Metformin as an Emerging Pollutant in the Aquatic Environment: Occurrence, Analysis, and Toxicity
Abstract
1. Introduction
2. Property, Production, and Usage of Metformin
3. Detection Methods of Metformin in Water
3.1. Liquid Chromatography
3.2. Electrochemical Analysis Techniques
3.3. Spectrophotometry
3.4. Capillary Electrophoresis
3.5. Thin-Layer Chromatography
3.6. Liquid Chromatography–Mass Spectrometry
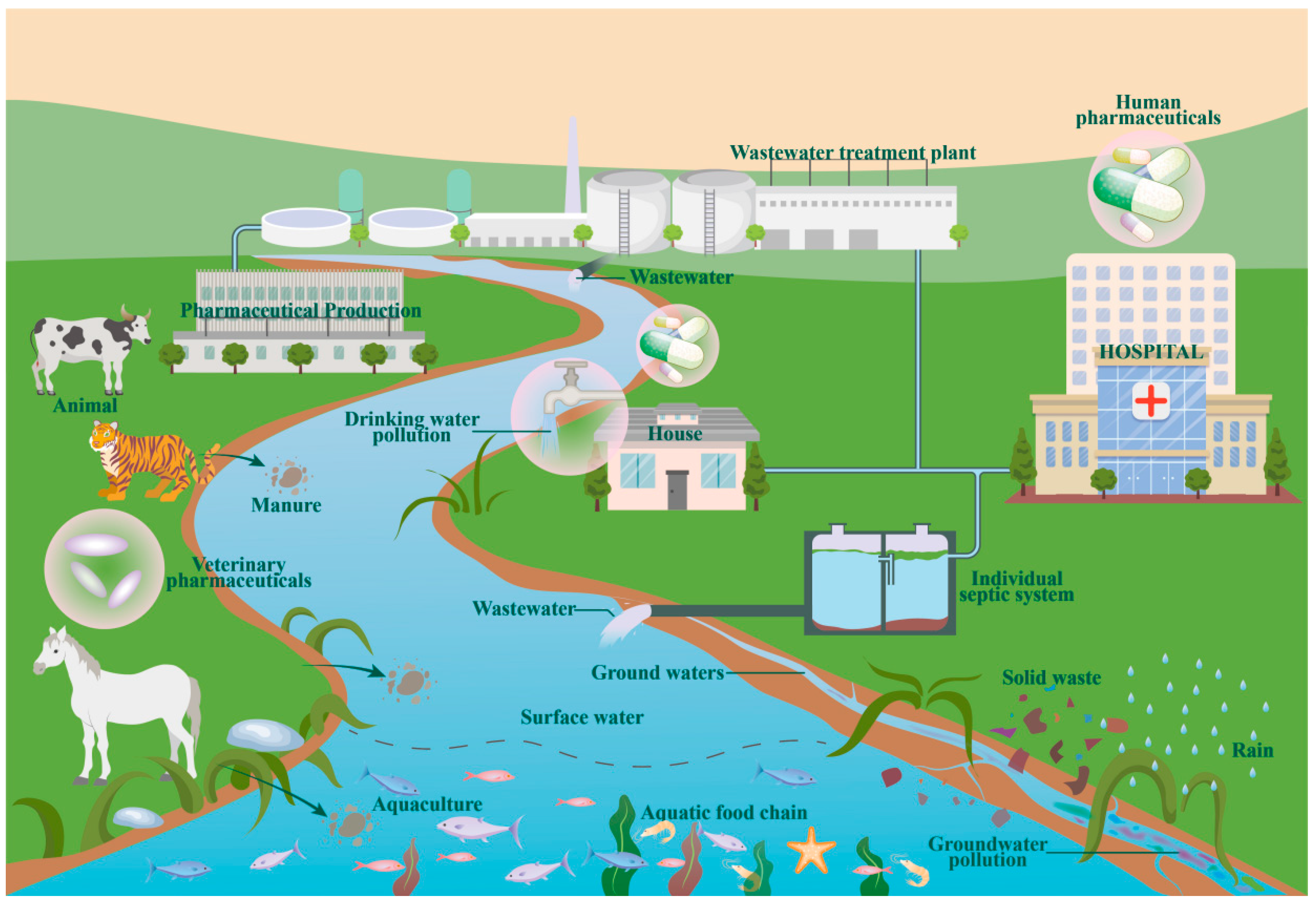
4. Sources and Distribution of Metformin in Water Bodies
4.1. Sources of Metformin in Water Bodies
4.2. Metformin Distribution in Water Bodies
5. Toxic Effects of Metformin on Aquatic Organisms
5.1. Mode of Entry into Organisms and Enrichment
5.2. Toxic Effects
5.2.1. Toxic Effects of Metformin in Fish
5.2.2. Effects of Metformin on Other Test Organisms
Daphnia
Rotifers
Chlorella
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scheen, A.J. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes Metab. 2020, 46, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Mohammed, I.; Bshesh, K.; Marei, I.; Ye, K.; Ding, H.; MacDonald, R.; Hollenberg, M.D.; Hill, M.A. Metformin: Is it a drug for all reasons and diseases? Metab.-Clin. Exp. 2022, 133, 155223. [Google Scholar] [CrossRef]
- Yan, J.-H.; Xiao, Y.; Tan, D.-Q.; Shao, X.-T.; Wang, Z.; Wang, D.-G. Wastewater analysis reveals spatial pattern in consumption of anti-diabetes drug metformin in China. Chemosphere 2019, 222, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Masoner, J.R.; Kolpin, D.W.; Furlong, E.T.; Cozzarelli, I.M.; Gray, J.L. Landfill leachate as a mirror of today’s disposable society: Pharmaceuticals and other contaminants of emerging concern in final leachate from landfills in the conterminous United