Tris (2-chloroisopropyl) phosphate and Tris (nonylphenyl) phosphite Promote Human Renal Cell Apoptosis through the ERK/CEPBA/Long Non-Coding RNA Cytoskeleton Regulator Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability Assay
2.3. Quantitative Real-Time PCR (qRT-PCR)
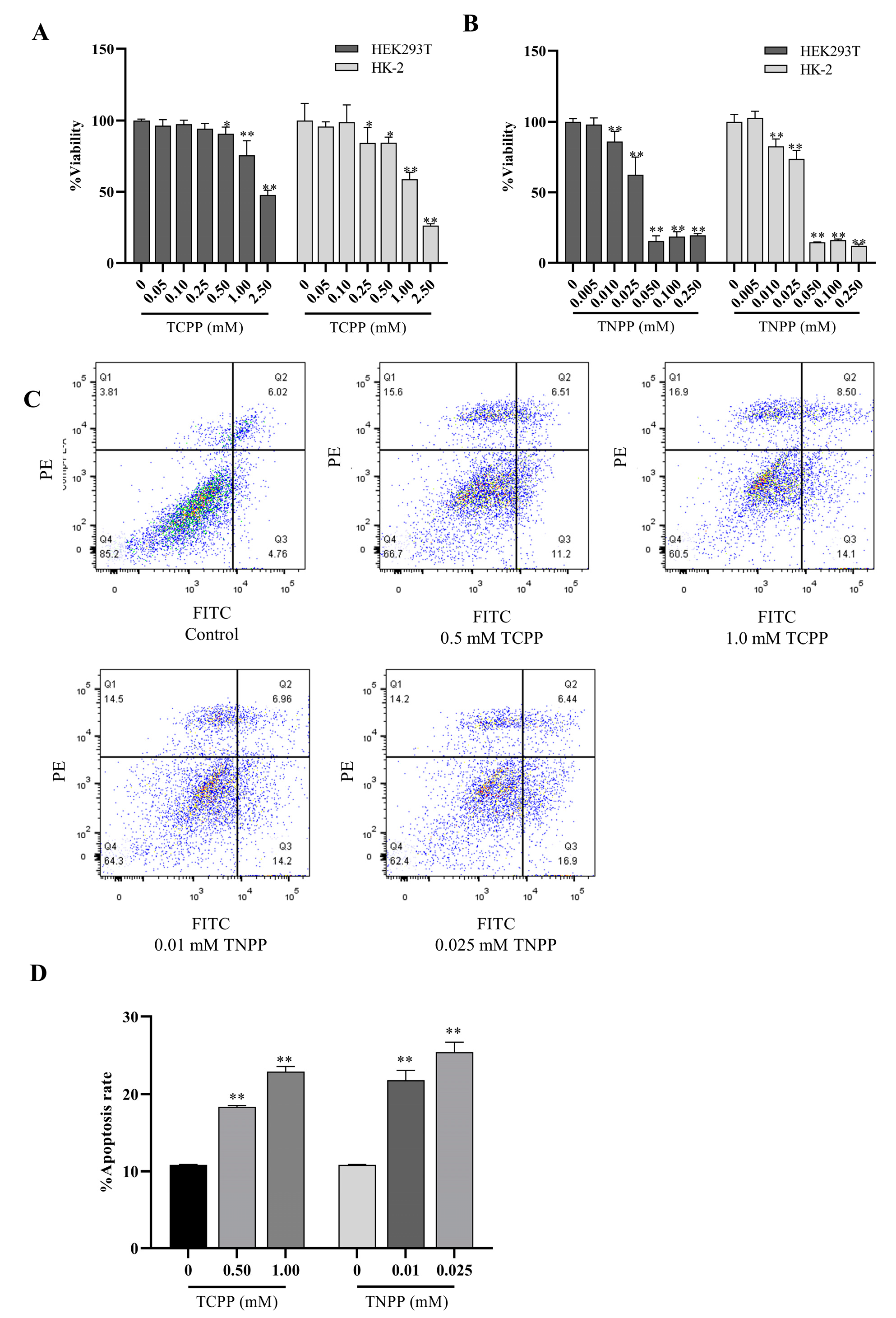
2.4. Apoptosis Detection
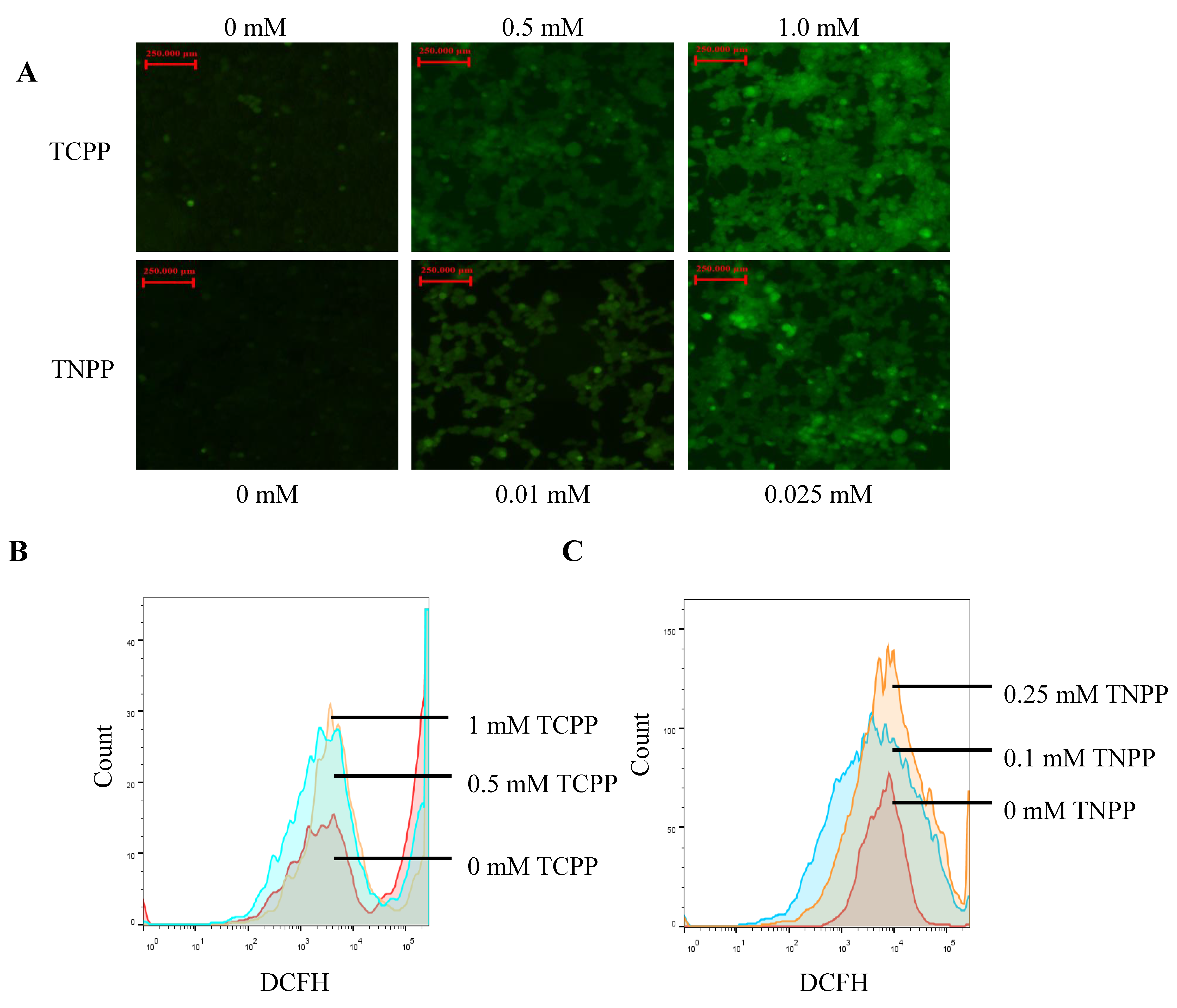
2.5. Reactive Oxygen Species (ROS) Measurement
2.6. Transcription Factor Prediction
2.7. siRNA Interference Assay
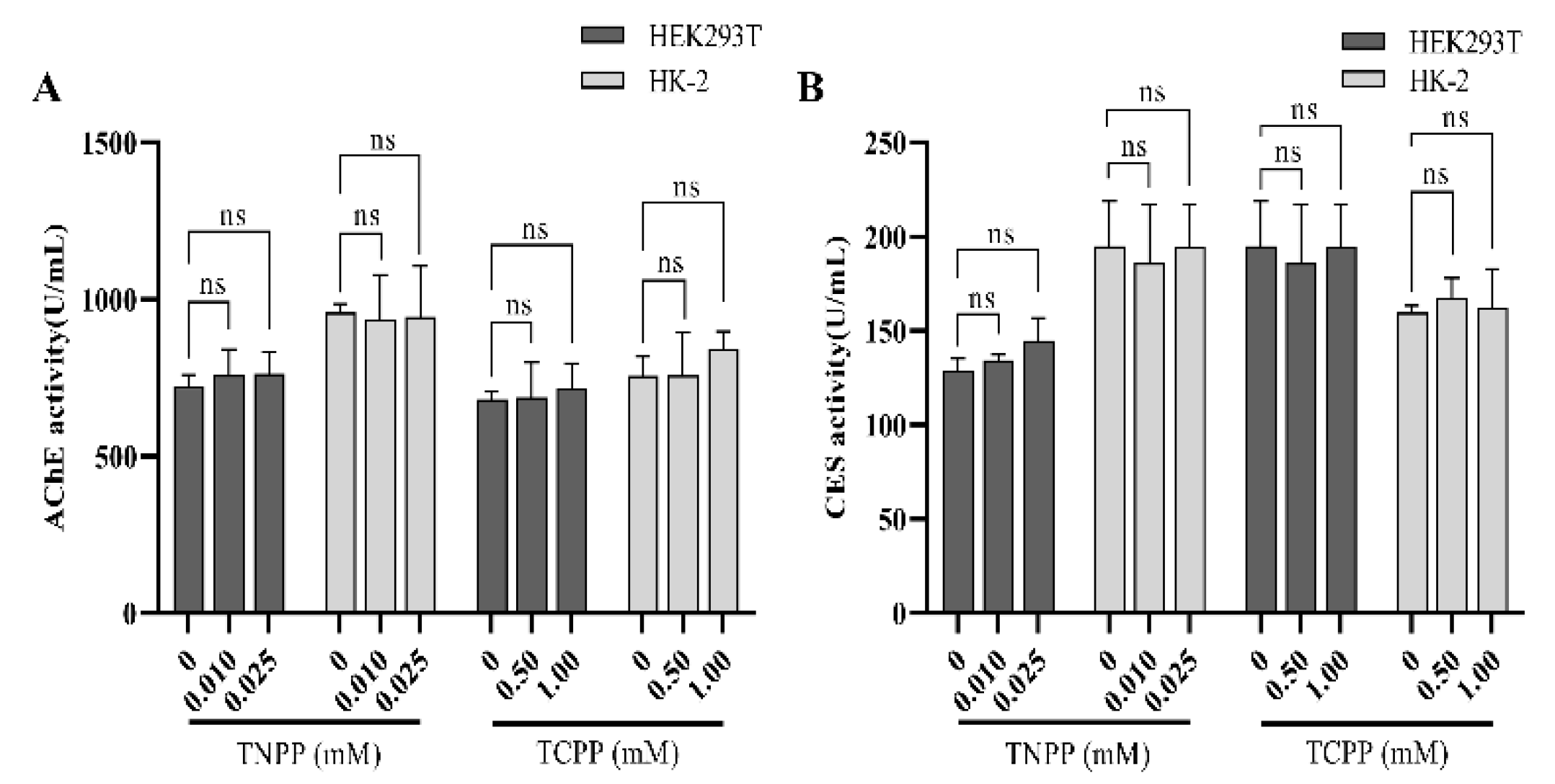
2.8. Acetylcholinesterase (AChE) and Carboxylesterase (CES) Activity
2.9. Dual Luciferase Assay
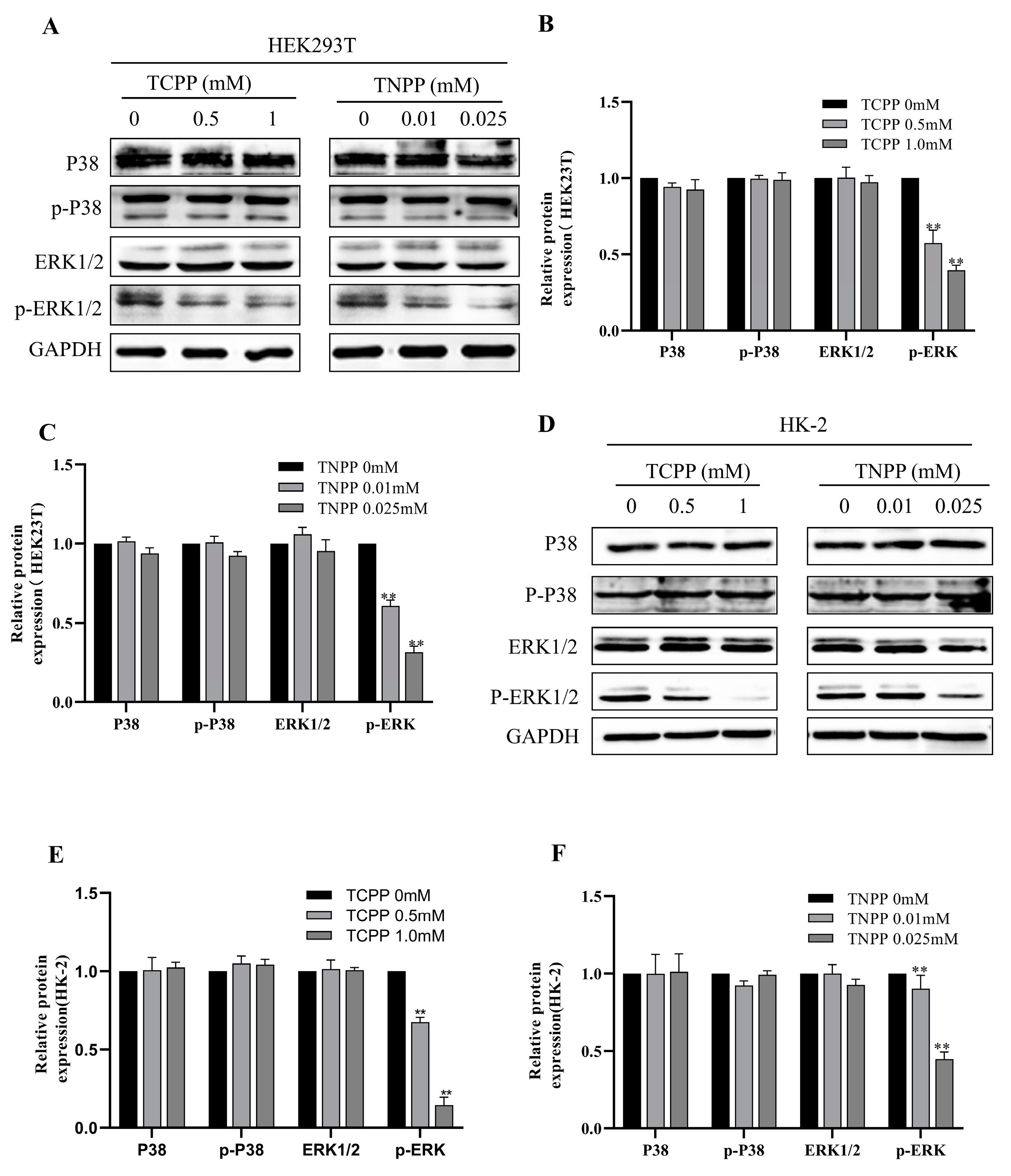
2.10. Western Blot
2.11. Statistical Analysis
3. Results
3.1. TCPP and TNPP Inhibited the Viability of HEK293T and HK-2 by Inducing Apoptosis
3.2. TCPP and TNPP Downregulated CYTOR and Overexpressed the Apoptosis of CYTOR-Attenuated Cells
3.3. TCPP and TNPP Increased the Cellular ROS Levels
3.4. TCPP and TNPP Had No Effect on Cellular Phosphotransferase Activity
3.5. CEBPA Is One of the CYTOR Transcription Factors
3.6. TCPP and TNPP Inhabited the Phosphorylation of ERK1/2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Gao, L.; Li, W.; Wang, Y.; Liu, J.; Cai, Y. Occurrence, distribution and seasonal variation of organophosphate flame retardants and plasticizers in urban surface water in Beijing, China. Environ. Pollut. 2016, 209, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wen, J.; Zeng, F.; Li, S.; Zhou, X.; Zeng, Z. Occurrence and distribution of organophosphate esters in urban soils of the subtropical city, Guangzhou, China. Chemosphere 2017, 175, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.G.; Zanini, N.C.; de