Progress in Research on the Role of the Thioredoxin System in Chemical Nerve Injury
Abstract
1. Introduction
2. Trx System
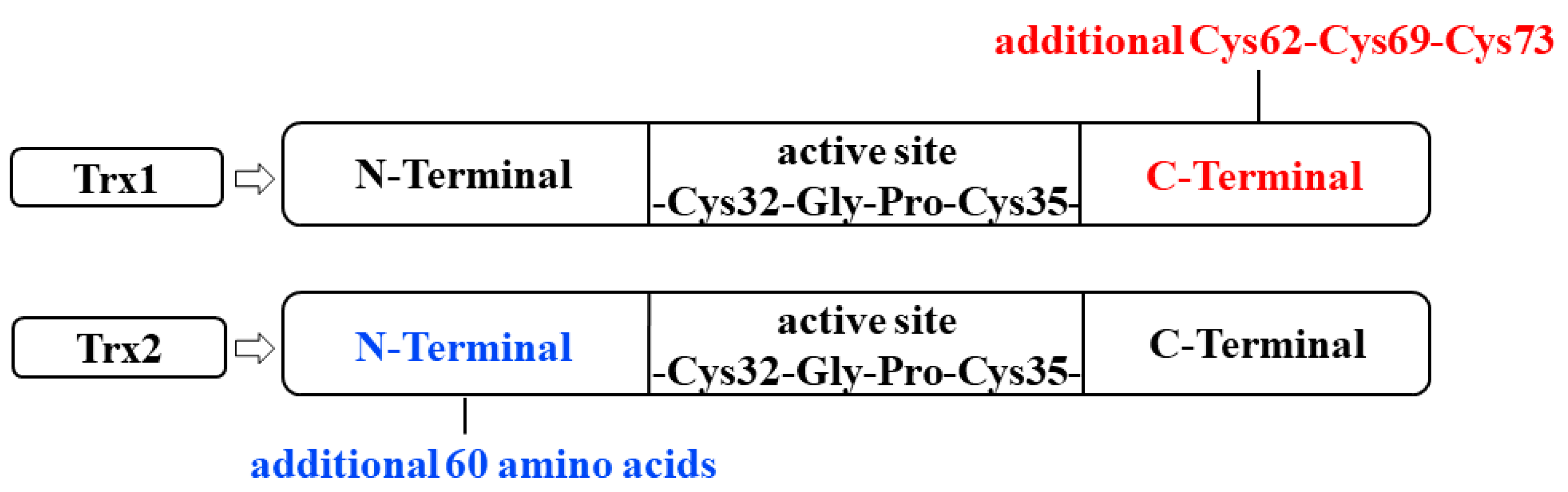
2.1. Trx
2.2. TrxR
2.3. NADPH
2.4. TXNIP
3. Chemicals Damaging Nerves by Disrupting Trx System Homeostasis
3.1. Chemicals Damaging Nerves through Abnormal Trx Expression
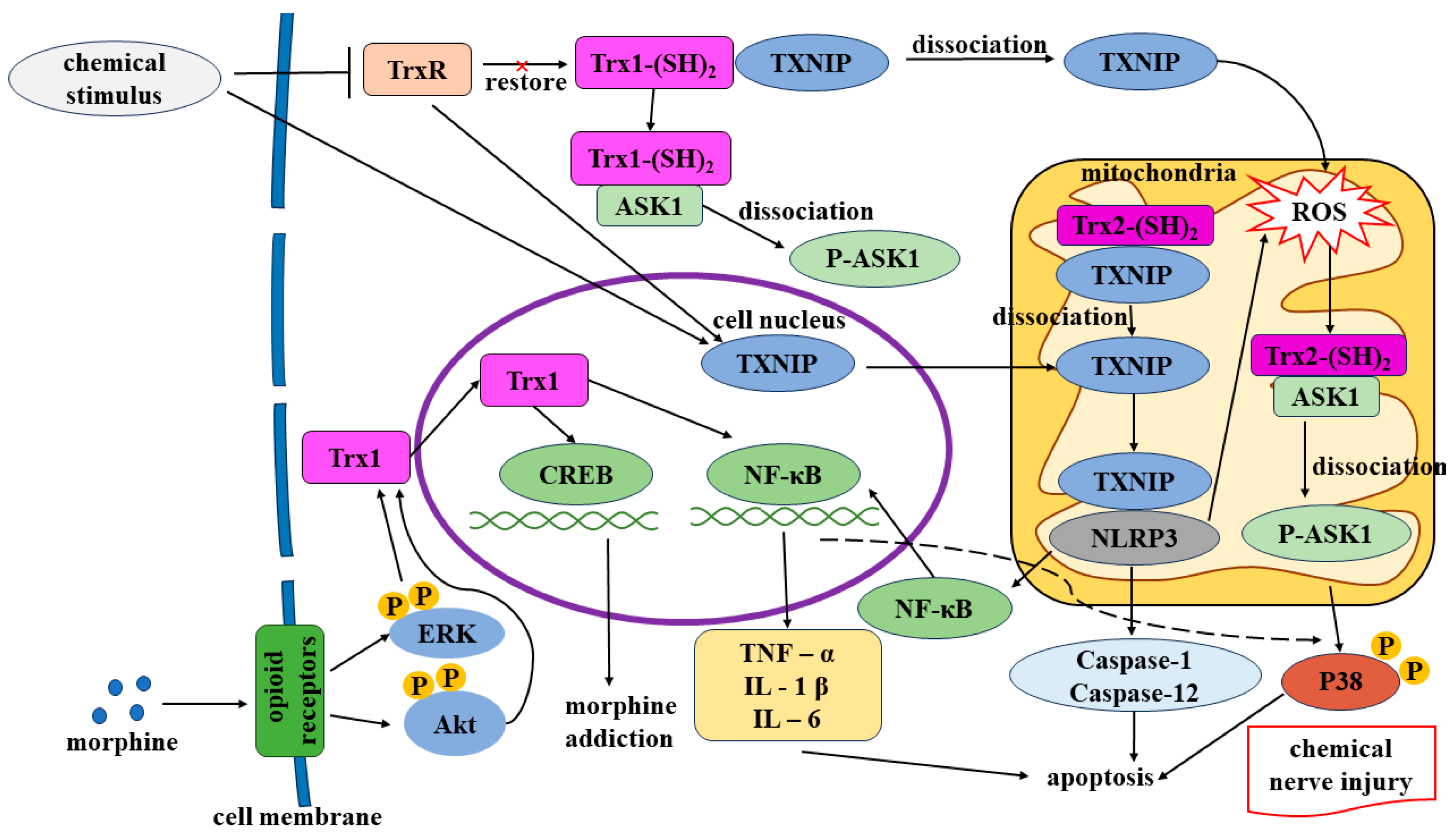
3.1.1. Morphine-Induced Overexpression of Trx Causes Nerve Damage
3.1.2. Methamphetamine-Induced Inhibition of Trx Expression Causes Nerve Damage
3.2. Chemicals Damaging Nerves through the Inhibition of TrxR Activity
3.2.1. Methylglyoxal Inhibits TrxR-Induced Nerve Damage
3.2.2. Metals and Metalloids Inhibit Trx and TrxR, Inducing Nerve Damage
3.3. Chemicals Damaging Nerves through the Activation of TXNIP
3.3.1. Streptozotocin-Induced Activation of TXNIP Causes Nerve Damage
3.3.2. Nitrous Oxide-Induced Activation of TXNIP Causes Nerve Damage
4. Regulation of Trx System Homeostasis Mitigates Nerve Damage
4.1. Upregulation of Trx Transcription and Expression Mitigates Nerve Damage
4.1.1. Sulforaphane-Induced Expression of Trx Mitigates Nerve Damage
4.1.2. N-Acetylcysteine-Induced Expression of Trx Mitigates Nerve Damage
4.2. Selenium Supplementation-Induced Enhancement of TrxR1 Activity Mitigates Nerve Damage
4.3. Inhibition of TXNIP Mitigates Nerve Damage
4.3.1. Vitamin D3-Induced Inhibition of TXNIP Mitigates Nerve Damage
4.3.2. Resveratrol-Induced Inhibition of TXNIP Expression Mitigates Nerve Damage
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AAPA | 2-acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulf anylthiocarbonylamino)phenylthiocarbamoylsulfanyl]propionic acid |
| AD | Alzheimer’s disease |
| Akt | Protein kinase B |
| ASK1 | Apoptotic signaling-regulated kinase 1 |
| ATP | Adenosine triphosphate |
| Bax | B-cell lymphoma-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| cAMP | Cyclic adenosine monophosphate |
| CHOP | CCAAT/enhancer-binding protein homologous protein |
| CRE | Cyclic adenosine monophosphate-responsive element |
| CREB | Cyclic adenosine monophosphate-responsive element-binding protein |
| CVNVGC | -Cys-Val-Asn-Val-Gly-Cys- |
| DM | Diabetes mellitus |
| DPN | Diabetic peripheral neuropathy |
| ERK | Extracellular signal-regulated kinase |
| GSH | Glutathione |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| JNK | Phosphorylated c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MPP+ | 1-methyl-4-phenylpyridinium ion |
| NAc | Nucleus accumbens |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear transcription factor-κB |
| NLRP3 | Pyrin domain-containing-3 |
| PD | Parkinson’s disease |
| PI3K | Phosphatidylinositol-3-kinase |
| ROS | Reactive oxygen species |
| TNF-α | Tumor necrosis factor-α |
