Evaluating the Impact of Airborne Fine Particulate Matter and Heavy Metals on Oxidative Stress via Vitamin Supplementation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Particulate Matter Assessment
2.3. Biomarker Measurements for Assessing Metal Exposure
2.4. Measuring Urinary Total Antioxidant Capacity and Oxidative Stress Markers
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Abdullahi, K.L.; Delgado-Saborit, J.M.; Harrison, R.M. Emissions and indoor concentrations of particulate matter and its specific chemical components from cooking: A review. Atmos. Environ. 2013, 71, 260–294. [Google Scholar] [CrossRef]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- Thomaidis, N.S.; Bakeas, E.B.; Siskos, P.A. Characterization of lead, cadmium, arsenic and nickel in PM(2.5) particles in the Athens atmosphere, Greece. Chemosphere 2003, 52, 959–966. [Google Scholar] [CrossRef]
- Choi, E.; Yi, S.-M.; Lee, Y.S.; Jo, H.; Baek, S.-O.; Heo, J.-B. Sources of airborne particulate matter-bound metals and spatial-seasonal variability of health risk potentials in four large cities, South Korea. Environ. Sci. Pollut. Res. Int. 2022, 29, 28359–28374. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Sun, C.; Qi, M.; Yu, X.; Zhao, W.; Li, X. Pollution Level and Health Risk Assessment of PM-Bound Metals in Baoding City Before and After the Heating Period. Int. J. Environ. Res. Public Health 2018, 15, 2286. [Google Scholar] [CrossRef]
- Pardo, M.; Porat, Z.; Rudich, A.; Schauer, J.J.; Rudich, Y. Repeated exposures to roadside particulate matter extracts suppresses pulmonary defense mechanisms, resulting in lipid and protein oxidative damage. Environ. Pollut. 2016, 210, 227–237. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, B.; Wang, H.; Zeng, H.; Wang, N.; Wang, M.; Wang, X.; Hao, Y.; Wang, Q.; Yang, W. The role of systemic inflammation and oxidative stress in the association of particulate air pollution metal content and early cardiovascular damage: A panel study in healthy college students. Environ. Pollut. 2023, 323, 121345. [Google Scholar] [CrossRef]
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope CA 3rd Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020, 70, 460–479. [Google Scholar] [CrossRef]
- Lee, C.W.; Vo, T.T.T.; Wu, C.Z.; Chi, M.C.; Lin, C.M.; Fang, M.L.; Lee, I.T. The Inducible Role of Ambient Particulate Matter in Cancer Progression via Oxidative Stress-Mediated Reactive Oxygen Species Pathways: A Recent Perception. Cancers 2020, 12, 2505. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Q.; Xu, Z.; Guo, X.; Wu, S. Association between short-term exposure to ambient particulate air pollution and biomarkers of oxidative stress: A meta-analysis. Environ. Res. 2020, 191, 110105. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, E.H.; Schauer, J.J.; Yi, S.M.; Heo, J. Reactive oxygen species (ROS) activity of ambient fine particles (PM(2.5)) measured in Seoul, Korea. Environ. Int. 2018, 117, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Gao, Y.; Kang, J.; Wang, W.; Yong, Y.L.; Qu, X.; Dang, X.; Shang, D.; Shao, Y.; et al. Fine particulate matter exposure disturbs autophagy, redox balance and mitochondrial homeostasis via JNK activation to inhibit proliferation and promote EMT in human alveolar epithelial A549 cells. Ecotoxicol. Environ. Saf. 2023, 262, 115134. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.H.; Devlin, R.B.; Rappold, A.G.; Case, M.W.; Diaz-Sanchez, D. Low levels of fine particulate matter increase vascular damage and reduce pulmonary function in young healthy adults. Part. Fibre Toxicol. 2020, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.Y.; Kim, A.; Lee, J.H.; Kim, S.M.; Lee, S.Y.; Hwang, K.K.; Lim, H.J.; Cho, M.C.; Kim, Y.D.; Bae, J.W.; et al. Positive Effect of Air Purifier Intervention on Baroreflex Sensitivity and Biomarkers of Oxidative Stress in Patients with Coronary Artery Disease: A Randomized Crossover Intervention Trial. Int. J. Environ. Res. Public Health 2022, 19, 7078. [Google Scholar] [CrossRef]
