Effects of Freeze-Thaw Cycles on Bioaccessibilities of Polycyclic Aromatic Hydrocarbons
Abstract
1. Introduction
2. Materials and Methods
2.1. Sorbents Preparation
2.2. Simulated Body Fluids Preparation
2.3. Soil Spiking and Aging
2.4. Desorption Kinetics Experiments
2.5. Sample Analysis and Data Calculation
2.5.1. Sample Analysis
2.5.2. Data Calculation
2.6. Quality Assurance and Quality Control
3. Results and Discussion
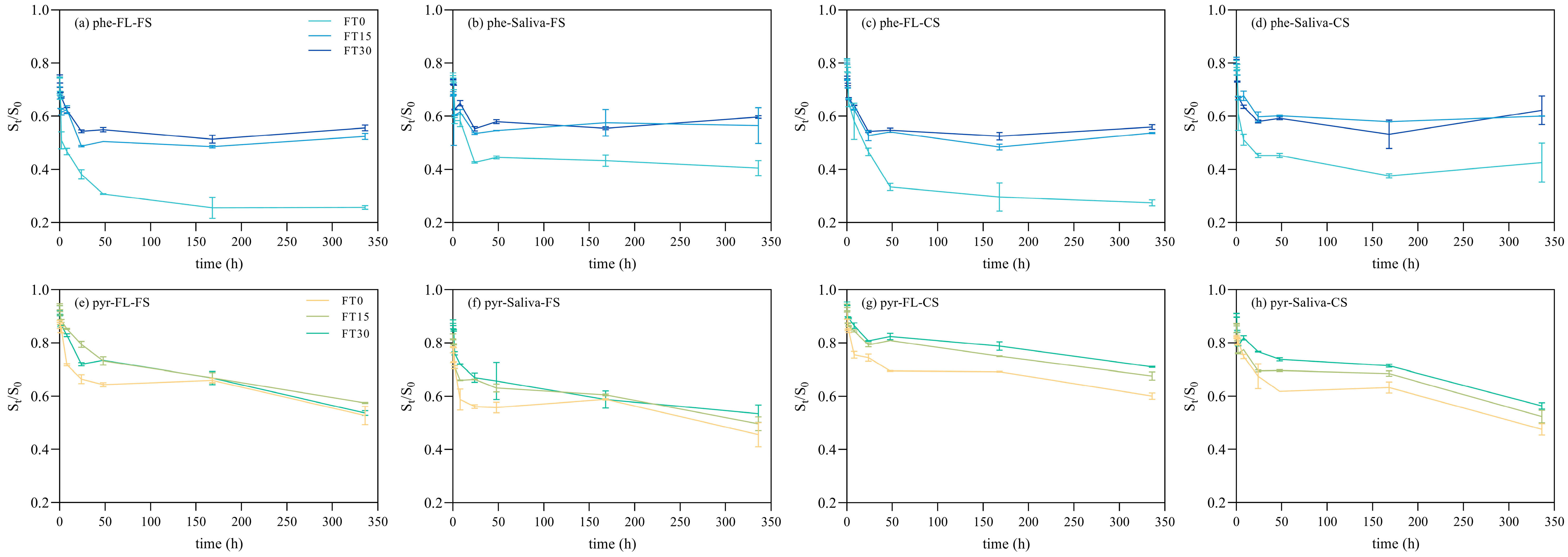
3.1. General Shape
3.2. Contribution of Soil Nature
3.3. Role of Hydrophobicity
3.4. Effect of Simulated Body Fluids
3.5. Impact of freeze-thaw Cycle
3.6. Implications of PAHs Desorption Kinetics and Aging on Health Risks
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahpoury, P.; Wnorowski, A.; Harner, T.; Saini, A.; Halappanavar, S. A Method for Measuring the Bioaccessibility of Polycyclic Aromatic Hydrocarbons in Cell Culture Media. Chemosphere 2024, 351, 141257. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Xiang, P.; He, R.-W.; Juhasz, A.; Ma, L.Q. Advances in in Vitro Methods to Evaluate Oral Bioaccessibility of PAHs and PBDEs in Environmental Matrices. Chemosphere 2016, 150, 378–389. [Google Scholar] [CrossRef]
- Ruby, M.V.; Lowney, Y.W.; Bunge, A.L.; Roberts, S.M.; Gomez-Eyles, J.L.; Ghosh, U.; Kissel, J.C.; Tomlinson, P.; Menzie, C. Oral Bioavailability, Bioaccessibility, and Dermal Absorption of PAHs from Soil—State of the Science. Environ. Sci. Technol. 2016, 50, 2151–2164. [Google Scholar] [CrossRef]
- Boisa, N.; Bird, G.; Brewer, P.A.; Dean, J.R.; Entwistle, J.A.; Kemp, S.J.; Macklin, M.G. Potentially Harmful Elements (PHEs) in Scalp Hair, Soil and Metallurgical Wastes in Mitrovica, Kosovo: The Role of Oral Bioaccessibility and Mineralogy in Human PHE Exposure. Environ. Int. 2013, 60, 56–70. [Google Scholar] [CrossRef]
- Kastury, F.; Smith, E.; Juhasz, A.L. A Critical Review of Approaches and Limitations of Inhalation Bioavailability and Bioaccessibility of Metal(Loid)s from Ambient Particulate Matter or Dust. Sci. Total Environ. 2017, 574, 1054–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Li, Y.; Zhang, R.; Gao, P.; Cui, X.; Ma, L.Q. Bioaccessibility of PAHs in Contaminated Soils: Comparison of Five in Vitro Methods with Tenax as a Sorption Sink. Sci. Total Environ. 2017, 601–602, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Lin, A.-J.; Zhu, Y.-G. Concentrations and Bioaccessibility of Polycyclic Aromatic Hydrocarbons in Wastewater–Irrigated Soil Using in Vitro Gastrointestinal Test. Environ. Sci. Pollut. Res.-Int. 2008, 15, 344–353. [Google Scholar] [CrossRef]