Souza, A.G.; Barbosa, R.F.; Rosa, D.S.; Mulinari, D.R. Halogen-Based Flame Retardants in Polyurethanes. In Materials and Chemistry of Flame-Retardant Polyurethanes Volume 1: A Fundamental Approach; ACS Publications: Washington, DC, USA, 2021; pp. 141–171. [Google Scholar]
- Ülkü, G.; Köken, N.; Akar, A.; Kızılcan, N.; Yaman, D. Tris (1-chloro-2-propyl) phosphate (TCPP) microcapsules for the preparation of flame-retardant rigid polyurethane foam. Polym.-Plast. Technol. Mater. 2021, 60, 562–570. [Google Scholar] [CrossRef]
- Li, W.; Shi, Y.; Gao, L.; Wu, C.; Liu, J.; Cai, Y. Occurrence, distribution and risk of organophosphate esters in urban road dust in Beijing, China. Environ. Pollut. 2018, 241, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, F.; Liu, M. Research progress of liquid phosphite antioxidants. Plast. Addit. 2020, 1–6, 29. [Google Scholar]
- Liu, R.; Mabury, S.A. Organophosphite Antioxidants in Indoor Dust Represent an Indirect Source of Organophosphate Esters. Environ. Sci. Technol. 2019, 53, 1805–1811. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Zhang, S.; Guo, M.; Chen, M.; Shi, L. Research progress on toxicity effects of organophosphate flame retardants. J. Ecotoxicol. 2018, 13, 19–30. [Google Scholar]
- Mottier, P.; Frank, N.; Dubois, M.; Tarres, A.; Bessaire, T.; Romero, R.; Delatour, T. LC-MS/MS analytical procedure to quantify tris (nonylphenyl) phosphite, as a source of the endocrine disruptors 4-nonylphenols, in food packaging materials. Food Addit. Contam. Part A 2014, 31, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shao, C.; Xu, M.; Ji, J.; Xie, Y.; Lei, Y.; Wang, X. Macrophage infiltration promotes invasiveness of breast cancer cells via activating long non-coding RNA UCA1. Int. J. Clin. Exp. Pathol. 2015, 8, 9052–9061. [Google Scholar]
