Variability in Microbial Communities Driven by Particulate Matter on Human Facial Skin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. 16S rRNA Gene Amplification and Sequencing Processing
2.3. Analysis of Particulate Matter Chemical Compositions
2.4. Electron Paramagnetic Resonance
2.5. Health Risk Assessment
| Model Parameters | Abbreviations | Values |
|---|---|---|
| Dermal adherence factors | AF | 2000 mg/m2 |
| Dermal absorption factor | ABS | 0.13 |
| Total exposed skin area | SA | m2 |
| Exposure duration | ED | 25 years |
| Exposure frequency | EF | 313 days/year |
| Body weight | BW | kg |
| Averaging time | AT | 25,550 days |
2.6. Statistical Analysis
3. Results
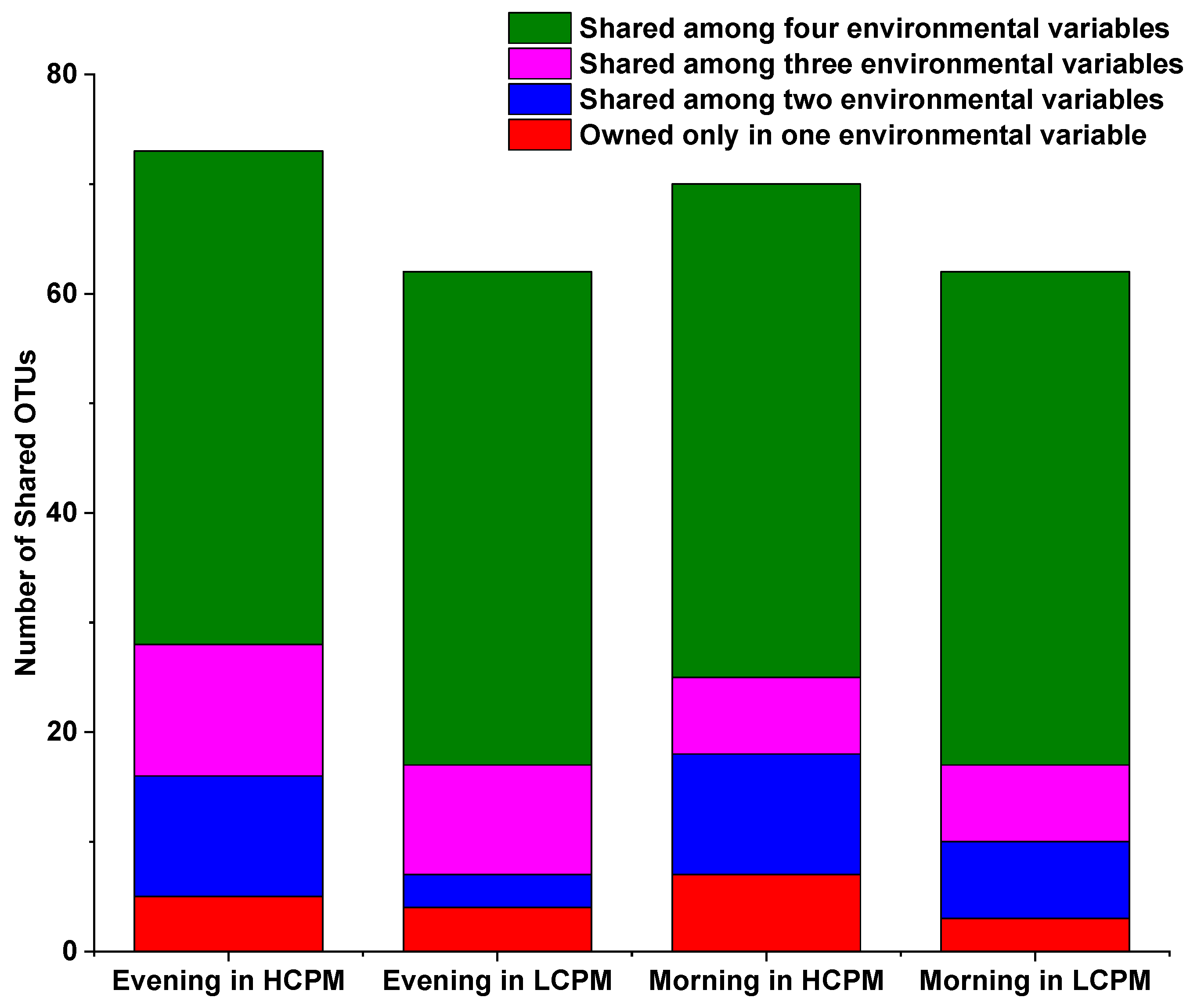
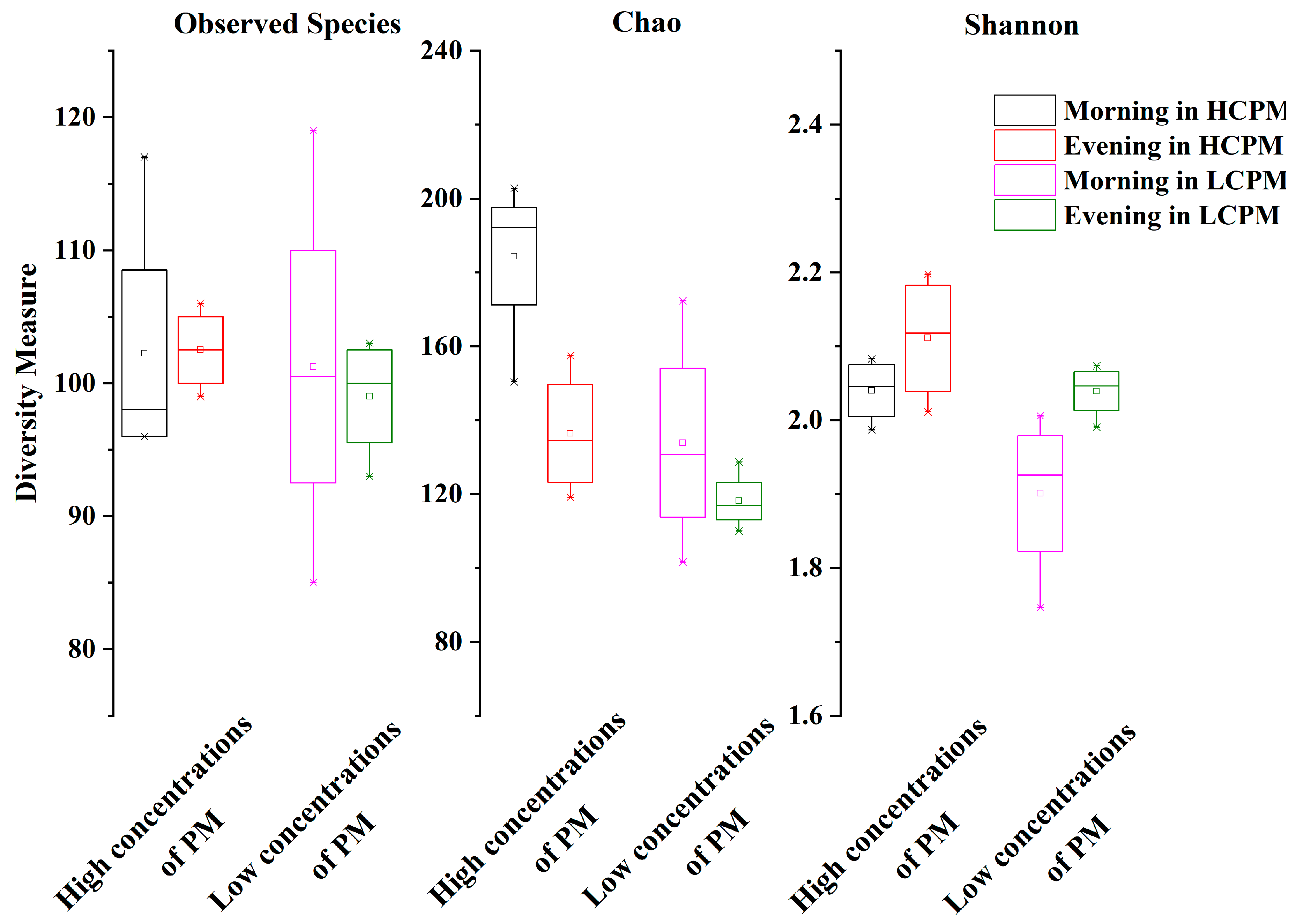
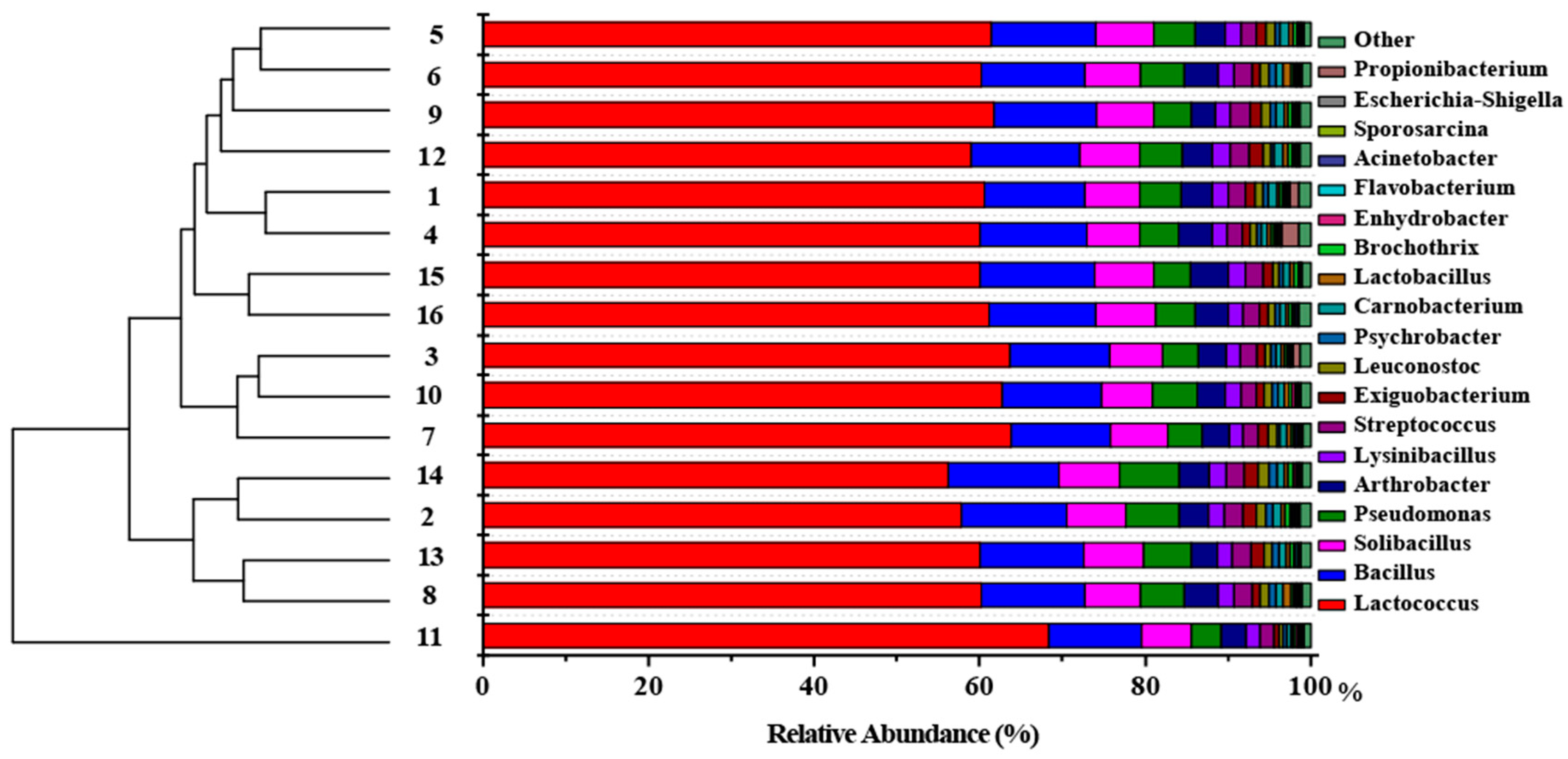
3.1. The Variability in Microbial Community Structure
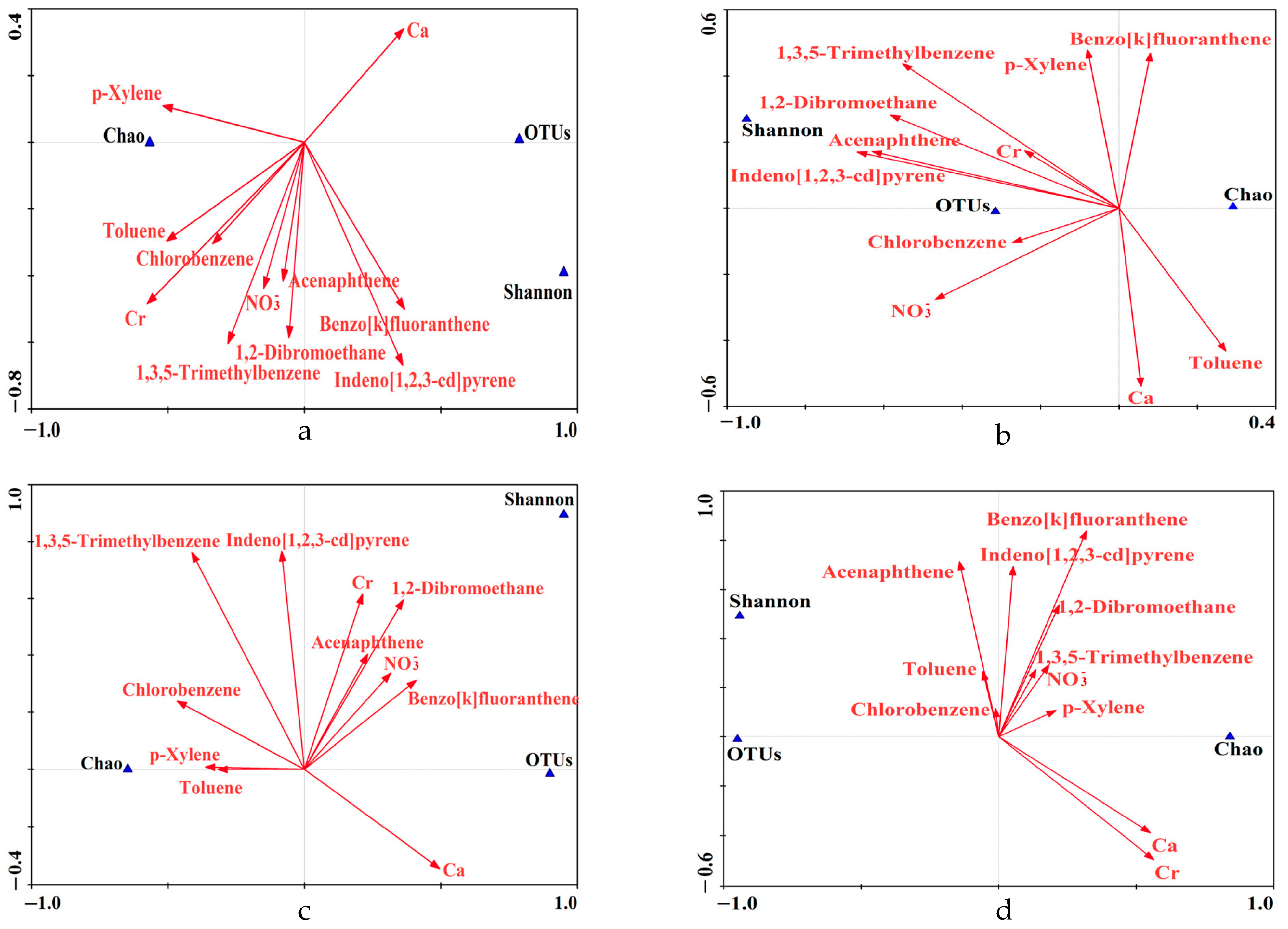
3.2. Relationships between Microbial Communities and Chemical Compositions
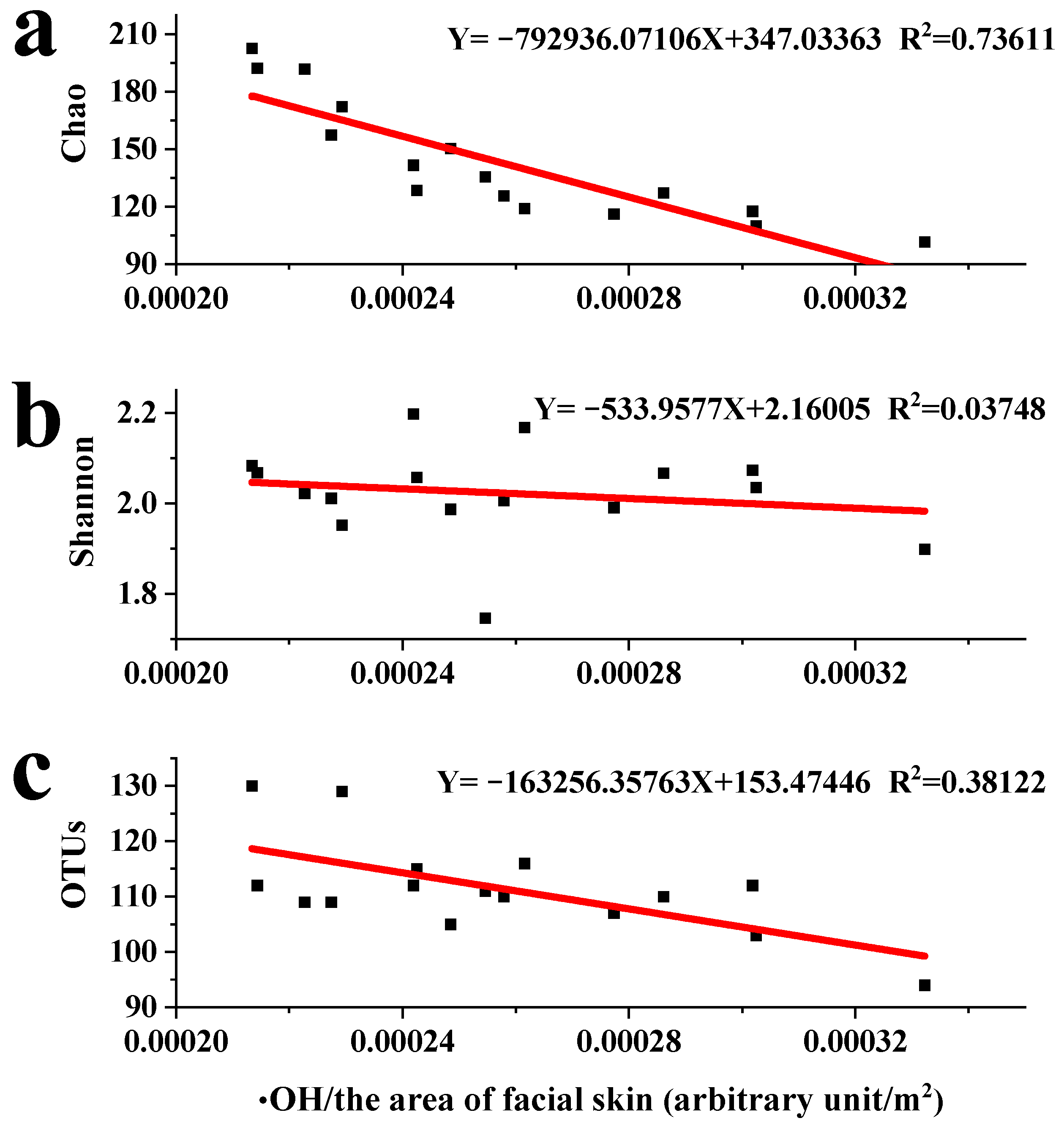
3.3. Reactive Species Influence Microbial Communities
3.4. Health Risks from PM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | particulate matter |
| US EPA | US Environmental Protection Agency |
| PAHs | polycyclic aromatic hydrocarbons |
| VOCs | volatile organic compounds |
| CCA | canonical correspondence analysis |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| ·OH | hydroxyl radicals |
| EPR | electron paramagnetic resonance |
| CNEMC | China National Environmental Monitoring Centre |
| PCR | polymerase chain reaction |
| Operational taxonomic units | OTUs |
| RDP | Ribosomal Database Project |
| DMPO | 5,5-dimethyl-1-pyrroline-N-oxide |
| HQ | hazard quotient |
| CR | carcinogenic risk |
| SF | slope factor |
| AF | dermal adherence factors |
| ABS | dermal absorption factor |
| SA | skin area |
| ED | exposure duration |
| EF | exposure frequency |
| BW | body weight |
| AT | averaging time |
| NaP | naphthalene |
| Acy | acenaphthylene |
| Acen | acenaphthene |
| Fl | fluorene |
