Temperature and Dissolved Oxygen Drive Arsenic Mobility at the Sediment—Water Interface in the Lake Taihu
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Experimental Mesocosms
2.3. Sampling and Analysis
2.4. Quality Assurance and Data Analyses
3. Results and Discussion
3.1. The Influence of Temperature Variations on the Redox Environment of Sediments under Different DO Levels
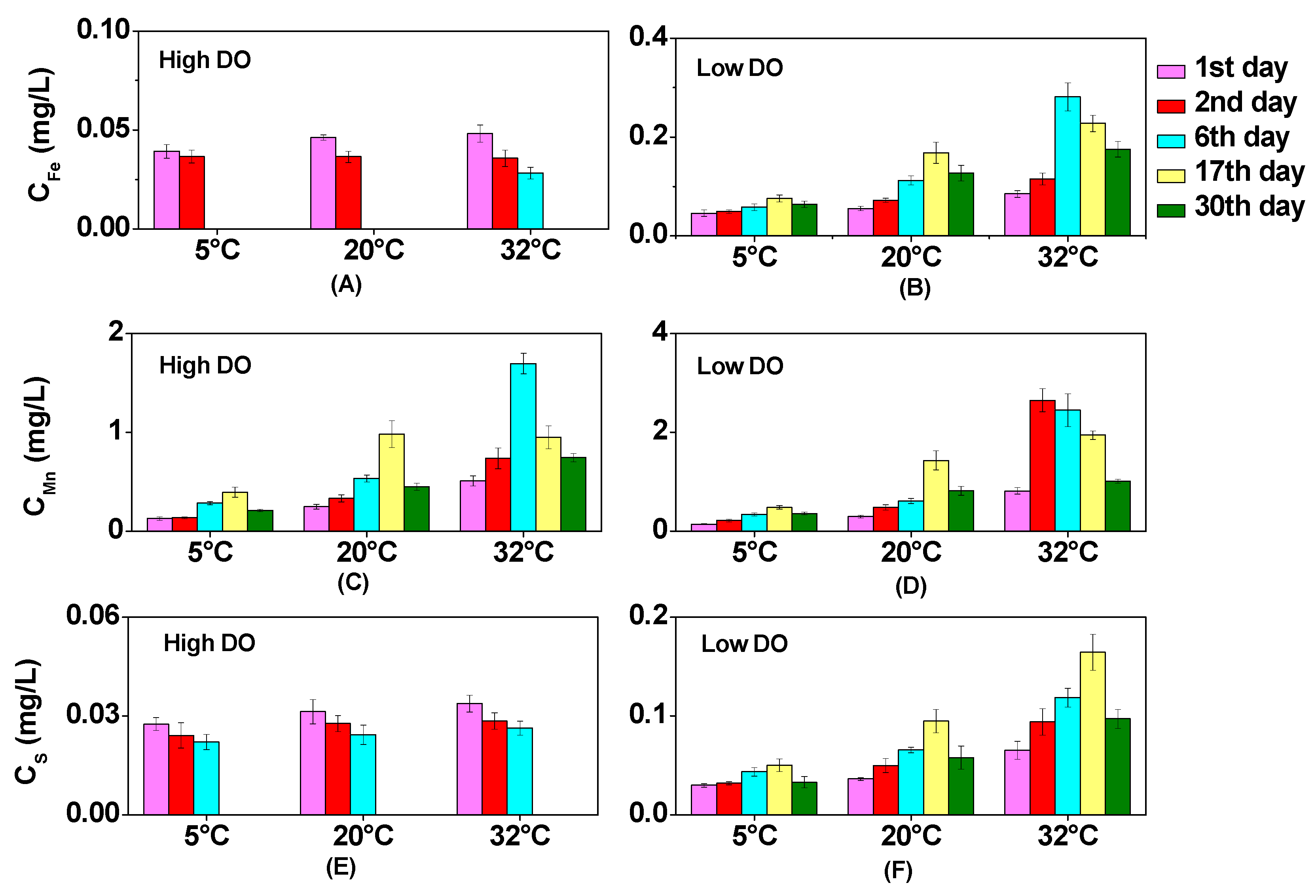
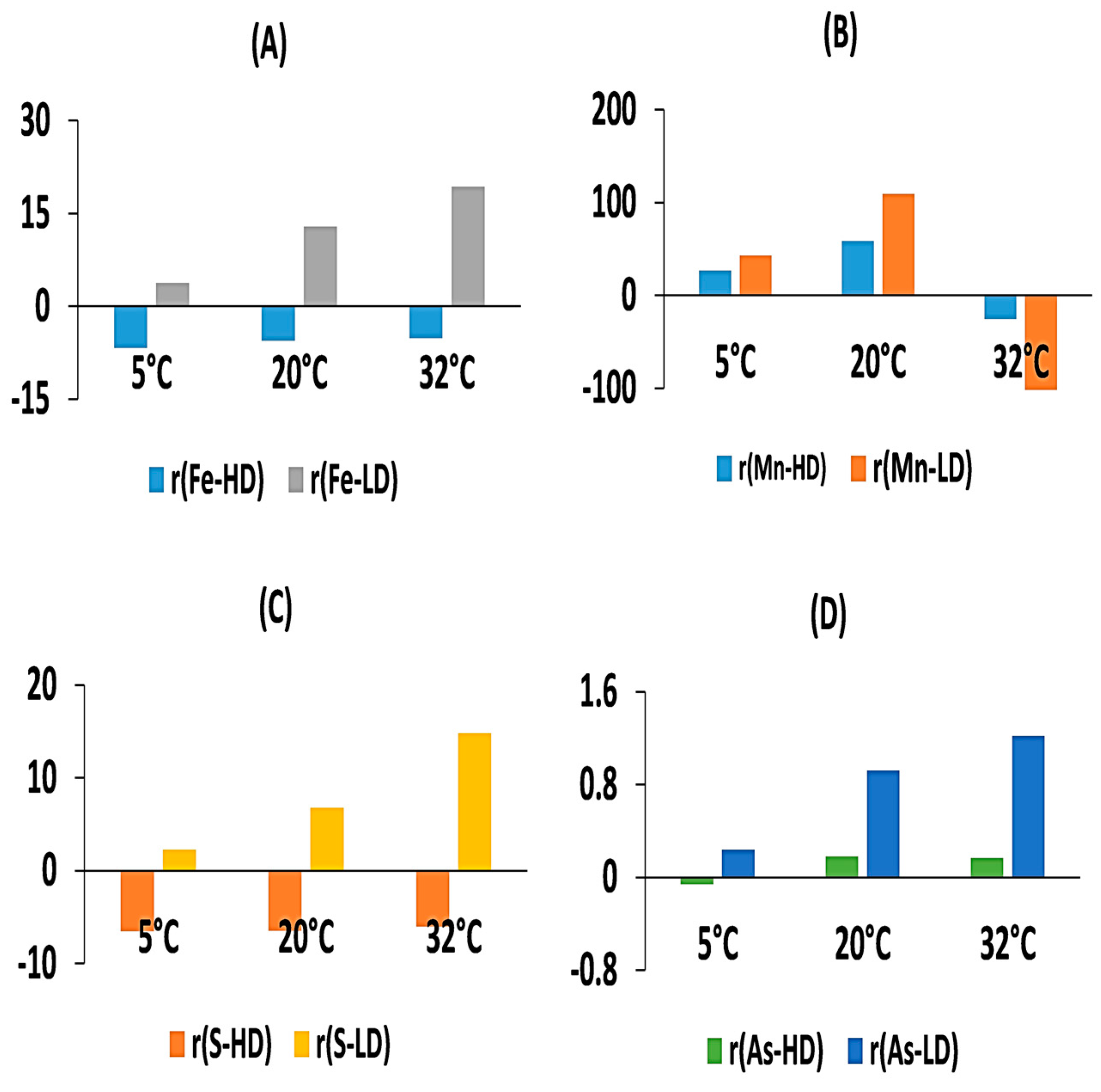
3.2. Effect of Temperature on Dissolved Fe, Mn, and S under Different DO Conditions
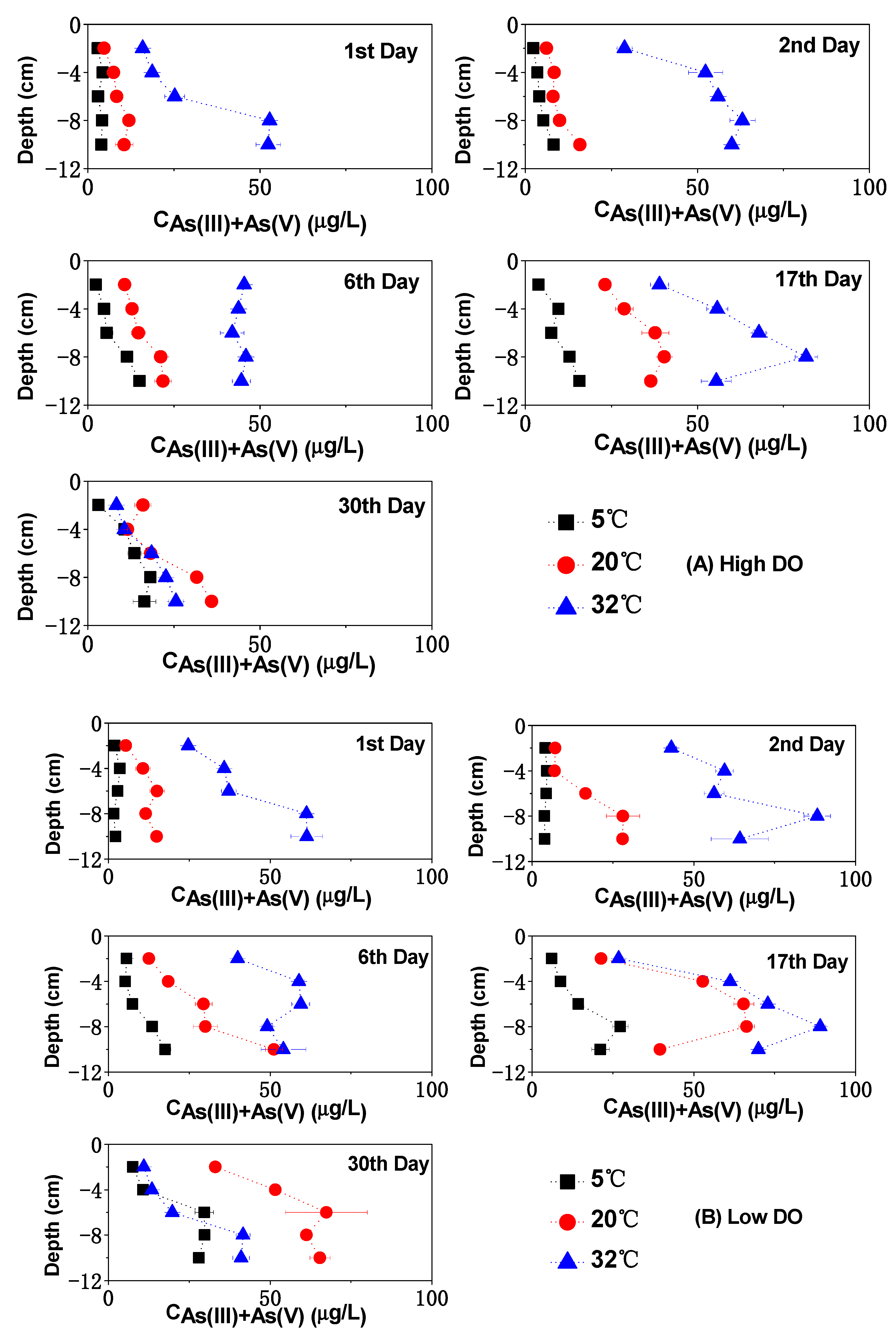
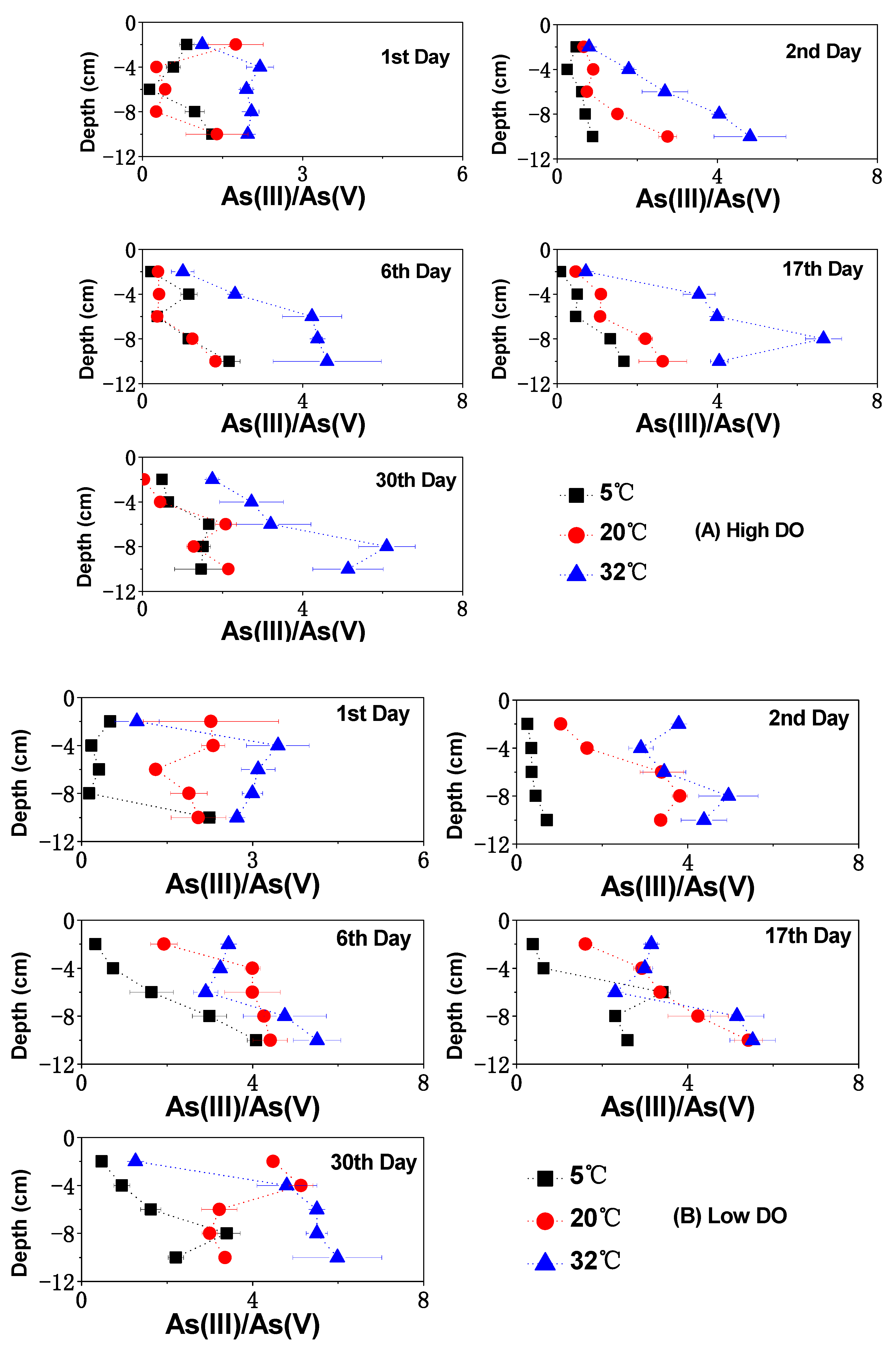
3.3. The Change of Dissolved As in Sediments
3.4. Variation of the Cumulative Release Rate of Each Element in Overlying Water
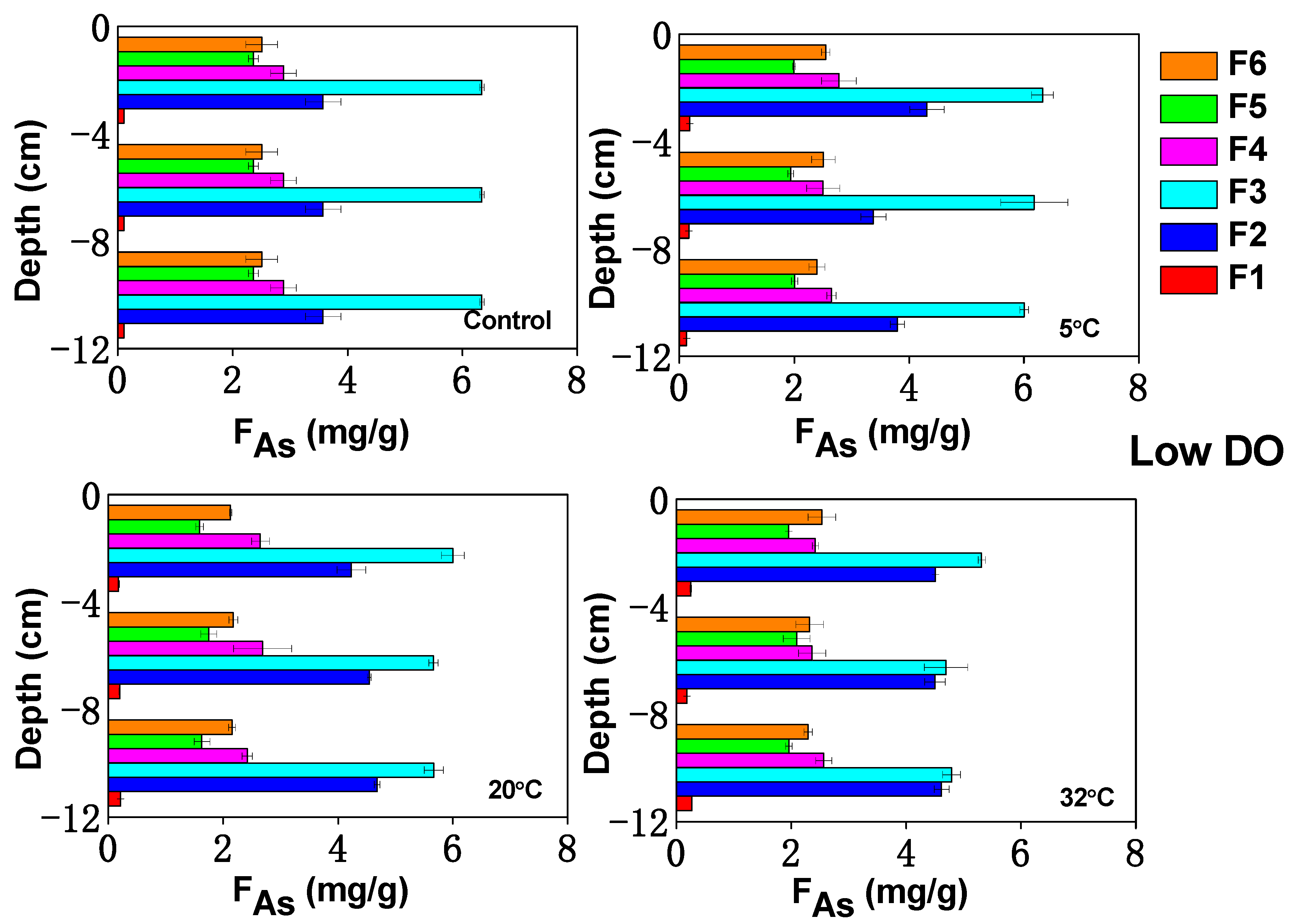
3.5. Influence of Temperature on As Fractions in Sediments under Varying DO Conditions
3.5.1. Ion Exchange As
3.5.2. Iron/Manganese (Hydrogen) Oxide Combined As
3.5.3. Organically Bound As
3.6. Controls of Temperature and DO on As Mobility in Sediment–Water Incubations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Bi, Y.; Mi, W.; Xie, S.; Ji, L. Land-use change caused by anthropogenic activities increase fluoride and arsenic pollution in groundwater and human health risk. J. Hazard Mater. 2021, 406, 124337. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Protecting Surface Water for Health: Identifying, Assessing and Managing Drinking-Water Quality Risks in Surface-Water Catchments; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Aftabtalab, A.; Rinklebe, J.; Shaheen, S.M.; Niazi, N.K.; Moreno-Jiménez, E.; Schaller, J.; Knorr, K.H. Review on the interactions of arsenic, iron (oxy)(hydr) oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 2022, 286, 131790. [Google Scholar] [CrossRef]
