The Effect of Different Thiamethoxam Concentrations on Riptortus pedestris Development and Fecundity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects and Soybean Plants
2.2. Toxicity Bioassay
2.3. Effects of Thiamethoxam on the F1 Generation of R. pedestris
2.4. Life Table Parameters
2.5. Statistical Analyses
3. Results
3.1. Toxicity Bioassay of Thiamethoxam
3.2. Effects of Thiamethoxam on the F1 Generation of R. pedestris
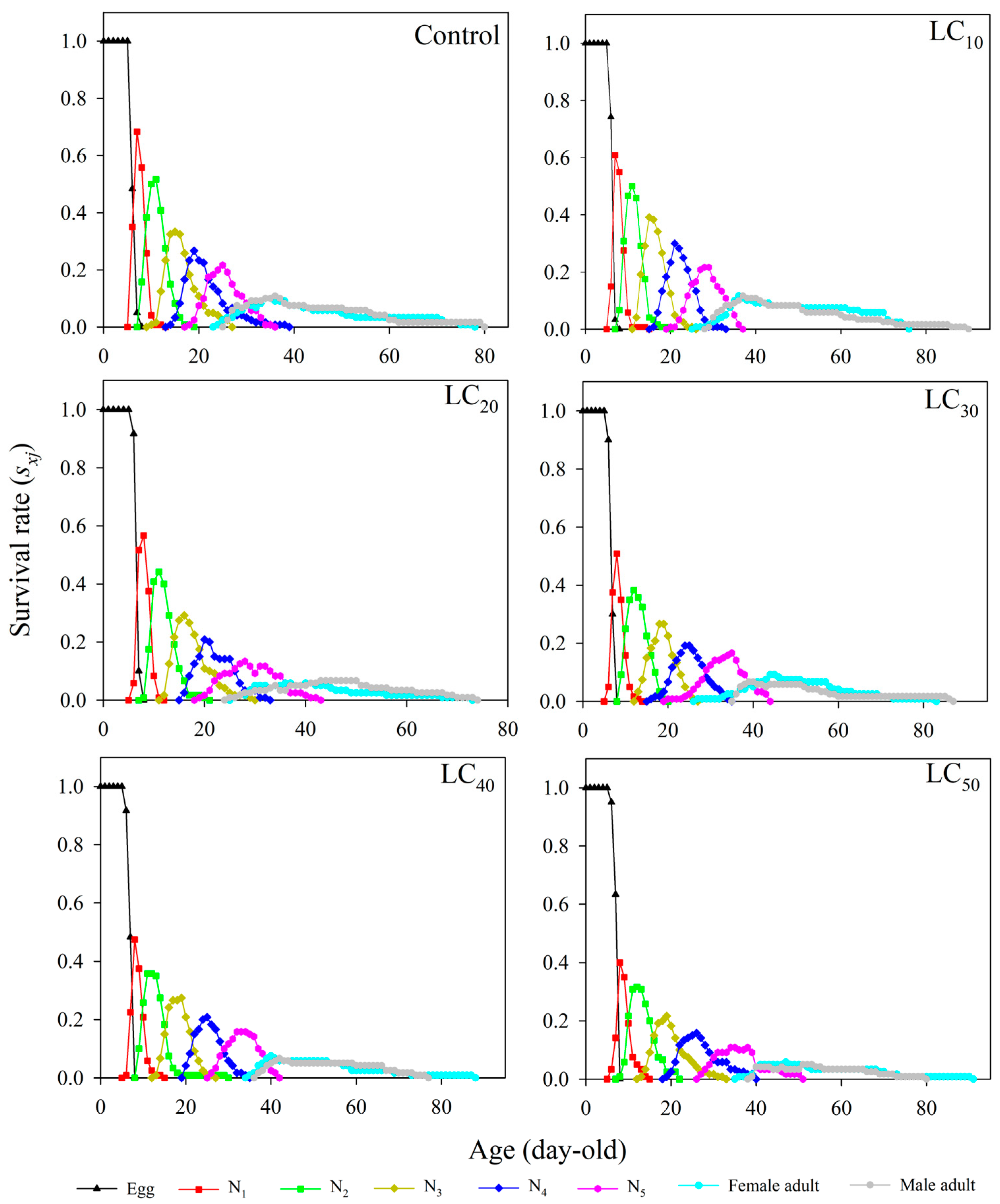
3.3. Age–Stage-Specific Survival Rate (Sxj)
3.4. Age-Specific Survivability and Age–Stage-Specific Fecundity
3.5. Age–Stage-Specific Life Expectancy
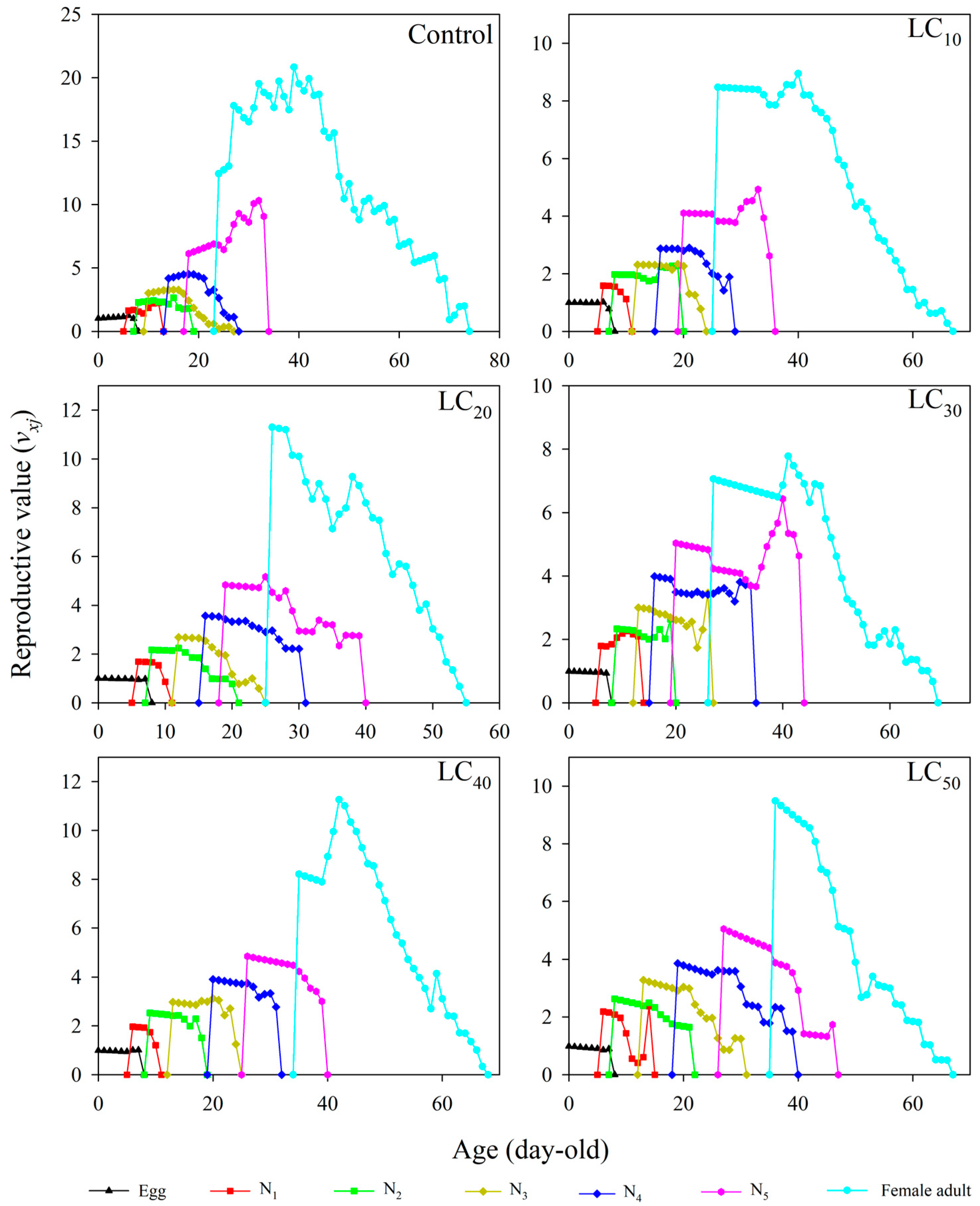
3.6. Age–Stage-Specific Reproductive Values
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyczewska, A.; Woźniak, E.; Gracz, J.; Kuczyński, J.; Twardowski, T. Towards food security: Current state and future prospects of agrobiotechnology. Trends Biotechnol. 2018, 36, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.S.; Maclean, I.M.D.; Gaston, K.J.; Bütikofer, L. Forecasting future crop suitability with microclimate data. Agric. Syst. 2021, 190, 103084. [Google Scholar] [CrossRef]
- Xiong, E.H.; Qu, X.L.; Li, J.F.; Liu, H.L.; Ma, H.; Zhang, D.; Chu, S.S.; Jiao, Y.Q. The soybean ubiquitin-proteasome system: Current knowledge and future perspective. Plant Genome 2023, 16, e20281. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.L.; Zhang, C.J.; Huang, Y.; Chen, H.F.; Zhou, X.; Cao, D. Genome editing technology and application in soybean improvement. Oil Crop Sci. 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, S.S.; Xu, M.L.; Cui, J. Current research on soybean pest management in China. Oil Crop Sci. 2018, 3, 215–227. [Google Scholar] [CrossRef]
- Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Zhou, Q.; Strelkov, S.E.; Conner, R.L.; McLaren, D.L.; Henriquez, M.A.; Harding, M.W.; Turnbull, G.D. First report of Phytophthora sojae causing root rot in soybean [Glycine max (L.) Merr.] in Alberta, Canada. Crop Protect. 2017, 91, 49–56. [Google Scholar] [CrossRef]
- Marchi-Werle, L.; Pereira, R.R.; Reese, J.C.; Reese, J.C.; Heng-Moss, T.M.; Hunt, T.E. Yield response of tolerant and susceptible soybean to the soybean aphid. Agron. J. 2017, 109, 1663–1669. [Google Scholar] [CrossRef]