| Phe | phenanthrene |
| Ant | anthracene |
| Flu | fluoranthene |
| Pyr | pyrene |
| BaA | benzo[a]anthracene |
| Chr | chrysene |
| BbF | benzo[b]fluoranthene |
| BkF | benzo[k]fluoranthene |
| BaP | benzo[a]pyrene |
| InP | indeno[1,2,3-cd]pyrene |
| DbA | dibenzo[a,h]anthracene |
| BgP | benzo[g,h,i]perylene |
| VIP | variable importance |
References
- Luo, H.; Guan, Q.; Lin, J.; Wang, Q.; Yang, L.; Tan, Z.; Wang, N. Air pollution characteristics and human health risks in key cities of northwest China. J. Environ. Manag. 2020, 269, 110791. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, R.; Corringham, T.; Gershunov, A.; Benmarhnia, T. Wildfire smoke impacts respiratory health more than fine particles from other sources: Observational evidence from Southern California. Nat. Commun. 2021, 12, 1493. [Google Scholar] [CrossRef] [PubMed]
- Jbaily, A.; Zhou, X.D.; Liu, J.; Lee, T.H.; Kamareddine, L.; Verguet, S.; Dominici, F. Air pollution exposure disparities across US population and income groups. Nature 2022, 601, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [PubMed]
- Grigorieva, E.; Lukyanets, A. Combined Effect of Hot Weather and Outdoor Air Pollution on Respiratory Health: Literature Review. Atmosphere 2021, 12, 790. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, Y.; Wang, J.N.; Xu, D.D.; Yin, Z.X.; Chen, H.S.; Lv, Y.B.; Luo, J.S.; Zeng, Y.; Liu, Y.; et al. All-cause mortality risk associated with long-term exposure to ambient PM2.5 in China: A cohort study. Lancet Public Health 2018, 3, E470–E477. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wei, Y.J.; Fang, Z.F. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Khan, M.D.H.; Jolly, Y.N.; Kabir, J.; Akter, S.; Salam, A. Assessing risk to human health for heavy metal contamination through street dust in the Southeast Asian Megacity: Dhaka, Bangladesh. Sci. Total Environ. 2019, 660, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef]