States. Environ. Toxicol. Chem. 2016, 35, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Rahman Ahmad, N. Facial visible: Spectrophotometric determination of metformin hydrochloride in glucosam tablets and industrial waste water: Application to content uniformity testing. Iraqi J. Pharm. 2012, 12, 75–85. [Google Scholar] [CrossRef]
- Armbruster, D.; Happel, O.; Scheurer, M.; Harms, K.; Schmidt, T.C.; Brauch, H.-J. Emerging nitrogenous disinfection byproducts: Transformation of the antidiabetic drug metformin during chlorine disinfection of water. Water Res. 2015, 79, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Shen, L.; Jiang, Z.; Gao, M.; Qiu, Y.; Qi, H.; Chen, C. NDMA formation during ozonation of metformin: Roles of ozone and hydroxyl radicals. Sci. Total Environ. 2021, 796, 149010. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; He, Y.; Yao, L.; Chen, J.; Zhu, S.; Rao, X.; Tang, P.; You, J.; Hua, G.; Zhang, L.; et al. Metformin chlorination byproducts in drinking water exhibit marked toxicities of a potential health concern. Environ. Int. 2021, 146, 106244. [Google Scholar] [CrossRef]
- He, Y.; Jin, H.; Gao, H.; Zhang, G.; Ju, F. Prevalence, production, and ecotoxicity of chlorination-derived metformin byproducts in Chinese urban water systems. Sci. Total Environ. 2022, 816, 151665. [Google Scholar] [CrossRef]
- Leading Chemical Substances Dispensed in England in 2020, by Number of Items; Statista, 2021. Available online: https://www.statista.com/statistics/378445/prescription-cost-analysis-top-twenty-chemicals-by-items-in-england/ (accessed on 12 May 2024).
- The ClinCalc DrugStats Database. The Top 200 Drugs of 2019. 2020. Available online: https://clincalc.com/DrugStats/Top200Drugs.aspx (accessed on 12 May 2024).