- Mayama, T.; Marr, A.K.; Kino, T. Differential Expression of Glucocorticoid Receptor Noncoding RNA Repressor Gas5 in Autoimmune and Inflammatory Diseases. Horm. Metab. Res. 2016, 48, 550–557. [Google Scholar] [CrossRef]
- Bhan, A.; Hussain, I.; Ansari, K.I.; Bobzean, S.A.; Perrotti, L.I.; Mandal, S.S. Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo. J. Steroid Biochem. Mol. Biol. 2014, 141, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Manevski, M.; Devadoss, D.; Long, C.; Singh, S.P.; Nasser, M.W.; Borchert, G.M.; Nair, M.N.; Rahman, I.; Sopori, M.; Chand, H.S. Increased expression of LASI lncRNA regulates the cigarette smoke and COPD associated airway inflammation and mucous cell hyperplasia. Front. Immunol. 2022, 13, 803362. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Mao, Z.-d.; Shi, Y.-j.; Liu, Z.-g.; Cao, Q.; Zhang, Q. Comprehensive analysis of miRNA-mRNA-lncRNA networks in non-smoking and smoking patients with chronic obstructive pulmonary disease. Cell. Physiol. Biochem. 2018, 50, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Zhang, H.; Wang, L.; Jie, S.; Zhao, Q.; Chen, F.; Yue, Y.; Wang, H.; Tian, L.; Xie, J. Long-term cadmium exposure impairs cognitive function by activating lnc-Gm10532/m6A/FIS1 axis-mediated mitochondrial fission and dysfunction. Sci. Total Environ. 2023, 858, 159950. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, L.; Liu, J.; Zhang, Z.; Wang, R.; Zhang, Q.; Li, H.; Xiang, J.; Fang, L.; Xu, P.; et al. LINC00152 induced by TGF-β promotes metastasis via HuR in lung adenocarcinoma. Cell Death Dis. 2022, 13, 772. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, Y.; Shi, M.H.; Wang, J.F.; Ran, X.Q. miR-96-5p attenuates malathion-induced apoptosis of human kidney cells by targeting the ER stress marker DDIT3. J. Environ. Sci. Health Part B 2020, 55, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, L.; Korabecny, J.; Musilek, K.; Nepovimova, E.; Malinak, D.; Kucera, T.; Dolezal, R.; Jun, D.; Soukup, O.; Kuca, K. Progress in acetylcholinesterase reactivators and in the treatment of organophosphorus intoxication: A patent review (2006–2016). Expert Opin. Ther. Pat. 2017, 27, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; McManus, M.; Kumar, N.; Awoyemi, O.; Crago, J. Comparative analyses of the neurobehavioral, molecular, and enzymatic effects of organophosphates on embryo-larval zebrafish (Danio rerio). Neurotoxicology Teratol. 2019, 73, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Belaid, A.; Methneni, N.; Nasri, E.; Bchir, S.; Anthonissen, R.; Verschaeve, L.; Le Tilly, V.; Lo Turco, V.; Di Bella, G.; Ben Mansour, H. Endocrine disruption, cytotoxicity and genotoxicity of an organophosphorus insecticide. Sustainability 2021, 13, 11512. [Google Scholar] [CrossRef]
- Bai, W.; Yang, J.; Yang, G.; Niu, P.; Tian, L.; Gao, A. Long non-coding RNA NR_045623 and NR_028291 involved in benzene hematotoxicity in occupationally benzene-exposed workers. Exp. Mol. Pathol. 2014, 96, 354–360. [Google Scholar] [CrossRef]
