Comparative Analysis of Therapeutic Efficacy and Adverse Reactions among Various Thrombolytic Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Thrombus Preparation
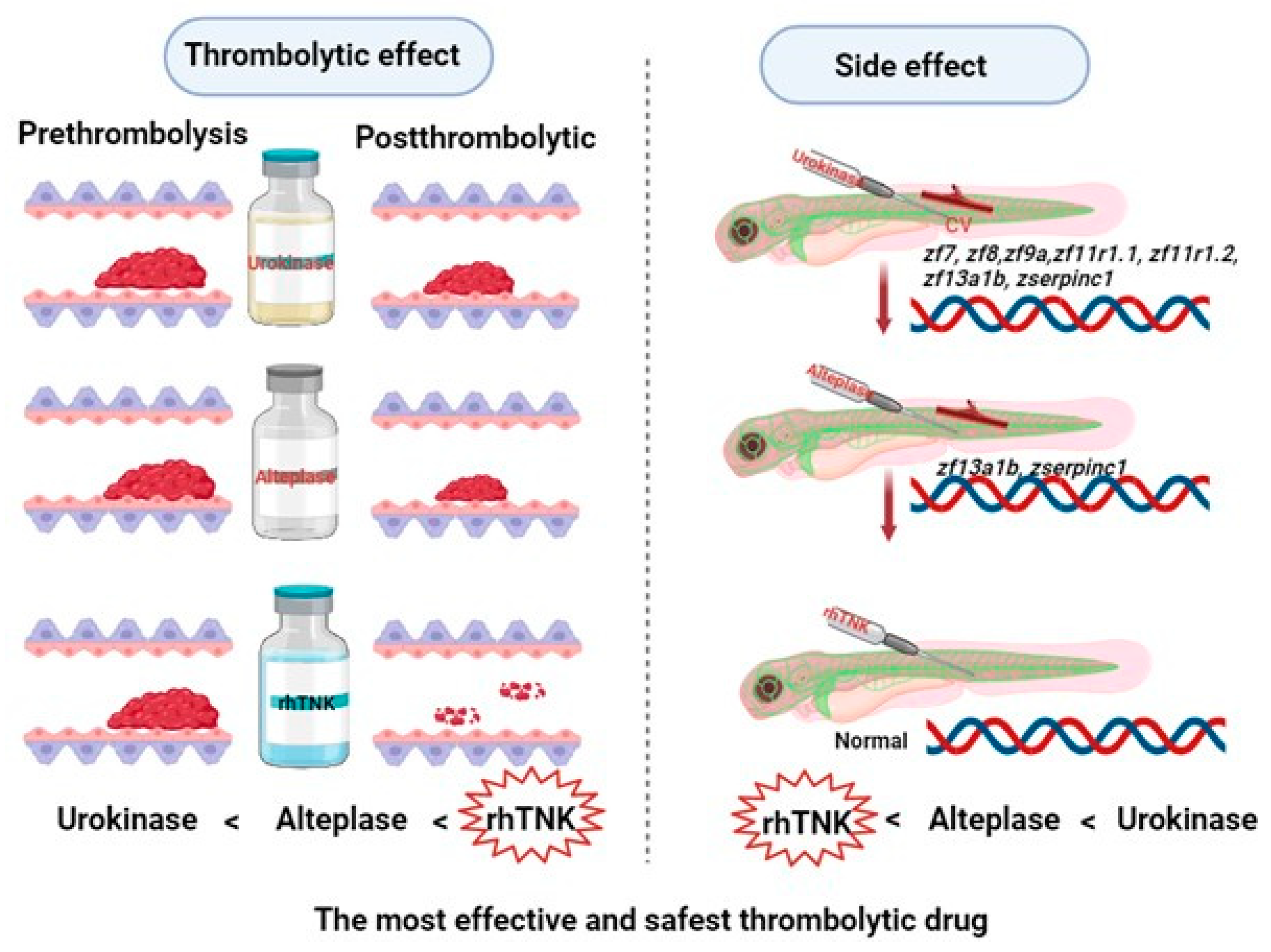
2.2. Extracorporeal Thrombolysis
2.3. Cell Culture
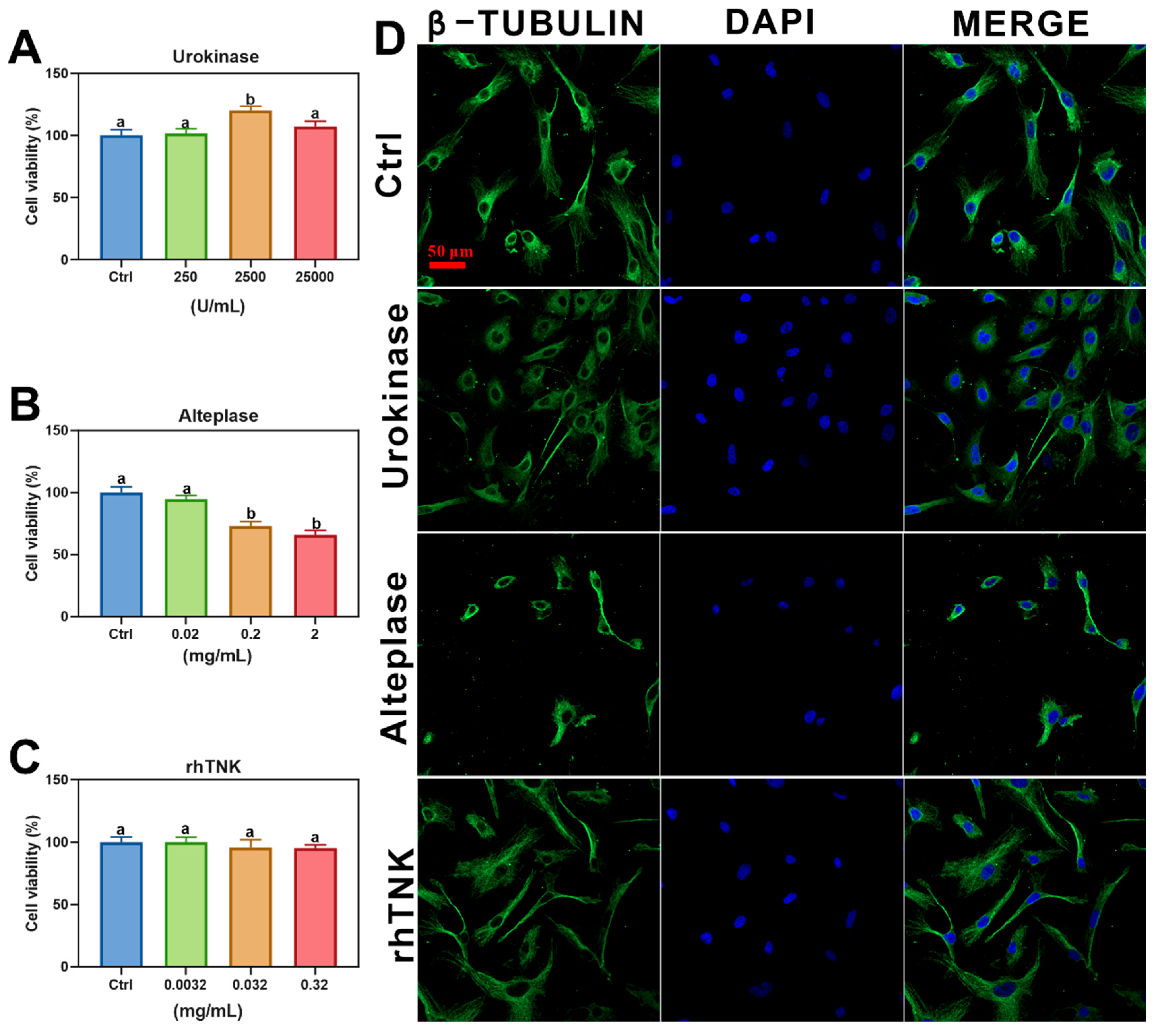
2.4. Cell Viability
2.5. Immunofluorescence
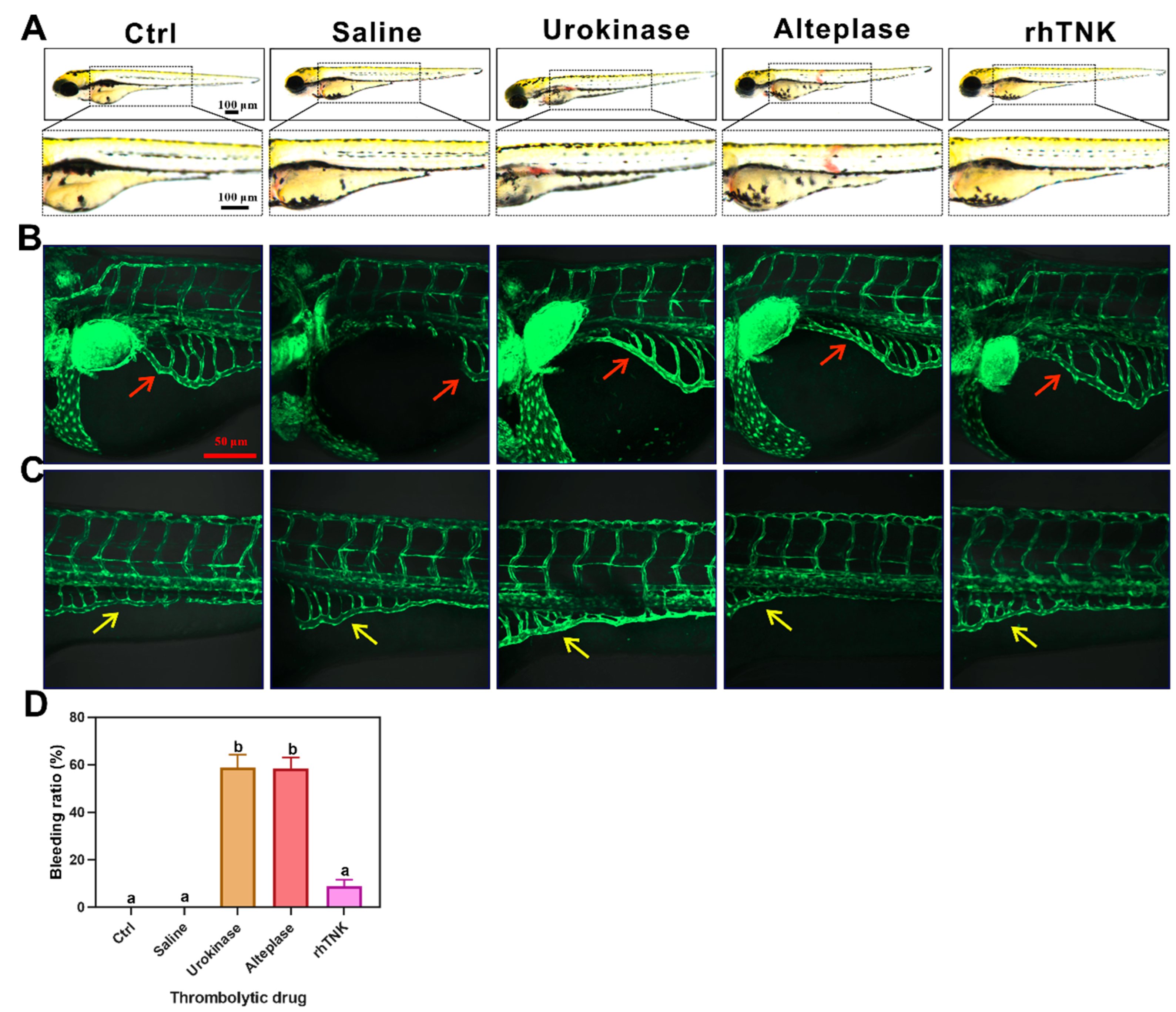
2.6. Zebrafish Culture
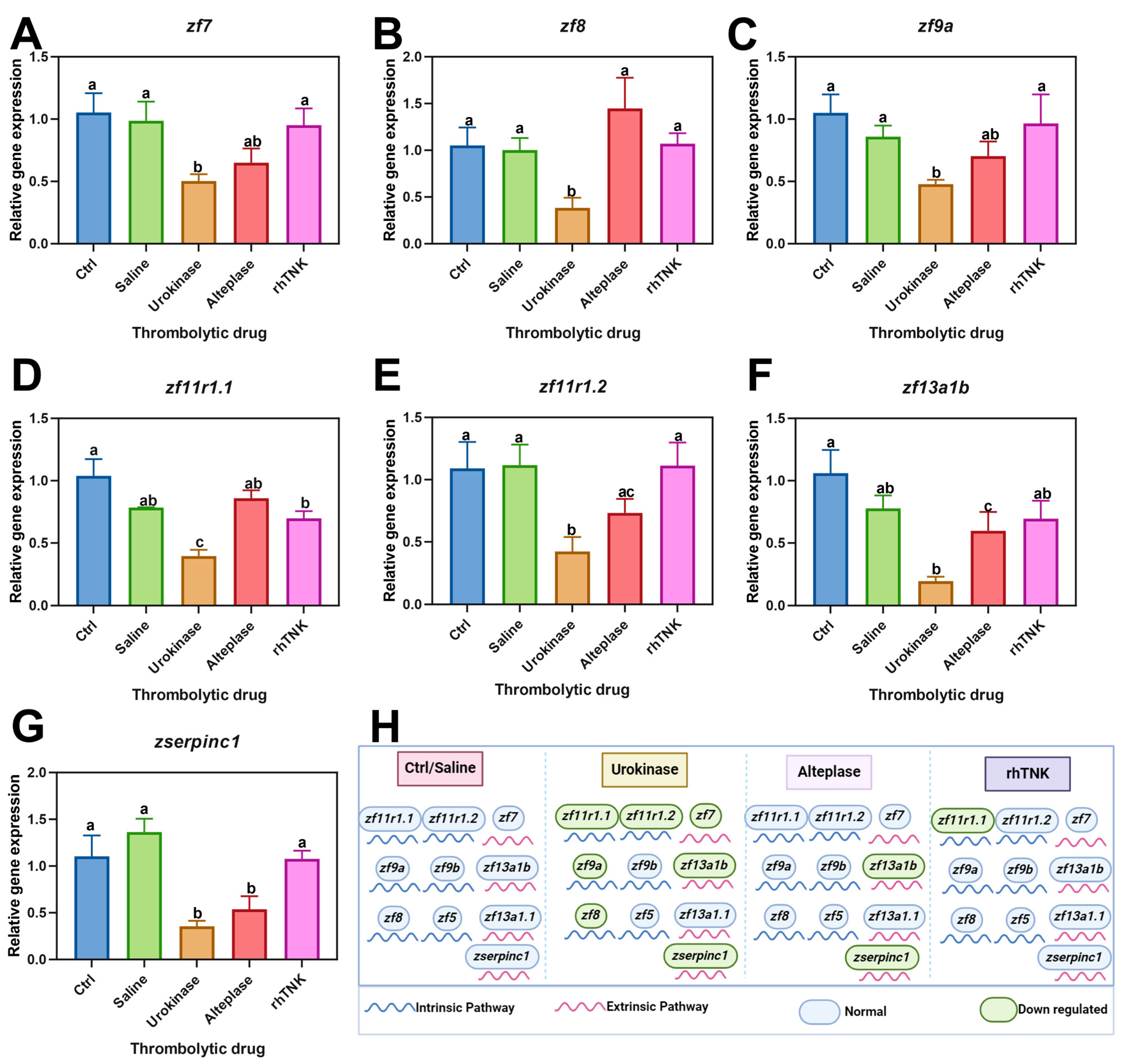
2.7. Real-Time Quantitative PCR (qPCR)
3. Results
3.1. In Vitro Thrombus Preparation
3.2. Comparison of the Effects of Different Thrombolytic Drugs on 24-Hour and 48-Hour Thrombi
3.3. Effects of Different Thrombolytic Drugs on AC16 Cells
3.4. Effects of the Three Thrombolytic Drugs on the Blood Vessels of Zebrafish
3.5. Impact of Three Thrombolytic Drug Injections on the Gene Expression Levels Associated with the Coagulation Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dauerman, H.L.; Ibanez, B. The Edge of Time in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Mechanic, O.J.; Gavin, M.; Grossman, S.A. Acute Myocardial Infarction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rehman, S.; Rehman, E.; Ikram, M.; Jianglin, Z. Cardiovascular disease (CVD): Assessment, prediction and policy implications. BMC Public Health 2021, 21, 1382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiological Features of Cardiovascular Disease in Asia. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Access Investigators. Management of acute coronary syndromes in developing countries: Acute Coronary Events—A multinational Survey of current management Strategies. Am. Heart J. 2011, 162, 852–859.e22. [Google Scholar] [CrossRef] [PubMed]