- Balakrishnan, A.; Sillanpaa, M.; Jacob, M.M.; Vo, D.-V.N. Metformin as an emerging concern in wastewater: Occurrence, analysis and treatment methods. Environ. Res. 2022, 213, 113613. [Google Scholar] [CrossRef]
- Gedawy, A.; Al-Salami, H.; Dass, C.R. Development and validation of a new analytical HPLC method for simultaneous determination of the antidiabetic drugs, metformin and gliclazide. J. Food Drug Anal. 2019, 27, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Song, J.F.; Luan, X.J.; Wang, Y.Y.; Shi, Q.Z. Determination of metformin based on amplification of its voltammetric response by a combination of molecular wire and carbon nanotubes. Anal. Bioanal. Chem. 2006, 386, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, S.; Mirceski, V.; Ciesielski, W.; Sokolowski, A.; Zakrzewski, R. Direct determination of metformin in urine by adsorptive catalytic square-wave voltammetry. J. Pharm. Biomed. Anal. 2007, 45, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Munde, M.K.; Kulkarni, N.S.; Sen, A.K.; Sen, D.B. A Novel Validated Stability Indicating Analytical Method for Simultaneous Quantification of Metformin Hydrochloride and Empagliflozin in Bulk and Marketed Formulation by HPTLC using Box-Wilson Experimental Design Approach. Indian J. Pharm. Educ. Res. 2020, 54, S644–S656. [Google Scholar] [CrossRef]
- Attimarad, M.; Nair, A.B.; Nagaraja, S.; Aldhubiab, B.E.; Venugopala, K.N.; Pottathil, S. Smart UV Derivative Spectrophotometric Methods for Simultaneous Determination of Metformin and Remogliflozin: Development, Validation and Application to the Formulation. Indian J. Pharm. Educ. Res. 2021, 55, S293–S302. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y.; Gupta, V.K. Analysis of metformin dosage formulations by capillary electrophoresis at nano scale detection. Comb. Chem. High Throughput Screen. 2007, 10, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Kale, D.; Kakde, R. Simultaneous Determination of Pioglitazone, Metformin, and Glimepiride in Pharmaceutical Preparations using HPTLC Method. JPC-J. Planar Chromatogr.-Mod. TLC 2011, 24, 331–336. [Google Scholar] [CrossRef]
- Al Bratty, M.; Alhazmi, H.A.; Javed, S.A.; Lalitha, K.G.; Asmari, M.; Woelker, J.; El Deeb, S. Development and Validation of LC-MS/MS Method for Simultaneous Determination of Metformin and Four Gliptins in Human Plasma. Chromatographia 2017, 80, 891–899. [Google Scholar] [CrossRef]
- Zhang, M.; Moore, G.A.; Lever, M.; Gardiner, S.J.; Kirkpatrick, C.M.J.; Begg, E.J. Rapid and simple high-performance liquid chromatographic assay for the determination of metformin in human plasma and breast milk. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2002, 766, 175–179. [Google Scholar] [CrossRef]
- Haq, I.; Cruz, A.G.; Di Masi, S.; Cowen, T.; Allcock, N.S.; Malitesta, C.; Mujahid, A.; Hussain, T.; Piletska, E.; Piletsky, S.A. Smart nano-actuators for electrochemical sensing of Metformin in human plasma. Sens. Actuators B-Chem. 2023, 376, 132928. [Google Scholar] [CrossRef]
- Alamgir, M.; Hayat, A.; Majidano, A.A.; Khuhawar, M.Y. Spectrophotometric Determination of Metformin in Pharmaceutical Preparations, Serum and Urine using Benzoin as Derivatizing Reagent. J. Chem. Soc. Pak. 2014, 36, 344–349. [Google Scholar]
- Goswami, L.; Mukhopadhyay, S.; Durgapal, S. Simultaneous Estimation of Metformin and Pioglitazone by Ultraviolet Spectrophotometry. Indian J. Pharm. Sci. 2010, 72, 508. [Google Scholar] [CrossRef] [PubMed]
