Removal of Aqueous Antimony and Arsenic by Iron-Loaded Coal Gasification Slag Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
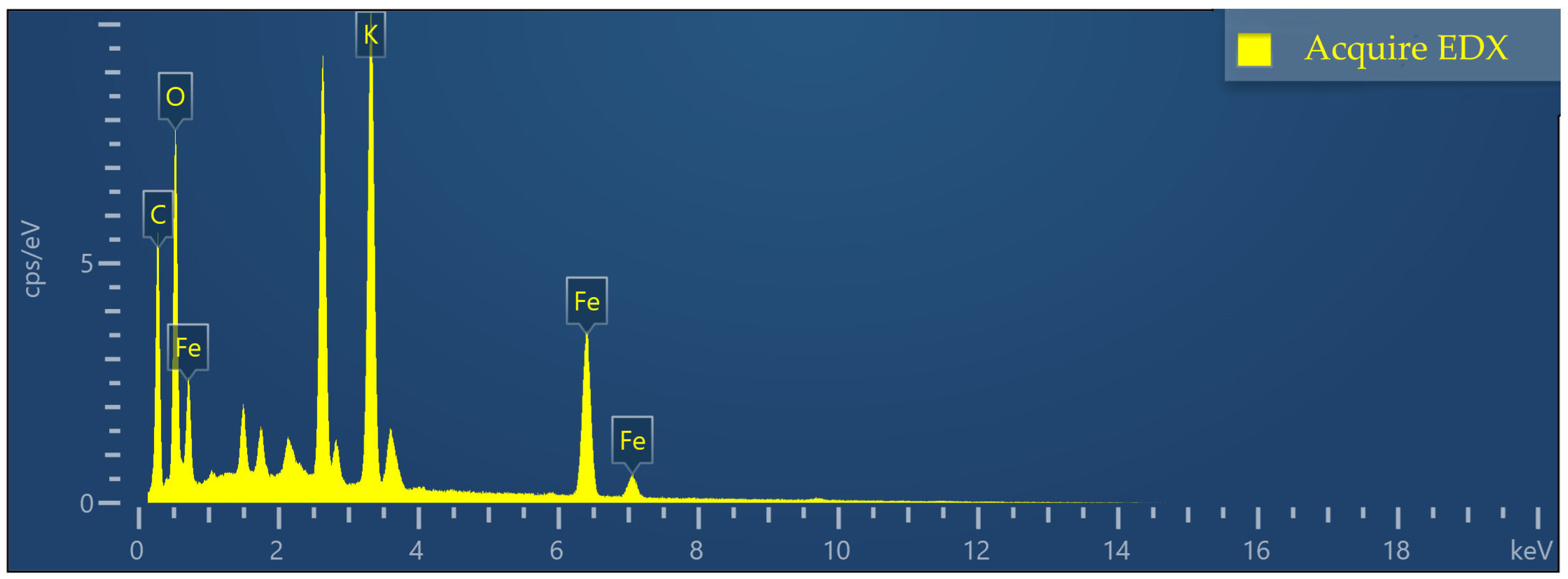
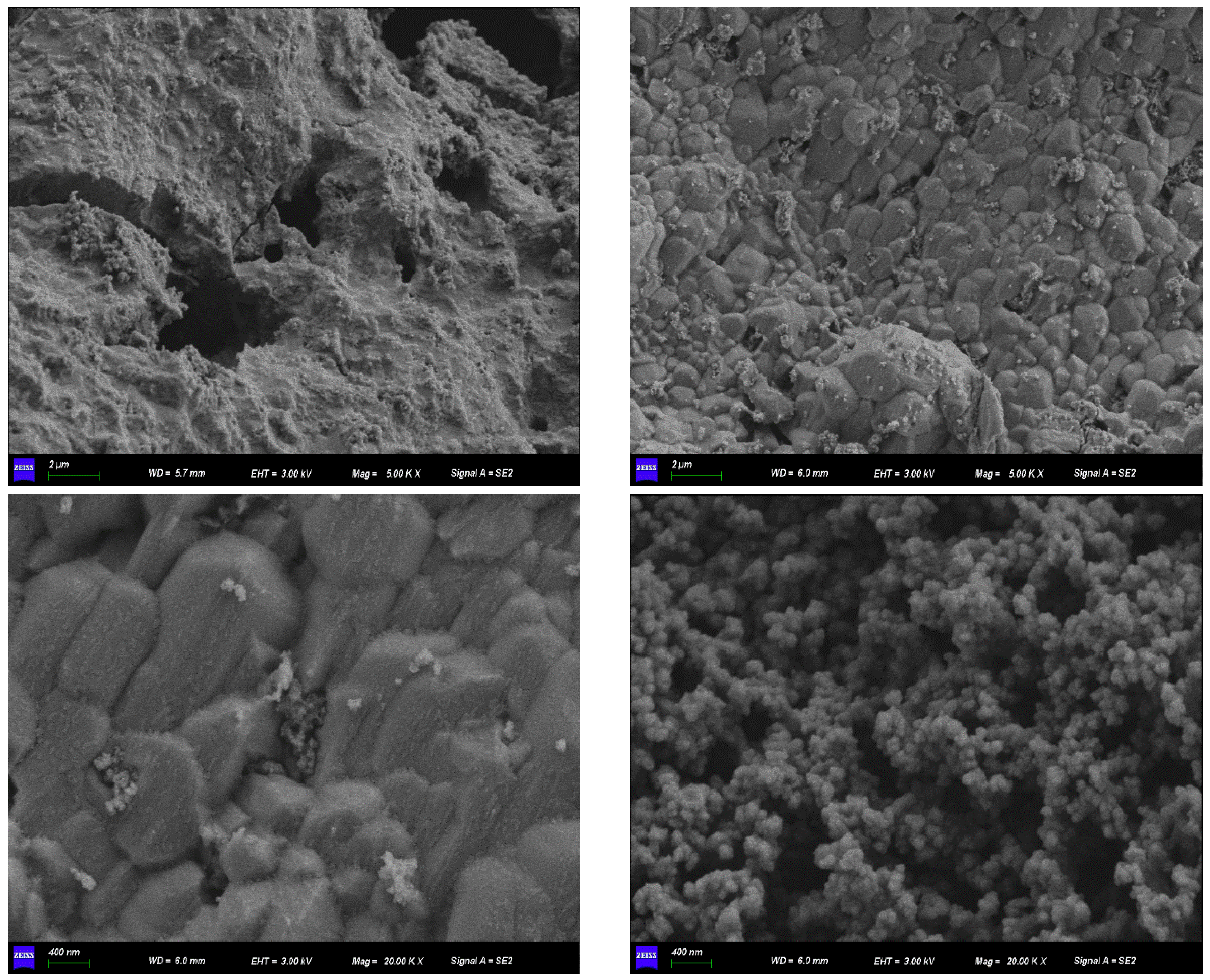
2.2. Characterization of Fe-GFS
2.3. Adsorption Experiment in Aqueous System
2.3.1. Adsorption Isotherms
2.3.2. Adsorption Kinetics
2.3.3. Adsorption Experiments at Different pH Values
2.3.4. Binary Adsorption of As and Sb
2.4. Data Analysis
3. Results and Discussion
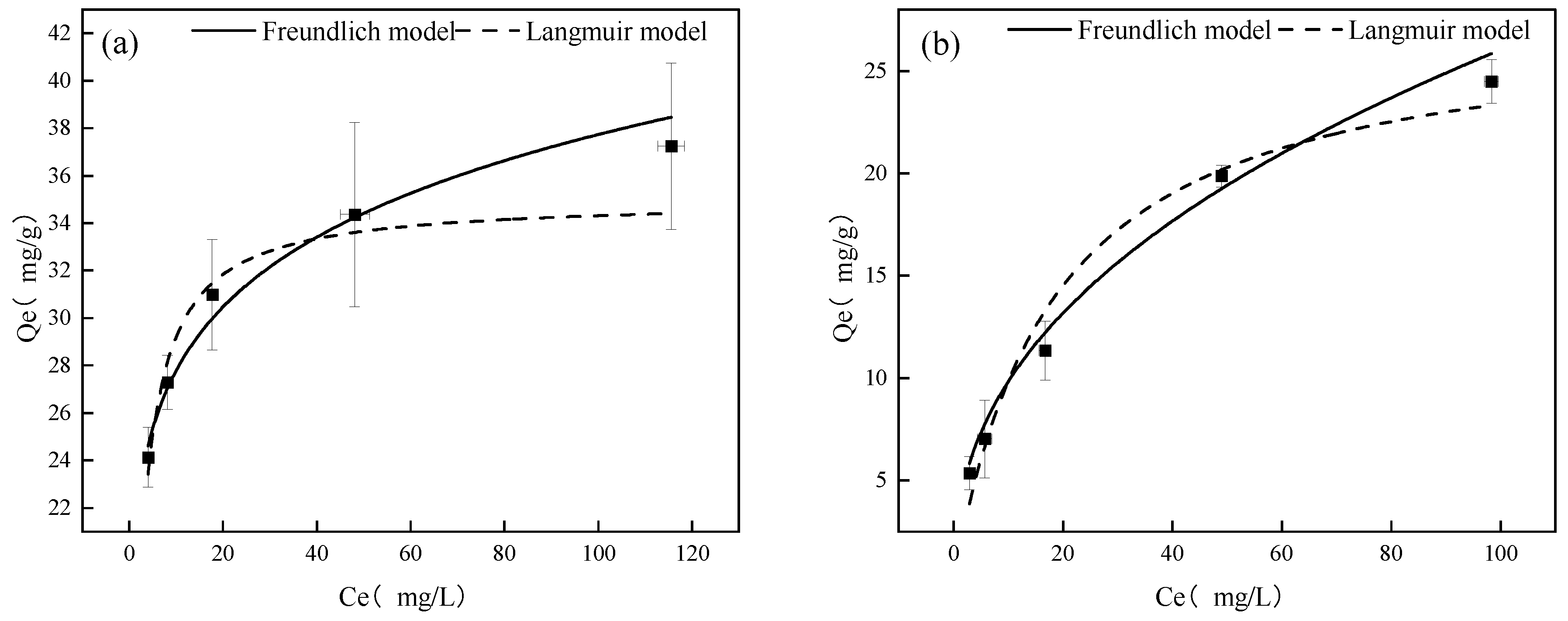
3.1. Adsorption Isotherms
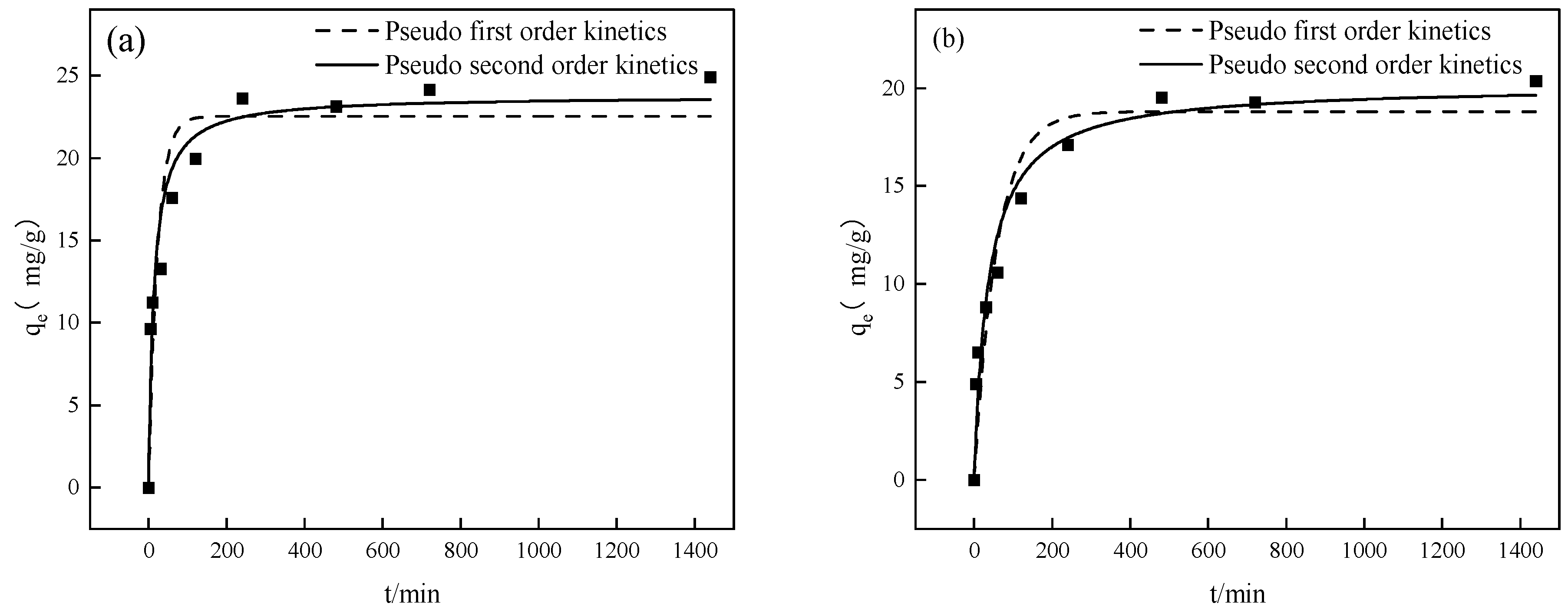
3.2. Adsorption Kinetics
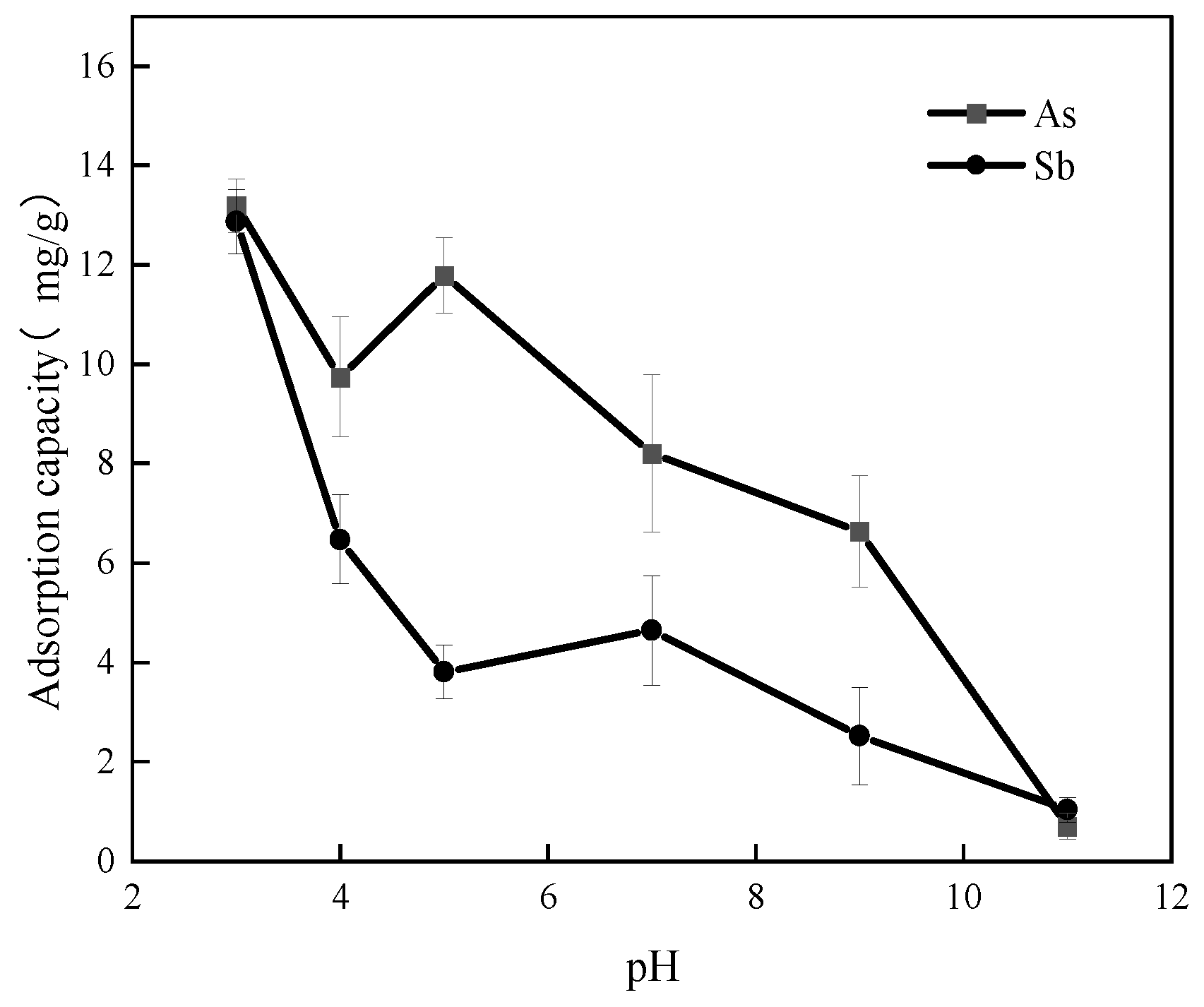
3.3. Effect of pH on As(V) and Sb(V) Adsorption
3.4. Adsorption in Binary Systems
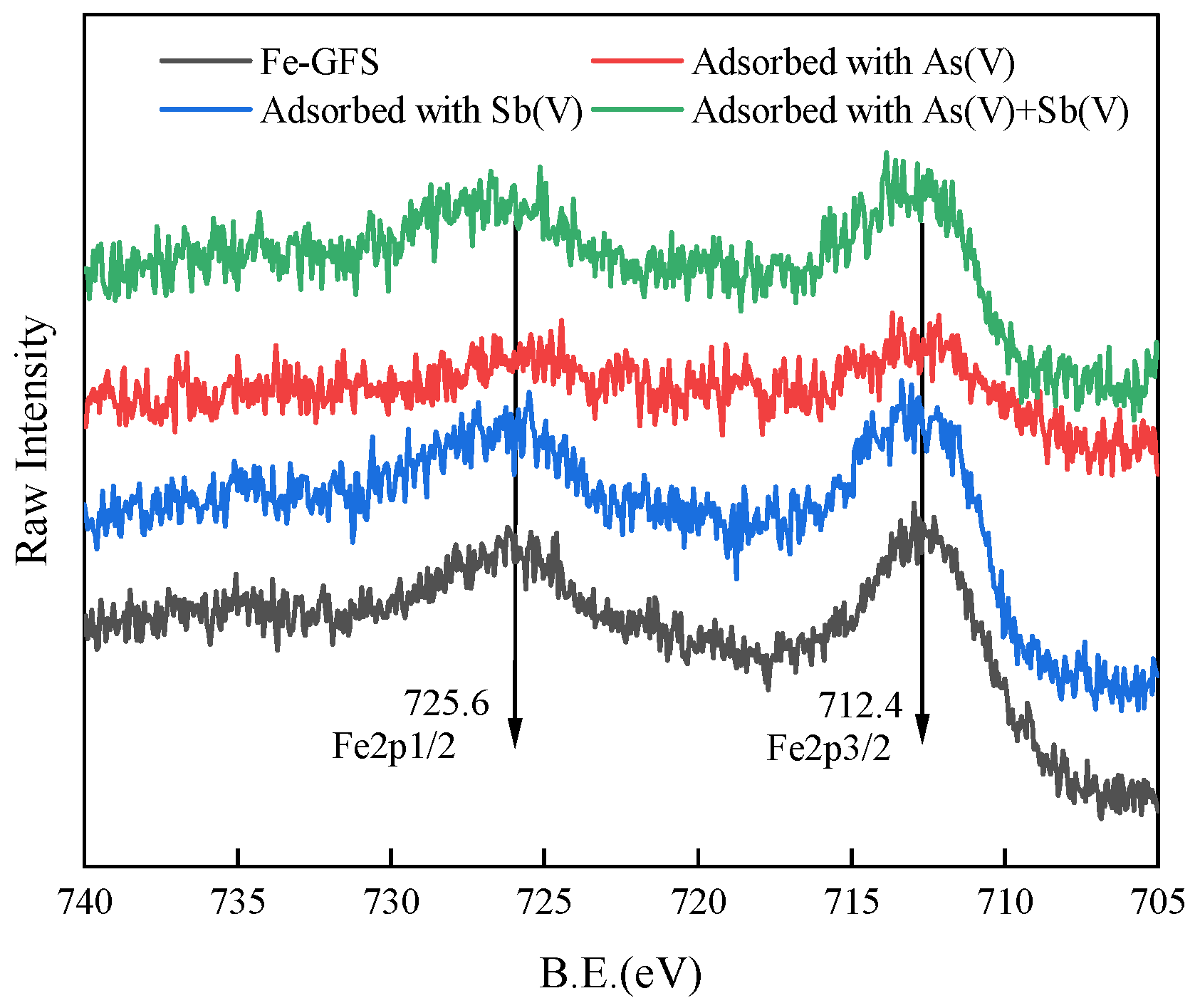
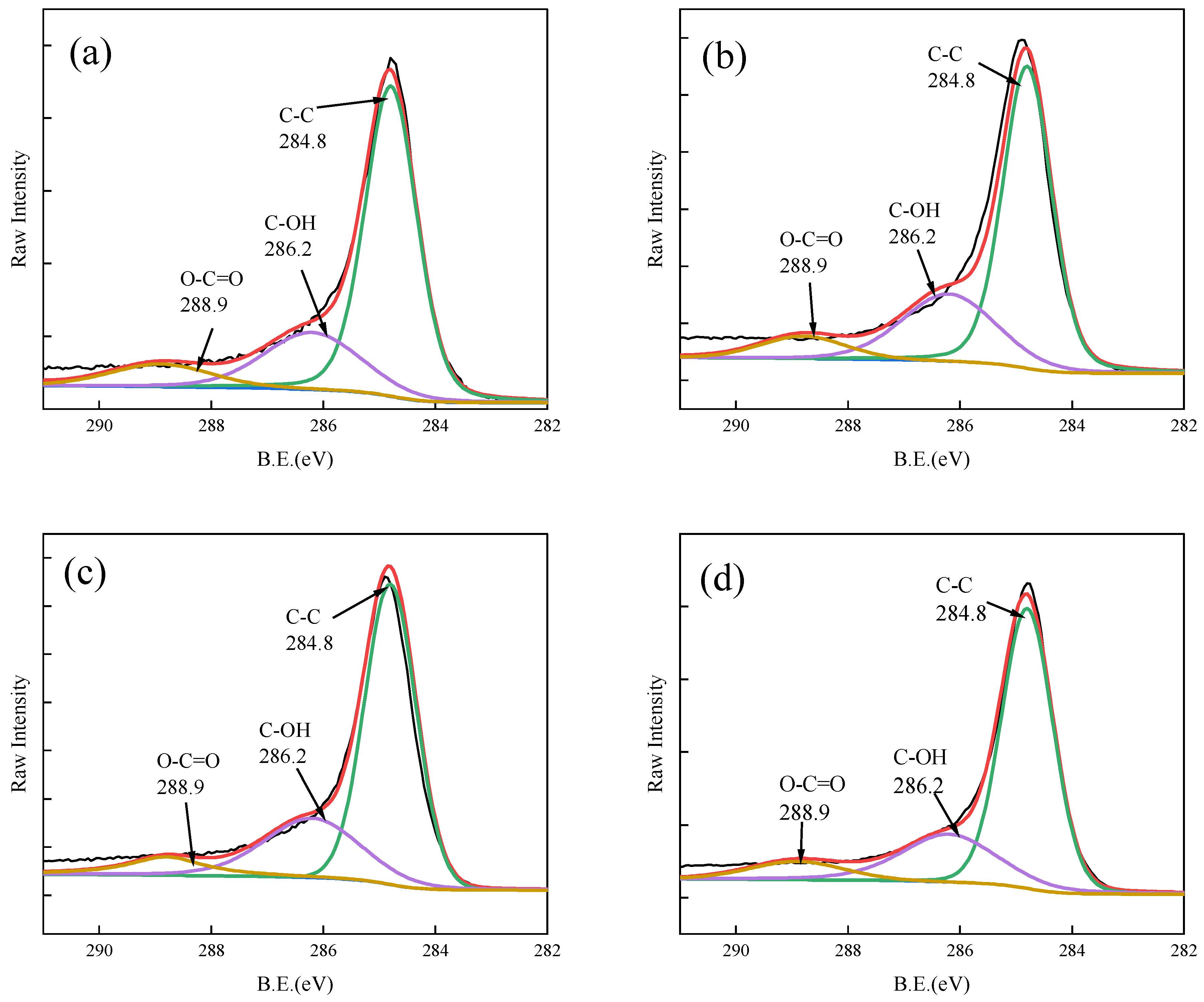
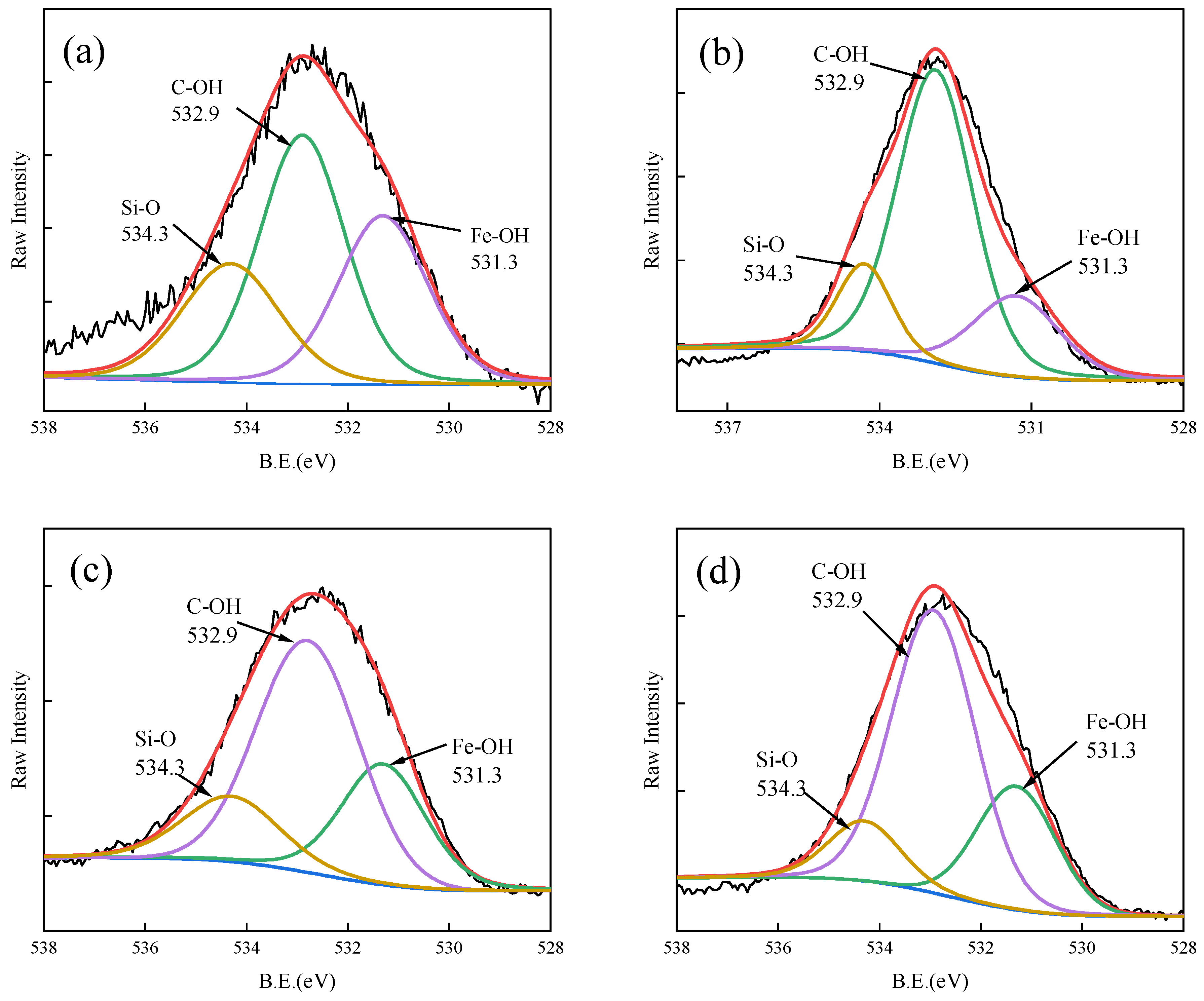
3.5. Adsorption Mechanism of As(V)/Sb(V) on Fe-GFS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bolan, N.; Kumar, M.; Singh, E.; Kumar, A.; Singh, L.; Kumar, S.; Keerthanan, S.; Hoang, S.A.; El-Naggar, A.; Vithanage, M.; et al. Antimony contamination and its risk management in complex environmental settings: A review. Environ. Int. 2022, 158, 106908. [Google Scholar] [CrossRef]
- Wilson, N.J.; Craw, D.; Hunter, K. Antimony distribution and environmental mobility at an historic antimony smelter site, New Zealand. Environ. Pollut. 2004, 129, 257–266. [Google Scholar] [CrossRef]
