Role of IRE1α/XBP1/CHOP/NLRP3 Signalling Pathway in Neonicotinoid Imidacloprid-Induced Pancreatic Dysfunction in Rats and Antagonism of Lycopene: In Vivo and Molecular Docking Simulation Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals and Ethics
2.3. Experimental Design
- -
- Group I: Rats received 0.5 mL/day of corn oil, which served as the vehicle used in preparing IMI and LYC.
- -
- -
- -
- Group IV: Rats were treated with a combination of IMI (45 mg/kg) and LYC (10 mg/kg).
2.4. Biochemical Parameters
2.4.1. Evaluation of Amylase Activity, Insulin, and Glucose Levels
2.4.2. Assessment of the Oxidative Stress Biomarkers and Inflammatory Mediators
2.5. Real-Time Quantitative PCR (qPCR) Analysis for ATF6, CHOP, XBP1, IRE-1α, BAX, and Casp-3 mRNA Expression
2.5.1. RNA Purification and cDNA Synthesis
2.5.2. mRNA Quantification
2.6. Histopathologic Study
2.6.1. Immunohistochemical Study
2.6.2. IHC Analysis
2.6.3. Computer-Assisted Digital Image Analysis (Digital Morphometric Study)
2.7. In Silico Study
2.8. Statistical Analysis
3. Results
3.1. Effect of LYC on Amylase Activity, Insulin, and Glucose Levels in IMI-Intoxicated Rats
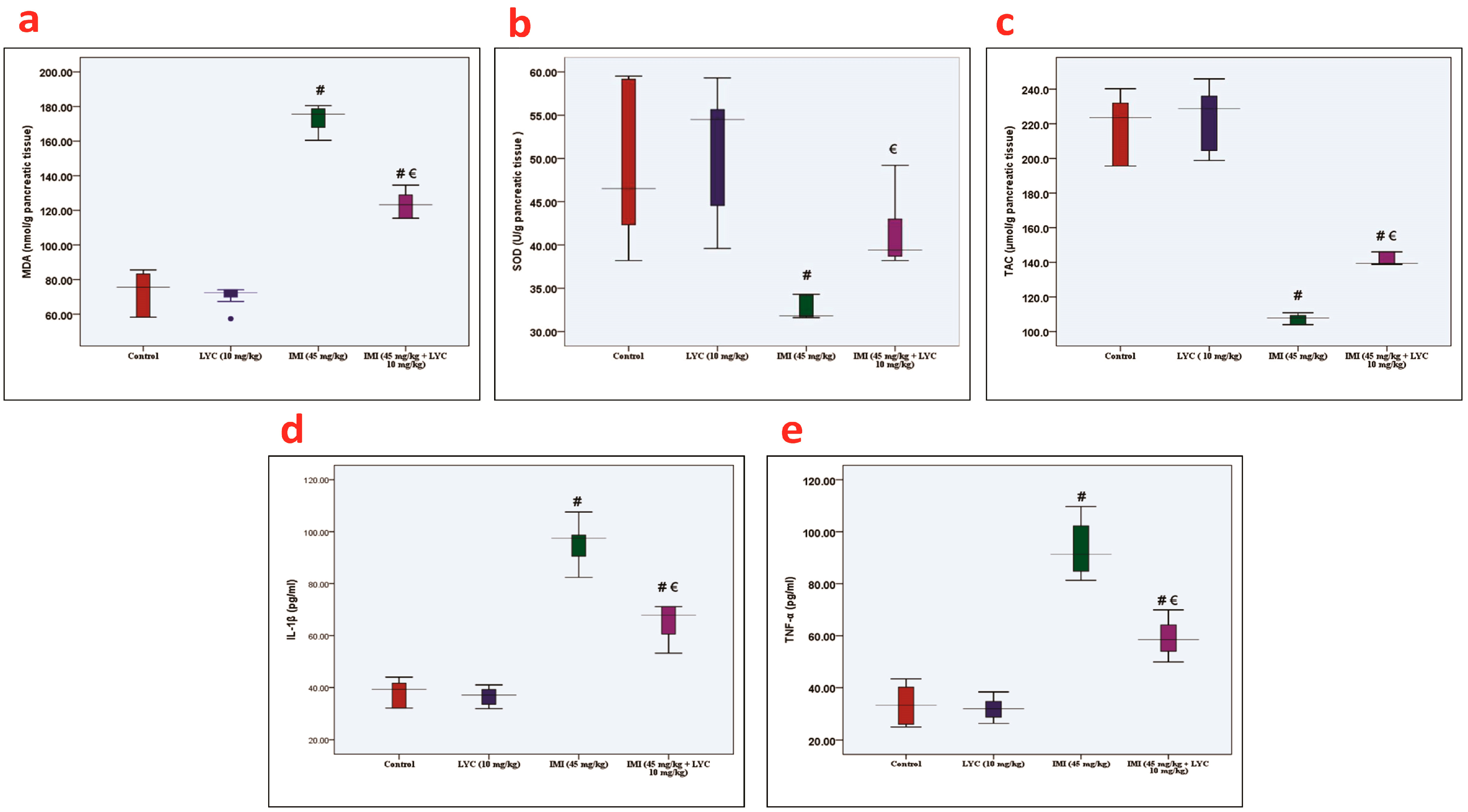
3.2. Effect of LYC on the Oxidative Stress Biomarkers and Inflammatory Mediators in IMI-Intoxicated Rats
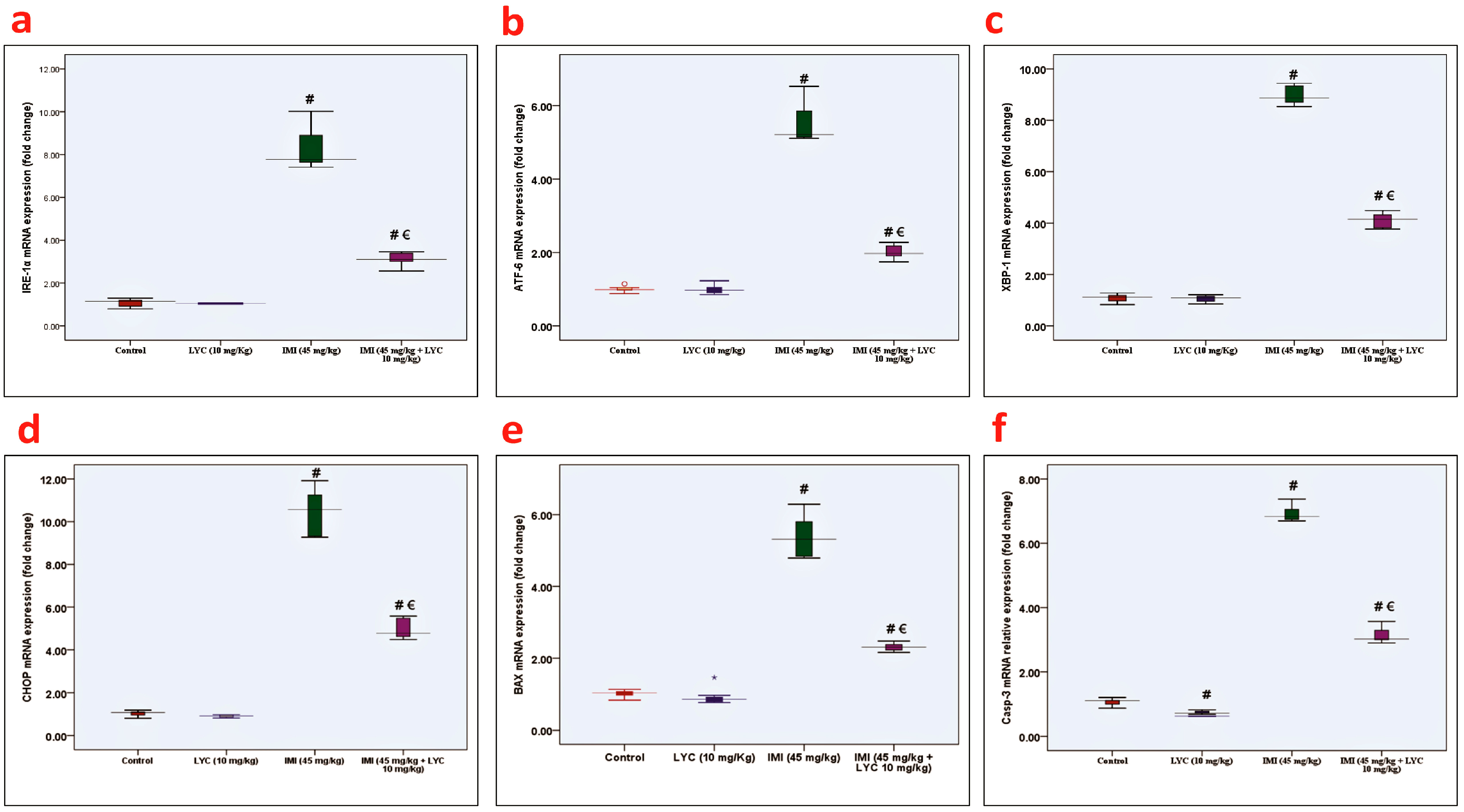
3.3. Effect of LYC on IRE-1α, ATF6, XBP1, CHOP, BAX, and Casp-3 mRNA Expression in IMI-Intoxicated Rats
3.4. Histopathological Examination
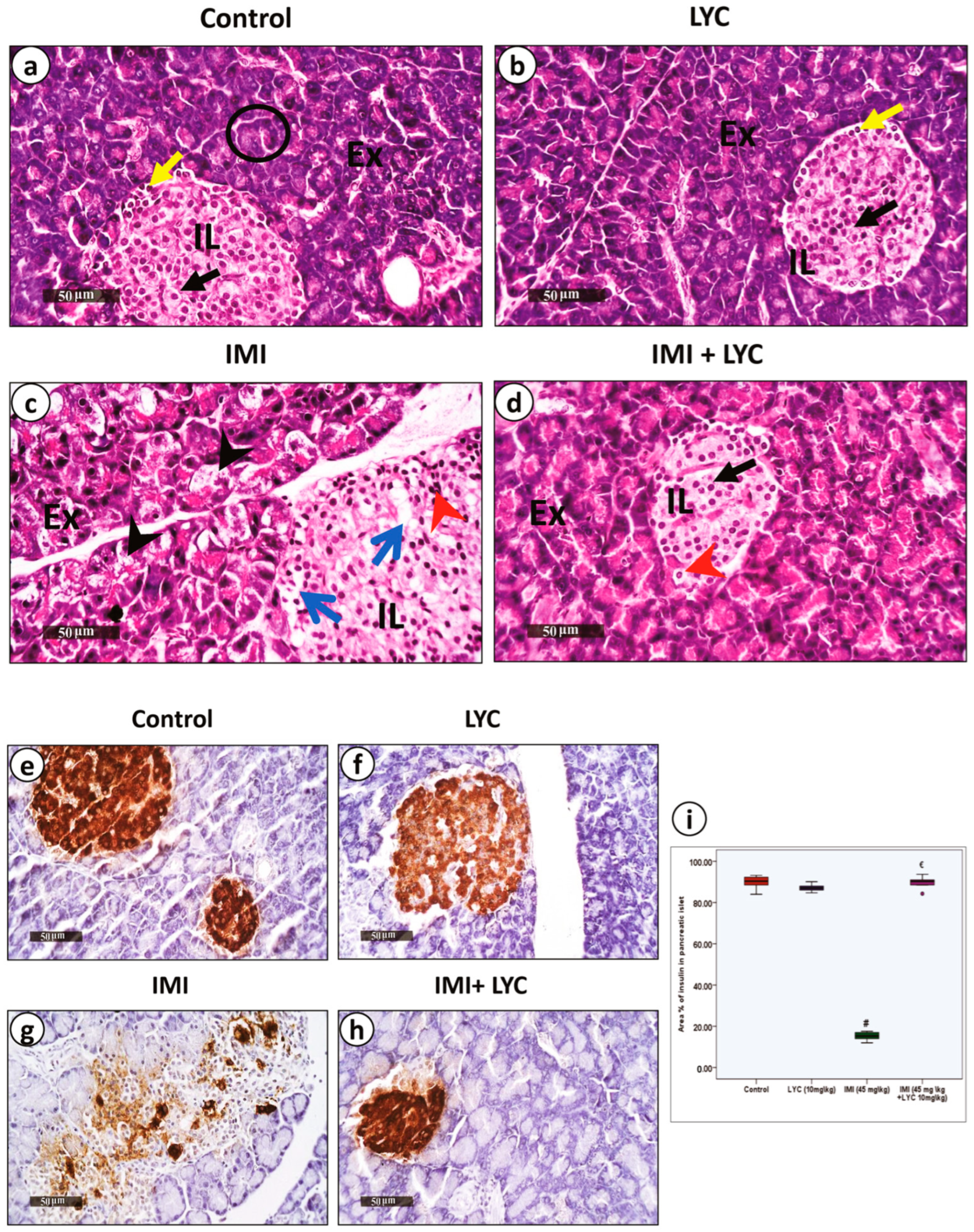
3.4.1. Lycopene Alleviated Pathological Changes and Restored Insulin Secretion after IMI Intoxication
3.4.2. Lycopene Downregulated Both Anti-NLRP3 and Anti-Caspase 1 Inflammatory Biomarkers
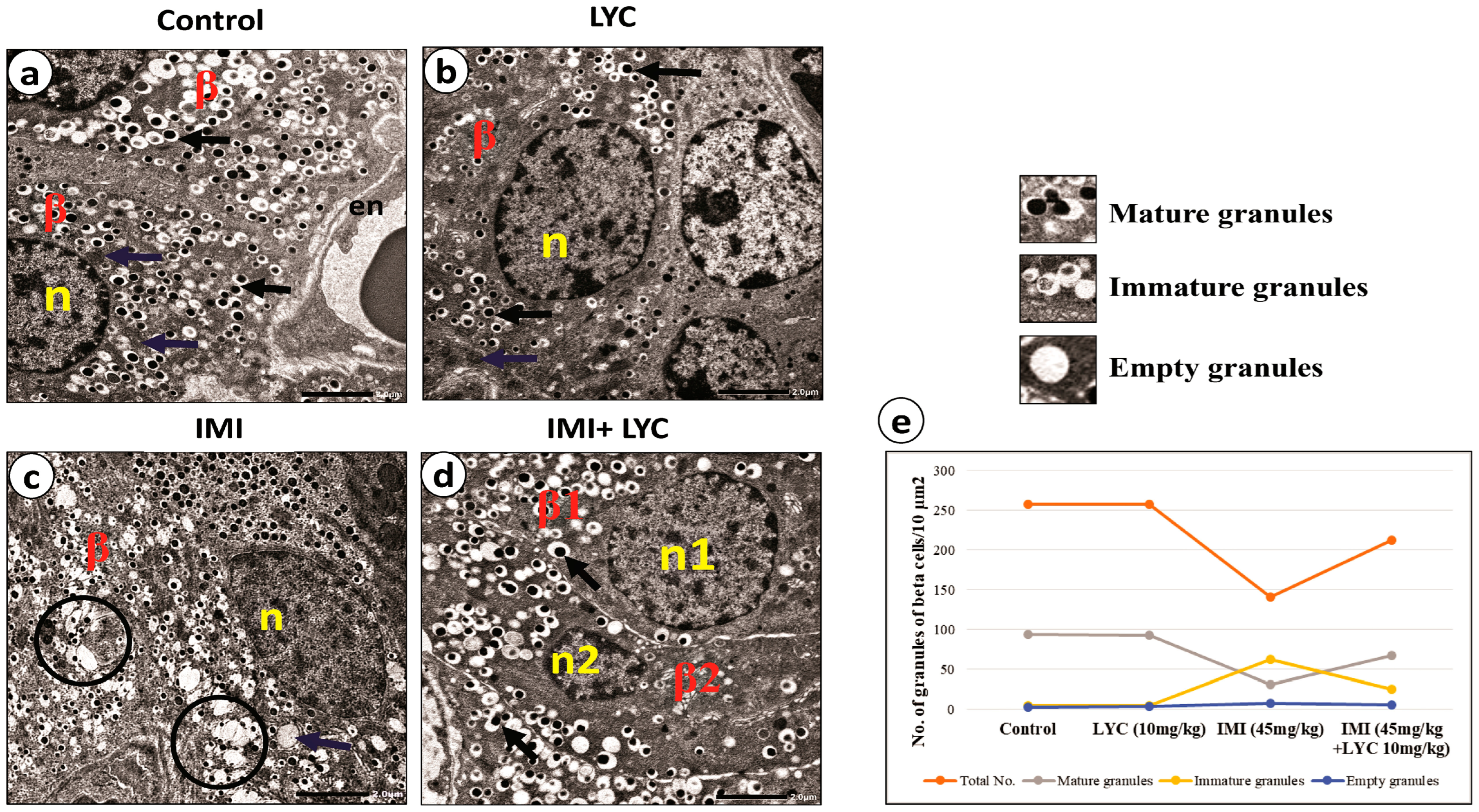
3.4.3. Lycopene Alleviated the Ultra-Structural Pathological Changes in Beta Cells after IMI Intoxication

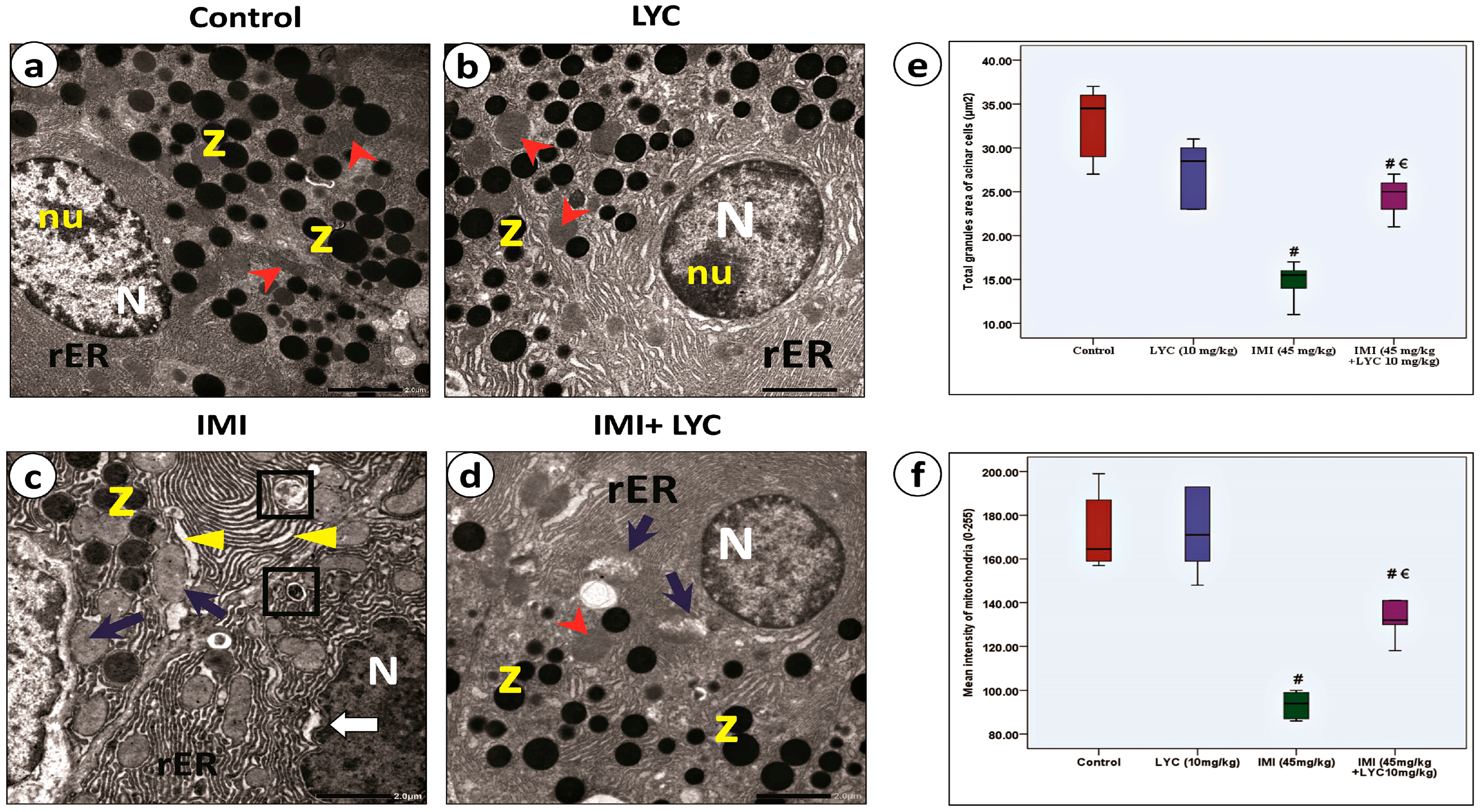
3.4.4. Lycopene Alleviated the Ultra-Structural Pathological Changes in Pancreatic Acinar Cells after IMI Intoxication
