Degradation of Sodium Acetate by Catalytic Ozonation Coupled with MnOx/NiOOH-Modified Fly Ash
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Catalysts
2.2. Characterization
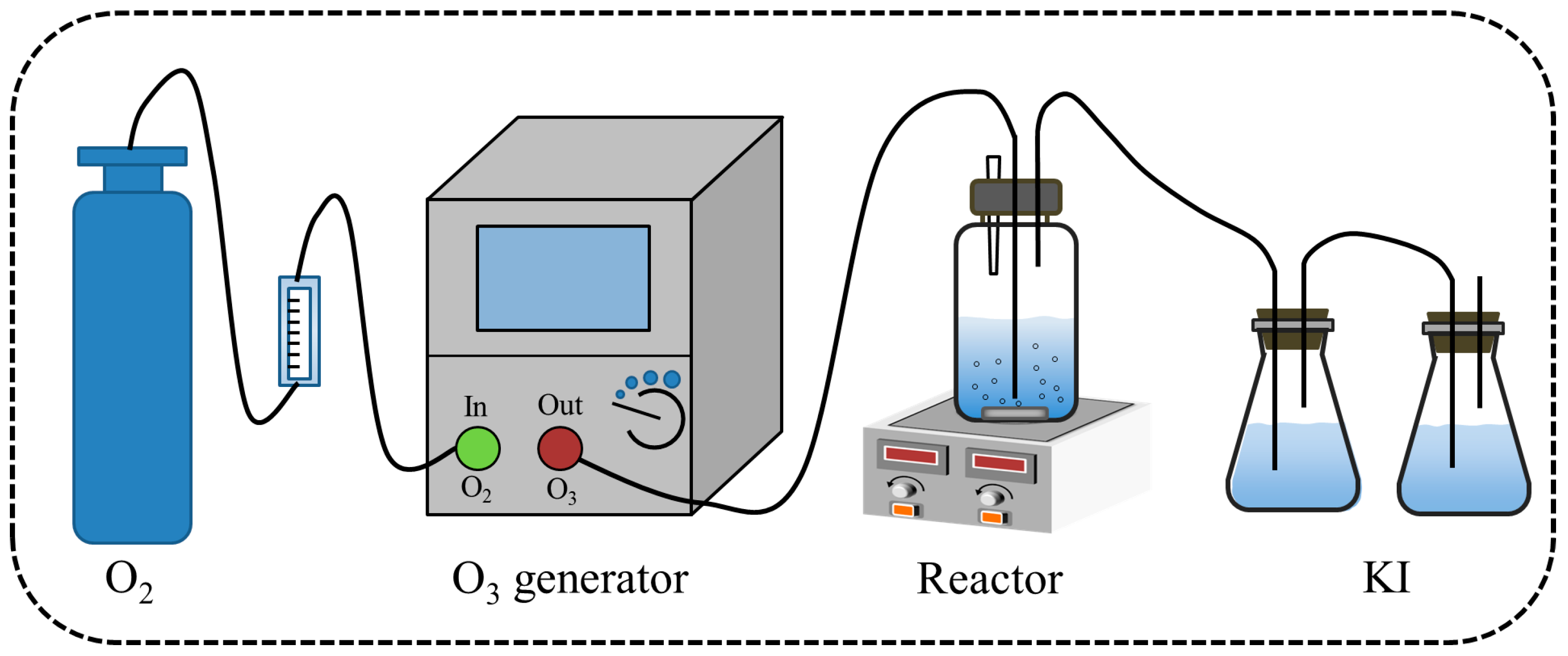
2.3. Catalytic Ozonation Reaction
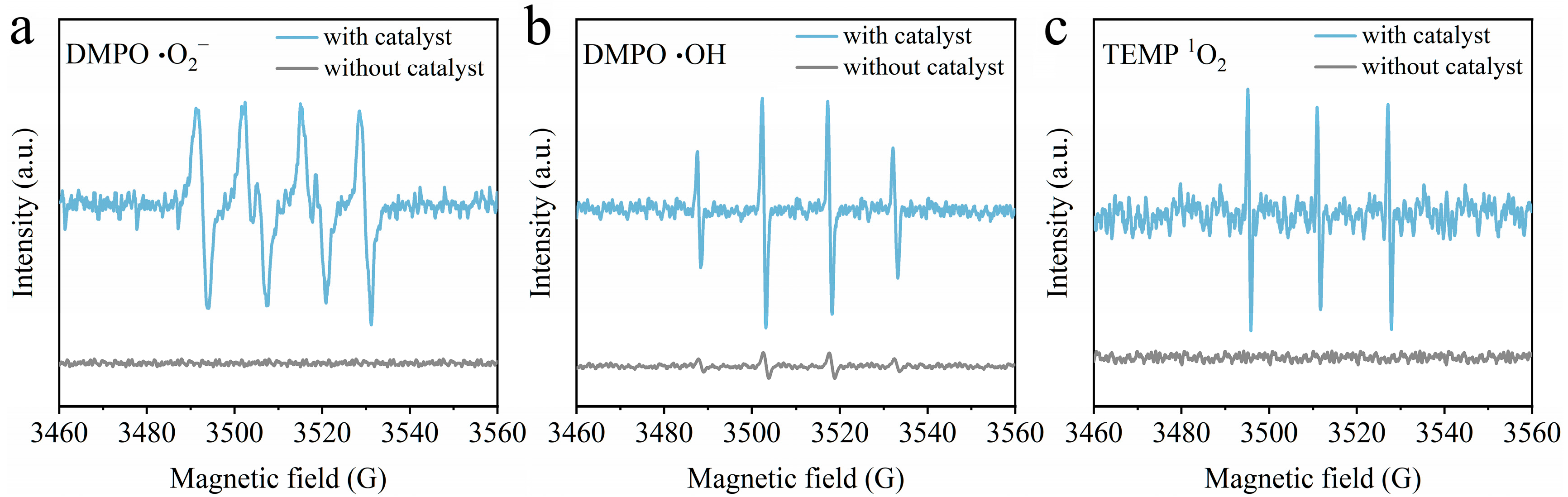
2.4. Reactive Species Detection
3. Results and Discussion
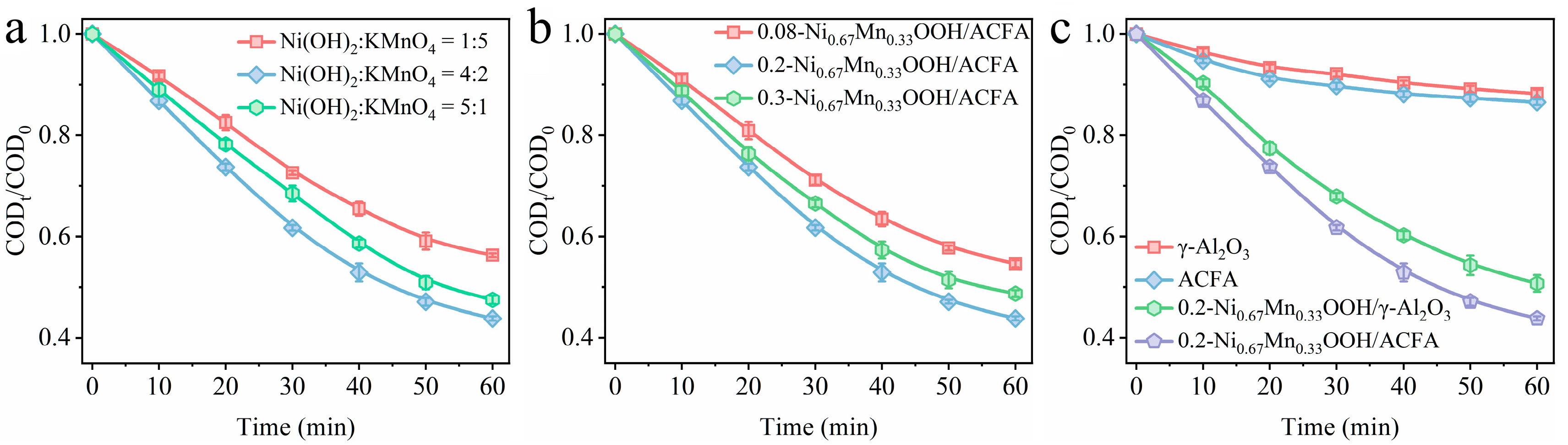
3.1. Catalytic Ozonation Performance of x-NiyMn1−yOOH/ACFA
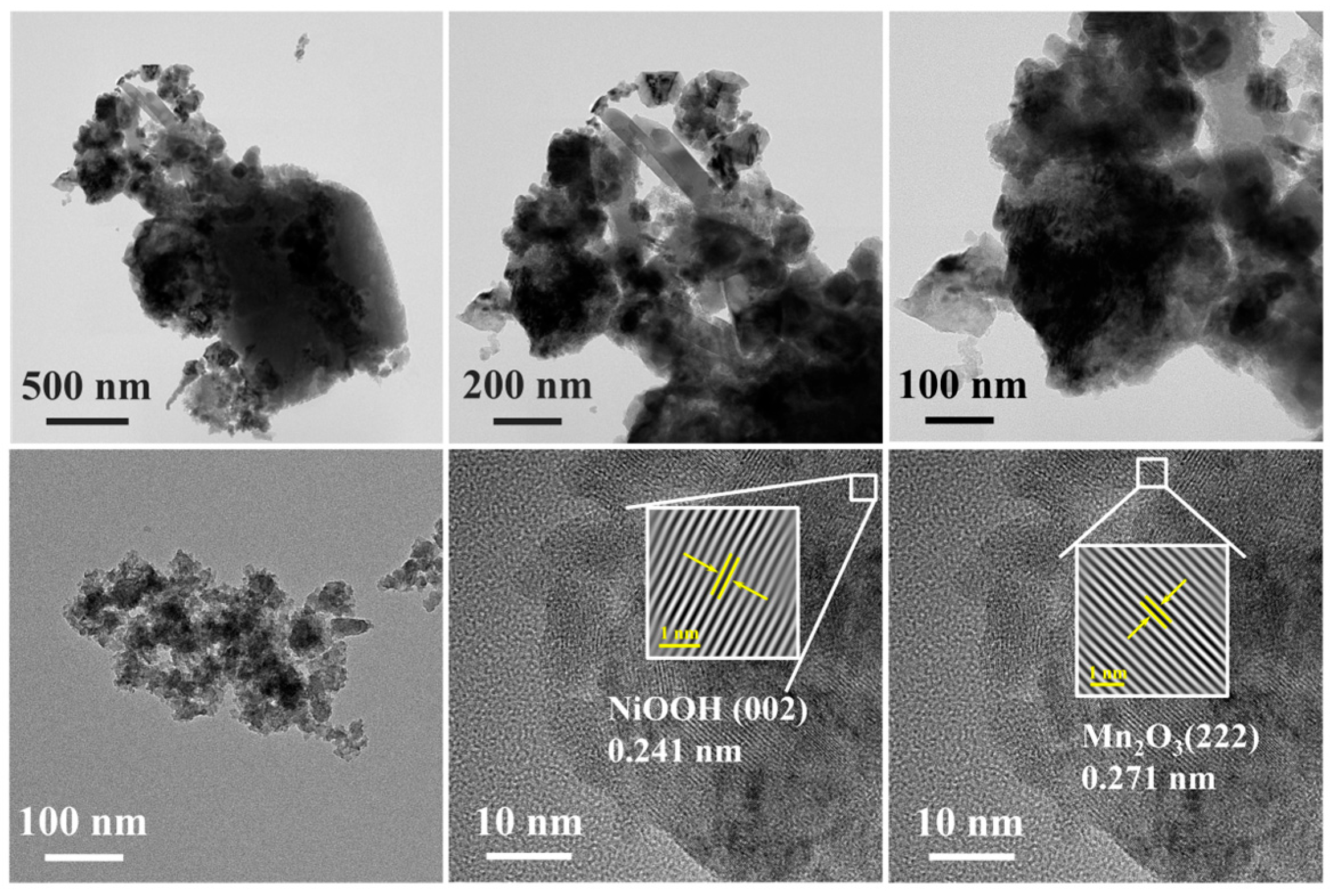
3.2. Morphology Characterization of x-NiyMn1−yOOH/ACFA Catalysts
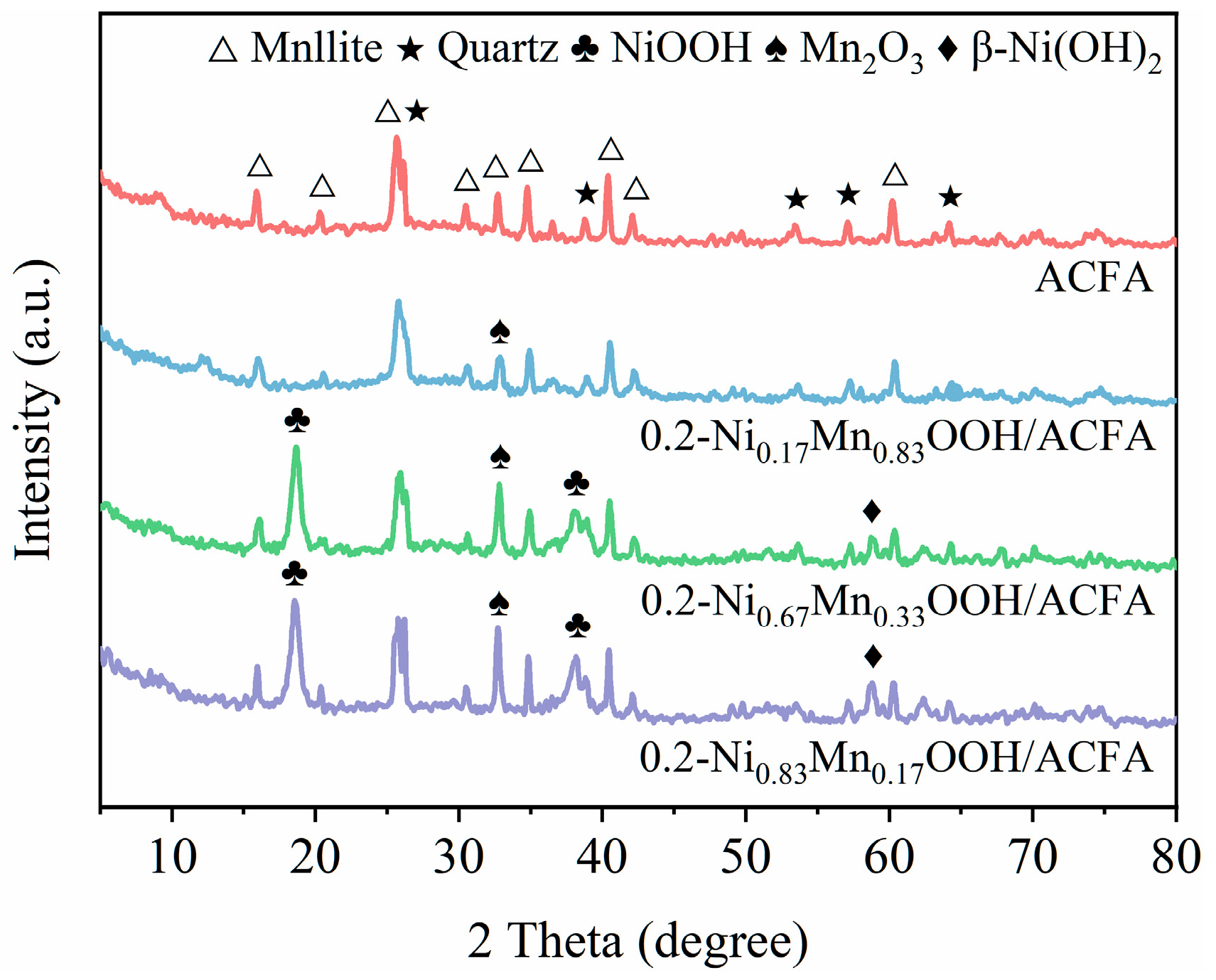
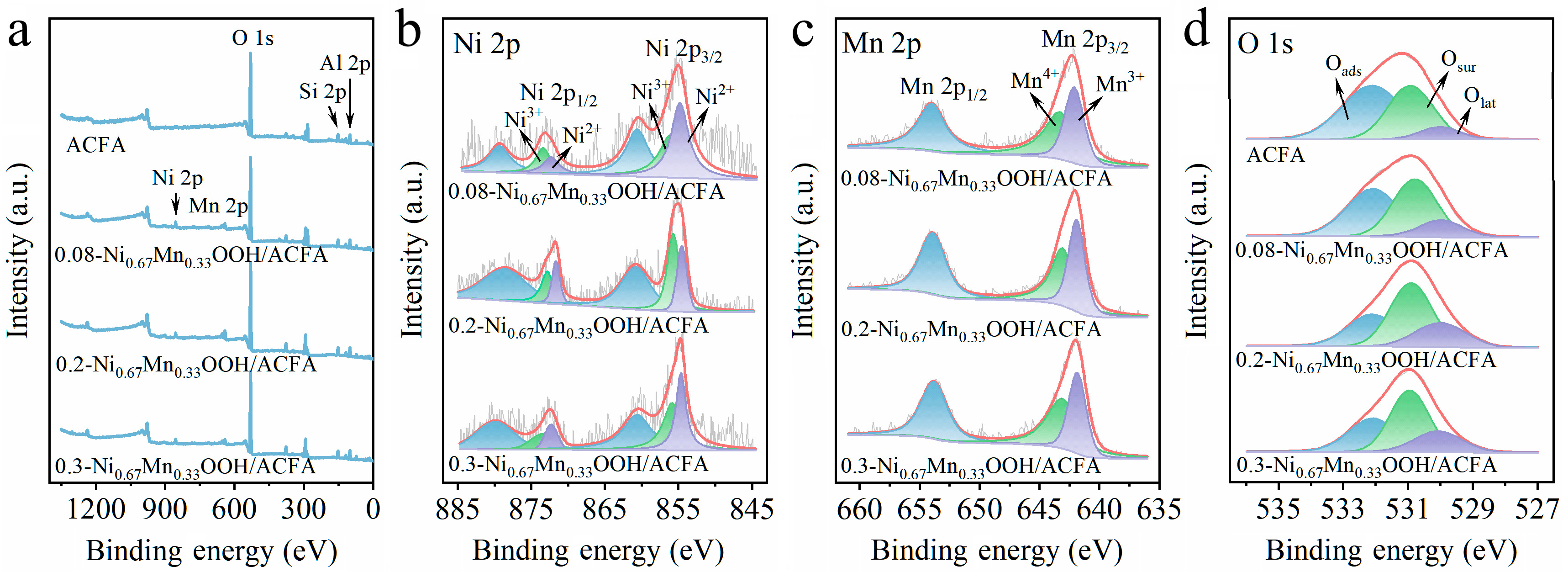
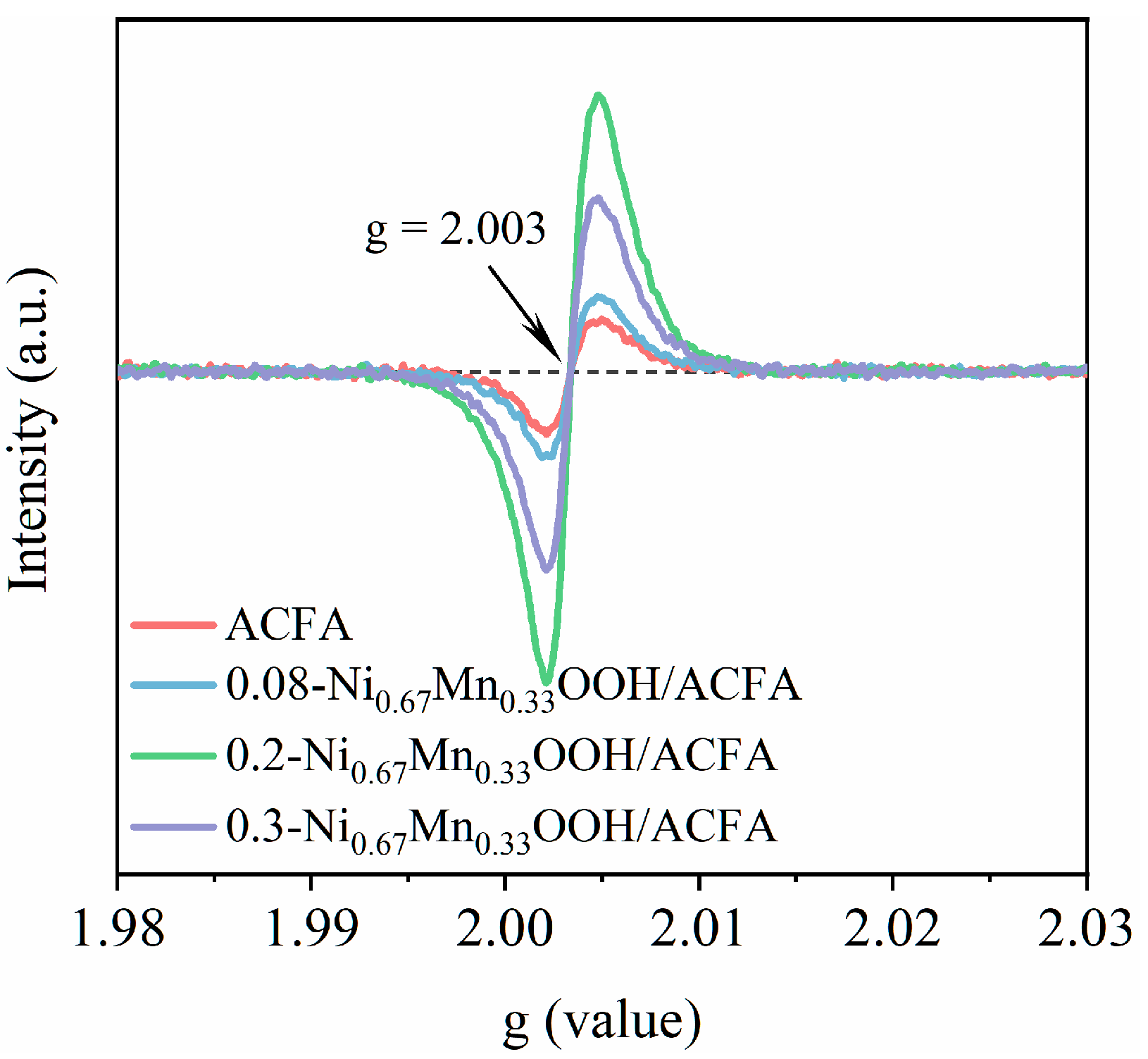
3.3. Surface Properties of x-NiyMn1−yOOH/ACFA
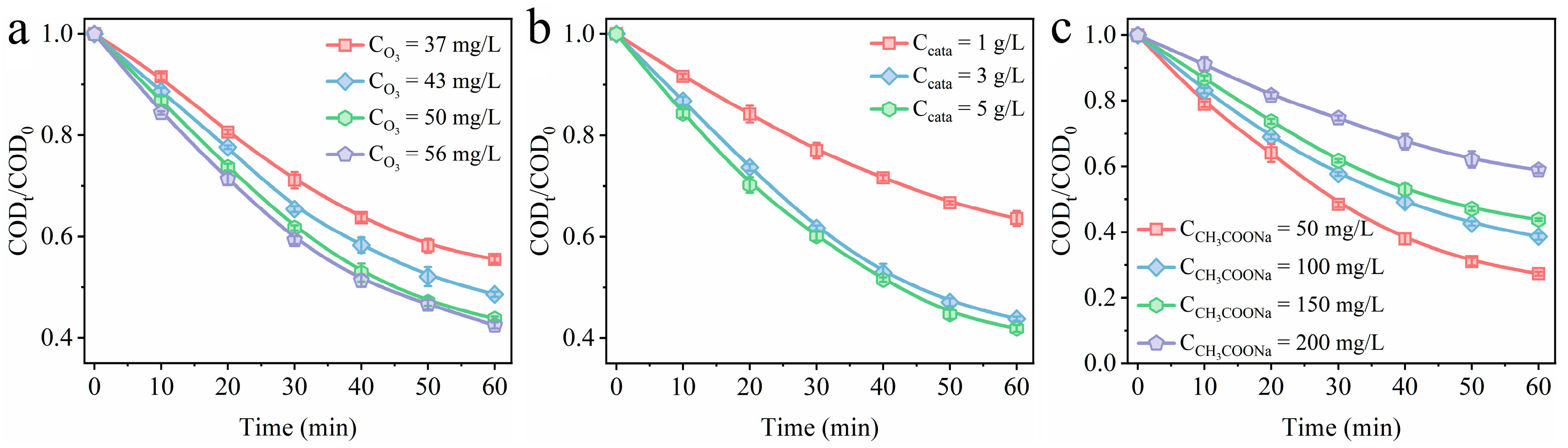
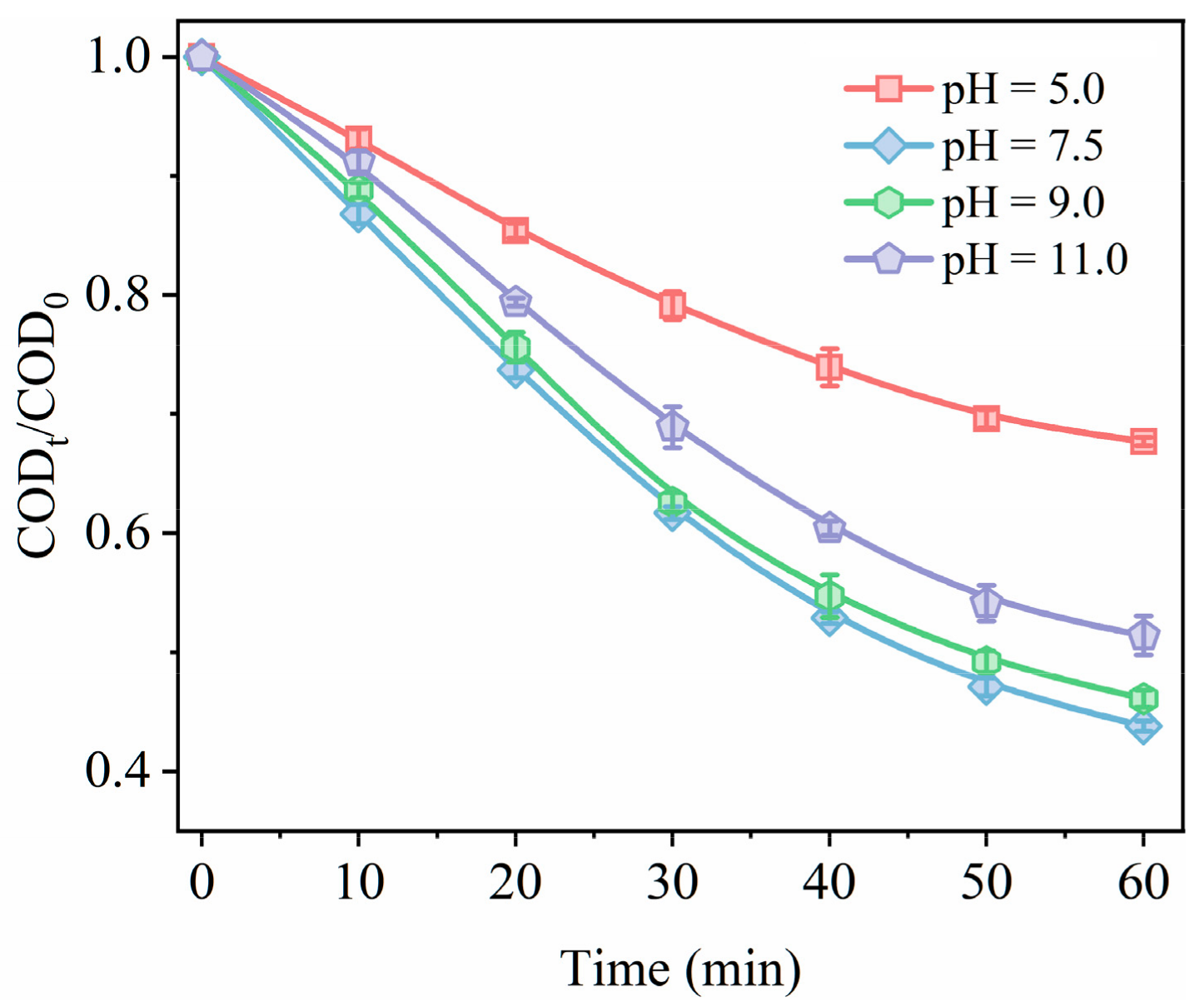
3.4. Effect of Reaction Parameters on Catalytic Degradation Efficiency
3.5. Study of Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.; et al. Synthesis of high quality zeolites from coal fly ash: Mobility of hazardous elements and environmental applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.; Sarker, P.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Yu, F.; Tang, W.; Liu, J.; Geng, M.; Yang, H. The synergistic adsorption-catalysis research of highly efficient interlayer constructed from coal fly ash for superior lithium-sulfur batteries. Chem. Eng. J. 2023, 465, 142795. [Google Scholar] [CrossRef]
- Gollakota, A.-R.-K.; Volli, V.; Shu, C.-M. Progressive utilisation prospects of coal fly ash: A review. Sci. Total Environ. 2019, 672, 951–989. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Sahraei, O.; Iliuta, M. Ni-based catalysts supported on acid/alkali-activated coal fly ash residue for improved glycerol steam reforming. Appl. Catal. B Environ. 2022, 301, 120791. [Google Scholar] [CrossRef]