- Zhou, J.; Nyirenda, M.T.; Xie, L.; Li, Y.; Zhou, B.; Zhu, Y.; Liu, H. Mine waste acidic potential and distribution of antimony and arsenic in waters of the Xikuangshan mine, China. Appl. Geochem. 2017, 77, 52–61. [Google Scholar] [CrossRef]
- Hiller, E.; Lalinská, B.; Chovan, M.; Jurkovič, L.; Klimko, T.; Jankulár, M.; Hovorič, R.; Šottník, P.; Fľaková, R.; Ženišová, Z.; et al. Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia. Appl. Geochem. 2012, 27, 598–614. [Google Scholar] [CrossRef]
- Ashley, P.M.; Craw, D.; Graham, B.P.; Chappell, D. Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand. J. Geochem. Explor. 2003, 77, 1–14. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic Toxicity: Molecular Targets and Therapeutic Agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef]
- Wu, F.; Fu, Z.; Liu, B.; Mo, C.; Chen, B.; Corns, W.; Liao, H. Health risk associated with dietary co-exposure to high levels of antimony and arsenic in the world’s largest antimony mine area. Sci. Total Environ. 2011, 409, 3344–3351. [Google Scholar] [CrossRef]
- Fu, X.; Song, X.; Zheng, Q.; Liu, C.; Li, K.; Luo, Q.; Chen, J.; Wang, Z.; Luo, J. Frontier Materials for Adsorption of Antimony and Arsenic in Aqueous Environments: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10824. [Google Scholar] [CrossRef]
- Zhu, J.; Baig, S.A.; Sheng, T.; Lou, Z.; Wang, Z.; Xu, X. Fe3O4 and MnO2 assembled on honeycomb briquette cinders (HBC) for arsenic removal from aqueous solutions. J. Hazard. Mater. 2015, 286, 220–228. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, J.; Sun, Z.; Yang, C.; Shi, D.; Li, S.; Li, H. Research progress on comprehensive utilization of coal gasification slag. Clean. Coal Technol. 2020, 26, 184–193. (In Chinese) [Google Scholar]