- Patel, K.S.; Pandey, P.K.; Martín-Ramos, P.; Corns, W.T.; Varol, S.; Bhattacharya, P.; Zhu, Y. A review on arsenic in the environment: Contamination, mobility, sources, and exposure. Rsc. Adv. 2023, 13, 8803–8821. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Zhou, Z.; Wehrli, B.; Friedl, G. Diagenetic cycling of arsenic in the sediments of eutrophic Baldeggersee, Switzerland. Appl. Geochem. 2003, 18, 1497–1506. [Google Scholar] [CrossRef]
- Schuh, C.E.; Jamieson, H.E.; Palmer, M.J.; Martin, A.J. Solid-phase speciation and post-depositional mobility of arsenic in lake sediments impacted by ore roasting at legacy gold mines in the Yellowknife area, Northwest Territories, Canada. Appl. Geochem. 2018, 91, 208–220. [Google Scholar] [CrossRef]
- Couture, R.M.; Shafei, B.; Van Cappellen, P.; Tessier, A.; Gobeil, C. Non-steady state modeling of arsenic diagenesis in lake sediments. Environ. Sci. Technol. Let. 2010, 44, 197–203. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Hudson-Edwards, K.A. Seasonal variations in arsenic mobility and bacterial diversity: The case study of Huangshui Creek, Shimen Realgar Mine, Hunan Province, China. Sci. Total Environ. 2020, 749, 142353. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, S.; Yeager, K.M.; Xi, B.; Zhang, J.; He, Z.; Ma, C.; Wu, F. Accumulation of arsenic, mercury and heavy metals in lacustrine sediment in relation to eutrophication: Impacts of sources and climate change. Ecolo. Indic. 2018, 93, 771–780. [Google Scholar] [CrossRef]
- Johnston, S.G.; Karimian, N.; Burton, E.D. Seasonal temperature oscillations drive contrasting arsenic and antimony mobilization in a mining-impacted river system. Water Resour. Res. 2020, 56, e2020WR028196. [Google Scholar] [CrossRef]
- Weber, F.A.; Hofacker, A.F.; Voegelin, A.; Kretzschmar, R. Temperature dependence and coupling of iron and arsenic reduction and release during flooding of a contaminated soil. Environ. Sci. Technol. Let. 2010, 44, 116–122. [Google Scholar] [CrossRef]
- Barral-Fraga, L.; Barral, M.T.; MacNeill, K.L.; Martiñá-Prieto, D.; Morin, S.; Rodríguez-Castro, M.C.; Tuulaikhuu, B.A.; Guasch, H. Biotic and abiotic factors influencing arsenic biogeochemistry and toxicity in fluvial ecosystems: A review. Int. J. Environ. Res. Public Health 2020, 17, 2331. [Google Scholar] [CrossRef]