- Tani, H.; Takeshita, J.; Aoki, H.; Abe, R.; Toyoda, A.; Endo, Y.; Miyamoto, S.; Gamo, M.; Torimura, M. Genome-wide gene expression analysis of mouse embryonic stem cells exposed to p-dichlorobenzene. J. Biosci. Bioeng. 2016, 122, 329–333. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Vlastos, D.; Dormousoglou, M.; Bouras, S.; Varela-Athanasatou, M.; Bekakou, I.-E. Genotoxic and Toxic Effects of the Flame Retardant Tris(Chloropropyl) Phosphate (TCPP) in Human Lymphocytes, Microalgae and Bacteria. Toxics 2022, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, K.; Li, X.; Li, X.; Chu, H.; Zhang, Q. Purity Assessment of Tripropyl Phosphate through Mass Balance and (1)H and (31)P Quantitative Nuclear Magnetic Resonance. Molecules 2024, 29, 1975. [Google Scholar] [CrossRef]
- Van Emon, J.M.; Pan, P.; van Breukelen, F. Effects of chlorpyrifos and trichloropyridinol on HEK 293 human embryonic kidney cells. Chemosphere 2018, 191, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Saquib, Q.; Siddiqui, M.; Al-Khedhairy, A. Organophosphorus flame-retardant tris(1-chloro-2-propyl)phosphate is genotoxic and apoptotic inducer in human umbilical vein endothelial cells. J. Appl. Toxicol. 2021, 41, 861–873. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Ji, C.; Wu, H.; Zhao, J.; Tang, J. Toxicological effects of tris (2-chloropropyl) phosphate in human hepatic cells. Chemosphere 2017, 187, 88–96. [Google Scholar] [CrossRef]
- Vercesi, A.E.; Kowaltowski, A.J.; Grijalba, M.T.; Meinicke, A.R.; Castilho, R.F. The role of reactive oxygen species in mitochondrial permeability transition. Biosci. Rep. 1997, 17, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhong, Y.; Yu, Z.; Shang, Y.; An, J. Triphenyl phosphate (TPP) and tris (2-chloroisopropyl) phosphate (TCPP) induced apoptosis and cell cycle arrest in HepG2 cells. SDRP J. Earth