| Trx | Thioredoxin |
| TrxR | Trx reductase |
| TXNIP | Trx-interacting protein |
| VTA | Ventral tegmental area |
References
- Pena, S.A.; Iyengar, R.; Eshraghi, R.S.; Bencie, N.; Mittal, J.; Aljohani, A.; Mittal, R.; Eshraghi, A.A. Gene Therapy for Neurological Disorders: Challenges and Recent Advancements. J. Drug Target. 2020, 28, 111–128. [Google Scholar] [CrossRef]
- Gunata, M.; Parlakpinar, H.; Acet, H.A. Melatonin: A Review of Its Potential Functions and Effects on Neurological Diseases. Rev. Neurol. 2020, 176, 148–165. [Google Scholar] [CrossRef]
- Yang, H.; Li, L.; Jiao, Y.; Zhang, Y.; Wang, Y.; Zhu, K.; Sun, C. Thioredoxin-1 Mediates Neuroprotection of Schisanhenol against MPP+-Induced Apoptosis via Suppression of ASK1-P38-NF-κB Pathway in SH-SY5Y Cells. Sci. Rep. 2021, 11, 21604. [Google Scholar] [CrossRef]
- Vincent, A.M.; Edwards, J.L.; McLean, L.L.; Hong, Y.; Cerri, F.; Lopez, I.; Quattrini, A.; Feldman, E.L. Mitochondrial Biogenesis and Fission in Axons in Cell Culture and Animal Models of Diabetic Neuropathy. Acta Neuropathol. 2010, 120, 477–489. [Google Scholar] [CrossRef]
- Ishrat, T.; Mohamed, I.N.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Ergul, A.; El-Remessy, A.B.; Fagan, S.C. Thioredoxin-Interacting Protein: A Novel Target for Neuroprotection in Experimental Thromboembolic Stroke in Mice. Mol. Neurobiol. 2015, 51, 766–778. [Google Scholar] [CrossRef]
- Bjørklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants 2022, 11, 2161. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, I.N.; Hafez, S.S.; Fairaq, A.; Ergul, A.; Imig, J.D.; El-Remessy, A.B. Thioredoxin-Interacting Protein Is Required for Endothelial NLRP3 Inflammasome Activation and Cell Death in a Rat Model of High-Fat Diet. Diabetologia 2014, 57, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D.F.D.; Abderrazak, A.; El Hadri, K.; Simmet, T.; Rouis, M. The Thioredoxin System as a Therapeutic Target in Human Health and Disease. Antioxid. Redox Signal. 2013, 19, 1266–1303. [Google Scholar] [CrossRef] [PubMed]
- Pekkari, K.; Holmgren, A. Truncated Thioredoxin: Physiological Functions and Mechanism. Antioxid. Redox Signal. 2004, 6, 53–61. [Google Scholar] [CrossRef]
- Laurent, T.C.; Moore, E.C.; Reichard, P. Enzymatic Synthesis of Deoxyribonucleotides. IV. Isolation and Characterization of Thioredoxin, the Hydrogen Donor from Escherichia coli B. J. Biol. Chem. 1964, 239, 3436–3444. [Google Scholar] [CrossRef]
- Xinastle-Castillo, L.O.; Landa, A. Physiological and Modulatory Role of Thioredoxins in the Cellular Function. Open Med. 2022, 17, 2021–2035. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Skrzydlewska, E. Thioredoxin-Dependent System. Application of Inhibitors. J. Enzym. Inhib. Med. Chem. 2021, 36, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Nasoohi, S.; Ismael, S.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and Neurodegenerative Diseases: Regulation and Implication. Mol. Neurobiol. 2018, 55, 7900–7920. [Google Scholar] [CrossRef] [PubMed]