- Jain, C.K.; Sharma, M.K. Adsorption of cadmium on bed sediments of river Hindon: Adsorption models and kinetics. Water Air Soil Pollut. 2002, 137, 1–19. [Google Scholar] [CrossRef]
- Barrett, P.M.; Hull, E.A.; Burkart, K.; Hargrave, O.; McLean, J.; Taylor, V.F.; Jackson, B.P.; Gawel, J.E.; Neumann, R.B. Contrasting As cycling in strongly and weakly stratified contaminated lakes: Evidence for temperature control on sediment-water arsenic fluxes. Limnol. Oceanogr. 2019, 64, 1333–1346. [Google Scholar] [CrossRef]
- Bonte, M.; van Breukelen, B.M.; Stuyfzand, P.J. Temperature-induced impacts on groundwater quality and arsenic mobility in anoxic aquifer sediments used for both drinking water and shallow geothermal energy production. Water Res. 2013, 47, 5088–5100. [Google Scholar] [CrossRef]
- Ni, P.; Guo, H.; Yuan, R.; Huang, Q. Arsenic Migration and Transformation in Aquifer Sediments under Successive Redox Oscillations. Procedia Earth Planet. Sci. 2017, 17, 384–387. [Google Scholar] [CrossRef]
- Duan, Y.; Schaefer, M.V.; Wang, Y.; Gan, Y.; Yu, K.; Deng, Y.; Fendorf, S. Experimental constraints on redox-induced arsenic release and retention from aquifer sediments in the central Yangtze River Basin. Sci. Total Environ. 2019, 649, 629–639. [Google Scholar] [CrossRef]
- Eberle, A.; Besold, J.; Kerl, C.F.; Lezama-Pacheco, J.S.; Fendorf, S.; Planer-Friedrich, B. Arsenic fate in peat controlled by the pH-dependent role of reduced sulfur. Environ. Sci. Technol. 2020, 54, 6682–6692. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sun, F.; Su, H.; Tao, Y.; Chang, H. Multiple risk assessment of heavy metals in surface water and sediment in Taihu Lake, China. Int. J. Environ. Res. Public Health 2022, 19, 13120. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Jiang, X.; Wang, K.; Xia, J.; Jiao, W.; Niu, Y.; Yu, H. Meta analysis of heavy metal pollution and sources in surface sediments of Lake Taihu, China. Sci. Total Environ. 2020, 700, 134509. [Google Scholar] [CrossRef]