Sci. Environ. Stud. 2018, 4, 490–501. [Google Scholar]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Espinoza, M.; Rivero Osimani, V.; Sánchez, V.; Rosenbaum, E.; Guiñazú, N. B-esterase determination and organophosphate insecticide inhibitory effects in JEG-3 trophoblasts. Toxicol. Vitr. 2016, 32, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.J.; Medina-Cleghorn, D.; Heslin, A.; King, S.M.; Orr, J.; Mulvihill, M.M.; Krauss, R.M.; Nomura, D.K. Organophosphorus flame retardants inhibit specific liver carboxylesterases and cause serum hypertriglyceridemia. ACS Chem. Biol. 2014, 9, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Moser, V.C.; Phillips, P.M.; Hedge, J.M.; McDaniel, K.L. Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and tris(2-chloro-2-ethyl)phosphate (TCEP). Neurotoxicol Teratol. 2015, 52, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhu, C.; Ye, C. LncRNA CYTOR attenuates sepsis-induced myocardial injury via regulating miR-24/XIAP. Cell Biochem. Funct. 2020, 38, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Wang, Z.-Q.; Wang, S.-H.; Li, C.; Zhu, Z.-G.; Quan, Z.-W.; Zhang, W.-J. Upregulation of long non-coding RNA LINC00152 by SP1 contributes to gallbladder cancer cell growth and tumor metastasis via PI3K/AKT pathway. Am. J. Transl. Res. 2016, 8, 4068. [Google Scholar] [PubMed]
- Ou, C.; Sun, Z.; He, X.; Li, X.; Fan, S.; Zheng, X.; Peng, Q.; Li, G.; Li, X.; Ma, J. Targeting YAP1/LINC00152/FSCN1 signaling axis prevents the progression of colorectal cancer. Adv. Sci. 2020, 7, 1901380. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhong, J.; Yu, P.; Zhao, Q.; Huang, T. YY1-regulated LINC00152 promotes triple negative breast cancer progression by affecting on stability of PTEN protein. Biochem. Biophys. Res. Commun. 