- Liu, S.; Chen, X.; Ai, W.; Wei, C. A new method to prepare mesoporous silica from coal gasification fine slag and its application in methylene blue adsorption. J. Clean. Prod. 2019, 212, 1062–1071. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Xu, D.; Tian, H.; Yang, H. Simultaneous adsorption of ammonia and phosphate using ferric sulfate modified carbon/zeolite composite from coal gasification slag. J. Environ. Manag. 2022, 305, 114404. [Google Scholar] [CrossRef]
- Bao, C.; Ling, Q.; Wu, C.; Fang, T.; Zhao, Y.; Sun, B.; Zhang, R.; Kong, Z.; Yang, H. Adsorption of heavy metal ions by modified coal gasification slag. Appl. Chem. Ind. 2016, 45, 630–633. (In Chinese) [Google Scholar]
- Deng, X.; Fan, Y.; Zhang, Q. Study on the Adsorption Performance of Cr6+ by Zr-Loaded Coal Gasification Waste Slag. World Sci. Res. J. 2020, 6, 27–34. [Google Scholar]
- Qi, P.; Pichler, T. Competitive adsorption of As(III), As(V), Sb(III) and Sb(V) onto ferrihydrite in multi-component systems: Implications for mobility and distribution. J. Hazard. Mater. 2017, 330, 142–148. [Google Scholar] [CrossRef]
- Wu, D.; Sun, S.-P.; He, M.; Wu, Z.; Xiao, J.; Chen, X.D.; Wu, W. DuoAs(V) and Sb(V) co-adsorption onto ferrihydrite: Synergistic effect of Sb(V) on As(V) under competitive conditions. Environ. Sci. Pollut. Res. 2018, 25, 14585–14594. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, J.; Wang, Q.; Leng, Z.; Geng, Y.; Sun, S.; Hou, H. Simultaneous adsorption of Cd and As by a novel coal gasification slag based composite: Characterization and application in soil remediation. Sci. Total Environ. 2023, 882, 163374. [Google Scholar] [CrossRef]