- Rosselló, X.; Huo, Y.; Pocock, S.; Van de Werf, F.; Chin, C.T.; Danchin, N.; Lee, S.W.; Medina, J.; Vega, A.; Bueno, H. Global geographical variations in ST-segment elevation myocardial infarction management and post-discharge mortality. Int. J. Cardiol. 2017, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Patel, A.; Li, X.; Du, X.; Wu, T.; Zhao, Y.; Feng, L.; Billot, L.; Peterson, E.D.; et al. CPACS-3 Investigators. Effect of a Quality of Care Improvement Initiative in Patients with Acute Coronary Syndrome in Resource-Constrained Hospitals in China: A Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Boersma, E.; Maas, A.C.; Deckers, J.W.; Simoons, M.L. Early thrombolytic treatment in acute myocardial infarction: Reappraisal of the golden hour. Lancet 1996, 348, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Alsomali, M.S.; Alateeq, M.A.; Abuzaid, S.E.; AlTurki, A.M.; Alnahdi, Y.A.; Koshan, M.A.; Alsamih, A.M.A.; Alsaif, A.M.; Alharbi, S.; Alharbi, M.A.S.; et al. Prehospital Fibrinolysis Therapy in Acute Myocardial Infarction: A Narrative Review. Cureus 2024, 16, e52045. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, G.; Natsuaki, M.; Goriki, Y.; Shinzato, K.; Nishihira, K.; Kuriyama, N.; Shimomura, M.; Inoue, Y.; Nishikido, T.; Hongo, H.; et al. Serum Albumin and Bleeding Events after Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction (from the HAGAKURE-ACS Registry). Am. J. Cardiol. 