- Manus, M.B.; Yu, J.J.; Park, L.P.; Mueller, O.; Windsor, S.C.; Horvath, J.E.; Nunn, C.L. Environmental influences on the skin microbiome of humans and cattle in rural Madagascar. Evol. Med. Public Health 2017, 2017, 144–153. [Google Scholar] [CrossRef]
- Chen, C.Y.; He, R.Q.; Cheng, Z.Y.; Han, M.Z.; Zha, Y.G.; Yang, P.S.; Yao, Q.; Zhou, H.; Zhong, C.F.; Ning, K. The Seasonal Dynamics and the Influence of Human Activities on Campus Outdoor Microbial Communities. Front. Microbiol. 2019, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- Flies, E.J.; Clarke, L.J.; Brook, B.W.; Jones, P. Urbanisation reduces the abundance and diversity of airborne microbes—But what does that mean for our health? A systematic review. Sci. Total Environ. 2020, 738, 140337. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Fu, K.; Hu, X.; Li, X.; Lai, Z.; Yuan, P. Relationships between Airborne Microbial Community Diversity, Heating Supply Patterns and Particulate Matter Properties. J. Environ. Chem. Eng. 2022, 10, 107309. [Google Scholar] [CrossRef]
- Sun, Y.J.; Xu, S.W.; Zheng, D.Y.; Li, J.; Tian, H.Z.; Wang, Y. Effects of haze pollution on microbial community changes and correlation with chemical components in atmospheric particulate matter. Sci. Total Environ. 2018, 637, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Du, P.R.; Du, R.; Ren, W.S.; Lu, Z.D.; Fu, P.Q. Seasonal variation characteristic of inhalable microbial communities in PM2.5 in Beijing city, China. Sci. Total Environ. 2018, 610, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, Y.; Zhao, X.; Wu, J.; Zhu, L.; Zhang, X.; Wei, Z.; Liu, Y.; He, P. Selective pressures of heavy metals on microbial community determine microbial functional roles during composting: Sensitive, resistant and actor. J. Hazard. Mater. 2020, 398, 122858. [Google Scholar] [CrossRef]
- Wu, M.; Guo, X.; Wu, J.; Chen, K. Effect of compost amendment and bioaugmentation on PAH degradation and microbial community shifting in petroleum-contaminated soil. Chemosphere 2020, 256, 126998. [Google Scholar] [CrossRef]