- Chiueh, C.; Lee, S.; Andoh, T.; Murphy, D. Induction of Antioxidative and Antiapoptotic Thioredoxin Supports Neuroprotective Hypothesis of Estrogen. Endocrine 2003, 21, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Noike, T.; Miwa, S.; Soeda, J.; Kobayashi, A.; Miyagawa, S. Increased Expression of Thioredoxin-1, Vascular Endothelial Growth Factor, and Redox Factor-1 Is Associated with Poor Prognosis in Patients with Liver Metastasis from Colorectal Cancer. Hum. Pathol. 2008, 39, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.G.; Flores, L.C.; Cunningham, G.M.; Cheng, C.; Allen, C.; Hubbard, G.B.; Bai, Y.; Saunders, T.L.; Ikeno, Y. Thioredoxin and Aging: What Have We Learned from the Survival Studies? Aging Pathobiol. Ther. 2020, 2, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Oberacker, T.; Kraft, L.; Schanz, M.; Latus, J.; Schricker, S. The Importance of Thioredoxin-1 in Health and Disease. Antioxidants 2023, 12, 1078. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Holmgren, A. Physiological Functions of Thioredoxin and Thioredoxin Reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zou, L.; Lu, J.; Holmgren, A. Selenocysteine in Mammalian Thioredoxin Reductase and Application of Ebselen as a Therapeutic. Free. Radic. Biol. Med. 2018, 127, 238–247. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Wirth, E.K.; Conrad, M.; Winterer, J.; Wozny, C.; Carlson, B.A.; Roth, S.; Schmitz, D.; Bornkamm, G.W.; Coppola, V.; Tessarollo, L.; et al. Neuronal Selenoprotein Expression Is Required for Interneuron Development and Prevents Seizures and Neurodegeneration. FASEB J. 2010, 24, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Evke, S.; Lee, M.; Melendez, J.A.; Begley, T.J. Epitranscriptomic Systems Regulate the Translation of Reactive Oxygen Species Detoxifying and Disease Linked Selenoproteins. Free. Radic. Biol. Med. 2019, 143, 573–593. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, I.; Ponniresan, V.K.; Govindasamy, K.; Prasad, N.R. Understanding the Survival Mechanisms of Deinococcus Radiodurans against Oxidative Stress by Targeting Thioredoxin Reductase Redox System. Arch. Microbiol. 2020, 202, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Guriev, N.; Pugovkina, N.; Lyublinskaya, O. Inhibition of Thioredoxin Reductase Activity Reduces the Antioxidant Defense Capacity of Human Pluripotent Stem Cells under Conditions of Mild but Not Severe Oxidative Stress. Biochem. Biophys. Res. Commun. 