- Ishimoto, M.; Iwata, D. Effects of the continuous cropping of soybean plants on the occurrence of soybean pod borer, Leguminivora glycinivorella (Lepidoptera: Tortricidae). Jpn. J. App. Entomol. Zool. 2018, 62, 239–247. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.X.; Guo, J.Q.; Penn, H.; Wu, T.T.; Li, L.; Jiang, H.; Chang, L.D.; Wu, C.X.; Han, T.F. Feeding of Riptortus pedestris on soybean plants, the primary cause of soybean staygreen syndrome in the Huang-Huai-Hai river basin. Crop J. 2019, 7, 360–367. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wang, M.; Wu, T.T.; Wu, C.X.; Jiang, B.J.; Guo, C.H.; Han, T.F. Physiological and molecular studies of staygreen caused by pod removal and seed injury in soybean. Crop J. 2016, 4, 435–443. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Guo, W.B.; Jiang, S.S.; Yan, D.K.; Shi, Y.; Wu, B.; Xin, X.Q.; Chen, L.; Cai, Y.P.; Zhang, H.H.; et al. Transcriptional profiling reveals a critical role for GmFT2a in soybean staygreen syndrome caused by the pest Riptortus pedestris. New Phytol. 2022, 237, 1876–1890. [Google Scholar] [CrossRef]
- Li, W.J.; Gao, Y.; Hu, Y.; Chen, J.; Zhang, J.P.; Shi, S.S. Field cage assessment of feeding damage by Riptortus pedestris on soybeans in China. Insects 2021, 12, 255. [Google Scholar] [CrossRef]
- Shan, S.Q.; Huang, Y.; Guo, C.Y.; Hu, B.; Zhang, H.H.; Li, Y.J.; Chen, J.P.; Wei, Z.Y.; Sun, Z.T. A salivary secretory protein from Riptortus pedestris facilitates pest infestation and soybean staygreen syndrome. Mol. Plant Pathol. 2023, 24, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Mei, R.; Yan, R.; Chen, H.Y.; Miao, D.; Cai, L.N.; Fan, J.Y.; Li, G.R.; Xu, R.; Lu, W.G.; et al. A new distinct geminivirus causes soybean stay-green disease. Mol. Plant 2022, 15, 927–930. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, M.X.; Wang, L.K.; Feng, H.; He, X.; Chang, S.; Wang, D.P.; Wang, L.; Yang, J.; An, G.Y.; et al. Whole-plant microbiome profiling reveals a novel geminivirus associated with soybean stay-green disease. Plant Biotechnol. J. 2022, 20, 2159–2173. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhang, Y.Y.; Lu, W.G.; Han, X.Y.; Yang, L.L.; Shi, Y.J.; Li, H.L.; Chen, L.L.; Liu, Y.Q.; Yang, X.; et al. Identifcation and characterization of a new geminivirus from soybean plants and determination of V2 as a pathogenicity factor and silencing suppressor. BMC Plant Biol. 2022, 22, 362. [Google Scholar] [CrossRef]
- Yin, J.L.; Hu, Z.Z.; Xu, S.Q.; Hong, X.; Qiu, Y.L.; Cheng, X.H.; Wang, L.Q.; Shen, W.L.; Zhi, H.J.; Li, K.; et al. Leafhopper transmits soybean stay-green associated virus to leguminous plants. Phytopathol. Res. 2023, 5, 17. [Google Scholar] [CrossRef]