- Chuaicham, C.; Inoue, T.; Balakumar, V.; Tian, Q.; Ohtani, B.; Sasaki, K. Visible light-driven ZnCr double layer oxide photocatalyst composites with fly ashes for the degradation of ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 106970. [Google Scholar] [CrossRef]
- Nadeem, N.; Yaseen, M.; Rehan, Z.; Zahid, M.; Shakoor, R.; Jilani, A.; Iqbal, J.; Rasul, S.; Shahid, I. Coal fly ash supported CoFe2O4 nanocomposites: Synergetic Fenton-like and photocatalytic degradation of methylene blue. Environ. Res. 2022, 206, 112280. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, M.; Liu, J.; Guo, B. Recent advances of SBA-15-based composites as the heterogeneous catalysts in water decontamination: A mini-review. J. Environ. Manag. 2020, 254, 109787. [Google Scholar] [CrossRef]
- Sun, X.; Xu, D.; Dai, P.; Liu, X.; Tan, F.; Guo, Q. Efficient degradation of methyl orange in water via both radical and non-radical pathways using Fe-Co bimetal-doped MCM-41 as peroxymonosulfate activator. Chem. Eng. J. 2020, 402, 125881. [Google Scholar] [CrossRef]
- Mohammadi, R.; Feyzi, M.; Joshaghani, M. Synthesis of ZnO-magnetic/ZSM-5 and its application for removal of disperse Blue 56 from contaminated water. Chem. Eng. Process Intensif. 2020, 153, 107969. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Xu, D.; Guo, Q. Activation of peroxymonosulfate by bimetallic CoMn oxides loaded on coal fly ash-derived SBA-15 for efficient degradation of Rhodamine B. Sep. Purif. Technol. 2021, 274, 119081. [Google Scholar] [CrossRef]
- Lincho, J.; Gomes, J.; Kobylanski, M.; Bajorowicz, B.; Zaleska-Medynska, A.; Martins, R. TiO2 nanotube catalysts for parabens mixture degradation by photocatalysis and ozone-based technologies. Process Saf. Environ. Prot. 2021, 152, 601–613. [Google Scholar] [CrossRef]