2023, 642, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.G.; Holmgren, A.; Arnér, E.S.J.; Schmidt, E.E. NADPH-Dependent and -Independent Disulfide Reductase Systems. Free. Radic. Biol. Med. 2018, 127, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Gao, W.; Fan, C.; Yuan, Y.; Ma, Q.; Li, Y.; Tang, M. NAD/NADP and the Role in Neurodegenerative Diseases by Mediating Oxidative Stress Molecular neurobiology. Prog. Mod. Biomed. 2018, 18, 4382–4385+4381. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Zhang, Y.; Yuan, Y.; Miao, Z.; Lu, K.; Zhang, X.; Ni, R.; Zhang, H.; Zhao, Y.; et al. ChemR23 Signaling Ameliorates Cognitive Impairments in Diabetic Mice via Dampening Oxidative Stress and NLRP3 Inflammasome Activation. Redox Biol. 2022, 58, 102554. [Google Scholar] [CrossRef]
- Sbai, O.; Djelloul, M.; Auletta, A.; Ieraci, A.; Vascotto, C.; Perrone, L. AGE-TXNIP Axis Drives Inflammation in Alzheimer’s by Targeting Aβ to Mitochondria in Microglia. Cell Death Dis. 2022, 13, 302. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-Oxide Induces Inflammation and Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells via Activating ROS-TXNIP-NLRP3 Inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Le, Q.; Liu, P.; Liu, C.; Wang, Z.; He, G.; Zheng, P.; Wang, F.; Ma, L. Morphine Coordinates SST and PV Interneurons in the Prelimbic Cortex to Disinhibit Pyramidal Neurons and Enhance Reward. Mol. Psychiatry 2021, 26, 1178–1193. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.-C.; Feng, Y.-M.; Zhao, L.; Li, K.; Wang, S.-D.; Song, J.-Y.; Bai, J. Thioredoxin-1 Expression Regulated by Morphine in SH-SY5Y Cells. Neurosci. Lett. 2012, 523, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, J.; Jia, X.; Zhu, W.; Zhao, L.; Li, S.; Xu, C.; Yang, C.; Wu, P.; Lu, L. Inhibition of Nuclear Factor-κB Impairs Reconsolidation of Morphine Reward Memory in Rats. Behav. Brain Res. 2011, 216, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yi, S.; Shi, W.; Zhang, G.; Wang, S.; Qi, Q.; Cong, B.; Li, Y. The Pathology of Morphine-Inhibited Nerve Repair and Morphine-Induced Nerve Damage Is Mediated via Endoplasmic Reticulum Stress. Front. Neurosci. 2021, 15, 618190. [Google Scholar] [CrossRef]
- Listos, J.; Łupina, M.; Talarek, S.; Mazur, A.; Orzelska-Górka, J.; Kotlińska, J. The Mechanisms Involved in Morphine Addiction: An Overview. Int. J. Mol. Sci. 2019, 20, 4302. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yang, Z.; Li, C.; Cai, M.; Zhou, D.; Zhang, Q.; Wei, Y.; Wang, T.; Liu, Y. NF-κB Signaling and Vesicle Transport Are Correlated with the Reactivation of the Memory Trace of Morphine Dependence. Diagn. Pathol. 2014, 9, 142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiao, H.-H.; Zhu, L.-N.; Wang, Y.; Hui, J.