- Cheng, R.; Yan, R.; Mei, R.; Wang, Y.D.; Niu, W.; Ai, H.; Qi, S.J.; Xu, M.X.; Yu, G.; Ye, W.W.; et al. Epidemiological evaluation and identification of the insect vector of soybean stay-green associated virus. Phytopathol. Res. 2023, 5, 20. [Google Scholar] [CrossRef]
- Kho, J.W.; Jung, M.; Lee, D.H. Evaluating the efficacy of two insect detection methods with Riptortus pedestris (Hemiptera: Alydidae): Portable harmonic radar system and fluorescent marking system. Pest Manag. Sci. 2019, 75, 224–233. [Google Scholar] [CrossRef]
- Ahn, J.J.; Choi, K.S. Population parameters and growth of Riptortus pedestris (Fabricius) (Hemiptera: Alydidae) under fluctuating temperature. Insects 2022, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.M.; Arifunnahar, M.; Mostafiz, M.M.; Ali, M.D.; Hossain, M.A.; Talamas, E.J. Hadronotus pubescens (Motschoulsky) (Hymenoptera, Scelionidae): Redescription, biological attributes, and parasitism on eggs of Riptortus pedestris (Fab.) (Hemiptera, Alydidae). J. Hymenopt. Res. 2022, 94, 139–161. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Hu, Y.L.; Tian, X.Y.; Wang, Y.Y.; Li, J.B.; Shi, S.S. Laboratory evaluation of leguminous plants for the development and reproduction of the bean bug Riptortus pedestris. Entomol. Sci. 2022, 5, e12525. [Google Scholar] [CrossRef]
- Yang, Y.T.; Lee, S.J.; Nai, Y.S.; Kim, S.; Kim, J.S. Up-regulation of carbon metabolism-related glyoxylate cycle and toxin production in Beauveria bassiana JEF-007 during infection of bean bug, Riptortus pedestris (Hemiptera: Alydidae). Fungal Biol. 2016, 120, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Win, T.; Lee, J.Y.; Woo, R.M.; Woo, S.D. Screening and evaluation of entomopathogenic fungi against the bean bug, Riptortus pedestris using multiple tools. Entomol. Res. 2022, 52, 493–503. [Google Scholar] [CrossRef]
- Song, J.; Lee, G.; Jung, J.; Monn, J.K.; Kim, S. Effect of soybean volatiles on the behavior of the bean bug, Riptortus pedestris. J. Chem. Ecol. 2022, 48, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lim, U.T. Occurrence and control method of Riptortus pedestris (Hemiptera: Alydidae): Korean perspectives. Korean J. Appl. Entomol. 2013, 52, 437–448. [Google Scholar] [CrossRef]
- Wang, Z.J.; Tian, X.Y.; Li, W.B.; Gao, Y.; Shi, S.S. Indoor biological activity and field effect of five kinds of insecticides to Riptortus pedestris. Agrochemicals 2020, 59, 537–540, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Hanson, A.A.; Menger-Anderson, J.; Silverstein, C.; Potter, B.D.; MacRae, I.; Hodgson, E.W.; Koch, R.L. Evidence for soybean aphid (Hemiptera: Aphididae) resistance to pyrethroid insecticides in the Upper Midwestern United States. J. Econ. Entomol. 2017, 110, 2235–2246. [Google Scholar] [CrossRef]