2019, 509, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, J.; Yoshida, Y.; Kominato, Y.; Auron, P.E. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 2011, 54, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Mehrpour, O.; Buhrmann, C.; Pourbagher-Shahri, A.M.; Shakibaei, M.; Samarghandian, S. Organophosphorus Compounds and MAPK Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 4258. [Google Scholar] [CrossRef]
- Hu, J.; Roy, S.K.; Shapiro, P.S.; Rodig, S.R.; Reddy, S.P.; Platanias, L.C.; Schreiber, R.D.; Kalvakolanu, D.V. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma. J. Biol. Chem. 2001, 276, 287–297. [Google Scholar] [CrossRef]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Yan, Y.; Gao, Y.; Wang, J.; Li, S. Tris (2-chloroisopropyl) phosphate and Tris (nonylphenyl) phosphite Promote Human Renal Cell Apoptosis through the ERK/CEPBA/Long Non-Coding RNA Cytoskeleton Regulator Axis. Toxics 2024, 12, 452. https://doi.org/10.3390/toxics12070452
Zhao H, Yan Y, Gao Y, Wang J, Li S. Tris (2-chloroisopropyl) phosphate and Tris (nonylphenyl) phosphite Promote Human Renal Cell Apoptosis through the ERK/CEPBA/Long Non-Coding RNA Cytoskeleton Regulator Axis. Toxics. 2024; 12(7):452. https://doi.org/10.3390/toxics12070452
Chicago/Turabian StyleZhao, Hu, Yi Yan, Yanyan Gao, Jiafu Wang, and Sheng Li. 2024. "Tris (2-chloroisopropyl) phosphate and Tris (nonylphenyl) phosphite Promote Human Renal Cell Apoptosis through the ERK/CEPBA/Long Non-Coding RNA Cytoskeleton Regulator Axis" Toxics 12, no. 7: 452. https://doi.org/10.3390/toxics12070452