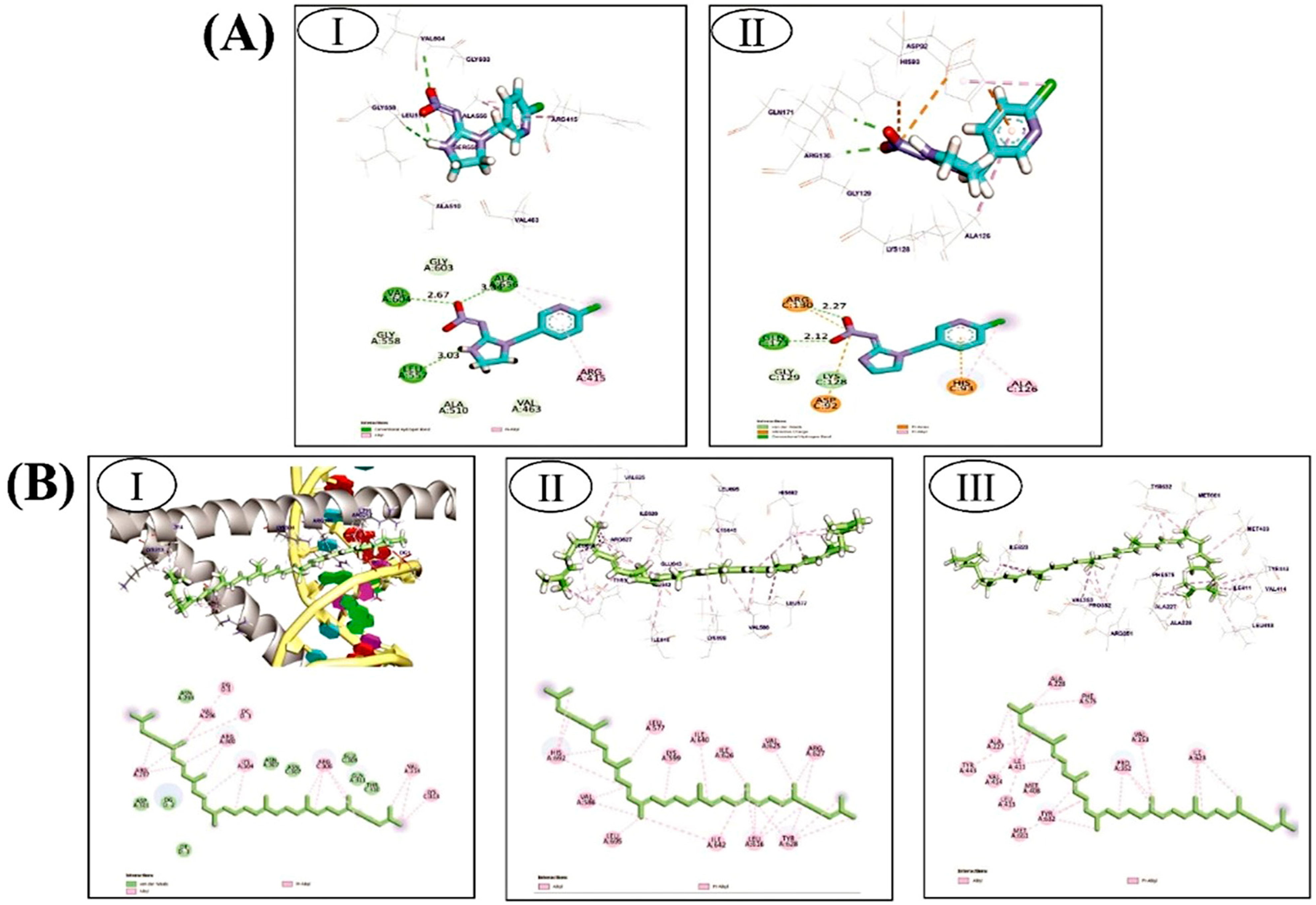
3.5. Molecular Docking Analysis
3.5.1. Imidaclopride against Nrf2/Keap1 Complex and PTEN Target Proteins
3.5.2. Lycopene against CHOP/IRE1-α/NLRP3 Pathway
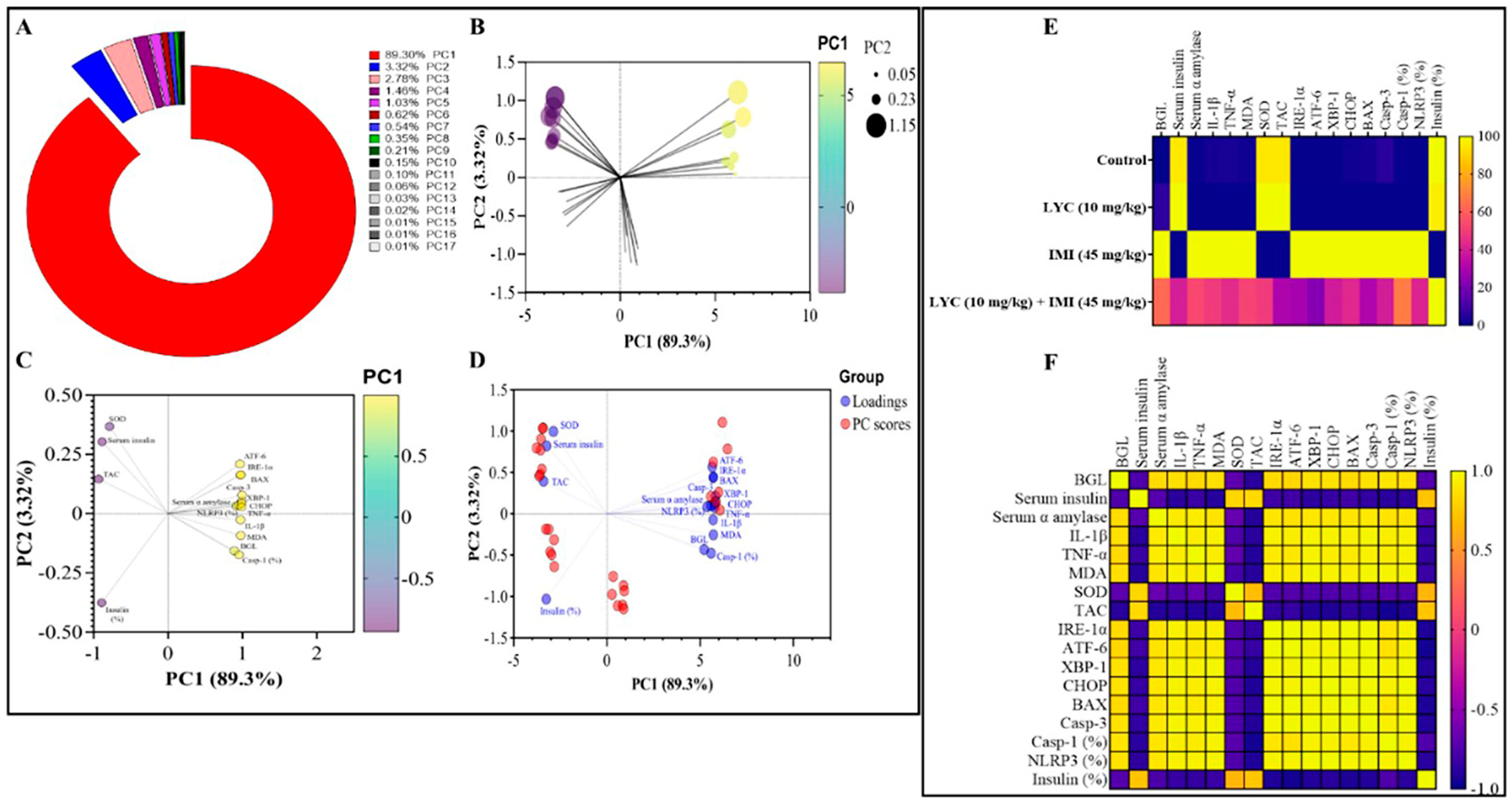
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuong, A.M.; Zhang, C.; Chen, A. Associations of neonicotinoids with insulin and glucose homeostasis parameters in US adults: NHANES 2015–2016. Chemosphere 2022, 286, 131642. [Google Scholar] [CrossRef] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 01582. [Google Scholar] [CrossRef] [PubMed]
- Karpińska, M.; Czauderna, M. Pancreas—Its Functions, Disorders, and Physiological Impact on the Mammals’ Organism. Front. Physiol. 2022, 13, 807632. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; ÖZtaŞ, E.; ÖZhan, G. Acetamiprid-induced Cyto- and Genotoxicity in the AR42J Pancreatic Cell Line. Turk. J. Pharm. Sci. 2020, 17, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Ren, J.; Hou, Y.; Han, Z.; Xiao, J.; Li, Y. Exposure Level of Neonicotinoid Insecticides in the Food Chain and the Evaluation of Their Human Health Impact and Environmental Risk: An Overview. Sustainability 2020, 12, 7523. [Google Scholar] [CrossRef]
- Nimako, C.; Ikenaka, Y.; Akoto, O.; Fujioka, K.; Taira, K.; Arizono, K.; Kato, K.; Takahashi, K.; Nakayama, S.M.M.; Ichise, T.; et al. Simultaneous quantification of imidacloprid and its metabolites in tissues of mice upon chronic low-dose administration of imidacloprid. J. Chromatogr. A 2021, 1652, 462350. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Pathe, C.S.; Vishwakarma, P.; Dhama, K.; Munjal, A. Evaluation of the ameliorative effects of Phyllanthus niruri on the deleterious insecticide imidacloprid in the vital organs of chicken embryos. J. Ayurveda Integr. Med. 2020, 11, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Zuščíková, L.; Bažány, D.; Greifová, H.; Knížatová, N.; Kováčik, A.; Lukáč, N.; Jambor, T. Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review. Toxics 2023, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pan, X.; Zhang, Y.-H.; Huang, T.; Cai, Y.-D. Analysis of Gene Expression Differences between Different Pancreatic Cells. ACS Omega 2019, 4, 6421–6435. [Google Scholar] [CrossRef]
- Khurana, A.; Saifi, M.A.; Godugu, C. Yttrium Oxide Nanoparticles Attenuate L-Arginine Induced Chronic Pancreatitis. Biol. Trace Elem. Res. 2023, 201, 3404–3417. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, N.; Yang, X.; Xia, Y.; Wu, D. Effects induced by polyethylene microplastics oral exposure on colon mucin release, inflammation, gut microflora composition and metabolism in mice. Ecotoxicol. Environ. Saf. 2021, 220, 112340. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Chun, J.; Vilaysane, A.; Clark, S.; French, G.; Bracey, N.A.; Trpkov, K.; Bonni, S.; Duff, H.J.; et al. Inflammasome-Independent NLRP3 Augments TGF-β Signaling in Kidney Epithelium. J. Immunol. 2013, 190, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Yao, X.; Jiang, L.; Qiu, T.; Wang, N.; Yang, L.; Gao, N.; Wang, Z.; Yang, G.; Liu, X.; et al. Inorganic arsenic induces pyroptosis and pancreatic β cells dysfunction through stimulating the IRE1α/TNF-α pathway and protective effect of taurine. Food Chem. Toxicol. 2019, 125, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.-Q.; Liu, R.-P.; Li, Y.-H.; Yao, X.-R.; Kim, N.-H.; Xu, Y.-N. Melatonin Supplementation during In Vitro Maturation of Porcine Oocytes Alleviates Oxidative Stress and Endoplasmic Reticulum Stress Induced by Imidacloprid Exposure. Animals 2023, 13, 2596. [Google Scholar] [CrossRef] [PubMed]
- Kanak, M.A.; Shahbazov, R.; Yoshimatsu, G.; Levy, M.F.; Lawrence, M.C.; Naziruddin, B. A small molecule inhibitor of NF-κB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J. Gastroenterol. 2017, 52, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, A.; Cielecka-Piontek, J.; Zalewski, P. The Importance of Antioxidant Activity for the Health-Promoting Effect of Lycopene. Nutrients 2023, 15, 3821. [Google Scholar] [CrossRef] [PubMed]
