TBHQ Alleviates Particulate Matter-Induced Pyroptosis in Human Nasal Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Treatment
2.3. Determination of Cell Viability
2.4. Pyroptotic Cell Death Assay
2.5. Quantitative Real-Time PCR
2.6. RNA Interference
2.7. Western Blotting
2.8. Immunofluorescent Staining
2.9. Oxidative Stress Assay
2.10. Supernatant ELISA Detection
2.11. Statistical Analysis
3. Results
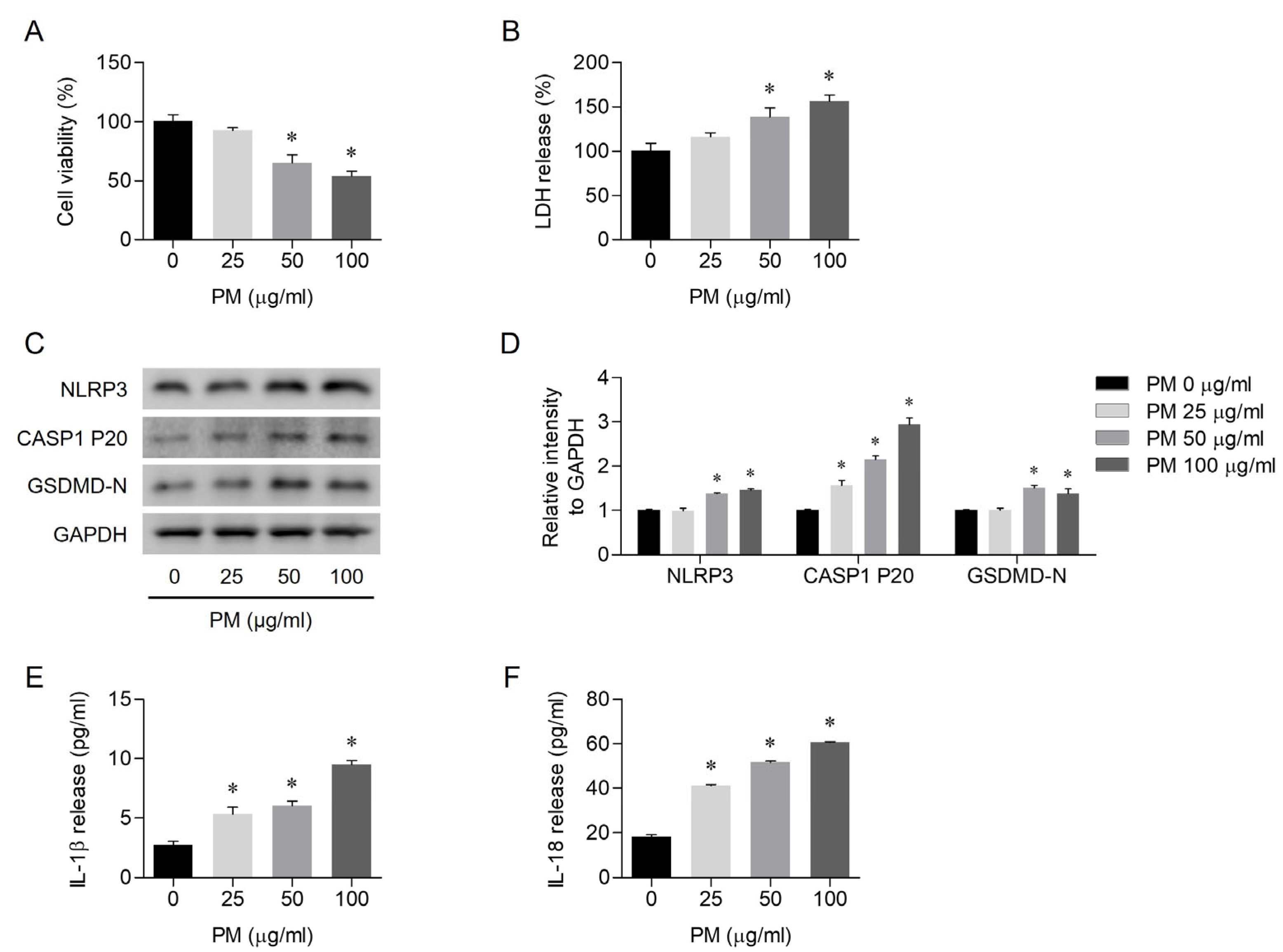
3.1. PM-Induced Pyroptosis in Human Nasal Epithelial Cells
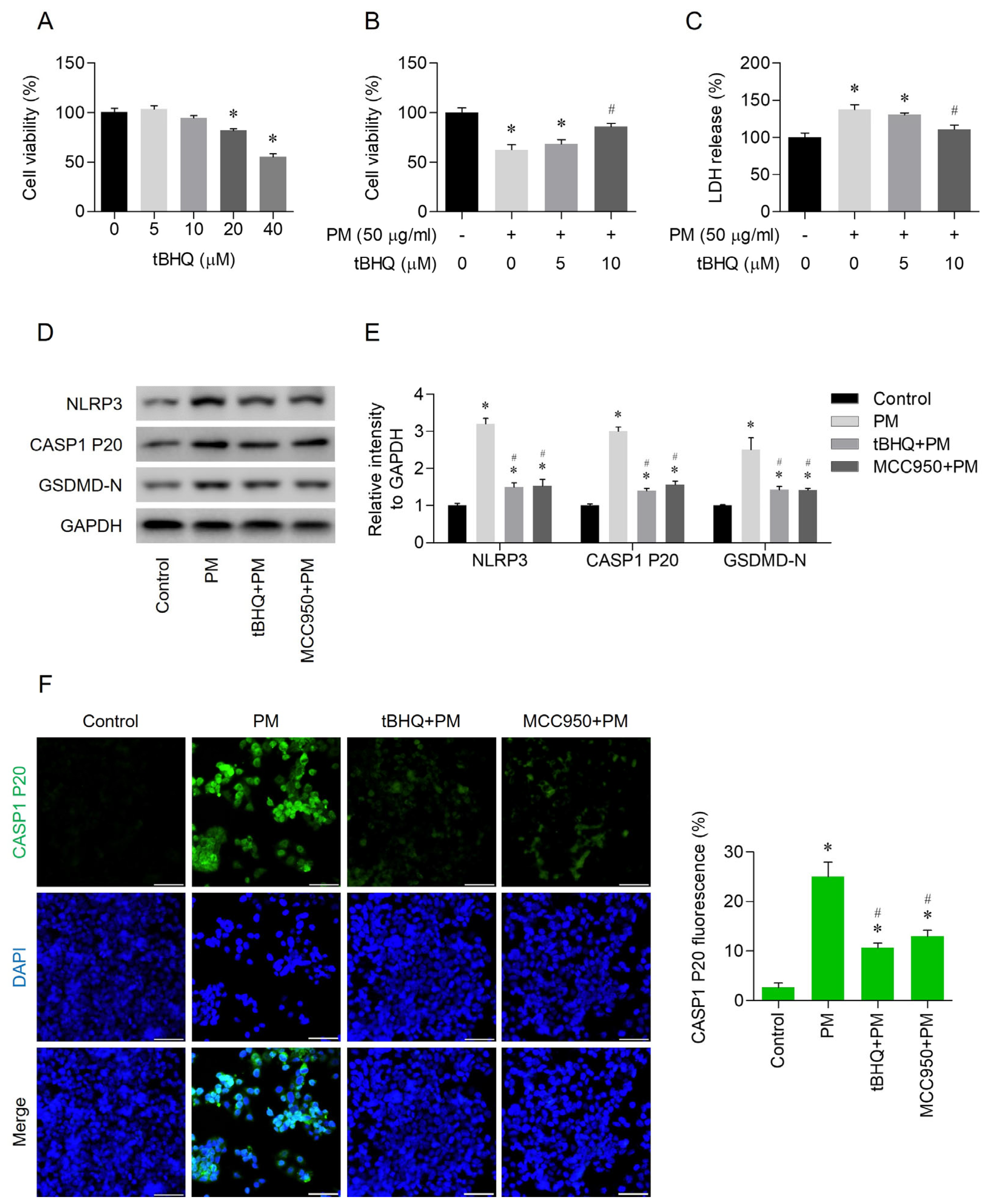
3.2. PM-Induced NLRP3 Inflammasome-Dependent Pyroptosis
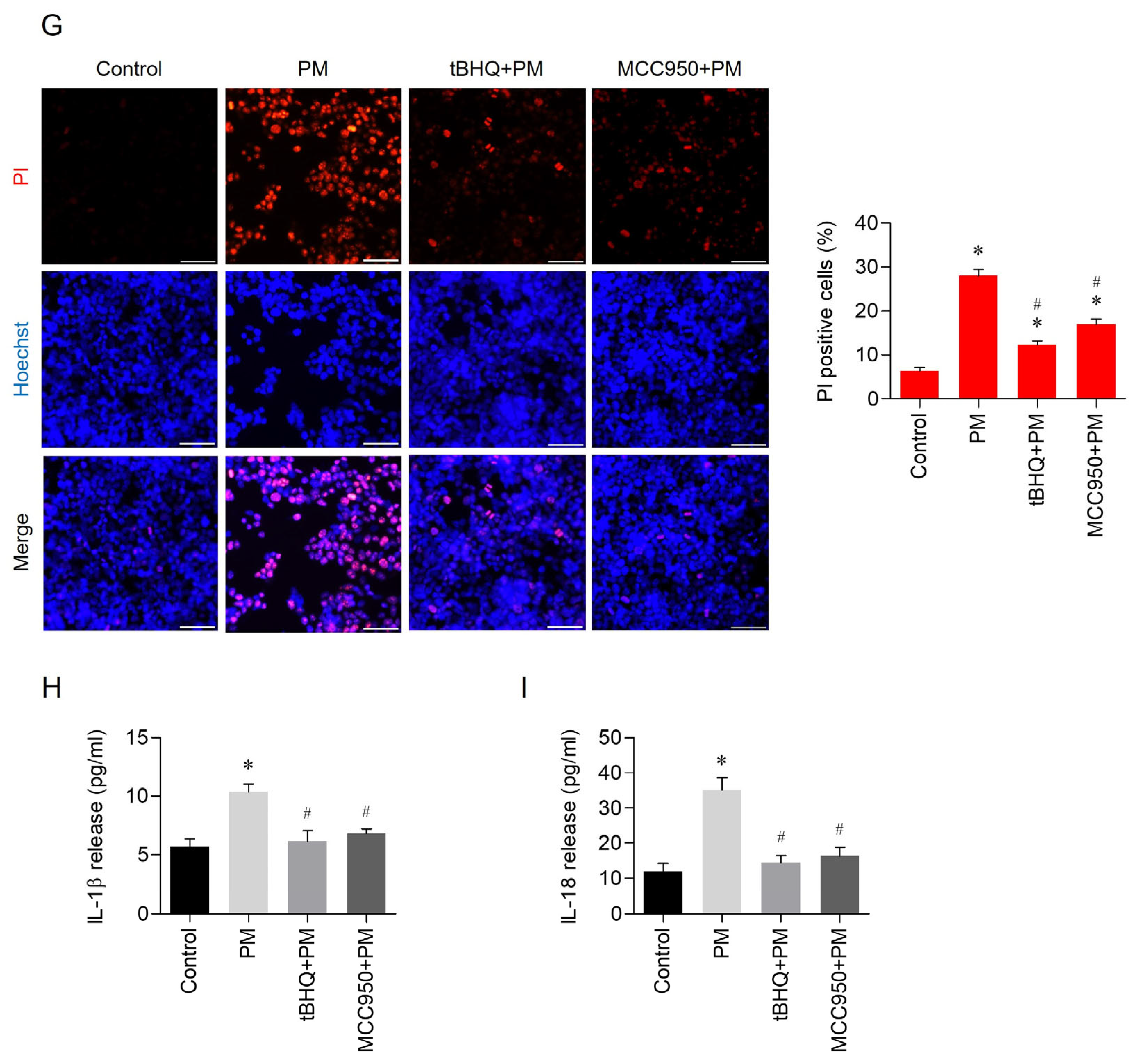
3.3. Effects of tBHQ on PM-Induced Pyroptosis
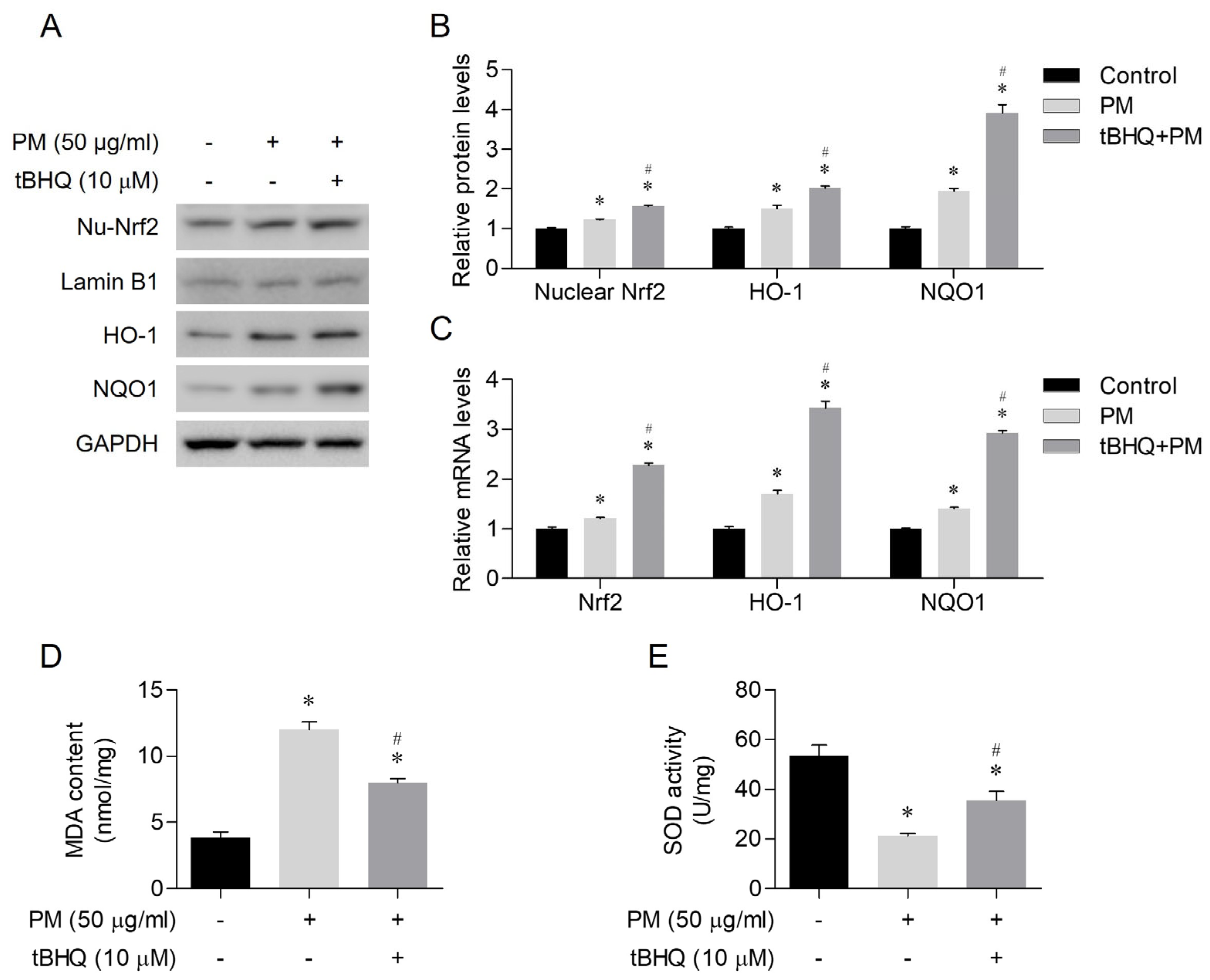
3.4. The Antioxidant Effects of tBHQ Via the Nrf2/HO-1 Pathway in PM-Treated Human Nasal Epithelial Cells
3.5. Nrf2 Knockdown Abolished the Pyroptosis-Inhibiting Effects of tBHQ
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef] [PubMed]
- Löndahl, J.; Massling, A.; Pagels, J.; Swietlicki, E.; Vaclavik, E.; Loft, S. Size-resolved respiratory-tract deposition of fine and ultrafine hydrophobic and hygroscopic aerosol particles during rest and exercise. Inhal. Toxicol. 2007, 19, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Oh, J.-M.; Choi, H.; Kim, S.W.; Kim, S.W.; Kim, B.G.; Cho, J.H.; Lee, J.; Lee, D.C. Activation of the Nrf2/HO-1 pathway by curcumin inhibits oxidative stress in human nasal fibroblasts exposed to urban particulate matter. BMC Complement. Med. Ther. 2020, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Choi, H.; Oh, J.