- McBride, S.G.; Osburn, E.D.; Lucas, J.M.; Simpson, J.S.; Brown, T.; Barrett, J.E.; Strickland, M.S. Volatile and Dissolved Organic Carbon Sources Have Distinct Effects on Microbial Activity, Nitrogen Content, and Bacterial Communities in Soil. Microb. Ecol. 2022, 85, 659–668. [Google Scholar] [CrossRef]
- Zhang, X.H.; Li, Z.Y.; Hu, J.M.; Yan, L.; He, Y.Y.; Li, X.; Wang, M.Y.; Sun, X.M.; Xu, H. The biological and chemical contents of atmospheric particulate matter and implication of its role in the transmission of bacterial pathogenesis. Environ. Microbiol. 2021, 23, 5481–5486. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zuo, Z.Q.; Li, H.; Xing, Y.X.; Cheng, D.; Guo, M.; Liu, T.; Zheng, M.; Yuan, Z.G.; Huang, X. In-situ advanced oxidation of sediment iron for sulfide control in sewers. Water Res. 2023, 240, 120077. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Han, R.; Zhang, W.; Zhang, H.; Zhao, L.; Chen, L.; Zhu, L. Advanced oxidation process/coagulation coupled with membrane distillation (AOP/Coag-MD) for efficient ammonia recovery: Elucidating biofouling control performance and mechanism. J. Hazard. Mater. 2024, 469, 134093. [Google Scholar] [CrossRef]
- Chen, H.; Du, R.; Zhang, Y.; Zhang, S.; Ren, W.; Du, P. Survey of background microbial index in inhalable particles in Beijing. Sci. Total Environ. 2021, 757, 143743. [Google Scholar] [CrossRef]
- Sun, Y.J.; Huang, Y.J.; Xu, S.W.; Li, J.; Yin, M.; Tian, H.Z. Seasonal Variations in the Characteristics of Microbial Community Structure and Diversity in Atmospheric Particulate Matter from Clean Days and Smoggy Days in Beijing. Microb. Ecol. 2022, 83, 568–582. [Google Scholar] [CrossRef]
- Qin, N.; Liang, P.; Wu, C.Y.; Wang, G.Q.; Xu, Q.; Xiong, X.; Wang, T.T.; Zolfo, M.; Segata, N.; Qin, H.L.; et al. Longitudinal survey of microbiome associated with particulate matter in a megacity. Genome Biol. 2020, 21, 55. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, H.; Zhang, H.; Zhang, X.; Zhou, M.; Lou, L.; Zheng, P.; Xi, C.; Hu, B. Temporal discrepancy of airborne total bacteria and pathogenic bacteria between day and night. Environ. Res. 2020, 186, 109540. [Google Scholar] [CrossRef] [PubMed]