- Zeng, Y.; Fan, Y.; Yan, X.; Zheng, J.; Chen, S.-J.; Mai, B.-X. In Vitro Oral and Inhalation Bioaccessibility of Hydrophobic Organic Contaminants (HOCs) in Airborne Particles and Influence of Relevant Parameters. Environ. Res. 2019, 170, 134–140. [Google Scholar] [CrossRef]
- Xie, S.-Y.; Lao, J.-Y.; Wu, C.-C.; Bao, L.-J.; Zeng, E.Y. In Vitro Inhalation Bioaccessibility for Particle–Bound Hydrophobic Organic Chemicals: Method Development, Effects of Particle Size and Hydrophobicity, and Risk Assessment. Environ. Int. 2018, 120, 295–303. [Google Scholar] [CrossRef]
- Wei, R.; Ni, J.; Guo, L.; Yang, L.; Yang, Y. The Effect of Aging Time on the Distribution of Pyrene in Soil Particle–Size Fractions. Geoderma 2014, 232–234, 19–23. [Google Scholar] [CrossRef]
- Kelsey, J.W.; Kottler, B.D.; Alexander, M. Selective Chemical Extractants To Predict Bioavailability of Soil–Aged Organic Chemicals. Environ. Sci. Technol. 1997, 31, 214–217. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.; Han, L.; Li, W.; Xu, L.; Hu, F.; Li, H. The Effects of Aging Time on the Fraction Distribution and Bioavailability of PAH. Chemosphere 2012, 86, 1072–1078. [Google Scholar] [CrossRef]
- Colombo, C.; Monhemius, A.J.; Plant, J.A. Platinum, Palladium and Rhodium Release from Vehicle Exhaust Catalysts and Road Dust Exposed to Simulated Lung Fluids. Ecotoxicol. Environ. Saf. 2008, 71, 722–730. [Google Scholar] [CrossRef]
- Dean, J.R.; Ma, R. Approaches to Assess the Oral Bioaccessibility of Persistent Organic Pollutants: A Critical Review. Chemosphere 2007, 68, 1399–1407. [Google Scholar] [CrossRef]
- Huang, L.; Pignatello, J. Improved extraction of atrazine and metolachlor in field soil samples. J. Assoc. Off. Anal. Chem. 1990, 73, 443–446. [Google Scholar] [CrossRef]
- Barnier, C.; Ouvrard, S.; Robin, C.; Morel, J.L. Desorption Kinetics of PAHs from Aged Industrial Soils for Availability Assessment. Sci. Total Environ. 2014, 470–471, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G.; van Noort, P.; Govers, H. Desorption kinetics of chlorobenzenes, polycyclic aromatic hydrocarbons, and polychlorinated biphenyls: Sediment extraction with Tenax and effects of contanct time and solute hydrophobicity. Environ. Toxicol. Chem. 1997, 16, 1351–1357. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; Xu, Y.; Tang, J. Aging Effects on Sorption–Desorption Behaviors of PAHs in Different Natural Organic Matters. J. Colloid Interface Sci. 2012, 382, 117–122. [Google Scholar] [CrossRef]
- Di, X.; Xiao, B.; Dong, H.; Wang, S. Implication of Different Humic Acid Fractions in Soils under Karst Rocky Desertification. CATENA 2019, 174, 308–315. [Google Scholar] [CrossRef]