- Dange, Y.D.; Honmane, S.M.; Bhinge, S.D.; Salunkhe, V.R.; Jadge, D.R. Development and Validation of UV-Spectrophotometric Method for Estimation of Metformin in Bulk and Tablet Dosage Form. Indian J. Pharm. Educ. Res. 2017, 51, S754–S760. [Google Scholar] [CrossRef]
- HaiYun, Z.; Yanhong, W.U.; Baomei, H.; Qinghua, H.; Bingyi, Y.; Zuanguang, C. Rapid determination of metformin hydrochloride in metformin hydrochloride tablets by capillary electrophoresis. Chem. Res. Appl. 2008, 20, 923–926. [Google Scholar]
- Wang, L.; Zhao, L.; Zhang, W.; Wu, Y.; Liang, Y.; Guo, G.; Wang, X. Investigation of metformin hydrochloride-bovine serum albumin interaction by narrow-bore capillary zone electrophoresis. Chem. Commun. 2022, 58, 2926–2929. [Google Scholar] [CrossRef] [PubMed]
- Attimarad, M. Multivariate optimization of a capillary zone electrophoresis assay method for simultaneous quantification of metformin and vildagliptin from a formulation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 401–407. [Google Scholar] [CrossRef]
- Strugaru, A.-M.; Mircea, C.; Agoroaei, L.; Botnariu, G.; Grigoriu, I.-C.; Marti, T.D.; Butnaru, E. Quantitative Determination of Metformin by Capillary Electrophoresis with UV Detection. Rev. De Chim. 2015, 66, 1448–1451. [Google Scholar]
- Doomkaew, A.; Prutthiwanasan, B.; Suntornsuk, L. Simultaneous analysis of metformin and cyanoguanidine by capillary zone electrophoresis and its application in a stability study. J. Sep. Sci. 2014, 37, 1687–1693. [Google Scholar] [CrossRef]
- Modi, D.K.; Patel, B.H. Simultaneous determination of metformin hydrochloride and glipizide in tablet formulation by HPTLC. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 28–39. [Google Scholar] [CrossRef]
- Abdelrahman, A.E.; Maher, H.M.; Alzoman, N.Z. HPTLC Method for the Determination of Metformin Hydrochloride, Saxagliptin Hydrochloride, and Dapagliflozin in Pharmaceuticals. Curr. Anal. Chem. 2020, 16, 609–619. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Kupkar, S.K.; Dharam, D.L.; Jangam, A.M.; Chaudhari, P.D. Development and Validation of RP-HPLC and HPTLC Methods for Simultaneous Estimation of Sitagliptin Phosphate and Metformin Hydrochloride in Bulk and Dosage form. Indian J. Pharm. Educ. Res. 2013, 47, 13–16. [Google Scholar]
- Modi, D.K.; Patel, B.H. Rapid and sensitive simultaneous estimation of metformin hydrochloride and pioglitazone hydrochloride in tablet formulation by HPTLC method. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 618–627. [Google Scholar] [CrossRef]
- El-Shoubashy, O.H.-E.; Beltagy, Y.A.E.M.; Issa, A.E.; El-Kafrawy, D.S. Comparative study of HPLC-DAD and HPTLC for the simultaneous determination of a new multitarget antidiabetic ternary mixture in combined tablets. Jpc-J. Planar Chromatogr. -Mod. TLC 2020, 33, 59–70. [Google Scholar] [CrossRef]
- Modi, D.K.; Parejiya, P.B.; Patel, B.H. A Simple and Sensitive HPTLC Method for Simultaneous Determination of Metformin Hydrochloride and Sitagliptin Phosphate in Tablet Dosage Form. J. Chem. 2013, 2013, 139561. [Google Scholar] [CrossRef]
- Georgita, C.; Sora, I.; Albu, F.; Monciu, C.M. Comparison of a LC/MS method with a LC/UV method for the determination of metformin in plasma samples. Farmacia 2010, 58, 158–169. [Google Scholar]