- Medunić, G.; Fiket, Ž.; Ivanić, M. Arsenic contamination status in Europe, Australia, and other parts of the world. In Arsenic in Drinking Water and Food; Springer: Singapore, 2020; pp. 183–233. [Google Scholar] [CrossRef]
- Burgess, W.G.; Hoque, M.A.; Michael, H.A.; Voss, C.I.; Breit, G.N.; Ahmed, K.M. Vulnerability of deep groundwater in the Bengal Aquifer System to contamination by arsenic. Nat. Geosci. 2010, 3, 83–87. [Google Scholar] [CrossRef]
- Bai, G.; Zhang, Y.; Yan, P.; Yan, W.; Kong, L.; Wang, L.; Wang, C.; Liu, Z.; Liu, B.; Ma, J.; et al. Spatial and seasonal variation of water parameters, sediment properties, and submerged macrophytes after ecological restoration in a long-term (6 year) study in Hangzhou west lake in China: Submerged macrophyte distribution influenced by environmental variables. Water Res. 2020, 186, 116379. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ding, S.; Zhao, H.; Chen, M.; Yang, D.; Li, C. Seasonal variations in spatial distribution, mobilization kinetic and toxicity risk of As in sediments of Lake Taihu, China. J. Hazard. Mater. 2024, 463, 132852. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yan, C.; Yang, F.; Zhen, Z.; Yang, J.; Chen, J.; Huang, Y.; Xiao, Y.; Zhang, W. The effects and mechanisms of ph and dissolved oxygen conditions on the release of arsenic at the sediment–water interface in Taihu Lake. Toxics 2023, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.T.; Owens, M.S.; Cornwell, J.C. Effect of sediment manipulation on the biogeochemistry of experimental sediment systems. J. Coastal Res. 2006, 22, 1539–1551. [Google Scholar] [CrossRef]
- Konsten, C.J.M.; Brinkman, R.; Andriesse, W. A field laboratory method to determine total potential and actual acidity in acid sulphate soils. In Selected Papers of the Dakar Symposium on Acid Sulphate Soils: Dakar, Senegal; International Institute for Land Reclamation and Improvement: Wageningen, The Netherlands, 1988; Volume 1986, pp. 106–134. [Google Scholar]
