Adsorption Behavior and Mechanisms of Trihalomethanes onto Virgin and Weathered Polyvinyl Chloride Microplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batch Adsorption Experiments
2.3. Analytical Methods
2.4. Adsorption Kinetics and Isotherm Models
2.5. Quality Control and Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization of Virgin and Weathered PVC MPs
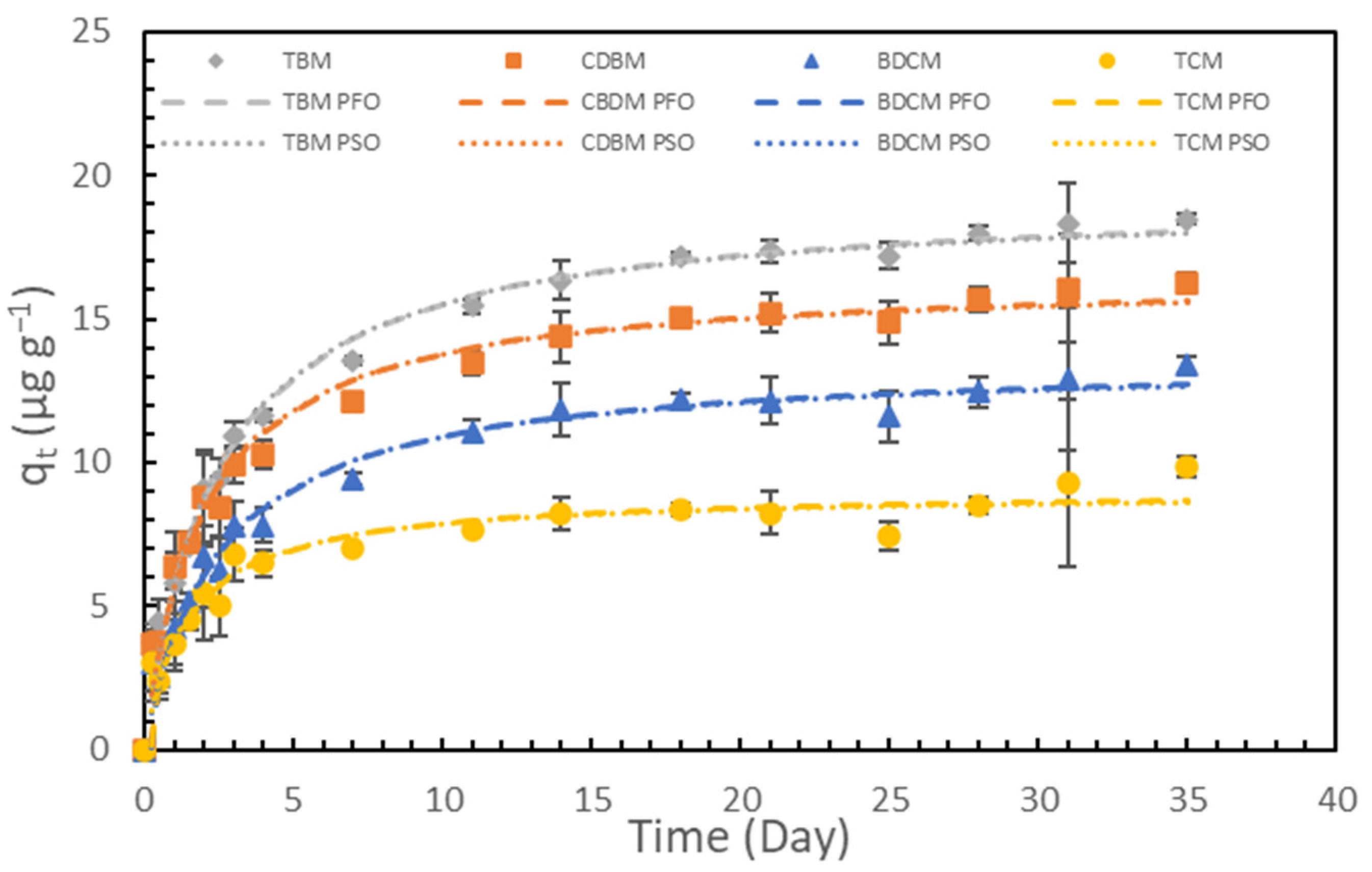
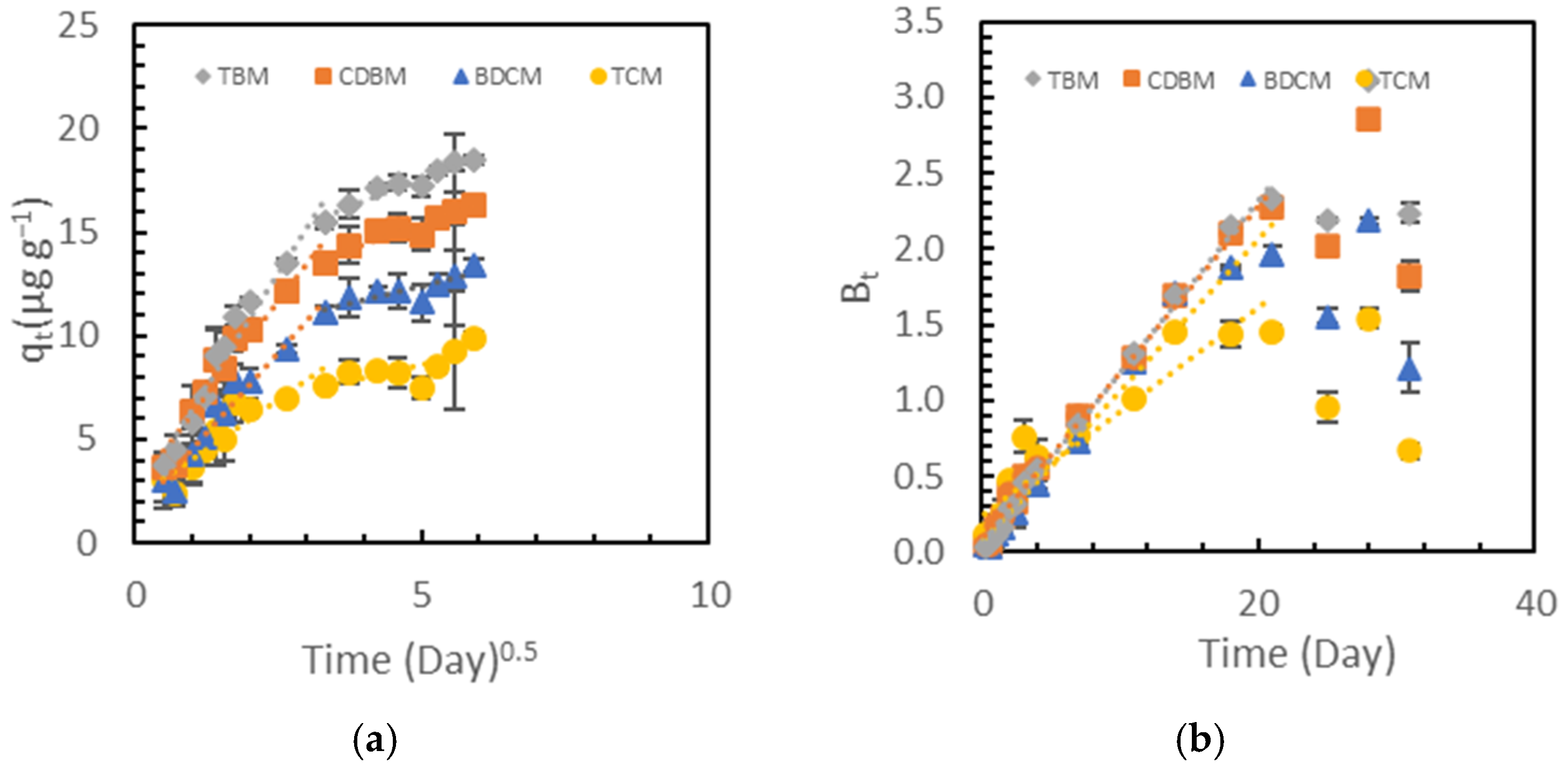
3.2. Adsorption Kinetics and Their Possible Relationships to Reported Mechanisms
3.3. Adsorption Isotherms for Virgin PVC MPs
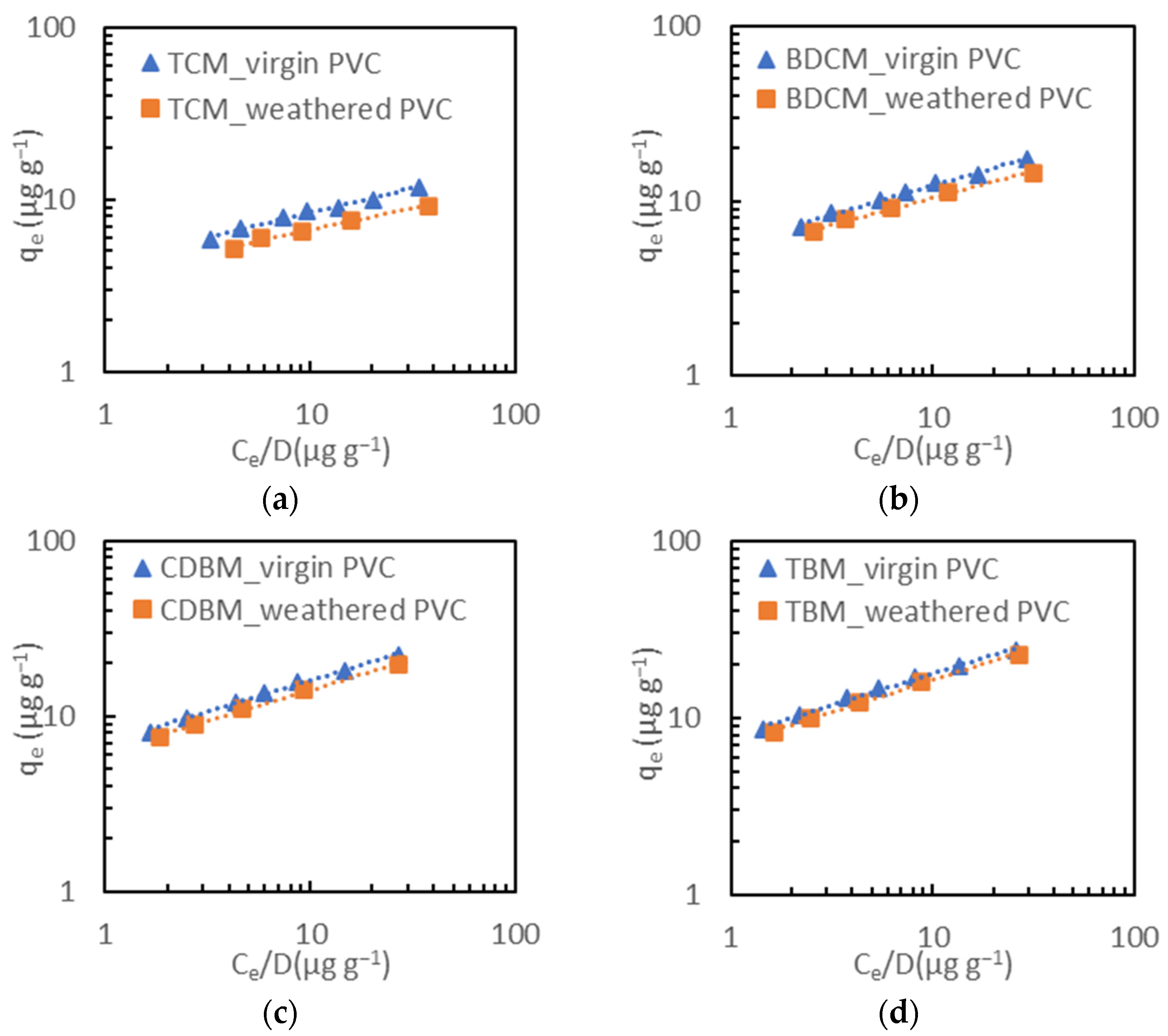
3.4. Impact of Weathering on Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Mason, S.A. Plastic Debris in 29 Great Lakes Tributaries: Relations to Watershed Attributes and Hydrology. Environ. Sci. Technol. 2016, 50, 10377–10385. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liang, W.; Liu, Q.X.; Fu, S.; Ma, C.; Chen, Q.; Su, L.; Craig, N.J.; Shi, H. Fish Ingest Microplastics Unintentionally. Environ. Sci. Technol. 2021, 55, 10471–10479. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef] [PubMed]
- WHO. Microplastics in Drinking Water; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Abdurahman, A.; Cui, K.; Wu, J.; Li, S.; Gao, R.; Dai, J.; Liang, W.; Zeng, F. Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis. Ecotoxicol. Environ. Saf. 2020, 198, 110658. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chen, C.; Wang, J. Sorption of sulfamethoxazole onto six types of microplastics. Chemosphere 2019, 228, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shim, W.J.; Kwon, J.H. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Seidensticker, S.; Grathwohl, P.; Lamprecht, J.; Zarfl, C. A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ. Sci. Eur. 2018, 30, 30. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, K.; Huang, X.; Liu, J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. 2016, 23, 8819–8826. [Google Scholar] [CrossRef]
- Bove, G.E.; Rogerson, P.A.; Vena, J.E. Case control study of the geographic variability of exposure to disinfectant byproducts and risk for rectal cancer. Int. J. Health Geogr. 2007, 61, 18. [Google Scholar] [CrossRef]
- Evlampidou, I.; Font-Ribera, L.; Rojas-Rueda, D.; Gracia-Lavedan, E.; Costet, N.; Pearce, N.; Vineis, P.; Jaakkola, J.J.K.; Delloye, F.; Makris, K.C.; et al. Trihalomethanes in Drinking Water and Bladder Cancer Burden in the European Union. Environ. Health Perspect. 2020, 128, 017001. [Google Scholar] [CrossRef] [PubMed]
- Babi, K.G.; Koumenides, K.M.; Nikolaou, A.D.; Makri, C.A.; Tzoumerkas, F.K.; Lekkas, T.D. Pilot study of the removal of THMs, HAAs and DOC from drinking water by GAC adsorption. Desalination 2007, 210, 215–224. [Google Scholar] [CrossRef]