- Yuan, L.; Shen, J.; Yan, P.; Chen, Z. Interface mechanisms of catalytic ozonation with amorphous iron silicate for removal of 4-chloronitrobenzene in aqueous solution. Environ. Sci. Technol. 2018, 52, 1429–1434. [Google Scholar] [CrossRef]
- Ghuge, S.-P.; Saroha, A.-K. Catalytic ozonation for the treatment of synthetic and industrial effluents—Application of mesoporous materials: A review. J. Environ. Manag. 2018, 211, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Kruanak, K.; Jarusutthirak, C. Degradation of 2,4,6-trichlorophenol in synthetic wastewater by catalytic ozonation using alumina supported nickel oxides. J. Environ. Chem. Eng. 2019, 7, 102825. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Wang, J.; Pan, J.; Gao, B.; Yue, Q. Catalytic ozonation performance and mechanism of Mn-CeOx@γ-Al2O3/O3 in the treatment of sulfate-containing hypersaline antibiotic wastewater. Sci. Total Environ. 2022, 807, 150867. [Google Scholar] [CrossRef]
- Rodríguez, J.; Poznyak, T.; Valenzuela, M.; Tiznado, H.; Chairez, I. Surface interactions and mechanistic studies of 2,4-dichlorophenoxyacetic acid degradation by catalytic ozonation in presence of Ni/TiO2. Chem. Eng. J. 2013, 222, 426–434. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.; Sambudi, N.; Bilad, M.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process Saf. Environ. Prot. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Lyu, C.; Zhang, L.; He, D.; Su, B.; Lyu, Y. Micrometer-sized NiOOH hierarchical spheres for enhanced degradation of sulfadiazine via synergistic adsorption and catalytic oxidation in peroxymonosulfate system. Chin. Chem. Lett. 2022, 33, 930–934. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Zhou, S.; Huang, R.; Huang, S.; Kuang, H.; Zeng, X.; Zhao, S. Activation of persulfate by MnOOH: Degradation of organic compounds by nonradical mechanism. Chemosphere 2021, 272, 129629. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; Zhong, H.; Sun, W.; Hu, L.; Luo, M. (Fe0.67Mn0.33)OOH riched in oxygen vacancies facilitated the PMS activation of modified EMR for refractory foaming agent removal from mineral processing wastewater. Chem. Eng. J. 2022, 441, 136024. [Google Scholar] [CrossRef]