- Lu, R.K. Analysis Method of Agricultural Chemistry in Soil; Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron (II) with 1, 10-phenanthroline in the presence of large amounts of iron (III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef]
- Teasdale, P.R.; Hayward, S.; Davison, W. In situ, high-resolution measurement of dissolved sulfide using diffusive gradients in thin films with computer-imaging densitometry. Anal. Chem. 1999, 71, 2186–2191. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Sun, G.X.; Lei, M.; Teng, M.; Liu, Y.X.; Chen, N.C.; Wang, L.H.; Carey, A.M.; Deacon, C.; Raab, A.; et al. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ. Sci. Technol. 2008, 42, 5008–5013. [Google Scholar] [CrossRef]
- Wang, S.; Cao, X.; Lin, C.; Chen, X. Arsenic content and fractionation in the surface sediments of the Guangzhou section of the Pearl River in Southern China. J. Hazard. Mater. 2010, 183, 264–270. [Google Scholar] [CrossRef]
- Pan, F.; Liu, H.; Guo, Z.; Li, Z.; Wang, B.; Gao, A. Geochemical behavior of phosphorus and iron in porewater in a mangrove tidal flat and associated phosphorus input into the ocean. Cont. Shelf Res. 2017, 150, 65–75. [Google Scholar] [CrossRef]
- Conant, R.T.; Drijber, R.A.; Haddix, M.L.; Parton, W.J.; Paul, E.A.; Plante, A.F.; Six, J.; Steinweg, J.M. Sensitivity of organic matter decomposition to warming varies with its quality. Glob. Change Biol. 2008, 14, 868–877. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Cai, Y.; Xiao, K.; Guo, Z.; Pan, F. High resolution dissolved heavy metals in sediment porewater of a small estuary: Distribution, mobilization and migration. Sci. Total Environ. 2023, 905, 167238. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H. Redox Cycling of Colloidal Macro-and Micro-Nutrients in a Monomictic Lake. Ph.D. Thesis, The University of Waikato, Hamilton, New Zealand, 2022. Available online: https://hdl.handle.net/10289/14988 (accessed on 20 June 2024).