- Wadie, W.; Mohamed, A.H.; Masoud, M.A.; Rizk, H.A.; Sayed, H.M. Protective impact of lycopene on ethinylestradiol-induced cholestasis in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 447–455. [Google Scholar] [CrossRef]
- Rajput, S.A.; Liang, S.-J.; Wang, X.-Q.; Yan, H.-C. Lycopene Protects Intestinal Epithelium from Deoxynivalenol-Induced Oxidative Damage via Regulating Keap1/Nrf2 Signaling. Antioxidants 2021, 10, 1493. [Google Scholar] [CrossRef]
- Choi, S.; Kim, H. The Remedial Potential of Lycopene in Pancreatitis through Regulation of Autophagy. Int. J. Mol. Sci. 2020, 21, 5775. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, C.; Yang, M.; Gan, D.; Fan, C.; Li, A.; Li, Q.; Zhao, J.; Zhu, L.; Lu, D. Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol. Biol. Rep. 2019, 46, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Leh, H.E.; Lee, L.K. Lycopene: A Potent Antioxidant for the Amelioration of Type II Diabetes Mellitus. Molecules 2022, 27, 2335. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.A.; Mohamed, A.A.-R.; Khater, S.I.; Noreldin, A.E.; Alosaimi, M.; Alansari, W.S.; Shamlan, G.; Eskandrani, A.A.; Awad, M.M.; El-Shaer, R.A.A.; et al. Enhancement of biochemical and genomic pathways through lycopene-loaded nano-liposomes: Alleviating insulin resistance, hepatic steatosis, and autophagy in obese rats with non-alcoholic fatty liver disease: Involvement of SMO, GLI-1, and PTCH-1 genes. Gene 2023, 883, 147670. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, M.; Elbadawy, M.; Saber, S.; Khalil, E.; Darweish, M.; Farag, A.; Elfadadny, A.; Alkafafy, M.; Soliman, A.M.; Elsayed, A. Allicin and Lycopene Possesses a Protective Effect against Methotrexate-induced Testicular Toxicity in Rats. Pak. Vet. J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ji, Y.; Wang, R.; Cheng, K.; Liu, L.; Wu, N.; Tang, Q.; Zheng, X.; Li, J.; Zhu, Z.; et al. Lycopene Ameliorates Hypoxic Pulmonary Hypertension via Suppression of Oxidative Stress. Oxidative Med. Cell. Longev. 2022, 2022, 9179427. [Google Scholar] [CrossRef]