- Xi, J.; He, M.; Wang, K.; Zhang, G. Adsorption of antimony(III) on goethite in the presence of competitive anions. J. Geochem. Explor. 2013, 132, 201–208. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Liao, L.; Wu, P. Study on the Mechanism of Modified Vinasse Biochar Adsorption of Sb(V) and As(V) in Water. Master’s Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Zhang, S.; Zhang, R. Study on the Removal Effect and Mechanism of Antimony and Arsenic in Acid Mining Drainage by Carbonate Rock Combined with AMD Iron Flocs. Master’s Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Wang, L.; Wang, J.-M.; Zhang, R.; Liu, X.; Song, G.; Chen, X.; Wang, Y.; Kong, J. Highly efficient As(V)/Sb(V) removal by magnetic sludge composite: Synthesis, characterization, equilibrium, and mechanism studies. RSC Adv. 2016, 6, 42876–42884. [Google Scholar] [CrossRef]
- Diquattro, S.; Garau, G.; Mangia, N.P.; Drigo, B.; Lombi, E.; Vasileiadis, S.; Castaldi, P. Mobility and potential bioavailability of antimony in contaminated soils: Short-term impact on microbial community and soil biochemical functioning. Ecotoxicol. Environ. Saf. 2020, 196, 110576. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; El-Naggar, A.; Wang, H.; Du Laing, G.; Alessi, D.S.; Ok, Y.S. Redox-induced mobilization of Ag, Sb, Sn, and Tl in the dissolved, colloidal and solid phase of a biochar-treated and un-treated mining soil. Environ. Int. 2020, 140, 105754. [Google Scholar] [CrossRef]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J. Environ. Manag. 2015, 151, 326–342. [Google Scholar] [CrossRef]
- Lan, B.; Wang, Y.; Wang, X.; Zhou, X.; Kang, Y.; Li, L. Aqueous arsenic (As) and antimony (Sb) removal by potassium ferrate. Chem. Eng. J. 2016, 292, 389–397. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, P. Removal of Antimony(V) By Adsorption of Iron-Modified Attapulgite Adsorbent. Master’s Thesis, Guizhou University, Guiyang, China, 2015. [Google Scholar]
- Xu, Z.; Jiang, H.; Yu, Y.; Xu, J.; Liang, J.; Zhou, L.; Hu, F. Activation and β-FeOOH modification of sepiolite in one-step hydrothermal reaction and its simulated solar light catalytic reduction of Cr(VI). Appl. Clay Sci. 2017, 135, 547–553. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Rui, X.; Chen, J.; Sim, D.; Shi, W.; Hng, H.H.; Lim, T.M.; Yan, Q. Synthesis of hexagonal-symmetry α-iron oxyhydroxide crystals using reduced graphene oxide as a surfactant and their Li storage properties. CrystEngComm 2012, 14, 147–153. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A. Thomas and Matthias Thommes Characterization of Porous Solids and Powders Superface Area, Pore Size and Density; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4020-2303-3. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China. Fuel 2013, 103, 258–268. [Google Scholar] [CrossRef]