- Zainudin, F.M.; Hasan, H.A.; Abdullah, S.R.S. An overview of the technology used to remove trihalomethane (THM), trihalomethane precursors, and trihalomethane formation potential (THMFP) from water and wastewater. J. Ind. Eng. Chem. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Qian, H.; Lin, Y.L.; Xu, B.; Wang, L.P.; Gao, Z.C.; Gao, N.Y. Adsorption of haloforms onto GACs: Effects of adsorbent properties and adsorption mechanisms. Chem. Eng. J. 2018, 349, 849–859. [Google Scholar] [CrossRef]
- Wong, H.; Mok, K.M.; Fan, X.J. Natural organic matter and formation of trihalomethanes in two water treatment processes. Desalination 2007, 210, 44–51. [Google Scholar] [CrossRef]
- Ateia, M.; Kanan, A.; Karanfil, T. Microplastics release precursors of chlorinated and brominated disinfection byproducts in water. Chemosphere 2020, 251, 126452. [Google Scholar] [CrossRef]
- Lee, Y.K.; Romera-Castillo, C.; Hong, S.; Hur, J. Characteristics of microplastic polymer-derived dissolved organic matter and its potential as a disinfection byproduct precursor. Water Res. 2020, 175, 115678. [Google Scholar] [CrossRef]
- Yan, Z.; Qian, H.; Yao, J.; Guo, M.; Zhao, X.; Gao, N.; Zhang, Z. Mechanistic insight into the role of typical microplastics in chlorination disinfection: Precursors and adsorbents of both MP-DOM and DBPs. J. Hazard. Mater. 2024, 462, 132716. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of microplastics affects their surface properties, thermal decomposition, additives leaching and interactions in simulated fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef]
- Udenby, F.A.; Almuhtaram, H.; McKie, M.J.; Andrews, R.C. Adsorption of fluoranthene and phenanthrene by virgin and weathered polyethylene microplastics in freshwaters. Chemosphere 2020, 307, 135585. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Zheng, T.; Calace, S.; Tharayil, N.; Pilla, S.; Karanfil, T. Sorption behavior of real microplastics (MPs): Insights for organic micropollutants adsorption on a large set of well-characterized MPs. Sci. Total Environ. 2020, 720, 137634. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zou, Y.; Geng, N.; Liu, J.; Hou, J.; Li, D.; Yang, C.; Li, Y. Investigation on the adsorption and desorption behaviors of antibiotics by degradable MPs with or without UV ageing process. J. Hazard. Mater. 2021, 401, 123363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Zhou, B.; Zhou, Y.; Dai, Z.; Zhou, Q.; Chriestie, P.; Luo, Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ. Pollut. 2018, 243, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mandoza-Hill, J.J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017; p. 57. ISBN 978-92-5-109882-0. Available online: https://openknowledge.fao.org/handle/20.500.14283/i7677en (accessed on 12 December 2021).
- Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui, S. A low-cost system to simulate environmental microplastic weathering. Mar. Pollut. Bull. 2019, 149, 110663. [Google Scholar] [CrossRef]
- Jiang, C. Abundance, Composition, and Factors Impacting the Formation of Microplastic-Associated Biofilm in Freshwaters. Master’s Thesis, University of Toronto, Toronto, ON, Canada, 2022. [Google Scholar]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—N-Nitrosodimethylamine; Health Canada: Ottawa, Canada, 2011; Available online: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-n-nitrosodimethylamine-ndma.html (accessed on 4 July 2021).
- Hyung, H.; Kim, J.H. Natural organic matter (NOM) adsorption to multi-walled carbon nanotubes: Effect of NOM characteristics and water quality parameters. Environ. Sci. Technol. 2008, 42, 4416–4421. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yuasa, A.; Ebie, K.; Azuma, Y.; Hagishita, T.; Matsui, Y. Factors affecting the adsorption capacity of dissolved organic matter onto activated carbon: Modified isotherm analysis. Water Res. 2002, 36, 4592–4604. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.J.; Voice, T.C.; Jodellah, A. Adsorption of humic substances: The effects of heterogeneity and system characteristics. J. Am. Water Work. Assoc. 1983, 75, 612–619. [Google Scholar] [CrossRef]
- Rice, E.E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American public health association: Washington, DC, USA, 2012; Available online: https://yabesh.ir/wp-content/uploads/2018/02/Standard-Methods-23rd-Perv.pdf (accessed on 30 May 2024).
- U. S. EPA. Method 551.1: Determination of Chlorination Disinfection Byproducts, Chlorinated Solvents, and Halogenated Pesticides/Herbicides in Drinking Water by Liquid-Liquid Extraction and Gas Chromatography With Electron-Capture Detection, Revision 1.0.; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1995. Available online: https://www.epa.gov/sites/default/files/2015-06/documents/epa-551.1.pdf (accessed on 30 May 2024).