- Hormazabal, V.R.; Ostensvik, O. Determination of metformin in cultivated plant species and soil by liquid chromatography-mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1630–1639. [Google Scholar]
- Gumieniczek, A.; Berecka-Rycerz, A.; Mroczek, T.; Wojtanowski, K. Determination of Chemical Stability of Two Oral Antidiabetics, Metformin and Repaglinide in the Solid State and Solutions Using LC-UV, LC-MS, and FT-IR Methods. Molecules 2019, 24, 4430. [Google Scholar] [CrossRef] [PubMed]
- Tisler, S.; Zwiener, C. Formation and occurrence of transformation products of metformin in wastewater and surface water. Sci. Total Environ. 2018, 628–629, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Axel Elizalde-Velazquez, G.; Manuel Gomez-Olivan, L. Occurrence, toxic effects and removal of metformin in the aquatic environments in the world: Recent trends and perspectives. Sci. Total Environ. 2020, 702, 134924. [Google Scholar] [CrossRef]
- Ogunbanwo, O.M.; Kay, P.; Boxall, A.B.; Wilkinson, J.; Sinclair, C.J.; Shabi, R.A.; Fasasi, A.E.; Lewis, G.A.; Amoda, O.A.; Brown, L.E. High Concentrations of Pharmaceuticals in a Nigerian River Catchment. Environ. Toxicol. Chem. 2022, 41, 551–558. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D., Jr. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, A.; Thakur, A.; Kumar, P.; Van-Huy, N.; Vo, D.-V.N.; Sharma, A.; Kumar, D. One-Pot Synthesis of Magnetite-ZnO Nanocomposite and Its Photocatalytic Activity. Top. Catal. 2020, 63, 1097–1108. [Google Scholar] [CrossRef]
- Ambrosio-Albuquerque, E.P.; Cusioli, L.F.; Bergamasco, R.; Sinopolis Gigliolli, A.A.; Lupepsa, L.; Paupitz, B.R.; Barbieri, P.A.; Borin-Carvalho, L.A.; de Brito Portela-Castro, A.L. Metformin environmental exposure: A systematic review. Environ. Toxicol. Pharmacol. 2021, 83, 103588. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, S.; Wang, D. Effects of metformin on body weight in polycystic ovary syndrome patients: Model-based meta-analysis. Expert Rev. Clin. Pharmacol. 2021, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Onisko, N.; Cao, J.D.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Metformin toxicities. Am. J. Emerg. Med. 2023, 72, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Comprehensive study of the antidiabetic drug metformin and its transformation product guanylurea in Greek wastewaters. Water Res. 2015, 70, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ruiz, R.; Pico, Y.; Alfarhan, A.H.; El-Sheikh, M.A.; Alshahrani, H.O.; Barcelo, D. Dataset of pesticides, pharmaceuticals and personal care products occurrence in wetlands of Saudi Arabia. Data Brief 2020, 31, 105776. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Berset, J.-D.; Wolschke, H.; Kuemmerer, K. Occurrence of the antidiabetic drug Metformin and its ultimate transformation product Guanylurea in several compartments of the aquatic cycle. Environ. Int. 2014, 70, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Scheurer, M.; Sacher, F.; Brauch, H.-J. Occurrence of the antidiabetic drug metformin in sewage and surface waters in Germany. J. Environ. Monit. 2009, 11, 1608–1613. [Google Scholar] [CrossRef]
- Kong, L.; Kadokami, K.; Hanh Thi, D.; Hong Thi Cam, C. Screening of 1300 organic micro-pollutants in groundwater from Beijing and Tianjin, North China. Chemosphere 2016, 165, 221–230. [Google Scholar] [CrossRef]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknecht, J.; Hernandez-Antonio, A.; Ramirez, A.I.; Barrios-Pina, H. Survey of 218 organic contaminants in groundwater derived from the world’s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef]