- Zhang, T.; Li, C.; Ma, J.; Tian, H.; Qiang, Z. Surface hydroxyl groups of synthetic α-FeOOH in promoting •OH generation from aqueous ozone: Property and activity relationship. Appl. Catal. B Environ. 2008, 82, 131–137. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, M.; Ben, Y.; Shen, J.; Qi, F.; Chen, Z. Efficiency and mechanism of atenolol decomposition in Co-FeOOH catalytic ozonation. J. Hazard. Mater. 2019, 365, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Luo, M.; Li, S.; Xia, D.; Tang, Z.; Hu, S.; Ye, S.; Sun, M.; He, C.; Shu, D. Efficient catalytic activity and bromate minimization over lattice oxygen-rich MnOOH nanorods in catalytic ozonation of bromide-containing organic pollutants: Lattice oxygen-directed redox cycle and bromate reduction. J. Hazard. Mater. 2021, 410, 124545. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Su, W.; Yang, Q.; Ni, J.; Tong, S. Synergetic mechanism for basic and acid sites of MgMxOy (M = Fe, Mn) double oxides in catalytic ozonation of p-hydroxybenzoic acid and acetic acid. Appl. Catal. B Environ. 2020, 279, 119346. [Google Scholar] [CrossRef]
- Niveditha, S.; Gandhimathi, R. Flyash augmented Fe3O4 as a heterogeneous catalyst for degradation of stabilized landfill leachate in Fenton process. Chemosphere 2020, 242, 125189. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Akhtar, M.; Bui, P.; Yang, O. Predominance of two dimensional (2D) Mn2O3 nanowalls thin film for high performance electrochemical supercapacitors. Chem. Eng. J. 2017, 330, 1240–1247. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, J.; Li, S.; Guo, Y.; Lian, F.; Wang, A.; Jin, Z.; Wang, W. Rational design of β-NiOOH nanosheet-sheathed CNTs as a highly efficient electrocatalyst for practical Li-S batteries. Acs Appl. Mater. Interfaces. 2021, 13, 58789–58798. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, B.; Lu, Q.; Xiao, L.; Ma, X.; Feng, Y.; Qian, Y. Cathode glow discharge electrolysis synthesis of flower-like β-Ni(OH)2 microsphere for high-performance supercapacitor. Chem. Eng. J. 2023, 453, 139769. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Wang, Z.; Sun, Y.; Liu, X.; Xiao, S.; Li, L.; Zhou, J. Magnetically separable NiFe2O4/sepiolite catalyst for enhanced ozonation treatment of quinoline and bio-treated coking wastewater in a catalytic ozonation system. Process Saf. Environ. Prot. 2022, 159, 422–432. [Google Scholar] [CrossRef]
- Ji, D.; Zhou, H.; Zhang, J.; Dan, Y.; Yang, H.; Yuan, A. Facile synthesis of a metal-organic framework-derived Mn2O3 nanowire coated three-dimensional graphene network for high-performance free-standing supercapacitor electrodes. J. Mater. Chem. A 2016, 4, 8283–8290. [Google Scholar] [CrossRef]
- Ren, X.; Tong, Y. The self-reconstruction of Co-modified bimetallic hydroxysulfide nanosheet arrays for efficient hydrazine assisted water splitting. Int. J. Hydrogen Energy 2024, 49, 489–497. [Google Scholar] [CrossRef]
- Sun, P.; Cheng, L.; Chen, S.; Xie, M.; Dong, F.; Dong, X. Nickel foam based monolithic catalyst supporting transition metal oxides for toluene combustion: Experimental and theoretical study of interfacial synergistic oxidation and water resistance. Chem. Eng. J. 2024, 483, 149176. [Google Scholar] [CrossRef]