-L.; Xie, W.-B.; Liu, C.; Chen, L.; Qiu, P.-M. Implications of Alpha-Synuclein Nitration at Tyrosine 39 in Methamphetamine-Induced Neurotoxicity in Vitro and in Vivo. Neural Regen. Res. 2019, 14, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, N.; Fan, W.; Ni, C.; Huang, M.; Bai, L.; Zhang, L.; Zhang, X.; Wen, Y.; Li, Y.; et al. Thioredoxin-1 Blocks Methamphetamine-Induced Injury in Brain through Inhibiting Endoplasmic Reticulum and Mitochondria-Mediated Apoptosis in Mice. NeuroToxicology 2020, 78, 163–169. [Google Scholar] [CrossRef]
- Kold-Christensen, R.; Johannsen, M. Methylglyoxal Metabolism and Aging-Related Disease: Moving from Correlation toward Causation. Trends Endocrinol. Metab. 2020, 31, 81–92. [Google Scholar] [CrossRef]
- Dafre, A.L.; Goldberg, J.; Wang, T.; Spiegel, D.A.; Maher, P. Methylglyoxal, the Foe and Friend of Glyoxalase and Trx/TrxR Systems in HT22 Nerve Cells. Free. Radic. Biol. Med. 2015, 89, 8–19. [Google Scholar] [CrossRef]
- Schmitz, A.E.; de Souza, L.F.; dos Santos, B.; Maher, P.; Lopes, F.M.; Londero, G.F.; Klamt, F.; Dafre, A.L. Methylglyoxal-Induced Protection Response and Toxicity: Role of Glutathione Reductase and Thioredoxin Systems. Neurotox. Res. 2017, 32, 340–350. [Google Scholar] [CrossRef]
- Lu, J.; Randell, E.; Han, Y.; Adeli, K.; Krahn, J.; Meng, Q.H. Increased Plasma Methylglyoxal Level, Inflammation, and Vascular Endothelial Dysfunction in Diabetic Nephropathy. Clin. Biochem. 2011, 44, 307–311. [Google Scholar] [CrossRef]
- Liu, C.; Cao, B.; Zhang, Q.; Zhang, Y.; Chen, X.; Kong, X.; Dong, Y. Inhibition of Thioredoxin 2 by Intracellular Methylglyoxal Accumulation Leads to Mitochondrial Dysfunction and Apoptosis in INS-1 Cells. Endocrine 2020, 68, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Peng, Y.; Li, J.; Holmgren, A.; Lu, J. Modulation of Thiol-Dependent Redox System by Metal Ions via Thioredoxin and Glutaredoxin Systems. Metallomics 2018, 10, 218–228. [Google Scholar] [CrossRef]
- Wang, H.; Abel, G.M.; Storm, D.R.; Xia, Z. Cadmium Exposure Impairs Adult Hippocampal Neurogenesis. Toxicol. Sci. 2019, 171, 501–514. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ren, Y.; Yang, R.; Guo, J.; Zong, Y.; Zhang, Q.; Zhao, J.; Zhang, W.; Xu, W.; et al. Selenite Ameliorates Cadmium-Induced Cytotoxicity Through Downregulation of ROS Levels and Upregulation of Selenoprotein Thioredoxin Reductase 1 in SH-SY5Y Cells. Biol. Trace Elem. Res. 2023, 201, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, Y.; Wang, Y.; Qi, H.; Chen, H.; Li, J.; Li, L. Cadmium-Induced Pyroptosis Is Mediated by PERK/TXNIP/NLRP3 Signaling in SH-SY5Y Cells. Environ. Toxicol. 