- Tang, X.; Wang, Z.; Ripepi, N.; Kang, B.; Yue, G. Adsorption Affinity of Different Types of Coal: Mean Isosteric Heat of Adsorption. Energy Fuels 2015, 29, 3609–3615. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Li, H.; Li, S.; Cheng, K.; Li, J.; Tsang, D.C.W. Corn straw-derived biochar impregnated with α-FeOOH nanorods for highly effective copper removal. Chem. Eng. J. 2018, 348, 191–201. [Google Scholar] [CrossRef]
- Wei, C.; Nan, Z. Effects of experimental conditions on one-dimensional single-crystal nanostructure of β-FeOOH. Mater. Chem. Phys. 2011, 127, 220–226. [Google Scholar] [CrossRef]
- Pham, T.T.; Ngo, H.H.; Tran, V.S.; Nguyen, M.K. Removal of As (V) from the aqueous solution by a modified granular ferric hydroxide adsorbent. Sci. Total Environ. 2020, 706, 135947. [Google Scholar] [CrossRef]
- Long, X.; Wang, X.; Guo, X.; He, M. A review of removal technology for antimony in aqueous solution. J. Environ. Sci. 2020, 90, 189–204. [Google Scholar] [CrossRef]
- McComb, K.A.; Craw, D.; McQuilan, A.J. ATR-IR Spectroscopic Study of Antimonate Adsorption to Iron Oxide. Langmuir 2007, 23, 12125–12130. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Dou, X.; Bolan, N.S.; Yang, J.E.; Ok, Y.S. Surface complexation modeling and spectroscopic evidence of antimony adsorption on iron-oxide-rich red earth soils. J. Colloid. Interface Sci. 2013, 406, 217–224. [Google Scholar] [CrossRef]
- Myneni, S.C.B.; Traina, S.J.; Waychunas, G.A.; Logan, T.J. Experimental and theoretical vibrational spectoroscopic evaluation of arsenate coordination in aqueous solutions, solids, and at mineral-water interfaces. Geochim. Cosmochim. Acta 1998, 62, 3285–3300. [Google Scholar] [CrossRef]