- Weber, W.J.; Huang, W. A Distributed Reactivity Model for Sorption by Soils and Sediments. 4. Intraparticle Heterogeneity and Phase–Distribution Relationships under Nonequilibrium Conditions. Environ. Sci. Technol. 1996, 30, 881–888. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, B. Effects of Geosorbent and Solution Properties on Sorption and Desorption of PAHs. Acta Geochim. 2021, 40, 212–224. [Google Scholar] [CrossRef]
- Xiao, B.; Huang, W. The Equilibria of Bisolute Sorption on Soil. Chemosphere 2011, 83, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Pignatello, J.J.; Gigliotti, B. Competitive Sorption between Atrazine and Other Organic Compounds in Soils and Model Sorbents. Environ. Sci. Technol. 1996, 30, 2432–2440. [Google Scholar] [CrossRef]
- Collins, C.D.; Craggs, M.; Garcia-Alcega, S.; Kademoglou, K.; Lowe, S. Towards a Unified Approach for the Determination of the Bioaccessibility of Organic Pollutants. Environ. Int. 2015, 78, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Stapleton, H.M. Evaluating the Bioaccessibility of Flame Retardants in House Dust Using an In Vitro Tenax Bead–Assisted Sorptive Physiologically Based Method. Environ. Sci. Technol. 2014, 48, 13323–13330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Brusseau, M.L. Cyclopentanol–Enhanced Solubilization of PolycycIic Aromatic Hydrocathns by Cyclodextrins. Environ. Sci. Technol. 1995, 29, 2346–2351. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, L.Y. Effects of ion strength on interaction between perylene and hydrophobic fractions of dissolved matter (DOM) isolated from lake water. Acta Mineral. Sin. 2016, 2, 295–300. (In Chinese) [Google Scholar] [CrossRef]
- Luo, X.M.; Liu, C.M. Effects of Ca2+ ionic strength on sorption of polycyclic aromatic hydrocarbons (PAHs) on soils and sediments in Yellow River Delta. Ecol. Environ. 2006, 15, 983–987. (In Chinese) [Google Scholar] [CrossRef]
- Duan, L.; Naidu, R. Effect of Ionic Strength and Index Cation on the Sorption of Phenanthrene. Water Air Soil Pollut. 2013, 224, 1700. [Google Scholar] [CrossRef]
- Gerstl, Z.; Yaron, B. Behavior of Bromacil and Napropamide in Soils: I. Adsorption and Degradation. Soil Sci. Soc. Am. J. 1983, 47, 474–478. [Google Scholar] [CrossRef]
- Gunnarsson, J.S.; Rosenberg, R. Eutrophication Increases the Association of PCB to Dissolved Organic Matter in Marine Microcosms. Mar. Pollut. Bull. 1996, 33, 100–111. [Google Scholar] [CrossRef]
- Brusseau, M.L.; Rao, P.S.C.; Gillham, R.W. Sorption Nonideality during Organic Contaminant Transport in Porous Media. Crit. Rev. Environ. Control 1989, 19, 33–99. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Xing, B. Mechanisms of Slow Sorption of Organic Chemicals to Natural Particles. Environ. Sci. Technol. 1996, 30, 1–11. [Google Scholar] [CrossRef]