- Daellenbach, K.R.; Uzu, G.; Jiang, J.H.; Cassagnes, L.E.; Leni, Z.; Vlachou, A.; Stefenelli, G.; Canonaco, F.; Weber, S.; Segers, A.; et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature 2020, 587, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Gunthe, S.S.; Liu, P.F.; Panda, U.; Raj, S.S.; Sharma, A.; Darbyshire, E.; Reyes-Villegas, E.; Allan, J.; Chen, Y.; Wang, X.; et al. Enhanced aerosol particle growth sustained by high continental chlorine emission in India. Nat. Geosci. 2021, 14, 77–84. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, Y.; Hasheminassab, S. Long-term source apportionment of PM2.5 across the contiguous United States (2000–2019) using a multilinear engine model. J. Hazard. Mater. 2024, 472, 134550. [Google Scholar] [CrossRef]
- Fan, S.M.; Xia, B.; Liu, W.X.; Yu, W.; Wu, Z.X.; Chen, S.B.; Liu, Q.H.; Chen, W.J.; Zhu, S.L.; Jin, M.; et al. Establishing an appropriate Z score regression equation for Chinese pediatric coronary artery echocardiography: A multicenter prospective cohort study. BMC Pediatr. 2021, 21, 429. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, Y.; Fan, X.; Ma, H.; Gu, W.R.; Sun, F.J. Evaluation of nine formulas for estimating the body surface area of children with hematological malignancies. Front. Pediatr. 2022, 10, 989049. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, A.; Kuo, K.; Gerken, K.; Cline, K.; Hespel, A.-M.; Cole, R.; Moon, R. Body mapping chart for estimation of percentage of body surface area in mesocephalic dogs. J. Vet. Emerg. Crit. Care 2022, 32, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Patil, A.; Vendrow, E.; Touponse, G.; Aboukhater, L.; Forrester, J.D.; Spain, D.A. Practical Computer Vision Application to Compute Total Body Surface Area Burn Reappraising a Fundamental Burn Injury Formula in the Modern Era. JAMA Surg. 2022, 157, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tripathi, S.; Chandra, R. Metagenomic analysis for profiling of microbial communities and tolerance in metal-polluted pulp and paper industry wastewater. Bioresour. Technol. 2021, 324, 124681. [Google Scholar] [CrossRef] [PubMed]