- Guisela B, Z.; Ohana N, D.A.; Dalvani S, D.; Fermin G, V.; Francisco HM, L.; Luis, N.G. Adsorption of arsenic anions in water using modified lignocellulosic adsorbents. Results Eng. 2022, 13, 100340. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Adsorption of sodium from saline water with natural and acid activated Ethiopian bentonite. Results Eng. 2022, 14, 100440. [Google Scholar] [CrossRef]
- Gora, S.L.; Andrews, S.A. Removal of natural organic matter and disinfection byproduct precursors from drinking water using photocatalytically regenerable nanoscale adsorbents. Chemosphere 2019, 218, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.S.; Roberts, P.V. Activated carbon adsorption of humic substances. I. Heterodisperse mixtures and desorption. J. Colloid Interface Sci. 1988, 122, 367–381. [Google Scholar] [CrossRef]
- Shi, Y.; Almuhtaram, H.; Andrews, R.C. Adsorption of Per- and Polyfluoroalkyl Substances (PFAS) and Microcystins by Virgin and Weathered Microplastics in Freshwater Matrices. Polymers 2023, 15, 3676. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of trace metals to plastic resin pellets in the marine environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Huang, K.; Whelton, A.J.; Shah, A.D. Formation and sorption of trihalomethanes from cross-linked polyethylene pipes following chlorinated water exposure. Environ. Sci. Water Res. Technol. 2020, 6, 2479–2491. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, X.; Wu, X.; Li, J.; Wang, H.; Gao, S. Effect of weathering on environmental behavior of microplastics: Properties, sorption and potential risks. Chemosphere 2020, 242, 125193. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liu, F.; Brookes, P.C.; Xu, J. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter. Environ. Pollut. 2018, 240, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Zhao, L.; Rong, L.; Xu, J.; Lian, J.; Wang, L.; Sun, H. Sorption of five organic compounds by polar and nonpolar microplastics. Chemosphere 2020, 257, 127206. [Google Scholar] [CrossRef]
- Morris, J.C.; Weber, W.J. Removal of biologically-resistant pollutants from water waters by adsorption. In Advances in Water Pollution Research; Elsevier: Amsterdam, The Netherlands, 1964; pp. 231–266. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere 2018, 193, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Kokate, S.; Parasuraman, K.; Prakash, H. Adsorptive removal of lead ion from water using banana stem scutcher generated in fiber extraction process. Results Eng. 2022, 14, 100439. [Google Scholar] [CrossRef]
- An, B. Cu(II) and As(V) adsorption kinetic characteristic of the multifunctional amino groups in chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II.; Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Tavlieva, M.P.; Genieva, S.D.; Georgieva, V.G.; Vlaev, L.T. Kinetic study of brilliant green adsorption from aqueous solution onto white rice husk ash. J. Colloid Interface Sci. 2013, 409, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Clint Sutherland and Chintanapalli Venkobachar. A diffusion-chemisorption kinetic model for simulating biosorption using forest macro-fungus, fomes fasciatus. Int. Res. J. Plant Sci. 2010. Available online: http://interesjournals.org/irjps/october-2010-vol-1-issue-4/a-diffusion-chemisorption-kinetic-model-for-simulating-biosorption-using-forest-macrofungus-fomes-fasciatus (accessed on 9 August 2021).
- Wang, H.; Masters, S.; Hong, Y.; Stallings, J.; Falkinham, J.O.; Edwards, M.A.; Pruden, A. Effect of Disinfectant, Water Age, and Pipe Material on Occurrence and Persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and Two Amoebas. Environ. Sci. Technol. 2012, 46, 11566–11574. [Google Scholar] [CrossRef] [PubMed]
- Cook, F.C.; Hartz, K.E. Adsorption of chlorinated methanes from aqueous solution on selected plastic adsorbents. J. Am. Water Work. Assoc. 1983, 75, 423–426. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Hüffer, T.; Weniger, A.K.; Hofmann, T. Sorption of organic compounds by aged polystyrene microplastic particles. Environ. Pollut. 2018, 18, 218–225. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.; Zhang, H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018, 237, 460–467. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Y.; Liu, R.; Yang, Y.; Liu, Y.; Xing, B. Aging characteristics of degradable and non-biodegradable microplastics and their adsorption mechanism for sulfonamides. Sci. Total Environ. 2023, 901, 166452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hao, C.; Zhang, M.; Lan, B. Enhanced Adsorption of Bromoform onto Microplastic Polyethylene Terephthalate Exposed to Ozonation and Chlorination. Molecules 2023, 28, 259. [Google Scholar] [CrossRef] [PubMed]
- Limousin, J.P.G. Gaudet, L. Charlet, S. Szenknect, V. Barthès, M. Krimissa, Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007; 22, 249–275. [Google Scholar] [CrossRef]
- Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document—Trihalomethanes; Health Canada: Ottawa, Canada, 2009; Available online: https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/healthy-living-vie-saine/water-trihalomethanes-eau/alt/water-trihalomethanes-eau-eng.pdf (accessed on 4 July 2021).