- Liang, L.; Cao, P.; Qin, X.; Wu, S.; Bai, H.; Chen, S.; Yu, H.; Su, Y.; Quan, X. Oxygen vacancies-driven nonradical oxidation pathway of catalytic ozonation for efficient water decontamination. Appl. Catal. B Environ. 2023, 325, 122321. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Wu, M.; Lin, K.; Xu, L.; Zeng, T.; Shi, H.; Zhang, M. Synergistic role of inherent calcium and iron minerals in paper mill sludge biochar for phosphate adsorption. Sci. Total Environ. 2022, 834, 155193. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zeng, D.; Zhang, Q.; Cui, R.; Hassan, M.; Dong, L.; Li, J.; He, Y. Single Mn atom anchored on N-doped porous carbon as highly efficient Fenton-like catalyst for the degradation of organic contaminants. Appl. Catal. B Environ. 2020, 279, 119363. [Google Scholar] [CrossRef]
- Zeng, T.; Tang, X.; Cai, X.; Jin, S.; Zhu, Y.; Xu, W.; Song, S.; Zhang, H. Fluorinated defect-engineered acetylenic polymers with separated active centers for switching the photosensitized activation pathway of peroxymonosulfate. Acs Catal. 2024, 14, 1405–1418. [Google Scholar] [CrossRef]
- Ma, N.; Ru, Y.; Weng, M.; Chen, L.; Chen, W.; Dai, Q. Synergistic mechanism of supported Mn-Ce oxide in catalytic ozonation of nitrofurazone wastewater. Chemosphere 2022, 308, 136192. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.; Li, L.; Bing, J.; Wang, Y.; Yan, H. Mechanism for enhanced degradation of clofibric acid in aqueous by catalytic ozonation over MnOx/SBA-15. J. Hazard. Mater. 2015, 286, 276–284. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, Z.; Ding, Y.; Ma, Y.; Fu, X.; Sun, J.; Ma, M.; Zhu, W.; Wang, J. In-situ synthesis of self-standing cobalt-doped nickel sulfide nanoarray as a recyclable and integrated catalyst for peroxymonosulfate activation. Appl. Catal. B Environ. 2022, 307, 121184. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Song, Y.; Sun, X.; Liu, W.; Duan, J.; Cai, Z. Synergistic effect of Fe and Ce on Fe doped CeO2 for catalytic ozonation of amoxicillin: Efficiency evaluation and mechanism study. Sep. Purif. Technol. 2023, 313, 123430. [Google Scholar] [CrossRef]