- Bukin, Y.S.; Galachyants, Y.P.; Morozov, I.V.; Bukin, S.V.; Zakharenko, A.S.; Zemskaya, T.I. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci. Data 2019, 6, 190007. [Google Scholar] [CrossRef]
- Li, Z.; Yang, W.; Xie, L.; Li, Y.; Liu, Y.; Sun, Y.; Bu, Y.; Mi, X.; Zhan, S.; Hu, W. Prominent role of oxygen vacancy for superoxide radical and hydroxyl radical formation to promote electro-Fenton like reaction by W-doped CeO2 composites. Appl. Surf. Sci. 2021, 549, 149262. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Wang, C.; Gu, L.; Chen, H.; Jana, D.; Feng, L.; Liu, J.; Wang, X.; Xu, P.; et al. Self-Assembled Single-Site Nanozyme for Tumor-Specific Amplified Cascade Enzymatic Therapy. Angew. Chem. Int. Ed. Engl. 2021, 60, 3001–3007. [Google Scholar] [CrossRef]
- Zhou, Q.; Song, C.; Wang, P.; Zhao, Z.; Li, Y.; Zhan, S. Generating dual-active species by triple-atom sites through peroxymonosulfate activation for treating micropollutants in complex water. Proc. Natl. Acad. Sci. USA 2023, 120, e2300085120. [Google Scholar] [CrossRef]
- Tao, H.; Wang, Y.J.; Liang, H.H.; Zhang, X.H.; Liu, X.P.; Li, J.L. Pollution characteristics of phthalate acid esters in agricultural soil of Yinchuan, northwest China, and health risk assessment. Environ. Geochem. Health 2020, 42, 4313–4326. [Google Scholar] [CrossRef]
- Santamouris, M.; Synnefa, A.; Asssimakopoulos, M.; Livada, I.; Pavlou, K.; Papaglastra, M.; Gaitani, N.; Kolokotsa, D.; Assimakopoulos, V. Experimental investigation of the air flow and indoor carbon dioxide concentration in classrooms with intermittent natural ventilation. Energy Build. 2008, 40, 1833–1843. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Integrated Risk Information System (Electronic Data Base). 2002. Available online: http://www.epa.gov/iris (accessed on 25 June 2024).