-M.; Lee, J.; Lee, J.; Lee, H.Y.; Kang, J.Y. Urban particulate matter regulates tight junction proteins by inducing oxidative stress via the Akt signal pathway in human nasal epithelial cells. Toxicol. Lett. 2020, 333, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Guo, Z.; Zhang, R.; Xu, J.; Dong, W.; Zhuang, G.; Deng, C. Airborne fine particulate matter induces oxidative stress and inflammation in human nasal epithelial cells. Tohoku J. Exp. Med. 2016, 239, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Folkmann, J.K.; Forchhammer, L.; Bräuner, E.V.; Danielsen, P.H.; Risom, L.; Loft, S. Air pollution, oxidative damage to DNA, and carcinogenesis. Cancer Lett. 2008, 266, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Osornio-Vargas, Á.R.; Bonner, J.C.; Alfaro-Moreno, E.; Martínez, L.; García-Cuellar, C.; Ponce-de-León Rosales, S.; Miranda, J.; Rosas, I. Proinflammatory and cytotoxic effects of Mexico City air pollution particulate matter in vitro are dependent on particle size and composition. Environ. Health Perspect. 2003, 111, 1289–1293. [Google Scholar] [CrossRef]
- Su, R.; Jin, X.; Zhang, W.; Li, Z.; Liu, X.; Ren, J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere 2017, 167, 444–453. [Google Scholar] [CrossRef]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Zheng, R.; Tao, L.; Jian, H.; Chang, Y.; Cheng, Y.; Feng, Y.; Zhang, H. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol. Environ. Saf. 2018, 163, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, N.; Huang, L.; Zhao, Y.; Shao, H.; Jin, Y.; Zhang, R.; Li, C.; Wu, W.; Wang, J. NLRP3 inflammasome activation is associated with PM2.5-induced cardiac functional and pathological injury in mice. Environ. Toxicol. 2019, 34, 1246–1254. [Google Scholar] [CrossRef]