2023, 38, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.H.; Bennett, G.J. Effects of Mitochondrial Poisons on the Neuropathic Pain Produced by the Chemotherapeutic Agents, Paclitaxel and Oxaliplatin. Pain 2012, 153, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Yumnamcha, T.; Devi, T.S.; Singh, L.P. Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1065. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, C.; Yan, B.; Shi, H.X.; Shi, Y.; Qu, L.; Liang, C.X. Expressions of Thioredoxin Interacting Protein/Nucleotide-binding Oligomerization Domain-like Receptor Protein 3 Inflammasome in the Sciatic Nerve of Streptozotocin-induced Diabetic Rats. J. Chin. Acad. Med. Sci. 2019, 41, 799–805. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, S.; Peng, M.; Wang, Z.; Ren, L.; Mu, S.; Zheng, M. Correlation Between Thioredoxin-Interacting Protein and Nerve Conduction Velocity in Patients With Type 2 Diabetes Mellitus. Front. Neurol. 2020, 11, 733. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, L.; Ma, X.; Li, S.; Xue, Y.; Yan, P.; Chen, M.; Wu, J. Recreational Nitrous Oxide Abuse: Prevalence, Neurotoxicity, and Treatment. Neurotox. Res. 2021, 39, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.-Y.; Kuo, C.-Y.; Chou, C.-C.; Kong, S.-S.; Hung, P.-C.; Tsai, H.-Y.; Chen, Y.-C.; Lin, J.-J.; Chou, I.-J.; Lin, K.-L.; et al. Recreational Nitrous Oxide Abuse Related Subacute Combined Degeneration of the Spinal Cord in Adolescents—A Case Series and Literature Review. Brain Dev. 2019, 41, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Swart, G.; Blair, C.; Lu, Z.; Yogendran, S.; Offord, J.; Sutherland, E.; Barnes, S.; Palavra, N.; Cremer, P.; Bolitho, S.; et al. Nitrous Oxide-Induced Myeloneuropathy. Eur. J. Neurol. 2021, 28, 3938–3944. [Google Scholar] [CrossRef]
- Berling, E.; Fargeot, G.; Aure, K.; Tran, T.H.; Kubis, N.; Lozeron, P.; Zanin, A. Nitrous Oxide-Induced Predominantly Motor Neuropathies: A Follow-up Study. J. Neurol. 2022, 269, 2720–2726. [Google Scholar] [CrossRef]
- Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines 2022, 10, 3186. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, G.; Sun, B.; Wang, S.; Lu, Y.; Xie, H. Activation of NLR Family, Domain of Pyrin Containing 3 Inflammasome by Nitrous Oxide through Thioredoxin-Interacting Protein to Induce Nerve Cell Injury. Bioengineered 2021, 12, 4768–4779. [Google Scholar] [CrossRef]
- Ren, X.; Li, C.; Liu, J.; Zhang, C.; Fu, Y.; Wang, N.; Ma, H.; Lu, H.; Kong, H.; Kong, L. Thioredoxin Plays a Key Role in Retinal Neuropathy Prior to Endothelial Damage in Diabetic Mice. Oncotarget 2017, 8, 61350–61364. [Google Scholar] [CrossRef]
- Tang, L.; Ren, X.; Han, Y.; Chen, L.; Meng, X.; Zhang, C.; Chu, H.; Kong, L.; Ma, H. Sulforaphane Attenuates Apoptosis of Hippocampal Neurons Induced by High Glucose via Regulating Endoplasmic Reticulum. Neurochem. Int. 2020, 136, 104728. [Google Scholar] [CrossRef]