- Yang, T.; Meng, L.; Han, S.; Hou, J.; Wang, S.; Wang, X. Simultaneous reductive and sorptive removal of Cr(VI) by activated carbon supported β-FeOOH. RSC Adv. 2017, 7, 34687–34693. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Zheng, Y.; Yin, M.; Wang, J.; Bolan, N.; Fan, F.; Yun, Z.; Zhou, C.; Yin, H.; et al. The fate of Sb(V) and As(V) during the aging of ferrihydrite. Chem. Eng. J. 2024, 479, 147671. [Google Scholar] [CrossRef]


| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | R2 | KF (mg(1−n)·Ln/g) | 1/n | R2 | |
| As(V) | 34.99 ± 1.56 | 0.51 ± 0.10 | 0.89606 | 20.47 ± 0.60 | 0.13 ± 0.011 | 0.96616 |
| Sb(V) | 27.61 ± 2.52 | 0.055 ± 0.018 | 0.97158 | 3.70 ± 0.58 | 0.42 ± 0.038 | 0.98406 |
| Material | Adsorption Conditions | Adsorption Capacity (mg/g) | Initial Concentration Range | |||
|---|---|---|---|---|---|---|
| T/(°C) | pH | Dose/(g/L) | As(V) | Sb(V) | ||
| Phosphogypsum-modified vinasse biochar [21] | 25 | 7.5 | 2.5 | 45.22 | 8.74 | 4–90 mg/L |
| Carbonate rock combined with AMD iron flocs [22] | 25 | 6.0 | 1.2 | 20.23 | 6.78 | Sb: 0.608–182.7 mg/L; As: 1.498–299.3 mg/L |
| Magnetic sludge composite [23] | 25 | 2.6 | 0.7 | 18.5 | 35.7 | 10–500 mg/L |
| Fe-GFS | 25 | 3 | 0.8 | 34.99 | 27.61 | 5–100 mg/L |
| Kinetic Model | Parameters | As(V) | Sb(V) |
|---|---|---|---|
| Pseudo-first-order kinetics | qe (mg/g) | 22.53 ± 1.33 | 18. 80 ± 1.08 |
| k1 (min−1) | 0.044 ± 0.013 | 0.017 ± 0.0040 | |
| R2 | 0.8593 | 0.9078 | |