- Munoz, M.O.; Bhattacharya, P.; Sracek, O.; Ramos, O.R.; Aguirre, J.Q.; Bundschuh, J.; Maity, J.P. Arsenic and other trace elements in thermal springs and in cold waters from drinking water wells on the Bolivian Altiplano. J. South. Am. Earth Sci. 2015, 60, 10–20. [Google Scholar] [CrossRef]
- Park, J.H.; Han, Y.S.; Ahn, J.S. Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream. Water Res. 2016, 106, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.S.; Tebo, B.M.; Mucci, A.; Sundby, B.; Luther III, G.W. Abundant porewater Mn(III) is a major component of the sedimentary redox system. Science 2013, 341, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Jiang, Y.; Jia, Y.; Lian, X.; Shang, C.; Zhao, M. Exogenous-organic-matter-driven mobilization of groundwater arsenic. Environ. Sci. Ecotechnol. 2023, 15, 100243. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, B.; Pan, F.; Fu, Y.; Guo, W.; Guo, Z.; Liu, H. Effects of manganese, iron and sulfur geochemistry on arsenic migration in the estuarine sediment of a small river in Xiamen, Southeast China. Environ. Pollut. 2022, 293, 118570. [Google Scholar] [CrossRef]
- Rickard, D.; Mussmann, M.; Steadman, J.A. Sedimentary sulfides. Elements 2017, 13, 117–122. [Google Scholar] [CrossRef]
- Otero, X.L.; Ferreira, T.O.; Vidal-Torrado, P.; Macías, F. Spatial variation in pore water geochemistry in a mangrove system (Pai Matos island, Cananeia-Brazil). Appl. Geochem. 2006, 21, 2171–2186. [Google Scholar] [CrossRef]
- Luek, J.L.; Thompson, K.E.; Larsen, R.K.; Heyes, A.; Gonsior, M. Sulfate reduction in sediments produces high levels of chromophoric dissolved organic matter. Sci. Rep. 2017, 7, 8829. [Google Scholar] [CrossRef]
- Gao, J.; Zheng, T.; Deng, Y.; Jiang, H. Microbially mediated mobilization of arsenic from aquifer sediments under bacterial sulfate reduction. Sci. Total Environ. 2021, 768, 144709. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, F.; Wu, J.; Gao, P.; Wang, Y.; Wang, J. Migration characteristics of arsenic in sediments under the influence of cascade reservoirs in Lancang River basin. J. Hydrol. 2022, 606, 127424. [Google Scholar] [CrossRef]
- Yao, Y.; Han, X.; Chen, Y.; Li, D. The variations of labile arsenic diffusion driven by algal bloom decomposition in eutrophic lake ecosystems. Sci. Total Environ. 2022, 842, 156703. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Jeelani, G.; Mukherjee, A.; Coomar, P. Arsenic fate in upper Indus river basin (UIRB) aquifers: Controls of hydrochemical processes, provenances and water-aquifer matrix interaction. Sci. Total Environ. 2021, 795, 148734. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Sanpawanitchakit, C.; Tempel, R.N. The oxidation and dissolution of arsenic-bearing sulfides. Can. Mineral. 2009, 47, 593–613. [Google Scholar] [CrossRef]
- Malakar, A.; Singh, R.; Westrop, J.; Weber, K.A.; Elofson, C.N.; Kumar, M.; Snow, D.D. Occurrence of arsenite in surface and groundwater associated with a perennial stream located in Western Nebraska, USA. J. Hazard. Mater. 2021, 416, 126170. [Google Scholar] [CrossRef] [PubMed]
- Barringer, J.L.; Reilly, P.A. Arsenic in groundwater: A summary of sources and the biogeochemical and hydrogeologic factors affecting As occurrence and mobility. Curr. Perspect. Contam. Hydrol. Water Resour. Sustain. 2013, 34, 83–116. [Google Scholar] [CrossRef]
- Khan, S.S.; Flora, S.J.S. Arsenic: Chemistry, occurrence, and exposure. In Handbook of As Toxicology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–49. [Google Scholar] [CrossRef]