2022, 165, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Heuser, R.R. The Role for Cardiologists in Stroke Intervention. Prog. Cardiovasc. Dis. 2017, 59, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Etzel, D.; Ehlers, R.; Walter, T.; Kazmaier, S.; Helber, U.; Hoffmeister, H.M. Combined thrombolysis with abciximab favourably influences platelet-leukocyte interactions and platelet activation in acute myocardial infarction. J. Thromb. Thrombolysis 2005, 20, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Semba, C.P.; Sugimoto, K.; Razavi, M.K. Society of Cardiovascular and Interventional Radiology (SCVIR). Alteplase and tenecteplase: Applications in the peripheral circulation. Tech. Vasc. Interv. Radiol. 2001, 4, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Chueh, J.; Gounis, M.J.; McCarthy, R.; McGarry, J.P.; McHugh, P.E.; Gilvarry, M. Mechanical behavior of in vitro blood clots and the implications for acute ischemic stroke treatment. J. Neurointerv. Surg. 2020, 12, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Plasenzotti, R.; Spiel, A.; Quehenberger, P.; Jilma, B. Interspecies differences in coagulation profile. Thromb. Haemost. 2008, 100, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.F.; Asai, D.J. Immunofluorescence Microscopy. Curr. Protoc. 2023, 3, e842. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.J.; Yang, Y.C.; Hsu, J.W.; Chang, W.T.; Chuang, Y.J.; Liau, I. Zebrafish model of photochemical thrombosis for translational research and thrombolytic screening in vivo. J. Biophotonics 2017, 10, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Yan, J.; Qian, W.; Song, C.; Zuo, Z.; He, C. Comparison of developmental toxicity of different surface modified CdSe/ZnS QDs in zebrafish embryos. J. Environ. Sci. 2021, 100, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kadir, R.R.A.; Bayraktutan, U. Urokinase Plasminogen Activator: A Potential Thrombolytic Agent for Ischaemic Stroke. Cell Mol. Neurobiol. 