- Aksay, E.; Yanturali, S.; Bayram, B.; Hocaoglu, N.; Kiyan, S. A rare side effect of metformin: Metformin-induced hepatotoxicity. Turk. J. Med. Sci. 2007, 37, 173–175. [Google Scholar]
- Chowdhury, W.; Lodhi, M.U.; Syed, I.A.; Ahmed, U.; Miller, M.; Rahim, M. Metformin-Induced Lactic Acidosis: A Case Study. Cureus 2018, 10, e2152. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Schroeder, P. Uptake, translocation and possible biodegradation of the antidiabetic agent metformin by hydroponically grown Typha latifolia. J. Hazard. Mater. 2016, 308, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ussery, E.; Bridges, K.N.; Pandelides, Z.; Kirkwood, A.E.; Bonetta, D.; Venables, B.J.; Guchardi, J.; Holdway, D. Effects of environmentally relevant metformin exposure on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2018, 205, 58–65. [Google Scholar] [CrossRef]
- Wei, J.; Qi, H.; Liu, K.; Zhao, C.; Bian, Y.; Li, G. Effects of Metformin on Life Span, Cognitive Ability, and Inflammatory Response in a Short-Lived Fish. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2020, 75, 2042–2050. [Google Scholar] [CrossRef]
- Li, S.; Hou, Y.; Liu, K.; Zhu, H.; Qiao, M.; Sun, X.; Li, G. Metformin Protects Against Inflammation, Oxidative Stress to Delay Poly I:C-Induced Aging-Like Phenomena in the Gut of an Annual Fish. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2022, 77, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.M.; DeCamp, L.; Birgisson, M.; Palace, V.P.; Kidd, K.A.; Parrott, J.L.; McMaster, M.E.; Alaee, M.; Blandford, N.; Ussery, E.J. Comparative Effects of Embryonic Metformin Exposure on Wild and Laboratory-Spawned Fathead Minnow (Pimephales promelas) Populations. Environ. Sci Technol 2022, 56, 10193–10203. [Google Scholar] [CrossRef]
- Parrott, J.L.; Restivo, V.E.; Kidd, K.A.; Zhu, J.; Shires, K.; Clarence, S.; Khan, H.; Sullivan, C.; Pacepavicius, G.; Alaee, M. Chronic Embryo-Larval Exposure of Fathead Minnows to the Pharmaceutical Drug Metformin: Survival, Growth, and Microbiome Responses. Environ. Toxicol. Chem. 2022, 41, 635–647. [Google Scholar] [CrossRef]
- Parrott, J.L.; Pacepavicius, G.; Shires, K.; Clarence, S.; Khan, H.; Gardiner, M.; Sullivan, C.; Alaee, M. Fathead minnow exposed to environmentally relevant concentrations of metformin for one life cycle show no adverse effects. Facets 2021, 6, 998–1023. [Google Scholar] [CrossRef]
- Sibiya, A.; Al-Ghanim, K.A.; Govindarajan, M.; Nicoletti, M.; Sachivkina, N.; Vaseeharan, B. Biochemical Patterns and Genotoxicity of the Endocrine Disruptor Metformin in the Freshwater Fish (Labeo rohita). Fishes 2023, 8, 380. [Google Scholar] [CrossRef]
- Taher, H.; Sabra, M.S.; El-Din, A.E.-D.S.; Sayed, A.E.-D.H. Hemato-biochemical indices alteration, oxidative stress, and immune suppression in the African catfish (Clarias gariepinus) exposed to metformin. Toxicol. Environ. Health Sci. 2022, 14, 361–369. [Google Scholar] [CrossRef]
- Rogall, E.T.; Jacob, S.; Triebskorn, R.; Schwartz, T. The impact of the anti-diabetic drug metformin on the intestinal microbiome of larval brown trout (Salmo trutta f. fario). Environ. Sci. Eur. 2020, 32, 65. [Google Scholar] [CrossRef]