- Degirmenci, U.; Wang, M.; Hu, J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells 2020, 9, 198. [Google Scholar] [CrossRef]
- Kong, L.; Liu, B.; Zhang, C.; Wang, B.; Wang, H.; Song, X.; Yang, Y.; Ren, X.; Yin, L.; Kong, H.; et al. The Therapeutic Potential of Sulforaphane on Light-Induced Photoreceptor Degeneration through Antiapoptosis and Antioxidant Protection. Neurochem. Int. 2016, 100, 52–61. [Google Scholar] [CrossRef]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-Acetylcysteine (NAC) in Neurological Disorders: Mechanisms of Action and Therapeutic Opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Nagakannan, P.; Iqbal, M.A.; Yeung, A.; Thliveris, J.A.; Rastegar, M.; Ghavami, S.; Eftekharpour, E. Perturbation of Redox Balance after Thioredoxin Reductase Deficiency Interrupts Autophagy-Lysosomal Degradation Pathway and Enhances Cell Death in Nutritionally Stressed SH-SY5Y Cells. Free Radic. Biol. Med. 2016, 101, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, H.; Huo, H.; Ma, F.; Zhao, M.; Han, Q.; Hu, L.; Li, Y.; Zhang, H.; Pan, J.; et al. N-Acetylcysteine Combined with Insulin Alleviates the Oxidative Damage of Cerebrum via Regulating Redox Homeostasis in Type 1 Diabetic Mellitus Canine. Life Sci. 2022, 308, 120958. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Selenium Homeostasis and Antioxidant Selenoproteins in Brain: Implications for Disorders in the Central Nervous System. Arch. Biochem. Biophys. 2013, 536, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef]
- Landel, V.; Stephan, D.; Cui, X.; Eyles, D.; Feron, F. Differential Expression of Vitamin D-Associated Enzymes and Receptors in Brain Cell Subtypes. J. Steroid Biochem. Mol. Biol. 2018, 177, 129–134. [Google Scholar] [CrossRef]
- Lin, F.-Y.; Lin, Y.-F.; Lin, Y.-S.; Yang, C.-M.; Wang, C.-C.; Hsiao, Y.-H. Relative D3 Vitamin Deficiency and Consequent Cognitive Impairment in an Animal Model of Alzheimer’s Disease: Potential Involvement of Collapsin Response Mediator Protein-2. Neuropharmacology 2020, 164, 107910. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Lu, Q.; Chen, W.; Li, J.; Li, C.; Zheng, Z. Vitamin D3 Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J. Diabetes Res. 2018, 2018, 8193523. [Google Scholar] [CrossRef]
- Han, L.; Xi, G.; Guo, N.; Guo, J.; Rong, Q. Expression and Mechanism of TXNIP/NLRP3 Inflammasome in Sciatic Nerve of Type 2 Diabetic Rats. Dis. Markers 2022, 2022, 9696303. [Google Scholar] [CrossRef]