- Schulz, A.J.; Mentz, G.B.; Sampson, N.R.; Dvonch, J.T.; Reyes, A.G.; Izumi, B. Effects of particulate matter and antioxidant dietary intake on blood pressure. Am. J. Public Health 2015, 105, 1254–1261. [Google Scholar] [CrossRef]
- Jiao, W.; Hagler, G.; Williams, R.; Sharpe, R.; Brown, R.; Garver, D.; Judge, R.; Caudill, M.; Rickard, J.; Davis, M.; et al. Community Air Sensor Network (CAIRSENSE) project: Evaluation of low-cost sensor performance in a suburban environment in the southeastern United States. Atmos. Meas. Tech. 2016, 9, 5281–5292. [Google Scholar] [CrossRef]
- Mukherjee, A.; Brown, S.G.; McCarthy, M.C.; Pavlovic, N.R.; Stanton, L.G.; Snyder, J.L.; D’Andrea, S.; Hafner, H.R. Measuring Spatial and Temporal PM(2.5) Variations in Sacramento, California, Communities Using a Network of Low-Cost. Sensors 2019, 19, 4701. [Google Scholar] [CrossRef]
- Environment, T.M.O. Annual Report of Ambient Air Quality in Korea. 3 December. Available online: https://www.airkorea.or.kr/eng (accessed on 3 December 2021).
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, A. Urinary uric acid and antioxidant capacity in children and adults with Down syndrome. Clin. Biochem. 2010, 43, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Guzmán, R.; López-Fernández, E.; Casado, A. Evaluation of the copper(II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: The CUPRAC-BCS assay. Anal. Biochem. 2009, 392, 37–44. [Google Scholar] [CrossRef]
- Agarwal, R.; Chase, S.D. Rapid, fluorimetric-liquid chromatographic determination of malondialdehyde in biological samples. J. Chromatogr. B 2002, 775, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Y.; Wang, T.; Ji, Q.; Jia, Q.; Meng, T.; Ma, S.; Zhang, Z.; Li, Y.; Chen, R.; et al. Ambient particulate matter compositions and increased oxidative stress: Exposure-response analysis among high-level exposed population. Environ.Int. 2021, 147, 106341. [Google Scholar] [CrossRef]
- Cauci, S.; Tavano, M.; Curcio, F.; Francescato, M. Biomonitoring of urinary metals in athletes according to particulate matter air pollution before and after exercise. Environ. Sci. Pollut. Res. 2022, 29, 26371–26384. [Google Scholar] [CrossRef]
- Kundu, S.; Stone, E.A. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ. Sci. Process Impacts 2014, 16, 1360–1370. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A. Cadmium & its adverse effects on human health. Indian J. Med. Res. 2008, 128, 557–564. [Google Scholar]
- Jarup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Sule, K.; Umbsaar, J.; Prenner, E.J. Mechanisms of Co, Ni, and Mn toxicity: From exposure and homeostasis to their interactions with and impact on lipids and biomembranes. Biochim. Et Biophys. Acta (BBA) Biomembr. 2020, 1862, 183250. [Google Scholar] [CrossRef]
- Patrick, L. Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med. Rev. 2006, 11, 2–22. [Google Scholar]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef]
- Dayan, A.D.; Paine, A.J. Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Hum. Exp. Toxicol. 2001, 20, 439–451. [Google Scholar] [CrossRef]
- Kerger, B.D.; Paustenbach, D.J.; Corbett, G.E.; Finley, B.L. Absorption and elimination of trivalent and hexavalent chromium in humans following ingestion of a bolus dose in drinking water. Toxicol. Appl. Pharmacol. 1996, 141, 145–158. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals. Academic Press: Cambridge, MA, USA, 2014.
