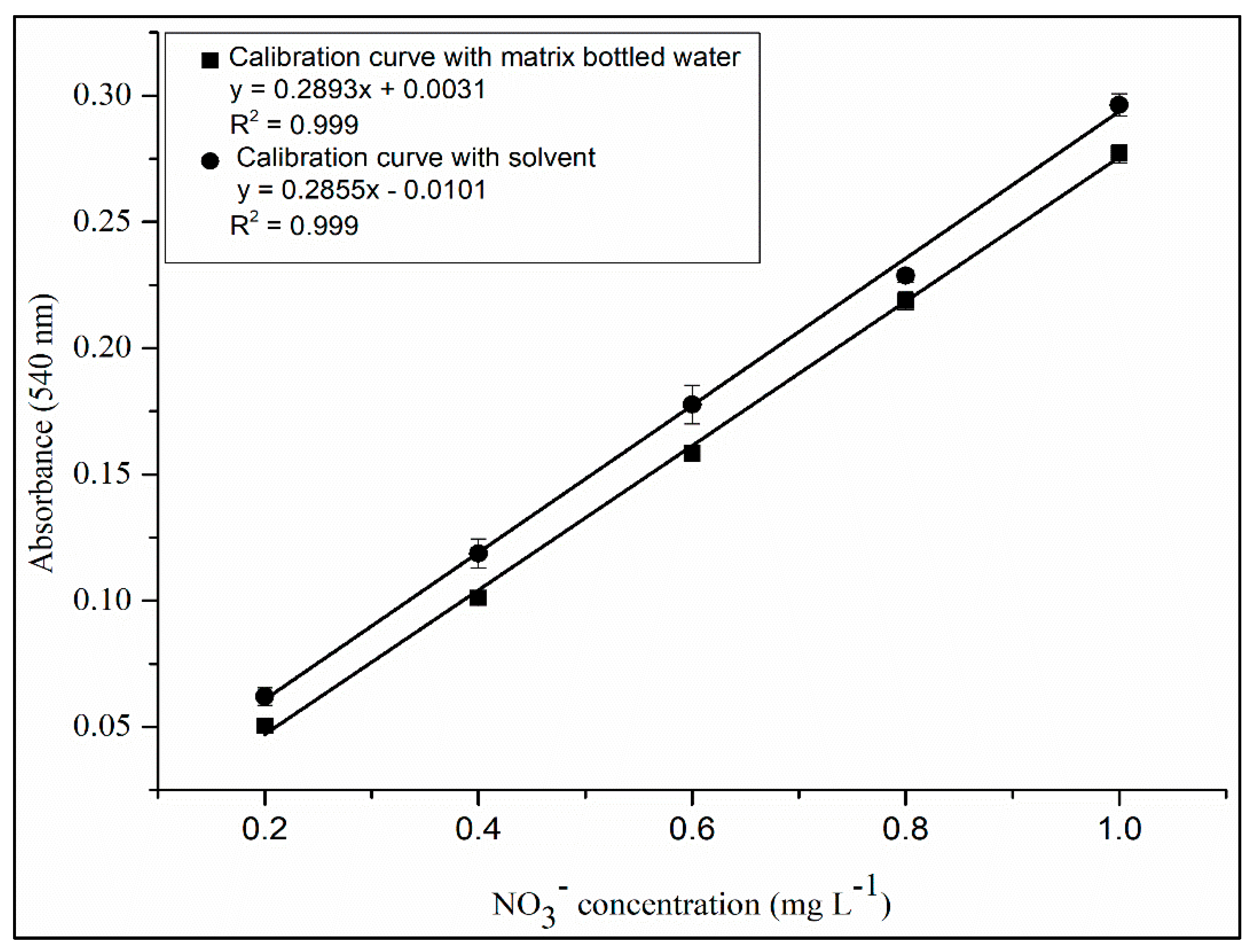
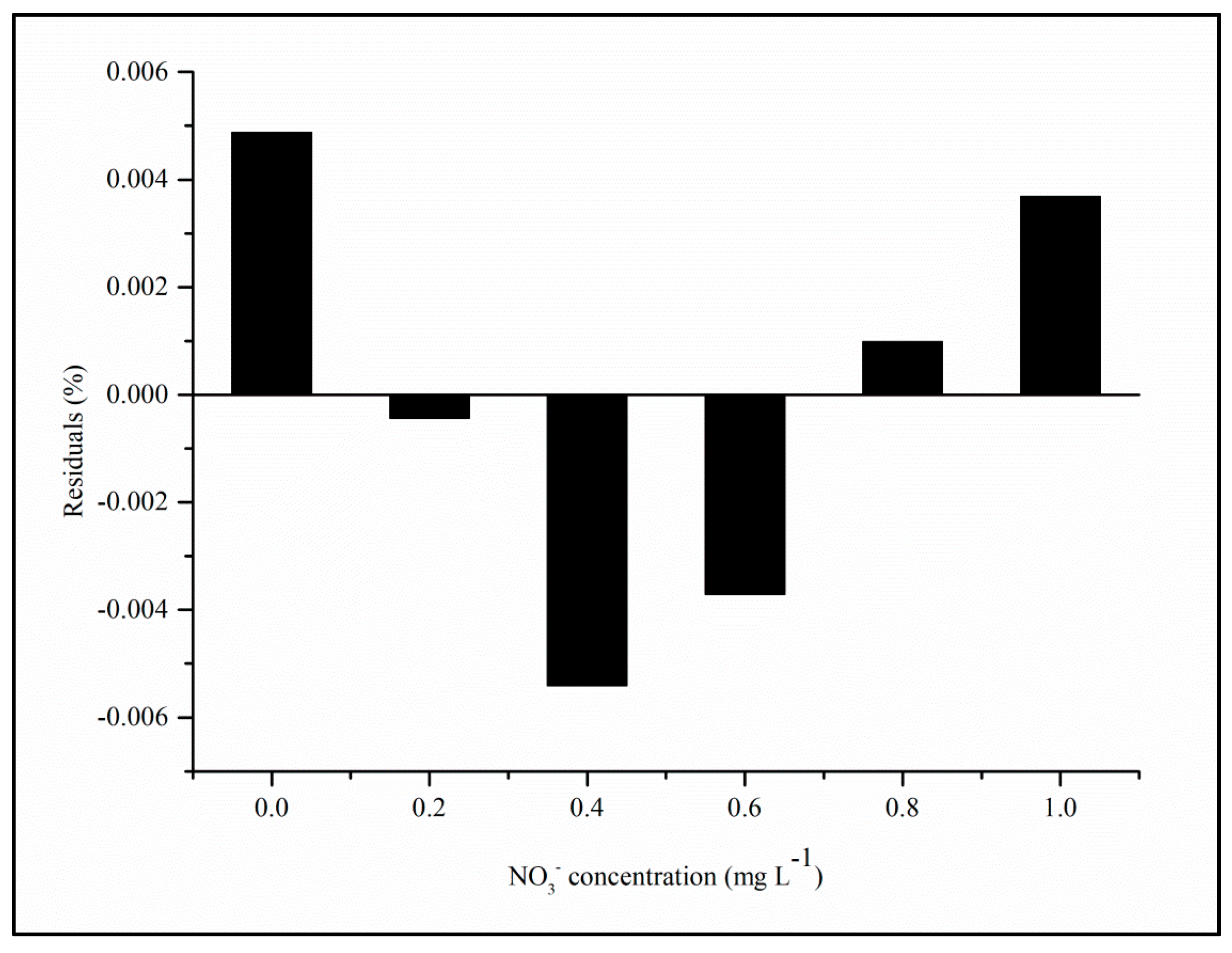
4.3. Linearity
4.4. Limit of Detection and Limit of Quantification
4.5. Bias
4.6. Uncertainty
4.7. Precision
4.8. Quantification of Nitrate in Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baird, C. (Ed.) Environmental Chemistry; WH Freeman and Company: New York, NY, USA, 2020. [Google Scholar]
- Svehla, G.; Suehla, G.; Vogel, A.I. (Eds.) Vogel’s Qualitative Inorganic Analysis, Longman: London, UK, 1996.
- Weyer, P.J.; Cerhan, J.R.; Kross, B.C.; Hallberg, G.R.; Kantamneni, J.; Breuer, G.; Jones, M.P.; Zheng, W.; Lynchet, C.F. Municipal drinking water nitrate level and cancer risk in older women: The Iowa Women’s Health Study. Epidemiology 2001, 12, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Parslow, L.; McKinney, P.; Cartwirght, R. Non-Hodgkin’s lymphoma and nitrate in drinking water: A study in Yorkshire. J. Epidemiol. Community Health 1999, 53, 353–354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cocco, P.; Broccia, G.; Aru, G.; Casula, P.; Muntoni, S.; Cantor, K.P.; Ward, M.H. Nitrate in community water supplies and incidence of non-Hodgkin’s lymphoma in Sardinia, Italy. J. Epidemiol. Community Health 2003, 57, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Swann, P.F.; Sci, J. The toxicology of nitrate, nitrite and n-nitroso compounds. J. Sci. Food Agric. 1975, 26, 1761–1770. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). National Primary Drinking Water Regulations; USEPA: Washington, DC, USA, 2009. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulation-table (accessed on 15 May 2024).