- Du, X.; Jiang, S.; Zeng, X.; Zhang, J.; Pan, K.; Zhou, J.; Xie, Y.; Kan, H.; Song, W.; Sun, Q. Air pollution is associated with the development of atherosclerosis via the cooperation of CD36 and NLRP3 inflammasome in ApoE-/-mice. Toxicol. Lett. 2018, 290, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, L.; Xing, C.; Luo, B.; Hu, C.; Song, M.; Niu, J.; Ruan, Y.; Sun, X.; Lei, Y. Airborne particulate matter (PM2.5) triggers cornea inflammation and pyroptosis via NLRP3 activation. Ecotoxicol. Environ. Saf. 2021, 207, 111306. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, L.; An, Z.; Song, J.; Wang, C.; Ma, Y.; Gu, Q.; Luo, Q.; Yang, W.; et al. Fine particulate matter exposure exacerbated nasal mucosal damage in allergic rhinitis mice via NLRP3 mediated pyroptosis. Ecotoxicol. Environ. Saf. 2021, 228, 112998. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Sargazi, S.; Moghadam, J.A.; Heidarpour, M. Protective effect of tert butyl hydroquinone on diazinon-induced oxidative stress in brain and heart of male rats. Sciences 2016, 18. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Li, W.; Xing, X.; Zhang, Y.; Zhao, L.; Sun, G.; Gao, X.-h.; Li, B. Antioxidant tert-butylhydroquinone ameliorates arsenic-induced intracellular damages and apoptosis through induction of Nrf2-dependent antioxidant responses as well as stabilization of anti-apoptotic factor Bcl-2 in human keratinocytes. Free Radic. Biol. Med. 2016, 94, 74–87. [Google Scholar] [CrossRef]