- Dooyema, C.A.; Neri, A.; Lo, Y.C.; Durant, J.; Dargan, P.I.; Swarthout, T.; Biya, O.; Gidado, S.O.; Haladu, S.; Sani-Gwarzo, N.; et al. Outbreak of fatal childhood lead poisoning related to artisanal gold mining in northwestern Nigeria, 2010. Environ. Health Perspect. 2012, 120, 601–607. [Google Scholar] [CrossRef]
- Liu, L.; Urch, B.; Szyszkowicz, M.; Evans, G.; Speck, M.; Van Huang, A.; Leingartner, K.; Shutt, R.H.; Pelletier, G.; Gold, D.R.; et al. Metals and oxidative potential in urban particulate matter influence systemic inflammatory and neural biomarkers: A controlled exposure study. Environ.Int. 2018, 121, 1331–1340. [Google Scholar] [CrossRef]
- Tan, C.; Lu, S.; Wang, Y.; Zhu, Y.; Shi, T.; Lin, M.; Deng, Z.; Wang, Z.; Song, N.; Li, S.; et al. Long-term exposure to high air pollution induces cumulative DNA damages in traffic policemen. Sci. Total Environ. 2017, 593–594, 330–336. [Google Scholar] [CrossRef]
- Eom, S.Y.; Seo, M.N.; Lee, Y.S.; Park, K.S.; Hong, Y.S.; Sohn, S.J.; Kim, Y.D.; Choi, B.S.; Lim, J.A.; Kwon, H.J.; et al. Low-Level Environmental Cadmium Exposure Induces Kidney Tubule Damage in the General Population of Korean Adults. Arch. Environ. Contam. Toxicol. 2017, 73, 401–409. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Vlahogianni, T.H.; Valavanidis, A. Heavy-metal effects on lipid peroxidation and antioxidant defence enzymes in mussels. Chem. Ecol. 2007, 23, 361–371. [Google Scholar] [CrossRef]
- Sen Gupta, R.; Sen Gupta, E.; Dhakal, B.K.; Thakur, A.R.; Ahnn, J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol. Cells 2004, 17, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Narbad, A.; Chen, W. Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients 2015, 7, 552–571. [Google Scholar] [CrossRef]
| Variables | Mean (SD) or N (%) |
|---|---|
| Number | 114 |
| Age, years | 61.5 (14.6) |
| Elderly, >65 years | 51 (44.7) |
| Sex, female | 81 (71.1) |
| Body mass index, kg/m2 | 25.4 (3.2) |
| Overweight or obese, >25 kg/m2 | 62 (54.4) |
| Ex-smoker | 33 (29.0) |
| Regular vitamin supplement taker * | 27 (32.4) |
| Chronic underlying disease, diagnosed | 70 (61.4) |
| Hypertension | 42 (36.8) |
| Diabetes | 27 (23.7) |
| Dyslipidemia | 21 (18.4) |
| Particulate matter exposure | |
| Personal PM2.5, µg/m3 | 16.9 (11.6) |