- Vojoudi, S.; Saber, M.; Hejazi, M.J.; Talaei-Hassanloui, R. Toxicity of chlorpyrifos, spinosad and abamectin on cotton bollworm, Helicoverpa armigera and their sublethal effects on fecundity and longevity. Bull. Insectol. 2011, 64, 189–193. [Google Scholar]
- Xu, L.; Zhao, J.; Xu, D.J.; Xu, G.C.; Gu, Z.Y.; Xiao, Z.; Dewer, Y.; Zhang, Y.N. Application of transcriptomic analysis to unveil the toxicity mechanisms of fall armyworm response after exposure to sublethal chlorantraniliprole. Ecotox. Environ. Saf. 2022, 230, 113145. [Google Scholar] [CrossRef]
- Guo, J.; An, J.; Chang, H.; Li, Y.; Dang, Z.; Wu, C.; Gao, Z. The lethal and sublethal effects of lambda-cyhalothrin and emamectin benzoate on the soybean pest Riptortus pedestris (Fabricius). Toxics 2023, 11, 971. [Google Scholar] [CrossRef]
- Sial, M.U.; Zhao, Z.Z.; Zhang, L.; Zhang, Y.N.; Mao, L.; Jiang, H.Y. Evaluation of insecticides induced hormesis on the demographic parameters of Myzus persicae and expression changes of metabolic resistance detoxification genes. Sci. Rep. 2018, 8, 16601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Li, L.X.; Li, X.H.; Li, W.B.; Gao, Y.; Li, J.B.; Shi, S.S. Cross-generational effects of different concentrations of thiamethoxam on Riptortus pedestris (Hemiptera: Alydidae) populations. Acta Entomol. Sin. 2023, 66, 277–291, (In Chinese with an English Abstract). [Google Scholar] [CrossRef]
- Tian, X.Y.; Gao, Y.; Ali, M.Y.; Li, X.H.; Hu, Y.L.; Li, W.B.; Wang, Z.J.; Shi, S.S.; Zhang, J.P. Impact of temperature on age–stage, two-sex life table analysis of a Chinese population of bean bug, Riptortus pedestris (Hemiptera: Alydidae). Agriculture 2022, 12, 1505. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2023. Available online: http://140.120.197.173/Ecology/ (accessed on 28 January 2023).
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Zhang, T.; Long, S.C.; Li, S.; Wang, C.; Chen, Q.; Chen, J.; Feng, Z.Y.; Cao, Y. Responses of Thrips hawaiiensis and Thrips flavus populations to elevated CO2 concentrations. J. Econ. Entomol. 2023, 116, 416–425. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.J.; Wang, D.; Yang, J.; Ding, N.; Shi, S.S. Effect of different plants on the growth and reproduction of Thrips flavus (Thysanoptera: Thripidae). Insects 2021, 12, 502. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, C.; Shen, Y.; Gao, H.; Zhang, G.; Liu, W.; Jiang, H.; Zhang, Y. Life table parameters of the tomato leaf miner Tuta absoluta (Lepidoptera: Gelechiidae) on five tomato cultivars in China. Insects 2024, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.B.; Dou, T.; Gao, F.T.; Wang, G.H.; Dong, Y.C.; Song, N.; An, S.H.; Yin, X.M.; Liu, X.Y.; Ren, Y.D. Sublethal effects of thiamethoxam on biological traits and detoxification enzyme activities in the small brown planthopper, Laodelphax striatellus (Fallén). J. Econ. Entomol. 2022, 115, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, L.L.; Yang, F.; Li, M.; Liu, X.M.; Wang, Y.; Lei, C.L.; Si, S.Y. Sublethal effects of thiamethoxam on the demographic parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2017, 110, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, L.L.; Yang, H.; Wang, Z.; Long, G.Y.; Jin, D.C. Sublethal effects of imidacloprid on the development, reproduction, and susceptibility of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Asia-Pac. Entomol. 2017, 20, 996–1000. [Google Scholar] [CrossRef]
- Mehdi, T.S.; Seyed, A.S. Sub-lethal effects of tiametoxam on life table parameters of the cabbage aphid, Brevicoryne brassicae (L.) (Hemiptera: Aphididae) under laboratory conditions. Arch. Phytopathol. Plant Prot. 2014, 47, 508–515. [Google Scholar] [CrossRef]
- Dai, W.; Li, Y.; Zhu, J.; Ge, G.L.; Yang, G.Q.; Liu, F. Selectivity and sublethal effects of some frequently-used biopesticides on the predator Cyrtorhinus lividipennis Reuter (Hemiptera: Miridae). J. Integr. Agr. 2019, 18, 124–133. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, S.; Yang, X.; Yan, H.; Wang, K.; Ba, X.; Wang, H. Sublethal effects of neonicotinoid insecticides on the development, body weight and economic characteristics of silkworm. Toxics 2023, 11, 402. [Google Scholar] [CrossRef]
- Lashkari, M.R.; Sahragard, A.; Ghadamyari, M. Sublethal effects of imidacloprid and pymetrozine on population growth parameters of cabbage aphid, Brevicoryne brassicae on rapeseed, Brassica napus L. Insect Sci. 2007, 14, 207–212. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Alam, M.B.; Chi, H.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Effects of sublethal doses of methyl benzoate on the life history traits and acetylcholinesterase (AChE) activity of Aphis gossypii. Agronomy 2020, 10, 1313. [Google Scholar] [CrossRef]
- Mao, K.K.; Ren, Z.J.; Li, W.H.; Liu, C.Y.; Wan, H. An insecticide resistance diagnostic kit for whitebacked planthopper Sogatella furcifera (Horvath). J. Pest. Sci. 2020, 94, 531–540. [Google Scholar] [CrossRef]