- Simmler, M.; Bommer, J.; Frischknecht, S.; Christl, I.; Kotsev, T.; Kretzschmar, R. Reductive solubilization of arsenic in a mining-impacted river floodplain: Influence of soil properties and temperature. Environ. Pollut. 2017, 231, 722–731. [Google Scholar] [CrossRef]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S.H. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef]
- Lors, C.; Tiffreau, C.; Laboudigue, A. Effects of bacByterial activities on the release of heavy metals from contaminated dredged sediments. Chemosphere 2004, 56, 619–630. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, N.; Wang, Y.; Zhang, D.; Xu, K.; Lv, X.; Long, Y. Arsenate microbial reducing behavior regulated by the temperature fields in landfills. Waste Manag. 2023, 168, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Guo, H.; Zhang, C.; Chen, D.; Ye, H. Variations and driving mechanism of dissolved arsenic in sediment porewater near wetland. Appl. Geochem. 2022, 137, 105185. [Google Scholar] [CrossRef]
- Yan, W.; He, X.; Chen, M.; Qian, B.; Li, M.; Yan, Y.; Lin, C.; Mao, Z. High arsenic pollution of the eutrophic Lake Taihu and its relationship with iron, manganese, and dissolved organic matter: High-resolution synchronous analysis. J. Hazard. Mater. 2024, 467, 133644. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Wang, S.; Che, F.; Zhang, Y.; Yang, P.; Zhang, J.; Liu, Y.; Guo, H.; Fu, Z. Adsorption and desorption of heavy metals at water sediment interface based on bayesian model. J. Environ. Manag. 2023, 329, 117035. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Wang, P.; Hu, B.; Wang, X.; Qian, J. Mobilization of colloids during sediment resuspension and its effect on the release of heavy metals and dissolved organic matter. Sci. Total Environ. 2023, 861, 160678. [Google Scholar] [CrossRef]
- Dai, M.; Benitez-Nelson, C. Colloidal organic carbon and 234Th in the Gulf of Maine. Mar. Chem. 2001, 74, 181–196. [Google Scholar] [CrossRef]
- Barringer, J.L.; Wilson, T.P.; Szabo, Z.; Bonin, J.L.; Fischer, J.M.; Smith, N.P. Diurnal variations in, and ninfluences on, concentrations of particulate and dissolved arsenic and metals in the mildly alkaline Wallkill River, New Jersey, USA. Environ. Geol. 2008, 53, 1183–1199. [Google Scholar] [CrossRef]
- Nimick, D.A.; Gammons, C.H.; Cleasby, T.E.; Madison, J.P.; Skaar, D.; Brick, C.M. Diel cycles in dissolved metal concentrations in streams: Occurrence and possible causes. Water Resour. Res. 2003, 39, 1247. [Google Scholar] [CrossRef]
- Parker, S.R.; Gammons, C.H.; Poulson, S.R.; DeGrandpre, M.D. Diel variations in stream chemistry and isotopic composition of dissolved inorganic carbon, upper Clark Fork River, Montana, USA. Appl. Geochem. 2007, 22, 1329–1343. [Google Scholar] [CrossRef]
- Yan, C.; Che, F.; Zeng, L.; Wang, Z.; Du, M.; Wei, Q.; Wang, Z.; Wang, D.; Zhen, Z. Spatial and seasonal changes of arsenic species in The Lake Taihu in relation to eutrophication. Sci. Tot. Environ. 2016, 563, 496–505. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.M.; Liu, H.Y.; Zhang, W.M. Source, migration, distribution, toxicological effects and remediation technologies of arsenic in groundwater in China. China Geol. 2023, 6, 476–493. [Google Scholar] [CrossRef]