| Ambient PM2.5, µg/m3 | 30.5 (15.2) |
| Biomarkers for exposure and oxidative stress † | |
| Blood Cd, µg/L | 0.97 (1.69) |
| Blood Pb, µg/dL | 0.85 (2.25) |
| Urinary Cr, µg/g creatinine | 0.52 (2.14) |
| Urinary Ni, µg/g creatinine | 5.60 (1.92) |
| Urinary TAC, mM UA equiv./mM creatinine | 1.98 (2.21) |
| Urinary MDA, µg/g creatinine | 4.60 (1.83) |
| Urinary 8-OHdG, µg/g creatinine | 6.91 (1.62) |
| Variables | Personal PM2.5 (µg/m3) | Blood Cd (µg/L) | Blood Pb (µg/dL) | Urinary Cr (µg/g creat.) | Urinary Ni (µg/g creat.) | Urinary TAC (mM UA equiv./mM creat.) | Urinary MDA (µg/g creat.) | Urinary 8-OHdG (µg/g creat.) | |
|---|---|---|---|---|---|---|---|---|---|
| Age | <65 | 17.16 (12.07) | 0.92 (1.79) | 0.97 (2.12) | 0.42 (1.92) | 4.72 (1.93) | 1.87 (2.34) | 4.25 (1.95) | 6.61 (1.48) |
| ≥65 | 16.48 (11.12) | 1.06 (1.53) | 0.70 (2.38) | 0.71 (2.28) | 7.25 (1.77) | 2.15 (2.05) | 5.08 (1.64) | 7.31 (1.77) | |
| p-value | 0.758 | 0.222 | 0.074 | 0.002 | 0.003 | 0.354 | 0.106 | 0.292 | |
| Sex | Male | 15.73 (10.94) | 0.77 (1.57) | 0.94 (1.96) | 0.45 (2.19) | 4.36 (2.06) | 1.77 (2.23) | 5.12 (2.00) | 6.94 (1.42) |
| Female | 17.32 (11.91) | 1.08 (1.70) | 0.81 (2.39) | 0.56 (2.12) | 6.32 (1.80) | 2.08 (2.21) | 4.41 (1.75) | 6.91 (1.69) | |
| p-value | 0.509 | 0.006 | 0.451 | 0.217 | 0.014 | 0.336 | 0.233 | 0.960 | |
| BMI | <25 | 15.78 (11.96) | 0.95 (1.67) | 0.89 (2.23) | 0.52 (2.18) | 5.71 (2.02) | 2.02 (2.19) | 4.3 (1.69) | 7.19 (1.56) |
| ≥25 | 17.76 (11.33) | 0.99 (1.72) | 0.82 (2.28) | 0.52 (2.13) | 5.50 (1.85) | 1.95 (2.24) | 4.88 (1.94) | 6.69 (1.66) | |
| p-value | 0.367 | 0.685 | 0.651 | 0.982 | 0.800 | 0.821 | 0.266 | 0.427 | |
| Smoking | Never | 17.24 (11.6) | 1.01 (1.73) | 0.81 (2.24) | 0.53 (2.18) | 5.73 (1.95) | 2.05 (2.09) | 4.60 (1.87) | 6.70 (1.65) |
| Ever | 15.91 (11.75) | 0.84 (1.51) | 1.00 (2.29) | 0.47 (2.02) | 5.11 (1.83) | 1.84 (2.53) | 4.61 (1.74) | 7.46 (1.54) | |
| p-value | 0.580 | 0.193 | 0.338 | 0.571 | 0.525 | 0.520 | 0.993 | 0.278 | |
| Chronic underlying disease | No | 15.83 (12.14) | 0.89 (1.80) | 0.83 (2.21) | 0.44 (1.86) | 4.84 (1.86) | 1.94 (2.33) | 3.87 (1.75) | 6.06 (1.63) |
| Yes | 17.50 (11.30) | 1.07 (1.54) | 0.86 (2.32) | 0.63 (2.37) | 6.60 (1.93) | 2.02 (2.15) | 5.14 (1.83) | 7.53 (1.58) | |