- Barbieri, P.A.; Mari-Ribeiro, I.P.; Lupepsa, L.; Sinopolis Gigliolli, A.A.; Paupitz, B.R.; de Melo, R.F.; de Souza Leite Mello, E.V.; De Brito Portela-Castro, A.L.; Borin-Carvalho, L.A. Metformin-induced alterations in gills of the freshwater fish (Astyanax lacustris) (Lutken, 1875) detected by histological and scanning electron microscopy. Ecotoxicology 2022, 31, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Ussery, E.J.; Nielsen, K.M.; Simmons, D.; Pandelides, Z.; Mansfield, C.; Holdway, D. An ‘omits approach to investigate the growth effects of environmentally relevant concentrations of guanylurea exposure on Japanese medaka (Oryzias latipes). Aquat. Toxicol. 2021, 232, 105761. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Akemann, C.; Shields, J.N.; Wu, C.-C.; Meyer, D.N.; Baker, B.B.; Pitts, D.K.; Baker, T.R. Developmental phenotypic and transcriptomic effects of exposure to nanomolar levels of metformin in zebrafish. Environ. Toxicol. Pharmacol. 2021, 87, 103716. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.A.; Domingues, I.; Arsenia Nogueira, A.J.; Kummrow, F. Ecotoxicological effects, water quality standards and risk assessment for the anti-diabetic metformin. Environ. Pollut. 2018, 243, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, G.; Reyes-Carrillo, G.I.; Sarma, S.S.S.; Nandini, S. Population level responses of rotifers (Brachionus calyciflorus and Plationus patulus) to the anti-diabetic drug, metformin. J. Environ. Biol. 2017, 38, 1213–1219. [Google Scholar] [CrossRef]
- Cummings, B.M.; Needoba, J.A.; Peterson, T.D. Effect of metformin exposure on growth and photosynthetic performance in the unicellular freshwater chlorophyte, Chlorella vulgaris. PLoS ONE 2018, 13, e0207041. [Google Scholar] [CrossRef]
| Detection Methods | Advantages | Limitations | Applicability | LOD | LOQ | References |
|---|---|---|---|---|---|---|
| High-performance liquid chromatography (HPLC) | High separation performance and good detection sensitivity; without the limitations of volatility and thermal stability of the analytes | Costly, sensitive to temperature changes, more time-consuming than gas chromatography | Metformin hydrochloride | 0.8 mg/L | 2.45 mg/L | [13] |
| Electrochemical analysis techniques | Highly sensitive and accurate, have a wide measuring range, and complete analysis quickly | Suffer from interference, heavy metal electrodes are toxic and not suitable for long-term work, reaction intermediates or products influence the results | Metformin | - | - | [14,15] |
| Spectrophotometry | Simple operation, high efficiency, easy maintenance, and high sensitivity | Not suitable for the determination of large quantities of substances | Metformin | 226 mg/L | 674.5 mg/L | [16,17] |
| Capillary electrophoresis | Simple operation, low sample volume, high separation efficiency, low cost, high separation capacity, high separation speed, and small feed volume | Less reproducible than HPLC in terms of migration time, injection precision, and detection sensitivity | Metformin | 60 mg/L | 100 mg/L | [18] |
| Thin-layer chromatography (TLC) | Ease of operation, simple equipment, and easy color development, Wide range of applications, Shorter time, higher resolution than paper chromatography, relatively cheap | Poor separation of biomolecules compared to HPLC | Metformin hydrochloride | 6160.85 ng per band | 18,669.26 ng per band | [19] |