- Rouwane, A.; Rabiet, M.; Bourven, I.; Grybos, M.; Mallet, L.; GJuibaud, G. Role of microbial reducing activity in antimony and arsenic release from an unpolluted wetland soil: A lab scale study using sodium azide as a microbial inhibiting agent. Environ. Chem. 2016, 13, 945–954. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Z.; Wang, J.; Hou, Q.; Zhang, Y. Impact of temperature on the aging mechanisms of arsenic in soils: Fractionation and bioaccessibility. Environ. Sci. Pollut. Res. 2016, 23, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, H.Y.; Liu, C.; Li, F.; Xu, X.; Wang, Q. Arsenic availability in rice from a mining area: Is amorphous iron oxide-bound As a source or sink? Environ. Pollut. 2015, 199, 95–101. [Google Scholar] [CrossRef]
- Majzlan, J.; Lalinská, B.; Chovan, M.; Bläß, U.; Brecht, B.; Göttlicher, J.; Steininger, R.; Hug, K.; Ziegler, K.; Gescher, J. A mineralogical, geochemical, and microbiogical assessment of the antimony-and arsenic-rich neutral mine drainage tailings near Pezinok, Slovakia. Am. Mineral. 2011, 96, 1–13. [Google Scholar] [CrossRef]
- Schilling, K.; Borch, T.; Rhoades, C.C.; Pallud, C.E. Temperature sensitivity of microbial Fe(III) reduction kinetics in subalpine wetland soils. Biogeochemistry 2019, 142, 19–35. [Google Scholar] [CrossRef]
- Larios, R.; Fernández-Martínez, R.; Álvarez, R.; Rucandio, I. Arsenic pollution and fractionation in sediments and mine waste samples from different mine sites. Sci. Total Environ. 2012, 431, 426–435. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Langenhoff, A.A.; Comans, R.N.; Sutton, N.B.; Rijnaarts, H.H. Effects of dissolved organic matter and nitrification on biodegradation of pharmaceuticals in aerobic enrichment cultures. Sci. Total Environ. 2018, 630, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.M.; Ptacek, C.J.; Blowes, D.W.; Gould, W.D.; Ma, J.; Landis, R.C.; Dyer, J.A. Role of organic carbon sources and sulfate in controlling net methylmercury production in riverbank sediments of the South River, VA (USA). Geomicrobiol. J. 2018, 35, 1–14. [Google Scholar] [CrossRef]
- Meunier, L.; Koch, I.; Reimer, K.J. Effects of organic matter and ageing on the bioaccessibility of arsenic. Environ. Pollut. 2011, 159, 2530–2536. [Google Scholar] [CrossRef]
- Aomi, S.; Tomoyuki, M. Functional effects of manganese and iron oxides on the dynamics of trace elements in soils with a special focus on As and cadmium: A review. Geoderma 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Pallud, C.; Rhoades, C.C.; Schneider, L.; Dwivedi, P.; Borch, T. Temperature-induced iron (III) reduction results in decreased dissolved organic carbon export in subalpine wetland soils, Colorado, USA. Geochim. Et Cosmochim. Acta. 2020, 280, 148–160. [Google Scholar] [CrossRef]


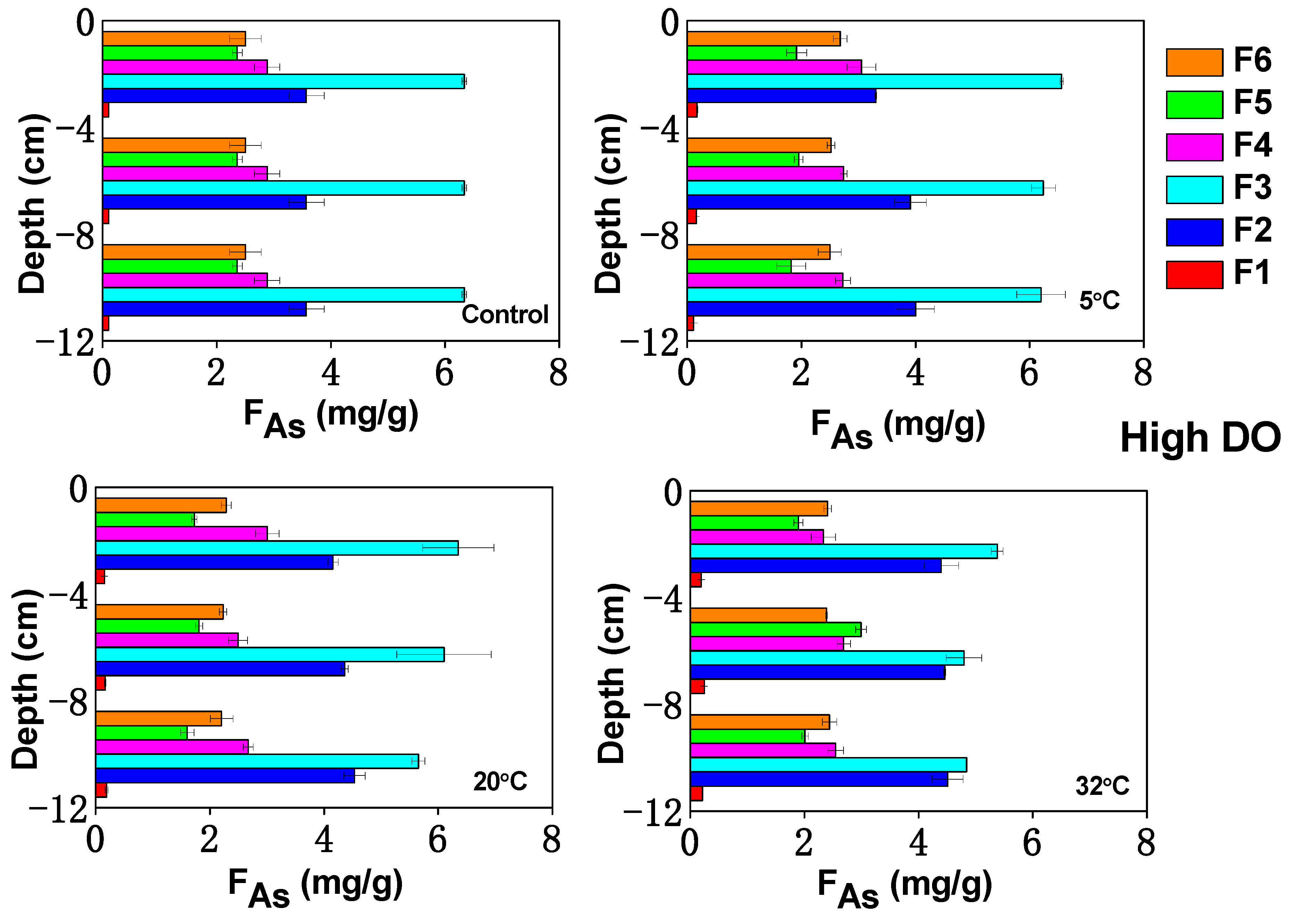
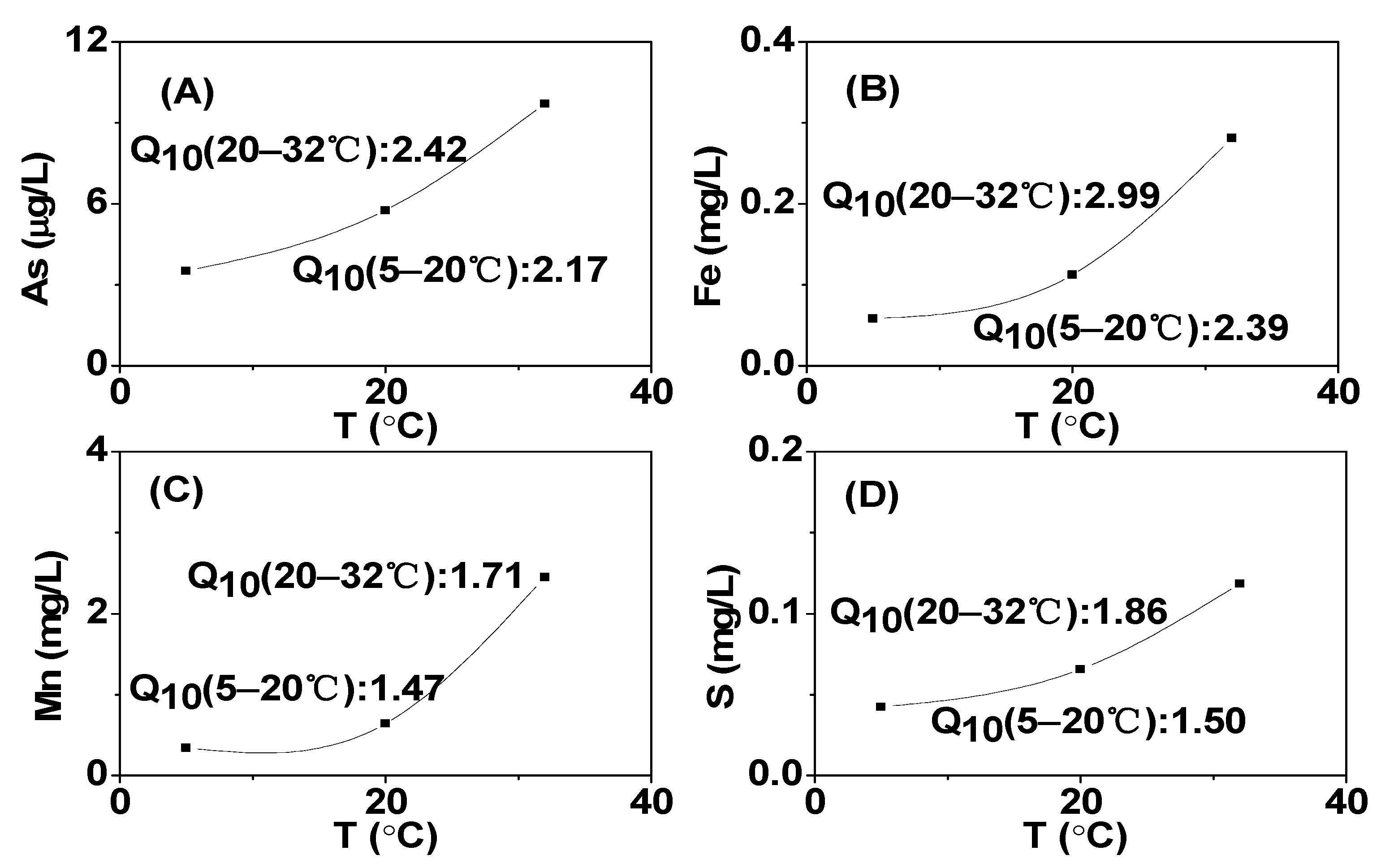
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Yang, F.; Chen, Y.; Chen, S.; Xu, M.; Gu, C. Temperature and Dissolved Oxygen Drive Arsenic Mobility at the Sediment—Water Interface in the Lake Taihu. Toxics 2024, 12, 471. https://doi.org/10.3390/toxics12070471
Zeng L, Yang F, Chen Y, Chen S, Xu M, Gu C. Temperature and Dissolved Oxygen Drive Arsenic Mobility at the Sediment—Water Interface in the Lake Taihu. Toxics. 2024; 12(7):471. https://doi.org/10.3390/toxics12070471
Chicago/Turabian StyleZeng, Liqing, Fan Yang, Yuyan Chen, Songmei Chen, Mei Xu, and Chongyu Gu. 2024. "Temperature and Dissolved Oxygen Drive Arsenic Mobility at the Sediment—Water Interface in the Lake Taihu" Toxics 12, no. 7: 471. https://doi.org/10.3390/toxics12070471
APA StyleZeng, L., Yang, F., Chen, Y., Chen, S., Xu, M., & Gu, C. (2024). Temperature and Dissolved Oxygen Drive Arsenic Mobility at the Sediment—Water Interface in the Lake Taihu. Toxics, 12(7), 471. https://doi.org/10.3390/toxics12070471