| Pseudo-second-order kinetics | qe (mg/g) | 23.77 ± 0.98 | 20.14 ± 0.91 |
| k2 (g/(mg·min)) | 0.0031 ± 0.00081 | 0.0014 ± 0.00034 | |
| R2 | 0.9433 | 0.9574 |
| Concentration/(mg/L) | Adsorption Capacity/mg/g | ||
|---|---|---|---|
| As(V) | Sb(V) | ||
| As(V)/Sb(V) | 100/0 | 12.21 ± 0.767 ab | - |
| 100/10 | 10.67 ± 0.677 a | 0 | |
| 100/20 | 11.29 ± 0.569 a | 0 | |
| 100/50 | 13.20 ± 0.448 b | 2.20 ± 0.103 | |
| 100/100 | 13.58 ± 1.680 b | 3.77 ± 0.227 | |
| 0/100 | - | 14.89 ± 0.952 c | |
| 10/100 | 1.95 ± 0.108 d | 8.74 ± 1.016 b | |
| 20/100 | 5.04 ± 0.517 c | 8.09 ± 1.576 b | |
| 50/100 | 10.61 ± 1.305 b | 6.89 ± 0.237 b | |
| 100/100 | 13.58 ± 1.680 a | 3.77 ± 0.227 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Z.; Zhou, C.; Hou, H.; Wang, J. Removal of Aqueous Antimony and Arsenic by Iron-Loaded Coal Gasification Slag Composite. Toxics 2024, 12, 440. https://doi.org/10.3390/toxics12060440
Leng Z, Zhou C, Hou H, Wang J. Removal of Aqueous Antimony and Arsenic by Iron-Loaded Coal Gasification Slag Composite. Toxics. 2024; 12(6):440. https://doi.org/10.3390/toxics12060440
Chicago/Turabian StyleLeng, Zheng, Changzhi Zhou, Hong Hou, and Junhuan Wang. 2024. "Removal of Aqueous Antimony and Arsenic by Iron-Loaded Coal Gasification Slag Composite" Toxics 12, no. 6: 440. https://doi.org/10.3390/toxics12060440
APA StyleLeng, Z., Zhou, C., Hou, H., & Wang, J. (2024). Removal of Aqueous Antimony and Arsenic by Iron-Loaded Coal Gasification Slag Composite. Toxics, 12(6), 440. https://doi.org/10.3390/toxics12060440