2020, 40, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Logallo, N.; Novotny, V.; Assmus, J.; Kvistad, C.E.; Alteheld, L.; Rønning, O.M.; Thommessen, B.; Amthor, K.F.; Ihle-Hansen, H.; Kurz, M.; et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR-TEST): A phase 3, randomised, open-label, blinded endpoint trial. Lancet Neurol. 2017, 16, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Warach, S.J. Evolving Thrombolytics: From Alteplase to Tenecteplase. Neurotherapeutics 2023, 20, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Ji, P.; Zhao, X.S.; Xu, H.; Yan, X.Y.; Yang, Q.; Yao, C.; Gao, R.L.; Wu, Y.F.; Qiao, S.B. Recombinant human TNK tissue-type plasminogen activator (rhTNK-tPA) versus alteplase (rt-PA) as fibrinolytic therapy for acute ST-segment elevation myocardial infarction (China TNK STEMI): Protocol for a randomised, controlled, non-inferiority trial. BMJ Open 2017, 7, e016838. [Google Scholar] [CrossRef] [PubMed]
- Callahan, K.P.; Malinin, A.I.; Gurbel, P.A.; Alexander, J.H.; Granger, C.B.; Serebruany, V.L. Platelet function and fibrinolytic agents: Two sides of a coin? Cardiology 2001, 95, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Roychoudhury, P.K. Production and purification of Urokinase: A comprehensive review. Protein Expr. Purif. 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bivard, A.; Zhao, H.; Churilov, L.; Campbell, B.C.V.; Coote, S.; Yassi, N.; Yan, B.; Valente, M.; Sharobeam, A.; Balabanski, A.H.; et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile Stroke Unit (TASTE-A): A phase 2, randomised, open-label trial. Lancet Neurol. 