- Mikolić, A.; Karačonji, I.B. Imidacloprid as reproductive toxicant and endocrine disruptor: Investigations in laboratory animals. Arch. Ind. Hyg. Toxicol. 2018, 69, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.A.I.; Badawy, M.E.I.; Abdel-Razik, R.K.; Younis, H.M.; Abo-El-Saad, M.M. Mitochondrial dysfunction and oxidative stress in liver of male albino rats after exposing to sub-chronic intoxication of chlorpyrifos, cypermethrin, and imidacloprid. Pestic. Biochem. Physiol. 2021, 178, 104938. [Google Scholar] [CrossRef]
- Hassanen, E.I.; Hussien, A.M.; Mehanna, S.; Ibrahim, M.A.; Hassan, N.H. Comparative assessment on the probable mechanisms underlying the hepatorenal toxicity of commercial imidacloprid and hexaflumuron formulations in rats. Environ. Sci. Pollut. Res. 2022, 29, 29091–29104. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saleh, A.M.; Mahdy, H.A.; El-Zahabi, M.A.; Mehany, A.B.M.; Khalifa, M.M.; Eissa, I.H. Design, synthesis, in silico studies, and biological evaluation of novel pyrimidine-5-carbonitrile derivatives as potential anti-proliferative agents, VEGFR-2 inhibitors and apoptotic inducers. RSC Adv. 2023, 13, 22122–22147. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C.; Suvarna, K.S. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Azab, E.F.E.; Alakilli, S.Y.M.; Saleh, A.M.; Alhassan, H.H.; Alanazi, H.H.; Ghanem, H.B.; Yousif, S.O.; Alrub, H.A.; Anber, N.; Elfaki, E.M.; et al. Actinidia deliciosa Extract as a Promising Supplemental Agent for Hepatic and Renal Complication-Associated Type 2 Diabetes (In Vivo and In Silico-Based Studies). Int. J. Mol. Sci. 2023, 24, 13759. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Bing, Q.; Lv, Z.; Xue, J.; Li, S.; Han, B.; Yang, Q.; Wang, X.; Zhang, Z. Imidacloprid-induced liver fibrosis in quails via activation of the TGF-β1/Smad pathway. Sci. Total Environ. 2020, 705, 135915. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-M.; Soliman, G.A.; Meza, J.L.; Islam, K.M.M.; Watanabe-Galloway, S. The influence of BMI on the association between serum lycopene and the metabolic syndrome. Br. J. Nutr. 2016, 115, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.R.; Awad, A.; Mohammed, H.H.; Nassan, M.A. Imidacloprid insecticide exposure induces stress and disrupts glucose homeostasis in male rats. Environ. Toxicol. Pharmacol. 2017, 55, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Hart, C.R.; Gries, K.J.; Parvizi, M.; Laurenti, M.; Man, C.D.; Moore, N.; Zhang, X.; Ryan, Z.; Polley, E.C.; et al. Adipose tissue macrophage populations and inflammation are associated with systemic inflammation and insulin resistance in obesity. Am. J. Physiol.-Endocrinol. Metab. 2021, 321, E105–E121. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.N.A.; AbdelMageed, M.A.; Assayed, M.E.M.; Gharib, H.S.A.-R.; Nasr, M.A.; Elshopakey, G.E.; Moniem, H.A.; Shahin, S.E.; Elhusseiny, E.; Ahmed, S.A.A. Imidacloprid induced growth, hematological, neuro-behavior, anti-oxidant, economic, genetic, and histopathological alterations in Clarias gariepinus: Alleviative role of dietary Hyphaene thebaica. Aquaculture 2023, 564, 739058. [Google Scholar] [CrossRef]
- Reinke, S.; Linge, M.; Diebner, H.H.; Luksch, H.; Glage, S.; Gocht, A.; Robertson, A.A.B.; Cooper, M.A.; Hofmann, S.R.; Naumann, R.; et al. Non-canonical Caspase-1 Signaling Drives RIP2-Dependent and TNF-α-Mediated Inflammation In Vivo. Cell Rep. 2020, 30, 2501–2511.e2505. [Google Scholar] [CrossRef]
- Adali, S.; Erbaş, O. Cellular Response to Endoplasmic Reticulum Stress: Focus on XBP, elF2, ATF4, and CHOP. J. Exp. Basic Med. Sci. 2023, 4, 122–133. [Google Scholar]