- Cheng, L.; Li, Y.; Fan, J.; Xie, M.; Liu, X.; Sun, P.; Dong, X. High efficiency photothermal synergistic degradation of toluene achieved through the utilization of a nickel foam loaded Pt-CeO2 monolithic catalyst. Sep. Purif. Technol. 2024, 333, 125742. [Google Scholar] [CrossRef]
- Li, K.; Tong, Y.; He, J.; Liu, X.; Chen, P. Anion-modulated CoP electrode as bifunctionalelectrocatalyst for anion-exchange membranehydrazine-assisted water electrolyser. Mater. Horiz. 2023, 10, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bian, C.; Li, Y.; Sun, P.; Xiao, Y.; Xiao, X.; Wang, W.; Dong, X. Aminobenzaldehyde convelently modified graphitic carbon nitride photocatalyst through Schiff base reaction: Regulating electronic structure and improving visible-light-driven photocatalytic activity for moxifloxacin degradation. J. Colloid Interface Sci. 2023, 630, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Tan, W.; Tang, X.; Yao, X.; Bao, Y.; Zhang, H.; Song, S.; Zeng, T. Unveiling the fundamental understanding of two dimensional π-conjugated FeN4+4 sites for boosting peroxymonosulfate activation. J. Mater. Chem. A 2024, 12, 11310–11321. [Google Scholar] [CrossRef]
- Bian, C.; Zhou, B.; Mo, F.; Liu, X.; Sun, P.; Dong, X. Post-synthetically covalent modification of g-C3N4 to regulate electronic structure and investigation of photocatalytic activity for eliminating antibiotics. Sep. Purif. Technol. 2023, 325, 124556. [Google Scholar] [CrossRef]
- Li, Y.; Sun, P.; Liu, T.; Cheng, L.; Chen, R.; Bi, X.; Dong, X. Efficient photothermal conversion for oxidation removal of formaldehyde using an rGO-CeO2 modified nickel foam monolithic catalyst. Sep. Purif. Technol. 2023, 311, 123236. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J. Catalytic ozonation for degradation of sulfamethazine using NiCo2O4 as catalyst. Chemosphere 2021, 268, 128840. [Google Scholar] [CrossRef]