2022, 21, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Donnan, G.; Nandurkar, H.; Ho, P. Global coagulation assays in hypercoagulable states. J. Thromb. Thrombolysis 2022, 54, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, K.; Aleksic, N.; Hannan, P.J.; Folsom, A.R. ARIC Study Inverstigators. Coagulation factors II, V, IX, X, XI, and XII, plasminogen, and alpha-2 antiplasmin and risk of coronary heart disease. J. Atheroscler. Thromb. 2010, 17, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kang, G.B.; Kang, D.E.; Hong, J.W.; Lee, M.G.; Kim, K.Y.; Han, J.W. A new manufacturing process to remove thrombogenic factors (II, VII, IX, X, and XI) from intravenous immunoglobulin gamma preparations. Biologicals 2016, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chirnside, A.; Urbaniak, S.J.; Prowse, C.V.; Keller, A.J. Coagulation abnormalities following intensive plasma exchange on the cell separator. II. Effects on factors I, II, V, VII, VIII, IX, X and antithrombin III. Br. J. Haematol. 1981, 48, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Mitsiakos, G.; Katsaras, G.N.; Pouliakis, A.; Papadakis, E.; Chatziioannidis, I.; Mitsiakou, C.; Gialamprinou, D.; Papacharalampous, E.; Kioumi, A.; Athanasiou, M.; et al. Neonatal haemostatic parameters in correlation to gestational age and birth weight. Int. J. Lab. Hematol. 2022, 44, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Bergmeier, W.; Stouffer, G.A.; Weitz, J.I. Therapeutic strategies for thrombosis: New targets and approaches. Nat. Rev. Drug Discov. 2020, 19, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Jha, V.; Min, J.K.; Cho, J. Protein disulfide isomerase in cardiovascular disease. Exp. Mol. Med. 2020, 52, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A.S. Thrombus Structural Composition in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Ariëns, R.A. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef]