- Pragg, C.; Mohammed, F.K. Distribution and health risk assessment of heavy metals in road dust from an industrial estate in Trinidad, West Indies. Int. J. Environ. Health Res. 2020, 30, 336–343. [Google Scholar] [CrossRef]
- Wang, R.; Liu, G.; Zhang, J. Variations of emission characterization of PAHs emitted from different utility boilers of coal-fired power plants and risk assessment related to atmospheric PAHs. Sci. Total Environ. 2015, 538, 180–190. [Google Scholar] [PubMed]
- Li, X.; Shang, X.; Luo, T.; Du, X.; Wang, Y.; Xie, Q.; Matsuura, N.; Chen, J.; Kadokami, K. Screening and health risk of organic micropollutants in rural groundwater of Liaodong Peninsula, China. Environ. Pollut. 2016, 218, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Mndela, M.; Madakadze, C.I.; Nherera-Chokuda, F.; Dube, S. Is the soil seed bank a reliable source for passive restoration of bush-cleared semi-arid rangelands of South Africa? Ecol. Process. 2020, 9, 1. [Google Scholar] [CrossRef]
- Borrego, S.; Vivar, I.; Molina, A. Air- and dustborne fungi in repositories of the National Archive of the Republic of Cuba. Microb. Cell 2022, 9, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Lau, F.; Zhang, T. Linking Microbial Community, Environmental Variables, and Methanogenesis in Anaerobic Biogas Digesters of Chemically Enhanced Primary Treatment Sludge. Environ. Sci. Technol. 2017, 51, 3982–3992. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Shu, H.-y.; Lin, X.-r.; Zhou, Q.-x.; Bramryd, T.; Shu, W.-s.; Huang, L.-n. Microbial community structure and function in sediments from e-waste contaminated rivers at Guiyu area of China. Environ. Pollut. 2018, 235, 171–179. [Google Scholar] [PubMed]
- Xu, C.; Chen, H.; Liu, Z.; Sui, G.; Li, D.; Kan, H.; Zhao, Z.; Hu, W.; Chen, J. The decay of airborne bacteria and fungi in a constant temperature and humidity test chamber. Environ. Int. 2021, 157, 106816. [Google Scholar] [CrossRef]
- Hou, Z.; Zhou, Q.; Mo, F.; Kang, W.; Ouyang, S. Enhanced carbon emission driven by the interaction between functional microbial community and hydrocarbons: An enlightenment for carbon cycle. Sci. Total Environ. 2023, 867, 161402. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Ma, K.; Tang, A.; Goulding, K.; Liu, X. Characteristics of airborne bacterial communities across different PM2.5 levels in Beijing during winter and spring. Atmos. Res. 2022, 273, 106179. [Google Scholar] [CrossRef]
- Meadow, J.F.; Altrichter, A.E.; Bateman, A.C.; Stenson, J.; Brown, G.Z.; Green, J.L.; Bohannan, B. Humans differ in their personal microbial cloud. PeerJ 2015, 3, e1258. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, Y.; Xie, W.; Lu, R.; Mu, F.; Bai, W.; Du, S. Temporal-spatial variations of fungal composition in PM2.5 and source tracking of airborne fungi in mountainous and urban regions. Sci. Total Environ. 2020, 708, 135027. [Google Scholar] [CrossRef]
- Kelly, L.W.; Nelson, C.E.; Haas, A.F.; Naliboff, D.S.; Calhoun, S.; Carlson, C.A.; Edwards, R.A.; Fox, M.D.; Hatay, M.; Johnson, M.D.; et al. Diel population and functional synchrony of microbial communities on coral reefs. Nat. Commun. 2019, 10, 1691. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zhang, T.; Su, J.; Zhao, L.L.; Wang, H.; Fang, X.M.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Structural Variation in the Bacterial Community Associated with Airborne Particulate Matter in Beijing, China, during Hazy and Nonhazy Days. Appl. Environ. Microbiol. 2018, 84, e00004-18. [Google Scholar] [CrossRef] [PubMed]
- Balato, A.; Cacciapuoti, S.; Di Caprio, R.; Marasca, C.; Masarà, A.; Raimondo, A.; Fabbrocini, G. Human Microbiome: Composition and Role in Inflammatory Skin Diseases. Arch. Immunol. Ther. Exp. 2019, 67, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Casterline, B.W.; Paller, A.S. Early development of the skin microbiome: Therapeutic opportunities. Pediatr. Res. 2021, 90, 731–737. [Google Scholar] [CrossRef]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef]
- Kelsic, E.D.; Zhao, J.; Vetsigian, K.; Kishony, R. Counteraction of antibiotic production and degradation stabilizes microbial communities. Nature 2015, 521, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Sperfeld, M.; Rauschenbach, C.; Diekert, G.; Studenik, S. Microbial community of a gasworks aquifer and identification of nitrate-reducing Azoarcus and Georgfuchsia as key players in BTEX degradation. Water Res. 2018, 132, 146–157. [Google Scholar]
- Gholami, F.; Mosmeri, H.; Shavandi, M.; Dastgheib, S.M.M.; Amoozegar, M.A. Application of encapsulated magnesium peroxide (MgO2) nanoparticles in permeable reactive barrier (PRB) for naphthalene and toluene bioremediation from groundwater. Sci. Total Environ. 2019, 655, 633–640. [Google Scholar] [CrossRef]
- Shu, W.; Zhang, Y.; Wen, D.; Wu, Q.; Liu, H.; Cui, M.-H.; Fu, B.; Zhang, J.; Yao, Y. Anaerobic biodegradation of levofloxacin by enriched microbial consortia: Effect of electron acceptors and carbon source. J. Hazard. Mater. 2021, 414 (Suppl. S4), 125520. [Google Scholar] [PubMed]