- Ayyanath, M.M.; Cutler, G.C.; Scott-Dupree, C.D.; Sibley, P.K. Transgenerational shifts in reproduction hormesis in green peach aphid exposed to low concentrations of imidacloprid. PLoS ONE 2013, 8, e74532. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Liu, Y.; Liu, B.; Lu, Y.H.; Xu, X.Y.; Qian, X.H.; Wu, K.M.; Desneux, N. Lethal and sublethal effects of cycloxaprid, a novel cis-nitromethylene neonicotinoid insecticide, on the mirid bug Apolygus lucorum. J. Pest Sci. 2014, 87, 731–738. [Google Scholar] [CrossRef]
- Planes, L.; Catalán, J.; Tena, A.; Porcuna, J.L.; Urbaneja, A. Lethal and sublethal effects of spirotetramat on the mealybug destroyer, Cryptolaemus montrouzieri. J. Pest Sci. 2013, 86, 321–327. [Google Scholar] [CrossRef]
- Wu, C.; Sun, T.; He, M.Y.; Zhang, L.; Zhang, Y.N.; Mao, L.G.; Zhu, L.Z.; Jiang, H.Y.; Zheng, Y.Q.; Liu, X.G. Sublethal toxicity, transgenerational effects, and transcriptome expression of the neonicotinoid pesticide cycloxaprid on demographic fitness of Coccinella septempunctata. Sci. Total Environ. 2022, 842, 156887. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Cheng, S.Y.; Desneux, N.; Gao, X.W.; Xiu, X.J.; Wang, F.L.; Hou, M.L. Transgenerational hormesis effects of nitenpyram on fitness and insecticide tolerance/resistance of Nilaparvata lugens. J. Pest Sci. 2022, 96, 161–180. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Xiao, D.; Li, J.Y.; Chen, Z.; Biondi, A.; Desneux, N.; Gao, X.W.; Song, D.L. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 2015, 24, 479–487. [Google Scholar] [CrossRef]
- Biondi, A.; Campolo, O.; Desneux, N.; Siscaro, G.; Palmeri, V.; Zappalà, L. Life stage-dependent susceptibility of Aphytis melinus DeBach (Hymenoptera: Aphelinidae) to two pesticides commonly used in citrus orchards. Chemosphere 2015, 128, 142–147. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, Z.Q.; Shen, Z.R.; Lu, J.; Xu, W.B. Sublethal effects of selected insecticides on fecundity and wing dimorphism of green peach aphid (Hom., Aphididae). J. Appl. Entomol. 2008, 132, 135–142. [Google Scholar] [CrossRef]
- Azzam, S.; Wang, F.; Wu, J.C.; Shen, J.; Wang, L.P.; Yang, G.Q.; Guo, Y.R. Comparisons of stimulatory effects of a series of concentrations of four insecticides on reproduction in the rice brown planthopper Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). Int. J. Pest Manag. 2009, 55, 347–358. [Google Scholar] [CrossRef]