- Li, T.; Yuan, D.; Yuan, J. Antithrombotic Drugs-Pharmacology and Perspectives. Adv. Exp. Med. Biol. 2020, 1177, 101–131. [Google Scholar] [PubMed]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef]
- Sawaguchi, Y.; Yamamoto, H.; Itou, S.; Tachibana, K.; Ohnuma, K.; Kamada, Y.; Nakajima, T. Proposal of an in vitro thrombus-growth model for evaluating anticoagulants. Drug Discov. Ther. 2022, 16, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, G.P.; Chokshi, A.; Rausch, M.K. Preparation and Mounting of Whole Blood Clot Samples for Mechanical Testing. Curr. Protoc. 2021, 1, e197. [Google Scholar] [CrossRef] [PubMed]
- Keyt, B.A.; Paoni, N.F.; Refino, C.J.; Berleau, L.; Nguyen, H.; Chow, A.; Lai, J.; Peña, L.; Pater, C.; Ogez, J.; et al. A faster-acting and more potent form of tissue plasminogen activator. Proc. Natl. Acad. Sci. USA 1994, 91, 3670–3674. [Google Scholar] [CrossRef] [PubMed]
- Sugioka, K.; Nishida, T.; Kodama-Takahashi, A.; Murakami, J.; Fukuda, M.; Matsuo, O.; Kusaka, S. Urokinase-type plasminogen activator promotes corneal epithelial migration and nerve regeneration. Exp. Eye Res. 2023, 233, 109559. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Wang, Y.B.; Ren, X.Y.; Wang, J.; Wang, H.S.; Jin, Y.H. Comparative Efficacy and Safety of Thrombolytic Agents for Pulmonary Embolism: A Bayesian Network Meta-Analysis. Pharmacology 2023, 108, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Schierling, W.; Bachleitner, K.; Kasprzak, P.; Betz, T.; Stehr, A.; Pfister, K. Safety aspect of intraoperative, local urokinase lysis in patients with acute lower limb ischemia. Clin. Hemorheol. Microcirc. 