- Gao, M.; Li, L.; Liu, J. Simultaneous removal of hydrogen sulfide and toluene in a bioreactor: Performance and characteristics of microbial community. J. Environ. Sci. 2011, 23, 353–359. [Google Scholar]
- Sun, F.-L.; Fan, L.-L.; Wang, Y.-S.; Huang, L.-Y. Metagenomic analysis of the inhibitory effect of chromium on microbial communities and removal efficiency in A2O sludge. J. Hazard. Mater. 2019, 368, 523–529. [Google Scholar] [PubMed]
- Araujo, A.S.F.; Miranda, A.R.L.; Pereira, A.P.d.A.; de Melo, W.J.; Melo, V.M.M.; Ventura, S.H.; Brito Junior, E.S.; de Medeiros, E.V.; Araujo, F.F.; Mendes, L.W. Microbial communities in the rhizosphere of maize and cowpea respond differently to chromium contamination. Chemosphere 2023, 313, 137417. [Google Scholar] [PubMed]
- Hahm, M.S.; Son, J.S.; Kim, B.S.; Ghim, S.Y. Comparative study of rhizobacterial communities in pepper greenhouses and examination of the effects of salt accumulation under different cropping systems. Arch. Microbiol. 2017, 199, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Brookes, P.C.; Wu, J. Addition impact of biochar from different feed stocks on microbial community and available concentrations of elements in a Psammaquent and a Plinthudult. J. Soil Sci. Plant Nutr. 2016, 16, 137–153. [Google Scholar]
- Albina, P.; Durban, N.; Bertron, A.; Albrecht, A.; Robinet, J.C.; Erable, B. Influence of Hydrogen Electron Donor, Alkaline pH, and High Nitrate Concentrations on Microbial Denitrification: A Review. Int. J. Mol. Sci. 2019, 20, 5163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, M.; Mur, L.; Shen, Q.; Guo, S. Unravelling the Roles of Nitrogen Nutrition in Plant Disease Defences. Int. J. Mol. Sci. 2020, 21, 572. [Google Scholar] [CrossRef]
- Huang, R.Y.; Tian, W.J.; Liu, Q.; Yu, H.B.; Jin, X.; Zhao, Y.G.; Zhou, Y.H.; Feng, G. Enhanced biodegradation of pyrene and indeno(1,2,3-cd)pyrene using bacteria immobilized in cinder beads in estuarine wetlands. Mar. Pollut. Bull. 2016, 102, 128–133. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, L.; Jin, C.; Xiao, N.; Jadeja, R.N.; Sun, T. Enzyme Responses to Phytoremediation of PAH-Contaminated Soil Using Echinacea purpurea (L.). Water Air Soil Pollut. 2014, 225, 2230. [Google Scholar]
- Fang, T.; Lakey, P.; Weber, R.J.; Shiraiwa, M. Oxidative Potential of Particulate Matter and Generation of Reactive Oxygen Species in Epithelial Lining Fluid. Environ. Sci. Technol. 2019, 53, 12784–12792. [Google Scholar] [CrossRef]
- Li, X.; Kuang, X.M.; Yan, C.; Ma, S.; Paulson, S.E.; Zhu, T.; Zhang, Y.; Zheng, M. Oxidative Potential by PM2.5 in the North China Plain: Generation of Hydroxyl Radical. Environ. Sci. Technol. 2019, 53, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Rui, D.; Lu, Z.; Ren, W.; Fu, P. Variation of Bacterial and Fungal Community Structures in PM2.5 Collected during the 2014 APEC Summit Periods. Aerosol Air Qual. Res. 2018, 18, 444–455. [Google Scholar] [CrossRef]
- Atzei, D.; Fermo, P.; Vecchi, R.; Fantauzzi, M.; Comite, V.; Valli, G.; Cocco, F.; Rossi, A. Composition and origin of PM2.5 in Mediterranean Countryside. Environ. Pollut. 2019, 246, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; He, Z.; Ni, S.; Chen, W.; Li, N.; Sun, S. Investigation of PM2.5 absorbed with heavy metal elements, source apportionment and their health impacts in residential houses in the North-east region of China. Sustain. Cities Soc. 2019, 51, 101690. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Li, Y.-C.; Amesho, K.T.T.; Chou, F.-C.; Cheng, P.-C. Characterization and quantification of PM2.5 emissions and PAHs concentration in PM2.5 from the exhausts of diesel vehicles with various accumulated mileages. Sci. Total Environ. 2019, 660, 188–198. [Google Scholar]
- Niu, Y.; Wang, F.; Liu, S.; Zhang, W. Source analysis of heavy metal elements of PM2.5 in canteen in a university in winter. Atmos. Environ. 2021, 244, 117879. [Google Scholar] [CrossRef]
- Idris, S.A.‘A.; Hanafiah, M.M.; Khan, M.F.; Hamid, H.H.A. Indoor generated PM2.5 compositions and volatile organic compounds: Potential sources and health risk implications. Chemosphere 2020, 255, 126932. [Google Scholar] [CrossRef]

| Samples Collection | Concentrations of PM2.5/μg/m3 | Concentrations of PM10/μg/m3 |
|---|---|---|
| High concentrations of PM | 139 | 183 |
| 207 | 290 | |
| 166 | 202 | |
| 160 | 196 | |
| Low concentrations of PM | 39 | 54 |
| 45 | 79 | |
| 27 | 50 | |
| 49 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, K.; Zhou, Q.; Wang, H. Variability in Microbial Communities Driven by Particulate Matter on Human Facial Skin. Toxics 2024, 12, 497. https://doi.org/10.3390/toxics12070497
Fu K, Zhou Q, Wang H. Variability in Microbial Communities Driven by Particulate Matter on Human Facial Skin. Toxics. 2024; 12(7):497. https://doi.org/10.3390/toxics12070497
Chicago/Turabian StyleFu, Kai, Qixing Zhou, and Heli Wang. 2024. "Variability in Microbial Communities Driven by Particulate Matter on Human Facial Skin" Toxics 12, no. 7: 497. https://doi.org/10.3390/toxics12070497