- Gevao, B.; Mordaunt, C.; Semple, K.T.; Piearce, T.G.; Jones, K.C. Bioavailability of Nonextractable (Bound) Pesticide Residues to Earthworms. Environ. Sci. Technol. 2001, 35, 501–507. [Google Scholar] [CrossRef]
- Xing, B. Sorption of Naphthalene and Phenanthrene by Soil Humic Acids. Environ. Pollut. 2001, 111, 303–309. [Google Scholar] [CrossRef]
- Hatzinger, P.; Alexander, M. Effect of aging of chemicals in soil on their biodegradability and extractability. Environ. Sci. Technol. 1995, 29, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.A.L. Soil freeze-thaw Cycle Experiments: Trends, Methodological Weaknesses and Suggested Improvements. Soil Biol. Biochem. 2007, 39, 977–986. [Google Scholar] [CrossRef]
- Kim, E. –A.; Lee, H.K.; Choi, J.H. Effects of a Controlled freeze-thaw Event on Dissolved and Colloidal Soil Organic Matter. Environ. Sci. Pollut. Res. 2017, 24, 1338–1346. [Google Scholar] [CrossRef]
- Tao, S.; Cui, Y.H.; Xu, F.L.; Li, B.G.; Cao, J.; Liu, W.X.; Schmitt, G.; Wang, X.J.; Shen, W.R.; Qing, B.P.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in Agricultural Soil and Vegetables from Tianjin. Sci. Total Environ. 2004, 320, 11–24. [Google Scholar] [CrossRef]
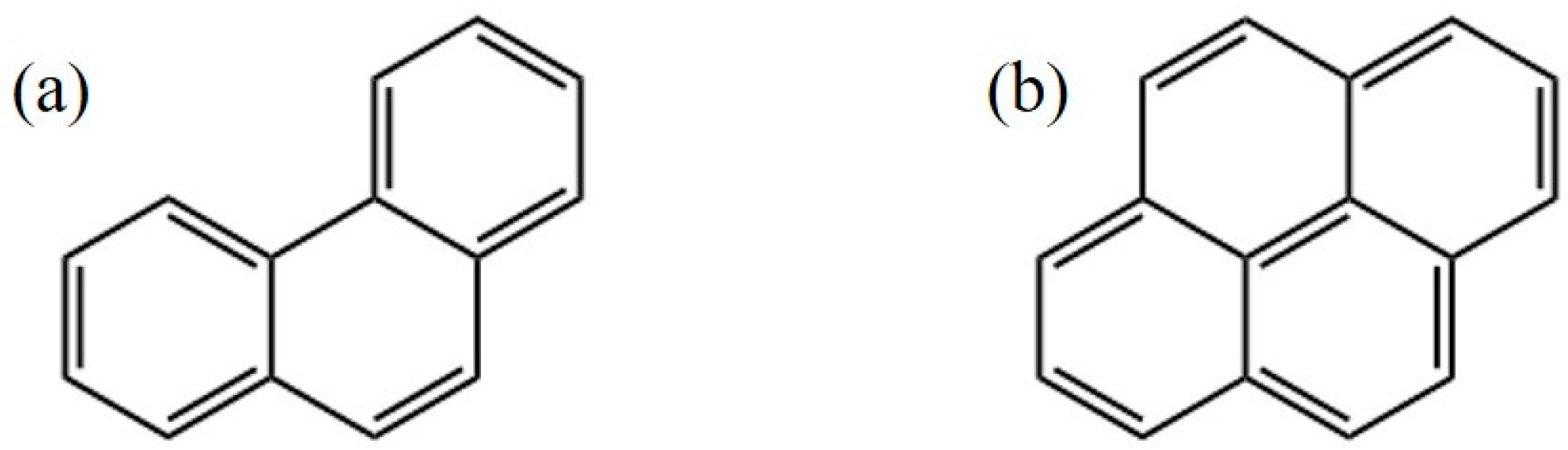


| Composition | Lung Fluid (mg L−1) | Saliva (mg L−1) |
|---|---|---|
| magnesium chloride | 95 | – |
| sodium chloride | 6019 | 290 |
| potassium chloride | 298 | 895 |
| disodium hydrogen phosphate | 126 | – |
| sodium sulphate | 63 | 570 |
| calcium chloride dihydrate | 368 | – |
| sodium acetate | 574 | – |
| sodium hydrogen carbonate | 2604 | – |
| sodium citrate dihydrate | 97 | – |
| potassium thiocyanate | – | 200 |
| sodium dihydrogen phosphate | – | 885 |
| urea | – | 200 |
| uric acid | – | 15 |
| α–amylase | – | 145 |
| mucin | – | 5 |
| pH | 7.2 ± 0.1 | 6.4 ± 0.1 |
| Compound | Group | Frap (%) | krap (h−1) | kslow (10−4 h−1) | |||
|---|---|---|---|---|---|---|---|
| FS | CS | FS | CS | FS | CS | ||
| phenanthrene | simulated lung fluid–FT0 | 57.90 | 50.24 | 1.23 | 0.73 | 12.60 | 13.10 |
| simulated lung fluid–FT15 | 42.91 | 39.07 | 3.44 | 1.91 | 3.47 | 4.26 | |
| simulated lung fluid–FT30 | 40.13 | 37.06 | 2.56 | 2.23 | 1.48 | 7.38 | |
| simulated saliva–FT0 | 62.29 | 47.72 | 1.20 | 0.99 | 6.03 | 16.10 | |
| simulated saliva–FT15 | 50.60 | 35.50 | 4.97 | 2.07 | 2.24 | 3.79 | |
| simulated saliva–FT30 | 43.12 | 34.00 | 4.06 | 2.49 | 2.45 | 2.48 | |
| Compound | Group | Frap (%) | krap (h−1) | kslow (10−4 h−1) | |||
