Performance and Mechanism of Co and Mn Loaded on Fe-Metal-Organic Framework Catalysts with Different Morphologies for Simultaneous Degradation of Acetone and NO by Photothermal Coupling
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.1.1. Preparation of Carriers
- (1)
- Preparation of octahedral shaped Fe-MOF
- (2)
- Preparation of Fe-MOF in stick form
2.1.2. Preparation of Catalysts with Different Carriers Loaded with Co and Mn
2.2. Catalyst Characterization
2.3. Catalytic Evaluation
3. Results and Discussion
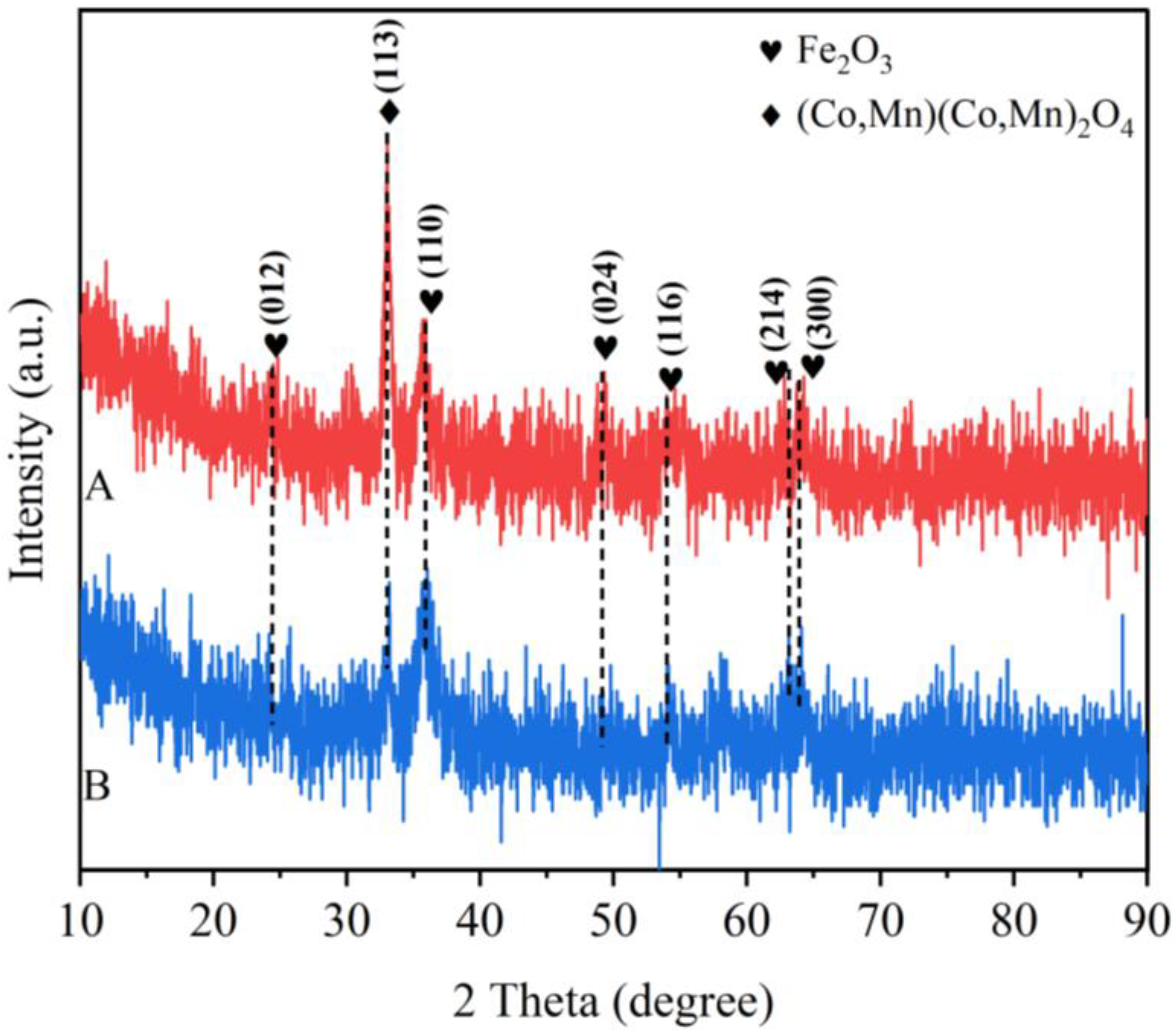
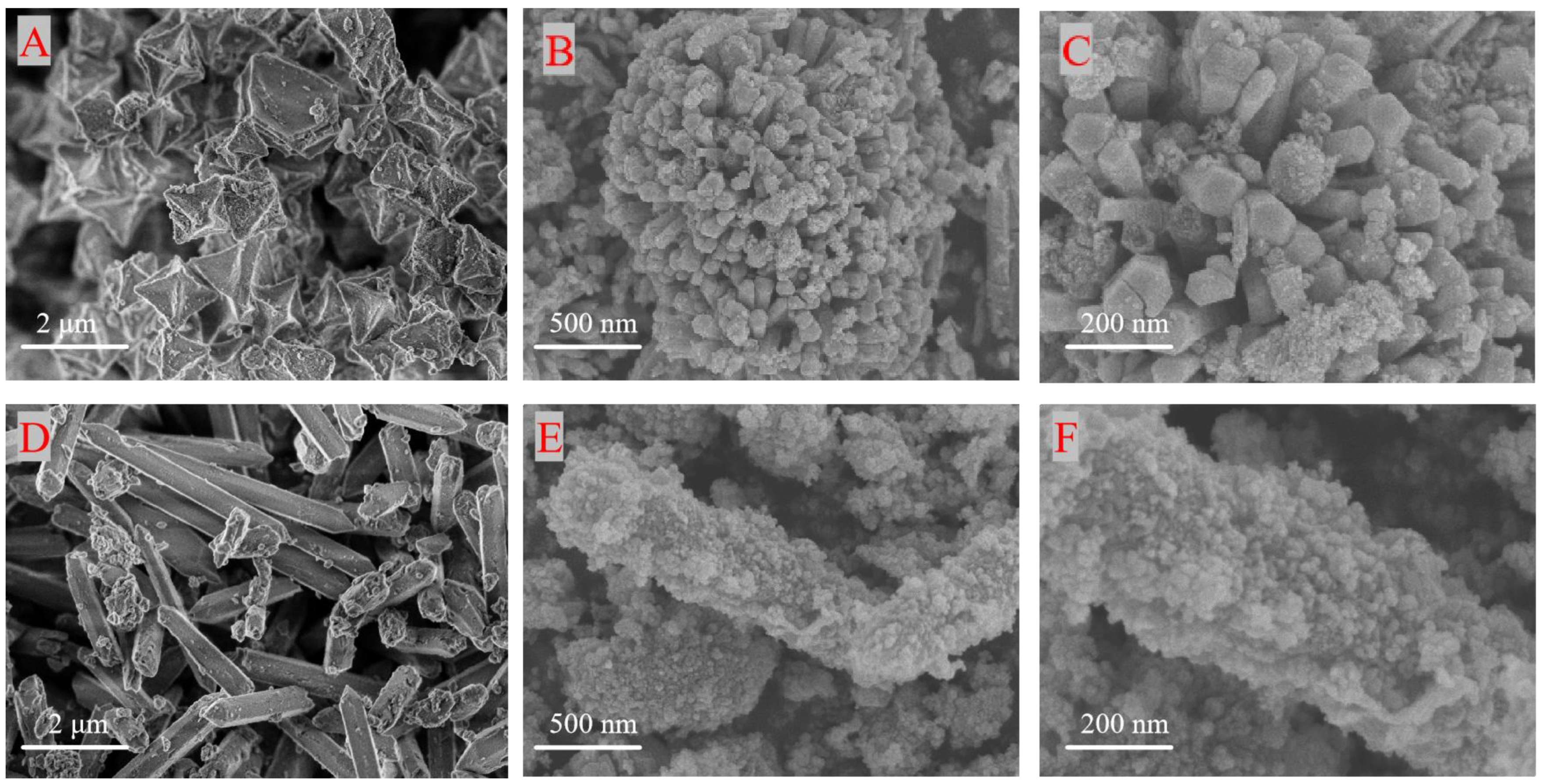
3.1. Structural Characterization of the Catalyst
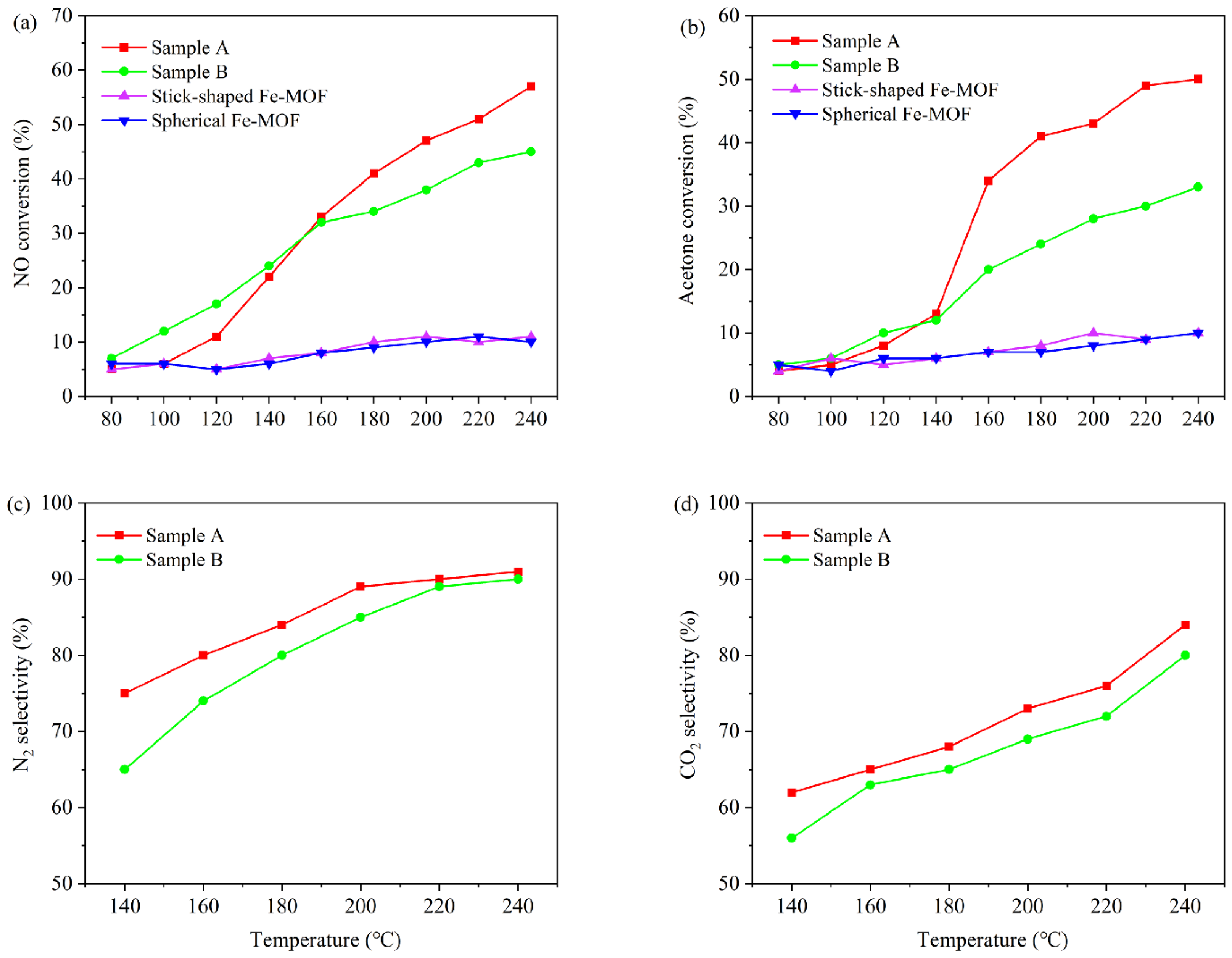
3.2. Analysis of the Photothermal Catalytic Activity of Catalysts
3.3. Analysis of Photothermal Catalysis Mechanism of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, L.; Yu, C.; Yang, K.; Fan, Q.; Ji, H. Recent advances in VOCs and CO removal via photothermal synergistic catalysis. Chin. J. Catal. 2021, 42, 1078–1095. [Google Scholar] [CrossRef]
- Chen, L.; Liao, Y.; Xin, S.; Song, X.; Liu, G.; Ma, X. Simultaneous removal of NO and volatile organic compounds (VOCs) by Ce/Mo doping-modified selective catalytic reduction (SCR) catalysts in denitrification zone of coal-fired flue gas. Fuel 2020, 262, 116485. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, Y.; He, Y.; Walters, W.W.; Ni, Q.; Liu, X.; Xu, G.; Shao, J.; Jiang, C. Substantial nitrogen oxides emission reduction from China due to COVID-19 and its impact on surface ozone and aerosol pollution. Sci. Total Environ. 2021, 753, 142238. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zhang, M.; Cai, S.C.; Yu, E.Q.; Chen, J.; Jia, H.P. Light-driven thermocatalysis/photo-thermocatalysis of VOCs: Recent advances and future perspectives. Environ. Eng. 2020, 38, 13–20. [Google Scholar]