| Samples | SBET (m2/g) | Vtotal (cm3/g) | Pore Size (nm) | ICP Test of Catalyst Elements | |
|---|---|---|---|---|---|
| Ni wt.% | Mn wt.% | ||||
| CFA | 1.47 | 0.0047 | 8.14 | \ | \ |
| ACFA | 11.37 | 0.0083 | 9.72 | \ | \ |
| 0.08-Ni0.67Mn0.33OOH/ACFA | 34.22 | 0.12 | 10.16 | 5.11 | 2.43 |
| 0.2-Ni0.67Mn0.33OOH/ACFA | 22.83 | 0.089 | 10.38 | 12.78 | 5.41 |
| 0.3-Ni0.67Mn0.33OOH/ACFA | 14.28 | 0.051 | 8.14 | 18.37 | 8.24 |
| γ-Al2O3 | 164.66 | 0.57 | 6.23 | \ | \ |
| 0.2-Ni0.67Mn0.33OOH/γ-Al2O3 | 122.49 | 0.38 | 5.71 | \ | \ |
| Samples | XPS Analysis | ||||
|---|---|---|---|---|---|
| Ni2+/Ni3+ | Mn3+/Mn4+ | Oads | Osur | Olat | |
| ACFA | – | – | 0.54 | 0.38 | 0.08 |
| 0.08-Ni0.67Mn0.33OOH/ACFA | 1.03 | 0.80 | 0.44 | 0.42 | 0.14 |
| 0.2-Ni0.67Mn0.33OOH/ACFA | 0.77 | 0.95 | 0.31 | 0.48 | 0.21 |
| 0.3-Ni0.67Mn0.33OOH/ACFA | 0.86 | 0.74 | 0.32 | 0.45 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Zhang, H.; Shao, S.; Xu, H.; Zhou, K.; Jiang, Y.; Sun, P. Degradation of Sodium Acetate by Catalytic Ozonation Coupled with MnOx/NiOOH-Modified Fly Ash. Toxics 2024, 12, 412. https://doi.org/10.3390/toxics12060412
Chen R, Zhang H, Shao S, Xu H, Zhou K, Jiang Y, Sun P. Degradation of Sodium Acetate by Catalytic Ozonation Coupled with MnOx/NiOOH-Modified Fly Ash. Toxics. 2024; 12(6):412. https://doi.org/10.3390/toxics12060412
Chicago/Turabian StyleChen, Ruifu, Hao Zhang, Shengyu Shao, Huajun Xu, Kaicheng Zhou, Yinzhi Jiang, and Pengfei Sun. 2024. "Degradation of Sodium Acetate by Catalytic Ozonation Coupled with MnOx/NiOOH-Modified Fly Ash" Toxics 12, no. 6: 412. https://doi.org/10.3390/toxics12060412
APA StyleChen, R., Zhang, H., Shao, S., Xu, H., Zhou, K., Jiang, Y., & Sun, P. (2024). Degradation of Sodium Acetate by Catalytic Ozonation Coupled with MnOx/NiOOH-Modified Fly Ash. Toxics, 12(6), 412. https://doi.org/10.3390/toxics12060412