- Li, X.; Liao, J.; Su, X.; Li, W.; Bi, Z.; Wang, J.; Su, Q.; Huang, H.; Wei, Y.; Gao, Y.; et al. Human Urine-Derived Stem Cells Protect against Renal Ischemia/Reperfusion Injury in a Rat Model via Exosomal miR-146a-5p Which Targets IRAK1. Theranostics 2020, 10, 9561–9578. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, W.; Chen, T.; Ren, X.; Zhu, J.; Hu, F.; Luo, J.; Xing, L.; Zhou, H.; Sun, J.; et al. miR-146a-5p Promotes Epithelium Regeneration against LPS-Induced Inflammatory Injury via Targeting TAB1/TAK1/NF-κB Signaling Pathway. Int. J. Biol. Macromol. 2022, 221, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Lan, T.; Wang, W.; Long, Y.; Yu, S.Y. Microglia Secrete miR-146a-5p-Containing Exosomes to Regulate Neurogenesis in Depression. Mol. Ther. 2022, 30, 1300–1314. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, Q.; Wang, J.-C.; Wang, J.; Liu, Y.; Zhu, L.-Y.; Xu, J.-X. Resveratrol Improves Diabetes-Induced Cognitive Dysfunction in Part through the miR-146a-5p/TXNIP Axis. Kaohsiung J. Med. Sci. 2023, 39, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Zanetti, G.; Megighian, A.; Scorzeto, M.; Fillo, S.; Shone, C.C.; Binz, T.; Rossetto, O.; Lista, F.; et al. Thioredoxin and Its Reductase Are Present on Synaptic Vesicles, and Their Inhibition Prevents the Paralysis Induced by Botulinum Neurotoxins. Cell Rep. 2014, 8, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Kato, H.; Iwashita, K.; Yamakage, H.; Kato, S.; Saito, S.; Ihara, M.; Nishimura, H.; Kawamoto, A.; Suganami, T.; et al. Taxifolin Suppresses Inflammatory Responses of High-Glucose-Stimulated Mouse Microglia by Attenuating the TXNIP-NLRP3 Axis. Nutrients 2023, 15, 2738. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Mesa-Herrera, F.; Marín, R. DHA and Its Elaborated Modulation of Antioxidant Defenses of the Brain: Implications in Aging and AD Neurodegeneration. Antioxidants 2021, 10, 907. [Google Scholar] [CrossRef]
- Jia, J.; Zeng, X.; Xu, G.; Wang, Z. The Potential Roles of Redox Enzymes in Alzheimer’s Disease: Focus on Thioredoxin. ASN Neuro 2021, 13, 1759091421994351. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Zhang, L.; He, Y.; Qi, C.; Li, F. Progress in Research on the Role of the Thioredoxin System in Chemical Nerve Injury. Toxics 2024, 12, 510. https://doi.org/10.3390/toxics12070510
Xu X, Zhang L, He Y, Qi C, Li F. Progress in Research on the Role of the Thioredoxin System in Chemical Nerve Injury. Toxics. 2024; 12(7):510. https://doi.org/10.3390/toxics12070510
Chicago/Turabian StyleXu, Xinwei, Lan Zhang, Yuyun He, Cong Qi, and Fang Li. 2024. "Progress in Research on the Role of the Thioredoxin System in Chemical Nerve Injury" Toxics 12, no. 7: 510. https://doi.org/10.3390/toxics12070510
APA StyleXu, X., Zhang, L., He, Y., Qi, C., & Li, F. (2024). Progress in Research on the Role of the Thioredoxin System in Chemical Nerve Injury. Toxics, 12(7), 510. https://doi.org/10.3390/toxics12070510