- Guo, Y.; Wen, M.; Li, G.; An, T. Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: A critical review. Appl. Catal. B Environ. 2021, 281, 119447. [Google Scholar] [CrossRef]
- Tang, Y.; Tao, Y.; Zhou, T.; Yang, B.; Wang, Q.; Zhu, Z.; Xie, A.; Luo, S.; Yao, C.; Li, X. Direct Z-scheme La1-xCexMnO3 catalyst for photothermal degradation of toluene. Environ. Sci. Pollut. Res. 2019, 26, 36832–36844. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.L.; Zhang, D.S. Study progress on the synergistic removal of Nitrogen Oxides and Volatile Organic Compounds. Energy Environ. Protect 2023, 37, 141–156. [Google Scholar]
- Yu, Y.L.; Weng, X.L.; Wu, Z.B. Reaction mechanism study of synergistic elimanation between NOx and chloroaromatics on CeWOx catalysts. Acta Sci. Circumst. 2022, 42, 351–365. [Google Scholar]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Van Heyst, B. An overview of photocatalyst immobilization methods for air pollution Remediation. Chem. Eng. J. 2020, 391, 123490. [Google Scholar] [CrossRef]
- Ma, R.; Sun, J.; Li, D.H.; Wei, J.J. Review of synergistic photo-thermo-catalysis: Mechanisms, materials and applications. Int. J. Hydrogen Energy 2020, 45, 30288–30324. [Google Scholar] [CrossRef]
- Chen, P.; Dong, F.; Ran, M.X.; Li, J.R. Synergistic photothermal catalytic NO purification of MnOx/g-C3N4: Enhanced performance and reaction mechanism. Chin. J. Catal. 2018, 39, 619–629. [Google Scholar] [CrossRef]