- Salvadó, L.; Palomer, X.; Barroso, E.; Vázquez-Carrera, M. Targeting endoplasmic reticulum stress in insulin resistance. Trends Endocrinol. Metab. 2015, 26, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.S.; Almeer, R.; Albasher, G.; Moneim, A.E.A. Lycopene mitigates arsenic-induced nephrotoxicity with activation of the Nrf2 pathway in mice. Toxin Rev. 2022, 41, 446–456. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 03083. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, H.; Chen, B.; Yue, R.; Zhou, Z.; Liu, Y.; Zhang, S.; Xu, L.; Wang, H.; Yu, Z. Lycopene Protects against Hypoxia/Reoxygenation Injury by Alleviating ER Stress Induced Apoptosis in Neonatal Mouse Cardiomyocytes. PLoS ONE 2015, 10, e0136443. [Google Scholar] [CrossRef] [PubMed]
- Albrahim, T.; Robert, A.A. Lycopene Effects on Metabolic Syndrome and Kidney Injury in Rats Fed a High-Fat Diet: An Experimental Study. ACS Omega 2022, 7, 30930–30938. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Hu, C.; Liu, J.; Wu, Z.; Duan, T.; Cao, Z. 1,25-(OH)D protects pancreatic beta cells against HO-induced apoptosis through inhibiting the PERK-ATF4-CHOP pathway. Acta Biochim. Et Biophys. Sin. 2020, 53, 46–53. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, S.; Zhang, J.; Xu, S.; Miao, Z. Cadmium induces endoplasmic reticulum stress-mediated apoptosis in pig pancreas via the increase of Th1 cells. Toxicology 2021, 457, 152790. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer | Size | Accession No. |
|---|---|---|---|---|
| IRE-1α | GCGCAGGTGCAATGACATAC | CTCTTCCACGTGTGTTGGGA | 178 | NM_001191926.1 |
| ATF6 | AAGTGAAGAACCATTACTTTATATC | TTTCTGCTGGCTATTTGT | 157 | NM_001107196.1 |
| XBP1 | TTACGAGAGAAAACTCATGGGC | GGGTCCAACTTGTCCAGAATGC | 289 | NM_001004210.2 |
| CHOP | CACAAGCACCTCCCAAAG | CCTGCTCCTTCTCCTTCAT | 158 | NM_001109986.1 |
| BAX | CGAATTGGCGATGAACTGGA | CAAACATGTCAGCTGCCACAC | 109 | NM_017059.2 |
| Casp-3 | GAGACAGACAGTGGAACTGACGATG | GGCGCAAAGTGACTGGATGA | 147 | NM_012922.2 |