- Cordeiro, E.M.G.; De Moura, I.L.T.; Fadini, M.A.M.; Guedes, R.N.C. Beyond selectivity: Are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis? Chemosphere 2013, 93, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Neonicotinoids and other insect nicotinic receptor competitive modulators: Progress and prospects. Annu. Rev. Entomol. 2018, 63, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.A.D.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, M.L.; Xiong, J.F. Raman and SERS spectra of thiamethoxam and the Ag3–thiamethoxam complex: An experimental and theoretical investigation. J. Environ. Sci. Health B 2019, 54, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, M.Y.A.; Gómez, V.L.; Ayala, L.A.; Arguello, E.; Conles, M.; Meneghelo, G.E.; Bareiro, J.; Peña Alvarenga, P.V.; González Vera, M.J. Efficacy of insecticides applied to soybean seeds for controlling lepidoptera caterpillars. J. Agric. Sci. 2022, 14, 135–144. [Google Scholar] [CrossRef]
- Lee, S.T.; Davis, J.A. The impact of thiamethoxam on the feeding and behavior of 2 soybean herbivore feeding guilds. J. Econ. Entomol. 2023, 116, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Eitchie, E.E.; Maisonneuve, F.; Scroggins, R.P.; Princz, J.I. Lethal and sublethal toxicity of thiamethoxam and clothianidin commercial formulations to soil invertebrates in a natural soil. Environ. Toxicol. Chem. 2019, 38, 2111–2120. [Google Scholar] [CrossRef]
- Hilton, M.J.; Jarvis, T.D.; Ricketts, D.C. The degradation rate of thiamethoxam in European field studies. Pest Manag. Sci. 2015, 72, 388–397. [Google Scholar] [CrossRef]


| Treatments | Development Duration of Developmental Stages of F1 Generation (Days) | APOP (Days) | TPOP (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Egg | First-Instar Nymph | Second-Instar Nymph | Third-Instar Nymph | Fourth-Instar Nymph | Fifth-Instar Nymph | Nymph Period | Total Pre-Adult Stage | Female Adult | Male Adult | |||
| Control | 6.56 ± 0.03 e | 2.52 ± 0.06 b | 4.49 ± 0.12 d | 4.86 ± 0.14 b | 4.90 ± 0.11 e | 6.97 ± 0.13 d | 23.74 ± 0.11 d | 29.09 ± 0.33 d | 15.09 ± 2.13 a | 16.09 ± 2.28 a | 3.80 ± 0.22 cd | 31.59 ± 0.94 c |
| LC10 | 6.79 ± 0.03 d | 2.52 ± 0.05 b | 4.71 ± 0.12 d | 5.41 ± 0.11 b | 5.93 ± 0.12 d | 7.93 ± 0.11 c | 26.50 ± 0.10 c | 32.76 ± 0.30 c | 14.02 ± 2.04 a | 15.04 ± 2.12 ab | 6.00 ± 0.30 a | 38.68 ± 1.00 b |
| LC20 | 7.00 ± 0.03 c | 2.70 ± 0.05 b | 4.84 ± 0.17 cd | 4.94 ± 0.13 b | 6.16 ± 0.24 cd | 8.17 ± 0.20 c | 26.81 ± 0.16 c | 33.12 ± 0.59 c | 11.01 ± 1.82 a | 13.01 ± 2.00 abc | 2.67 ± 0.20 d | 33.33 ± 1.19 c |