- Liotta, L.F.; Wu, H.; Pantaleo, G.; Venezia, A.M. Co3O4 nanocrystals and Co3O4-MnOx binary oxides for CO, CH4 and VOC oxidation at low temperatures: A review. Catal. Sci. Technol. 2013, 3, 3058–3102. [Google Scholar] [CrossRef]
- Ko, S.J.; Tang, X.L.; Gao, F.Y.; Zhou, Y.S.; Zhang, R.C.; Liu, H.H. The optimization of hydrothermal synthesis of MnxCo3-xO4/GC catalyst for low temperature NH3-SCR/using design of experiments. J. Chem. Technol. Biotechnol. 2021, 96, 2965–2975. [Google Scholar] [CrossRef]
- Liao, L.; Ding, X.; Li, J.; Huang, L.; Zhang, M.; Fan, Y.; Zhou, X.; Zhang, Y.; Mo, S.; Xie, Q.; et al. Constructing MOFs-derived Co3O4 microsphere with atomic p-n homojunction as an efficient photothermal catalyst for boosting ethyl acetate oxidation under light irradiation. Sep. Purif. Technol. 2023, 309, 122939. [Google Scholar] [CrossRef]
- Wen, Q.; Li, D.; Li, H.; Long, M.; Gao, C.; Wu, L.; Song, F.; Zhou, J. Synergetic effect of photocatalysis and peroxymonosulfate activated by Co/Mn-MOF-74@g-C3N4 Z-scheme photocatalyst for removal of tetracyclin hydrochloride. Sep. Purif. Technol. 2023, 313, 123518. [Google Scholar] [CrossRef]
- Lei, J.; Wang, P.; Wang, S.; Li, J.; Xu, Y.; Li, S. Enhancement effect of Mn doping on Co3O4 derived from Co-MOF for toluene catalytic oxidation. Chin. J. Chem. Eng. 2022, 52, 1–9. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, Y.; Li, C.; Niu, D.; Chu, Q.; Xiong, W.; Li, X. Synthesis of Bimetallic MOF-74-CoMn Catalyst and Its Application in Selective Catalytic Reduction of NO with CO. Acta Chim. Sin. 2019, 77, 758–764. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Huang, X.; Qiu, S.; Liu, X.; Liu, L.; Zhao, M.; Qu, J.; Zou, J.; Zhang, J. Organic pollutants removal from aqueous solutions using metal-organic frameworks (MOFs) as adsorbents: A review. J. Environ. Chem. Eng. 2023, 11, 111217. [Google Scholar] [CrossRef]