|---|---|---|---|---|---|---|---|
| FS | CS | FS | CS | FS | CS | ||
| pyrene | simulated lung fluid–FT0 | 41.58 | 32.87 | 0.93 | 5.79 | 4.20 | 1.90 |
| simulated lung fluid–FT15 | 31.31 | 24.80 | 1.73 | 0.37 | 5.14 | 3.37 | |
| simulated lung fluid–FT30 | 30.38 | 23.00 | 0.85 | 0.83 | 3.16 | 2.81 | |
| simulated saliva–FT0 | 39.00 | 27.60 | 0.68 | 6.90 | 3.99 | 2.08 | |
| simulated saliva–FT15 | 27.11 | 19.28 | 1.43 | 0.66 | 1.73 | 2.22 | |
| simulated saliva–FT30 | 26.43 | 18.50 | 0.93 | 1.12 | 1.23 | 1.56 | |
| Soil | Organic Fractions | Organic Constituents | Particle Size | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FA | HA | Humin | TOC | C/N * | H/C * | Sand | Silt | Clay | |
| (g kg−1) | (g kg−1) | (g kg−1) | (wt%) | (%) | (%) | (%) | |||
| FS | 2.30 | 7.90 | 11.47 | 2.01 | 15.02 | 9.11 | 13.4 | 17.7 | 68.9 |
| CS | 5.17 | 6.70 | 14.97 | 3.12 | 10.81 | 4.00 | 24.8 | 52.4 | 22.8 |
| Compound | Soil | Simulated Body Fluid | freeze-thaw Cycle | Bioaccessibility Range |
|---|---|---|---|---|
| phenanthrene | FS | lungf fluid | 0 | 25.41–75.46% |
| 15 | 30.17–51.44% | |||
| 30 | 25.01–48.62% | |||
| saliva | 0 | 24.23–59.50% | ||
| 15 | 31.25–46.49% | |||
| 30 | 26.10–44.69% | |||
| CS | lungf fluid | 0 | 18.82–72.58% | |
| 15 | 19.05–51.58% | |||
| 30 | 19.16–47.47% | |||
| saliva | 0 | 20.86–62.41% | ||
| 15 | 19.17–46.75% | |||
| 30 | 19.45–42.03% |
| Compound | Soil | Simulated Body Fluid | freeze-thaw Cycle | Bioaccessibility Range |
|---|---|---|---|---|
| pyrene | FS | lungf fluid | 0 | 22.73–54.37% |
| 15 | 17.48–50.30% | |||
| 30 | 11.98–46.47% | |||
| saliva | 0 | 17.46–52.42% | ||
| 15 | 13.33–47.65% | |||
| 30 | 8.87–43.66% | |||
| CS | lungf fluid | 0 | 11.80–47.23% | |
| 15 | 5.61–46.30% | |||
| 30 | 6.87–42.48% | |||
| saliva | 0 | 8.55–38.89% | ||
| 15 | 6.87–32.40% | |||
| 30 | 5.02–28.99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Wu, Z. Effects of Freeze-Thaw Cycles on Bioaccessibilities of Polycyclic Aromatic Hydrocarbons. Toxics 2024, 12, 413. https://doi.org/10.3390/toxics12060413
Dong H, Wu Z. Effects of Freeze-Thaw Cycles on Bioaccessibilities of Polycyclic Aromatic Hydrocarbons. Toxics. 2024; 12(6):413. https://doi.org/10.3390/toxics12060413
Chicago/Turabian StyleDong, Hui, and Ze Wu. 2024. "Effects of Freeze-Thaw Cycles on Bioaccessibilities of Polycyclic Aromatic Hydrocarbons" Toxics 12, no. 6: 413. https://doi.org/10.3390/toxics12060413
APA StyleDong, H., & Wu, Z. (2024). Effects of Freeze-Thaw Cycles on Bioaccessibilities of Polycyclic Aromatic Hydrocarbons. Toxics, 12(6), 413. https://doi.org/10.3390/toxics12060413