| act-b | AACCTTCTTGCAGCTCCTCC | CCATACCCACCATCACACCC | 193 | NM_031144.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Gazzar, W.B.; Bayoumi, H.; Youssef, H.S.; Ibrahim, T.A.; Abdelfatah, R.M.; Gamil, N.M.; Iskandar, M.K.; Abdel-Kareim, A.M.; Abdelrahman, S.M.; Gebba, M.A.; et al. Role of IRE1α/XBP1/CHOP/NLRP3 Signalling Pathway in Neonicotinoid Imidacloprid-Induced Pancreatic Dysfunction in Rats and Antagonism of Lycopene: In Vivo and Molecular Docking Simulation Approaches. Toxics 2024, 12, 445. https://doi.org/10.3390/toxics12070445
El Gazzar WB, Bayoumi H, Youssef HS, Ibrahim TA, Abdelfatah RM, Gamil NM, Iskandar MK, Abdel-Kareim AM, Abdelrahman SM, Gebba MA, et al. Role of IRE1α/XBP1/CHOP/NLRP3 Signalling Pathway in Neonicotinoid Imidacloprid-Induced Pancreatic Dysfunction in Rats and Antagonism of Lycopene: In Vivo and Molecular Docking Simulation Approaches. Toxics. 2024; 12(7):445. https://doi.org/10.3390/toxics12070445
Chicago/Turabian StyleEl Gazzar, Walaa Bayoumie, Heba Bayoumi, Heba S. Youssef, Tayseer A. Ibrahim, Reham M. Abdelfatah, Noha M. Gamil, Mervat K. Iskandar, Amal M. Abdel-Kareim, Shaymaa M. Abdelrahman, Mohammed A. Gebba, and et al. 2024. "Role of IRE1α/XBP1/CHOP/NLRP3 Signalling Pathway in Neonicotinoid Imidacloprid-Induced Pancreatic Dysfunction in Rats and Antagonism of Lycopene: In Vivo and Molecular Docking Simulation Approaches" Toxics 12, no. 7: 445. https://doi.org/10.3390/toxics12070445
APA StyleEl Gazzar, W. B., Bayoumi, H., Youssef, H. S., Ibrahim, T. A., Abdelfatah, R. M., Gamil, N. M., Iskandar, M. K., Abdel-Kareim, A. M., Abdelrahman, S. M., Gebba, M. A., Mohamed, M. A., Mokhtar, M. M., Kharboush, T. G., Bayoumy, N. M., Alomar, H. A., & Farag, A. A. (2024). Role of IRE1α/XBP1/CHOP/NLRP3 Signalling Pathway in Neonicotinoid Imidacloprid-Induced Pancreatic Dysfunction in Rats and Antagonism of Lycopene: In Vivo and Molecular Docking Simulation Approaches. Toxics, 12(7), 445. https://doi.org/10.3390/toxics12070445