- Agência Nacional de Vigilância Sanitária (ANVISA). Portaria Consolidação n° 5, de 28 de Setembro de 2017: Consolidação Das Normas Sobre Ações e Serviços de Saúde do Sistema Único de Saúde; ANVISA: Brasilia, Brazil, 2017. [Google Scholar]
- Agência Nacional de Vigilância Sanitária (ANVISA). Resolução RDC 274, de 22 de Setembro de 2005, Regulamento Técnico Para Água Engarrafada e Gelo; ANVISA: Brasilia, Brazil, 2005. [Google Scholar]
- Souto, M.A.M.; Okada, M.M.; Okada, I.A.; Dovidauskas, S. A determinação de nitrato em águas por espectrofotometria UV: Usos e precauções. Rev. Inst. Adolfo Lutz 2006, 65, 66–70. [Google Scholar] [CrossRef]
- Clesceri, L.; Greenberg, A.; Eaton, A. (Eds.) Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA; Water Environment Federation: Washington, DC, USA, 1999. [Google Scholar]
- Colman, B.P. Environmental Monitoring and Assessment. Understanding and eliminating iron interference in colorimetric nitrate and nitrite analysis. Environ. Monit. Assess. 2010, 165, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Yu, L.J.; Liu, Y.; Lin, L.; Lu, R.G.; Zhu, J.P.; He, L.; Lu, Z.L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Wierzbicka, E. Novel methods of nitrate and nitrite determination—A review. J. Elem. 2019, 25, 97–106. [Google Scholar] [CrossRef]
- Department of Health and Human Services (HHS)/Agency of Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Merino, L. Development and validation of a method for determination of residual nitrite/nitrate in foodstuffs and water after zinc reduction. Food Anal. Methods 2009, 2, 212–220. [Google Scholar] [CrossRef]
- Murray, E.; Nesterenko, E.P.; Margaret, M.; Morrin, A.; Diamond, D.; Moore, B. A colorimetric method for use within portable test kits for nitrate determination in various water matrices. Anal. Methods 2017, 9, 680–687. [Google Scholar] [CrossRef]
- Ellis, P.S.; Shabani, A.M.H.; Gentle, B.S.; Mckelvie, I.D. Field measurement of nitrate in marine and estuarine waters with a flow analysis system utilizing on-line zinc reduction. Talanta 2011, 84, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Guia de Validação e Controle de Qualidade Analítica: Fármacos em Produtos Para Alimentação e Medicamentos Veterinários; MAPA: Brasilia, Brazil, 2011. [Google Scholar]
- Agência Nacional de Vigilância Sanitária (ANVISA). Resolução RDC 166, de 24 de Julho de 2017, Dispõe Sobre a Validação de Métodos Analíticos e Dá Outras Providências; ANVISA: Brasilia, Brazil, 2017. [Google Scholar]
- Instituto Nacional de Qualidade e Normalização Industrial (INMETRO). Guidance on Validation of Chemical Test Methods, DOQ-CGCRE-008; Revisão 9; INMETRO: Brasilia, Brazil, 2020. Available online: http://inmetro.gov.br/credenciamento/organismos/doc_organismos.asp?torganismo=calibensaios (accessed on 15 May 2024).
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics. 2014. Available online: https://www.eurachem.org/images/stories/workshops/2016_05_MV/pdf/abstr/p03_eurachem_validation_guide.pdf (accessed on 15 May 2024).
- Lourenço, F.R.; Mello, G. (Eds.) Guia Eurachem/CITAC Definindo e Utilizando a Incerteza alvo em Medições Químicas (Versão em Português). 2020. Available online: https://www.eurachem.org/images/stories/Guides/pdf/STMU_2020_PT.pdf (accessed on 15 May 2024).