- World Health Organization. Health Effects of Particulate Matter: Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia. World Health Organization. Regional Office for Europe. 2013. Available online: https://iris.who.int/handle/10665/344854 (accessed on 5 March 2024).
- Brown, J.S.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 2013, 10, 12. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne particulate matter and human health: Toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health Part C 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Mo, J.-H. Association of particulate matter with ENT diseases. Clin. Exp. Otorhinolaryngol. 2019, 12, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Pénard-Morand, C.; Raherison, C.; Charpin, D.; Kopferschmitt, C.; Lavaud, F.; Caillaud, D.; Annesi-Maesano, I. Long-term exposure to close-proximity air pollution and asthma and allergies in urban children. Eur. Respir. J. 2010, 36, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mady, L.J.; Schwarzbach, H.L.; Moore, J.A.; Boudreau, R.M.; Willson, T.J.; Lee, S.E. Air Pollutants May Be Environmental Risk Factors in Chronic Rhinosinusitis Disease Progression; International Forum of Allergy & Rhinology; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 377–384. [Google Scholar]
- Ramanathan, M., Jr.; London, N.R., Jr.; Tharakan, A.; Surya, N.; Sussan, T.E.; Rao, X.; Lin, S.Y.; Toskala, E.; Rajagopalan, S.; Biswal, S. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am. J. Respir. Cell Mol. Biol. 2017, 57, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bergsbaken, T.; Fink, S.L.; Cookson, B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009, 7, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Muñoz-Planillo, R.; Núñez, G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manji, G.A.; Grenier, J.M.; Al-Garawi, A.; Merriam, S.; Lora, J.M.; Geddes, B.J.; Briskin, M.; DiStefano, P.S.; Bertin, J. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 2002, 277, 29874–29880. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Gao, L.; Qin, J.X.; Shi, J.Q.; Jiang, T.; Wang, F.; Xie, C.; Gao, Q.; Zhi, N.; Dong, Q.; Guan, Y.T. Fine particulate matter exposure aggravates ischemic injury via NLRP3 inflammasome activation and pyroptosis. CNS Neurosci. Ther. 2022, 28, 1045–1058. [Google Scholar] [CrossRef]
- Xi, M.; Shen, D.; Dai, P.; Han, G.; Li, C. TBHQ alleviates pyroptosis and necroptosis in chicken alveolar epithelial cells induced by fine particulate matter from broiler houses. Poult. Sci. 2022, 101, 101593. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Akhlaghi, A.P.; Alizadeh, A.M.; Dehghan, P.; Maleki, P. Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review. Toxicol. Rep. 2022, 9, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Dolatabadi, J.E.N.; Kashanian, S. A review on DNA interaction with synthetic phenolic food additives. Food Res. Int. 2010, 43, 1223–1230. [Google Scholar] [CrossRef]