2021, 78, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hanaway, J.; Torack, R.; Fletcher, A.P.; Landau, W.M. Intracranial bleeding associated with Urokinase therapy for acute ischemic hemispheral stroke. Stroke 1976, 7, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Doelare, S.A.N.; Oukrich, S.; Ergin, K.; Jongkind, V.; Wiersema, A.M.; Lely, R.J.; Ebben, H.P.; Yeung, K.K.; Hoksbergen, A.W.J. Collaborators. Major Bleeding during Thrombolytic Therapy for Acute Lower Limb Ischaemia: Value of Laboratory Tests for Clinical Decision Making, 17 Years of Experience. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 398–404. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Zheng, N.; Li, M.; Zhang, Z.; Huang, D.; Xiao, M.; Chen, D.; He, C.; Zuo, Z.; Chen, X. Comparative Analysis of Therapeutic Efficacy and Adverse Reactions among Various Thrombolytic Agents. Toxics 2024, 12, 458. https://doi.org/10.3390/toxics12070458
Xie C, Zheng N, Li M, Zhang Z, Huang D, Xiao M, Chen D, He C, Zuo Z, Chen X. Comparative Analysis of Therapeutic Efficacy and Adverse Reactions among Various Thrombolytic Agents. Toxics. 2024; 12(7):458. https://doi.org/10.3390/toxics12070458
Chicago/Turabian StyleXie, Chenxi, Naying Zheng, Mingmei Li, Zhiyang Zhang, Dongqin Huang, Meizhu Xiao, Dongdong Chen, Chengyong He, Zhenghong Zuo, and Xintan Chen. 2024. "Comparative Analysis of Therapeutic Efficacy and Adverse Reactions among Various Thrombolytic Agents" Toxics 12, no. 7: 458. https://doi.org/10.3390/toxics12070458