| LC30 | 7.19 ± 0.04 b | 3.12 ± 0.09 a | 5.57 ± 0.13 ab | 6.14 ± 0.12 a | 7.09 ± 0.20 bc | 9.35 ± 0.12 b | 31.27 ± 0.13 b | 38.26 ± 0.48 b | 13.04 ± 1.98 ab | 10.03 ± 1.78 bc | 4.00 ± 0.34 bcd | 42.33 ± 0.77 ab |
| LC40 | 7.51 ± 0.04 a | 2.53 ± 0.06 b | 5.46 ± 0.13 bc | 6.20 ± 0.09 a | 7.21 ± 0.16 b | 9.58 ± 0.11 b | 30.98 ± 0.11 b | 38.58 ± 0.25 b | 10.05 ± 1.76 ab | 9.06 ± 1.72 c | 5.40 ± 0.40 ab | 43.40 ± 0.29 a |
| LC50 | 7.57 ± 0.05 a | 2.69 ± 0.09 b | 6.24 ± 0.21 a | 6.68 ± 0.22 a | 8.52 ± 0.30 a | 10.93 ± 0.22 a | 35.06 ± 0.21 a | 41.98 ± 0.66 a | 7.12 ± 1.48 b | 8.12 ± 1.71 c | 5.00 ± 0.40 abc | 44.00 ± 0.53 a |
| Treatments | Intrinsic Rate of Increase (r) (per Day) | Finite Rate of Increase (λ) (per Day) | Net Reproductive Rate (R0) (per Offspring Individual) | Mean Generation Time (T) (Days) | Gross Reproductive Rate (per Female) |
|---|---|---|---|---|---|
| Control | 0.0236 ± 0.0126 a | 1.0239 ± 0.0128 a | 2.8250 ± 1.2880 a | 43.9912 ± 3.4052 bc | 23.3942 ± 11.1263 a |
| LC10 | −0.0014 ± 0.0095 ab | 0.9986 ± 0.0094 ab | 0.9333 ± 0.3796 ab | 49.3404 ± 3.8272 ab | 5.9912 ± 2.1338 b |
| LC20 | −0.0047 ± 0.0151 ab | 0.9953 ± 0.0147 ab | 0.8250 ± 0.3839 ab | 41.2273 ± 3.5944 c | 6.6078 ± 3.0136 b |
| LC30 | −0.0070 ± 0.0122 ab | 0.9931 ± 0.0119 ab | 0.7000 ± 0.3264 ab | 51.1990 ± 3.9177 ab | 6.4627 ± 3.2590 b |
| LC40 | −0.0098 ± 0.0098 ab | 0.9903 ± 0.0096 ab | 0.5917 ± 0.2643 b | 53.6654 ± 2.2354 a | 6.1293 ± 2.4077 b |
| LC50 | −0.0174 ± 0.0135 b | 0.9828 ± 0.0131 b | 0.4083 ± 0.2178 b | 51.5577 ± 2.4927 ab | 4.3263 ± 2.3479 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, S.; Li, L.; Chen, L.; Gao, Y.; Yuan, M.; Wang, Y.; Shi, S. The Effect of Different Thiamethoxam Concentrations on Riptortus pedestris Development and Fecundity. Toxics 2024, 12, 460. https://doi.org/10.3390/toxics12070460
Wang Z, Wang S, Li L, Chen L, Gao Y, Yuan M, Wang Y, Shi S. The Effect of Different Thiamethoxam Concentrations on Riptortus pedestris Development and Fecundity. Toxics. 2024; 12(7):460. https://doi.org/10.3390/toxics12070460
Chicago/Turabian StyleWang, Zijie, Song Wang, Lixia Li, Lei Chen, Yu Gao, Ming Yuan, Yueying Wang, and Shusen Shi. 2024. "The Effect of Different Thiamethoxam Concentrations on Riptortus pedestris Development and Fecundity" Toxics 12, no. 7: 460. https://doi.org/10.3390/toxics12070460
APA StyleWang, Z., Wang, S., Li, L., Chen, L., Gao, Y., Yuan, M., Wang, Y., & Shi, S. (2024). The Effect of Different Thiamethoxam Concentrations on Riptortus pedestris Development and Fecundity. Toxics, 12(7), 460. https://doi.org/10.3390/toxics12070460