| p-value | 0.456 | 0.099 | 0.844 | 0.030 | 0.030 | 0.787 | 0.014 | 0.018 | |
| Vitamin supplements | Non-taker | 17.61 (12.49) | 0.97 (1.70) | 0.88 (2.22) | 0.52 (2.25) | 5.47 (1.96) | 1.94 (2.20) | 4.65 (1.87) | 6.79 (1.57) |
| Regular taker | 15.29 (9.48) | 0.98 (1.68) | 0.78 (2.34) | 0.53 (1.91) | 5.91 (1.85) | 2.08 (2.26) | 4.51 (1.74) | 7.18 (1.72) | |
| p-value | 0.318 | 0.918 | 0.520 | 0.919 | 0.623 | 0.666 | 0.801 | 0.566 | |
| Dependent Variables * | Personal PM2.5 | Ambient PM2.5 | ||
|---|---|---|---|---|
| Beta † | p-Value | Beta † | p-Value | |
| Blood Cd, µg/L | 0.004 | 0.388 | 0.001 | 0.933 |
| Blood Pb, µg/dL | 0.005 | 0.505 | 0.009 | 0.226 |
| Urinary Cr, µg/g creatinine | 0.006 | 0.377 | 0.004 | 0.505 |
| Urinary Ni, µg/g creatinine | 0.007 | 0.228 | 0.004 | 0.368 |
| Urinary TAC, mM UA equiv./mM creatinine | −0.005 | 0.493 | −0.003 | 0.535 |
| Explanatory Variables | Model for MDA * | Model for 8-OHdG * | ||
|---|---|---|---|---|
| Beta † | p-Value | Beta † | p-Value | |
| Personal PM2.5, µg/m3 | 0.005 | 0.414 | 0.002 | 0.542 |
| Ambient PM2.5, µg/m3 | 0.006 | 0.246 | 0.006 | 0.053 |
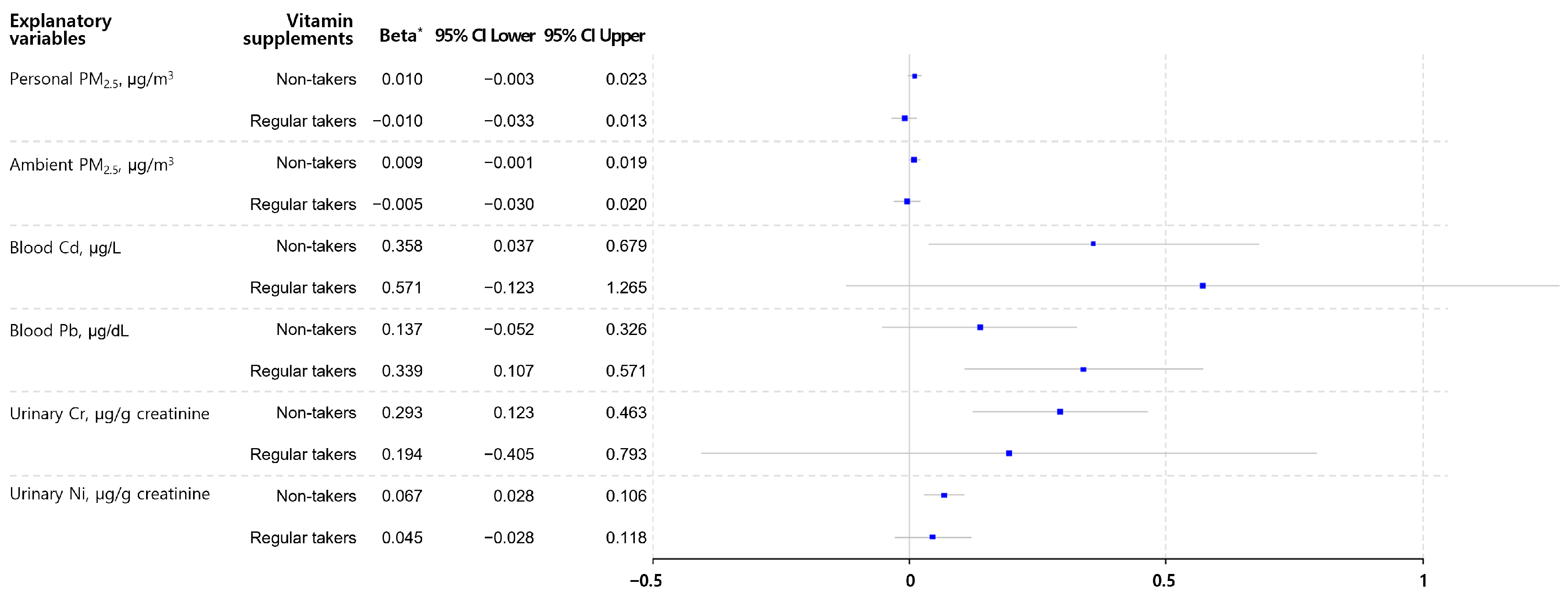
| Blood Cd, µg/L | 0.412 | 0.006 | −0.001 | 0.993 |
| Blood Pb, µg/L | 0.194 | 0.011 | 0.013 | 0.843 |
| Urinary Cr, µg/g creatinine | 0.282 | 0.001 | 0.080 | 0.275 |
| Urinary Ni, µg/g creatinine | 0.064 | <0.001 | 0.003 | 0.818 |
| Urinary TAC, mM UA equiv./mM creatinine | 0.025 | 0.495 | 0.015 | 0.539 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.; Hong, S.; Kim, Y.-D.; Lee, D.-I.; Eom, S.-Y. Evaluating the Impact of Airborne Fine Particulate Matter and Heavy Metals on Oxidative Stress via Vitamin Supplementation. Toxics 2024, 12, 465. https://doi.org/10.3390/toxics12070465
Lee E, Hong S, Kim Y-D, Lee D-I, Eom S-Y. Evaluating the Impact of Airborne Fine Particulate Matter and Heavy Metals on Oxidative Stress via Vitamin Supplementation. Toxics. 2024; 12(7):465. https://doi.org/10.3390/toxics12070465
Chicago/Turabian StyleLee, Eunji, Seonmi Hong, Yong-Dae Kim, Dae-In Lee, and Sang-Yong Eom. 2024. "Evaluating the Impact of Airborne Fine Particulate Matter and Heavy Metals on Oxidative Stress via Vitamin Supplementation" Toxics 12, no. 7: 465. https://doi.org/10.3390/toxics12070465