- Li, J.; Johnson, D.; Calkins, M.; Wright, L.; Svendsen, C.; Johnson, J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol. Sci. 2005, 83, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Shen, C.; Zhang, X.; Sun, S.; Cho, M.; Sun, C.; Song, Z. tert-Butylhydroquinone (tBHQ) protects hepatocytes against lipotoxicity via inducing autophagy independently of Nrf2 activation. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2014, 1841, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Turley, A.E.; Zagorski, J.W.; Rockwell, C.E. The Nrf2 activator tBHQ inhibits T cell activation of primary human CD4 T cells. Cytokine 2015, 71, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.E.; Timme-Laragy, A.R.; Karchner, S.I.; Stegeman, J.J. Nrf2 and Nrf2-related proteins in development and developmental toxicity: Insights from studies in zebrafish (Danio rerio). Free Radic. Biol. Med. 2015, 88, 275–289. [Google Scholar] [CrossRef]
- Sporn, M.B.; Liby, K.T. NRF2 and cancer: The good, the bad and the importance of context. Nat. Rev. Cancer 2012, 12, 564–571. [Google Scholar] [CrossRef]
- van Muiswinkel, F.L.; Kuiperij, H.B. The Nrf2-ARE Signalling pathway: Promising drug target to combat oxidative stress in neurodegenerative disorders. Curr. Drug Targets-CNS Neurol. Disord. 2005, 4, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.-L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Navarro, L.; Angosto-Bazarra, D.; Pelegrín, P.; Baroja-Mazo, A.; Cuevas, S. NLRP3 Inflammasome and Pyroptosis in Liver Pathophysiology: The Emerging Relevance of Nrf2 Inducers. Antioxidants 2022, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Luo, T.; Weng, A.; Huang, X.; Yao, Y.; Fu, Z.; Li, Y.; Liu, A.; Li, X.; Chen, D.; et al. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Through Enhancing Nrf2 Signaling. Front. Immunol. 2020, 11, 580593. [Google Scholar] [CrossRef]
- Zou, Y.; Luo, X.; Feng, Y.; Fang, S.; Tian, J.; Yu, B.; Li, J. Luteolin prevents THP-1 macrophage pyroptosis by suppressing ROS production via Nrf2 activation. Chem. Biol. Interact. 2021, 345, 109573. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Choi, H.; Oh, J.-M.; Kim, S.W.; Kim, S.W.; Kim, B.G.; Cho, J.H.; Lee, J.; Lee, D.C. TBHQ Alleviates Particulate Matter-Induced Pyroptosis in Human Nasal Epithelial Cells. Toxics 2024, 12, 407. https://doi.org/10.3390/toxics12060407
Kim J-S, Choi H, Oh J-M, Kim SW, Kim SW, Kim BG, Cho JH, Lee J, Lee DC. TBHQ Alleviates Particulate Matter-Induced Pyroptosis in Human Nasal Epithelial Cells. Toxics. 2024; 12(6):407. https://doi.org/10.3390/toxics12060407
Chicago/Turabian StyleKim, Ji-Sun, Hyunsu Choi, Jeong-Min Oh, Sung Won Kim, Soo Whan Kim, Byung Guk Kim, Jin Hee Cho, Joohyung Lee, and Dong Chang Lee. 2024. "TBHQ Alleviates Particulate Matter-Induced Pyroptosis in Human Nasal Epithelial Cells" Toxics 12, no. 6: 407. https://doi.org/10.3390/toxics12060407
APA StyleKim, J.-S., Choi, H., Oh, J.-M., Kim, S. W., Kim, S. W., Kim, B. G., Cho, J. H., Lee, J., & Lee, D. C. (2024). TBHQ Alleviates Particulate Matter-Induced Pyroptosis in Human Nasal Epithelial Cells. Toxics, 12(6), 407. https://doi.org/10.3390/toxics12060407