| THM Compound | Pseudo First Order (Non-Linear) | Pseudo Second Order (Non-Linear) | ||||
|---|---|---|---|---|---|---|
| qe (µg g−1) | K1 (day−1) | R2 | qe (µg g−1) | K2 (g(µg day)−1) | R2 | |
| TCM | 9.0 ± 0.5 | 0.076 ± 0.004 | 0.845 | 9.0 ± 0.4 | 0.082 ± 0.008 | 0.910 |
| BDCM | 13.6 ± 0.6 | 0.029 ± 0.002 | 0.947 | 13.6 ± 0.6 | 0.031 ± 0.003 | 0.971 |
| CDBM | 16.5 ± 0.5 | 0.030 ± 0.001 | 0.945 | 16.4 ± 0.5 | 0.032 ± 0.001 | 0.976 |
| TBM | 19.3 ± 0.3 | 0.021 ± 0.001 | 0.963 | 19.2 ± 0.3 | 0.022 ± 0.000 | 0.984 |
| Isotherm Model | THM Compound | Parameters | ||
|---|---|---|---|---|
| K (L g−1) | Intercept | R2 | ||
| Linear isotherm model (Henry) | TCM | 0.30 | −5.85 | 0.618 |
| BDCM | 0.35 | −1.92 | 0.899 | |
| CDBM | 0.40 | 1.40 | 0.974 | |
| TBM | 0.36 | 4.39 | 0.993 | |
| KL | qmax (µg g−1) | R2 | ||
| Langmuir (nonlinear form) | TCM | 0.00012 | 1766 | 0.553 |
| BDCM | 0.00007 | 4798 | 0.889 | |
| CDBM | 0.00060 | 730 | 0.970 | |
| TBM | 0.00558 | 108 | 0.990 | |
| KF | 1/n | R2 | ||
| Freundlich (nonlinear form) | TCM | 0.01 | 1.86 | 0.669 |
| BDCM | 0.13 | 1.21 | 0.910 | |
| CDBM | 0.52 | 0.95 | 0.971 | |
| TBM | 1.11 | 0.78 | 0.994 | |
| K′ | 1/n′ | R2 | ||
| Modified Freundlich (nonlinear form) | TCM | 1.91 | 0.36 | 0.904 |
| BDCM | 4.17 | 0.29 | 0.990 | |
| CDBM | 6.06 | 0.26 | 0.987 | |
| TBM | 7.51 | 0.23 | 0.963 | |
| THM Compound | PVC | K′ | 1/n′ | R2 |
|---|---|---|---|---|
| TCM | Virgin | 4.326 | 0.287 | 0.989 |
| Weathered | 3.710 | 0.253 | 0.991 | |
| BDCM | Virgin | 5.693 | 0.332 | 0.992 |
| Weathered | 5.116 | 0.308 | 0.995 | |
| CDBM | Virgin | 7.059 | 0.356 | 0.994 |
| Weathered | 6.244 | 0.353 | 0.996 | |
| TBM | Virgin | 7.823 | 0.355 | 0.996 |
| Weathered | 7.082 | 0.363 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Neema, P.; Andrews, S. Adsorption Behavior and Mechanisms of Trihalomethanes onto Virgin and Weathered Polyvinyl Chloride Microplastics. Toxics 2024, 12, 450. https://doi.org/10.3390/toxics12070450
Li Y, Neema P, Andrews S. Adsorption Behavior and Mechanisms of Trihalomethanes onto Virgin and Weathered Polyvinyl Chloride Microplastics. Toxics. 2024; 12(7):450. https://doi.org/10.3390/toxics12070450
Chicago/Turabian StyleLi, Yi, Paragi Neema, and Susan Andrews. 2024. "Adsorption Behavior and Mechanisms of Trihalomethanes onto Virgin and Weathered Polyvinyl Chloride Microplastics" Toxics 12, no. 7: 450. https://doi.org/10.3390/toxics12070450