| Liquid chromatography–mass spectrometry (LC–MS) | High sensitivity and specificity, more accurate identification of compound structure, and reliable quantitative and qualitative results | High instrumentation and maintenance costs | Metformin | - | 17.8 ng/L | [20] |
| Fish | Concentration | Negative Impact | References |
|---|---|---|---|
| Nothobranchius guentheri | 2 mg/g (food) | -Increased longevity -Improvement of cognitive skills -Suppression of the inflammatory response | [58] |
| 2 mg/g (food) | -Delayed aging -Resistance to oxidative stress and inflammation | [59] | |
| Pimephales promelas | 5 and 50 μg/L | -Energy homeostasis disorders and visual effects | [60] |
| 0.02, 3.44, 33.6, 269 μg/L | -microbial flora disorder | [61] | |
| 3.0, 31, 322 μg/L | -Delayed onset of reproduction 9–10 d | [62] | |
| Labeo rohita | 40 and 80 μg/L | -Increased reactive oxygen species and free radical production, DNA damage | [63] |
| Clarias gariepinus | 10 and 50 mg/L | -Suppression of immunity in exposed treated fish can be through activation of lymphocytes and monocytes -Induces cellular oxidative stress by reducing superoxide dismutase (SOD) antioxidant enzymes and total antioxidant capacity (TAC) -Increased expression of the inflammatory mediatorsinterleukin-6 (IL-6) and interleukin-1β (IL-1β) | [64] |
| Salmo trutta f. fario | 1, 10, 100, and 1000 μg/L | -In vitro expression of fish pathogen virulence genes induced by metformin and the effect of metformin on microbiome composition in juvenile brown trout (Oncorhynchus mykiss) | [65] |
| Astyanax lacustris | 50, 100, 1000, and 10,000 μg/L | -Plate fusion, capillary dilatation and proliferation, and disappearance of microridges, observed morphological changes that may interfere with gill physiology | [66] |
| Oryzias latipes | 1, 3.2, 10, 32, and 100 ng/L | -Alteration of many important pathways associated with the overall health of ELS fish, including biomolecular metabolism, cellular energetics, nervous system function/development, cellular communication and structure, and reactive oxygen species detoxification | [67] |
| Danio rerio | 1, 10, 100, 1000, and 10,000 ng/L | -Motor activity was affected and several genes involved in neurological and cardiovascular development were differently expressed after exposure to metformin. | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Shao, Y.; Zhang, Y.; Liu, Z.; Zhao, Z.; Xu, R.; Ding, J.; Li, W.; Wang, B.; Zhang, H. Metformin as an Emerging Pollutant in the Aquatic Environment: Occurrence, Analysis, and Toxicity. Toxics 2024, 12, 483. https://doi.org/10.3390/toxics12070483
Zheng Y, Shao Y, Zhang Y, Liu Z, Zhao Z, Xu R, Ding J, Li W, Wang B, Zhang H. Metformin as an Emerging Pollutant in the Aquatic Environment: Occurrence, Analysis, and Toxicity. Toxics. 2024; 12(7):483. https://doi.org/10.3390/toxics12070483
Chicago/Turabian StyleZheng, Yueyue, Yongjian Shao, Yinan Zhang, Zhiquan Liu, Zirui Zhao, Ranyun Xu, Jiafeng Ding, Wenbing Li, Binhao Wang, and Hangjun Zhang. 2024. "Metformin as an Emerging Pollutant in the Aquatic Environment: Occurrence, Analysis, and Toxicity" Toxics 12, no. 7: 483. https://doi.org/10.3390/toxics12070483
APA StyleZheng, Y., Shao, Y., Zhang, Y., Liu, Z., Zhao, Z., Xu, R., Ding, J., Li, W., Wang, B., & Zhang, H. (2024). Metformin as an Emerging Pollutant in the Aquatic Environment: Occurrence, Analysis, and Toxicity. Toxics, 12(7), 483. https://doi.org/10.3390/toxics12070483