- Brito, N.M.; Júnior, O.P.A.; Polese, L.; Ribeiro, M.L. Validação de métodos analíticos: Estratégia e discussão. Pestic. Rev. Ecotoxicologia Meio Ambiente 2003, 13, 129–146. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. (Eds.) Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Person: London, UK, 2010. [Google Scholar]
- Guedes, T.A.; Ivanqui, L.I.; Martins, A.B.T. Comparando equações de regressão em dados de saúde. Acta Sci. 2001, 23, 1531–1535. [Google Scholar]
- Milazzo, G.; Caroli, S.; Sharma, V.K. Tables of Standard Electrode Potentials; John Wiley & Sons, Chichester, UK, 1978.
- Atkins, P.; Jones, L. (Eds.) Chemical Principles; W. H. Freeman: New York, NY, USA, 2009. [Google Scholar]
- Giustarini, D.; Milzani, A.; Donne, I.D.; Rossi, R. Detection of S-nitrosothiols in biological fluids: A comparison of most widely applied methodologies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 85, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C. (Ed.) Quantitative Chemical Analysis; WH Freeman: New York, NY, USA, 1999. [Google Scholar]
- Urbansky, E.T.; Schock, M.R. Understanding, deriving, and computing buffer capacity. J. Chem. Educ. 2000, 77, 1640–1644. [Google Scholar] [CrossRef]
- Gujarati, D.N.; Porter, D.C. (Eds.) Basic Econometrics; McGraw Hill Inc.: New York, NY, USA, 2009. [Google Scholar]


| Level | Mean Concentration (mg L−1) | Recovery (%) | RSD (%) |
|---|---|---|---|
| Low (0.3 mg L−1) | 0.27 | 86.35 | 4.15 |
| Medium (0.6 mg L−1) | 0.52 | 85.9 | 2.75 |
| High (1.0 mg L−1) | 0.85 | 84.61 | 1.1 |
| Sample | Nitrate Mean Concentration (mg L−1) | Uncertainty (mg L−1) | Relative Uncertainty (%) | RSD (%) |
|---|---|---|---|---|
| A | 3.38 | 0.08 | 2.39 | 1.06 |
| B | 0.12 | 0.08 | 71.58 | 3.95 |
| C | 1.73 | 0.15 | 8.56 | 1.91 |
| D | <LoQ | - | - | - |
| E | 2.01 | 0.15 | 7.31 | 1.80 |
| F | 0.79 | 0.08 | 10.32 | 0.88 |
| G | 19.37 | 0.08 | 0.42 | 0.31 |
| H | 2.36 | 0.08 | 3.39 | 0.44 |
| I | 28.20 | 0.08 | 0.28 | 1.69 |
| J | 4.79 | 0.08 | 1.67 | 1.15 |
| K | 4.01 | 0.08 | 2 | 1.03 |
| L * | 0.13 | 0.08 | 66.18 | 3.66 |
| M | 2.87 | 0.08 | 2.80 | 0.48 |
| N | 5.04 | 0.08 | 1.59 | 0.82 |
| O | 2.94 | 0.08 | 2.73 | 0.93 |
| P | 5.74 | 0.08 | 1.40 | 2.01 |
| Q | 0.89 | 0.08 | 9.24 | 1.58 |
| R | 12.21 | 0.08 | 0.66 | 0.59 |
| S | 0.91 | 0.08 | 9.08 | 1.17 |
| T | 11.06 | 0.08 | 0.72 | 1.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Ascenção, W.D.; Augusto, C.C.; de Melo, V.H.S.; Batista, B.L. A Simple, Ecofriendly, and Fast Method for Nitrate Quantification in Bottled Water Using Visible Spectrophotometry. Toxics 2024, 12, 383. https://doi.org/10.3390/toxics12060383
da Ascenção WD, Augusto CC, de Melo VHS, Batista BL. A Simple, Ecofriendly, and Fast Method for Nitrate Quantification in Bottled Water Using Visible Spectrophotometry. Toxics. 2024; 12(6):383. https://doi.org/10.3390/toxics12060383
Chicago/Turabian Styleda Ascenção, Wellington Diego, Caroline Cristine Augusto, Vitor Hugo Soares de Melo, and Bruno Lemos Batista. 2024. "A Simple, Ecofriendly, and Fast Method for Nitrate Quantification in Bottled Water Using Visible Spectrophotometry" Toxics 12, no. 6: 383. https://doi.org/10.3390/toxics12060383
APA Styleda Ascenção, W. D., Augusto, C. C., de Melo, V. H. S., & Batista, B. L. (2024). A Simple, Ecofriendly, and Fast Method for Nitrate Quantification in Bottled Water Using Visible Spectrophotometry. Toxics, 12(6), 383. https://doi.org/10.3390/toxics12060383