- Park, J.; Koo, J.Y.; Choi, H.C. Solvent-Effected Coordination Variation of Flexible Ligands to Cu(II) for the Formation of 1D and 2D Secondary Building Units for Metal-Organic Frameworks. Inorg. Chem. 2021, 60, 5376–5382. [Google Scholar] [CrossRef]
- Xie, S.Z.; Qin, Q.J.; Liu, H.; Jin, L.J.; Li, B. MOF-74-M (M = Mn, Co, Ni, Zn, MnCo, MnNi, and MnZn) for Low-Temperature NH3-SCR and In Situ DRIFTS Study Reaction Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 48476–48485. [Google Scholar] [CrossRef]
- Oar-Arteta, L.; Wezendonk, T.; Sun, X.; Kapteijn, F.; Gascon, J. Metal organic frameworks as precursors for the manufacture of advanced catalytic materials. Mater. Chem. Front. 2017, 1, 1709–1745. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Shi, Y.; Liu, G.; Li, G.P. Mesoporous Co3O4@carbon omposites derived from microporous cobalt-based porous coordination polymers for enhanced electrochemical properties in supercapacitors. RSC Adv. 2016, 6, 18465–18470. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Liu, P.; Shi, Y.; Liu, G.; Li, J. Hierarchical porous carbons derived from microporous zeolitic metal azolate frameworks for supercapacitor electrodes. Mater. Res. Bull. 2017, 88, 62–68. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, Z.; Dong, F.; Zhang, J. Controlled porous hollow Co3O4 polyhedral nanocages derived from metal-organic frameworks (MOFs) for toluene catalytic oxidation. Mol. Catal. 2019, 463, 77–86. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, T.; Li, G.; Wojtas, L.; Huo, Q.; Eddaoudi, M.; Liu, Y. From Metal-Organic Squares to Porous Zeolite-like Supramolecular Assemblies. J. Am. Chem. Soc. 2010, 132, 18038–18041. [Google Scholar] [CrossRef] [PubMed]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Su, Y.; Lu, T.; Zhang, L.; Wu, L.; Lv, Y. MOFs-derived dodecahedra porous Co3O4: An efficient cataluminescence sensing material for H2S. Sens. Actuators B Chem. 2018, 258, 349–357. [Google Scholar] [CrossRef]
- Li, J.-R.; Wang, F.-K.; He, C.; Huang, C.; Xiao, H. Catalytic total oxidation of toluene over carbon-supported Cu-Co oxide catalysts derived from Cu-based metal organic framework. Powder Technol. 2020, 363, 95–106. [Google Scholar] [CrossRef]
- Huang, C.; Liu, R.; Yang, W.Y.; Li, Y.P.; Huang, J.S.; Zhu, H.J. Enhanced catalytic activity of MnCo-MOF-74 for highly selective aerobic oxidation of substituted toluene. Inorg. Chem. Front. 2018, 5, 1923–1932. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.Y.; Huang, L.; Zhang, J.P.; Zhang, D.S. Rational Design of High-Performance DeNOx Catalysts Based on MnxCo3-xO4 Nanocages Derived from Metal–Organic Frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, Y.; Zuo, J.; Feng, X.; Wang, X.; Zhang, T.; Zhang, K.; Jiang, L. Insights into the high performance of Mn-Co oxides derived from metal-organic frameworks for total toluene oxidation. J. Hazard. Mater. 2018, 349, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Li, M.R.; Wang, Y.H.; Fan, Y.M.; Liao, L.; Zhou, X.B.; Mo, S.P.; Wang, H.Q. Controllable synthesis various morphologies of 3D hierarchical MnOx-TiO2 nanocatalysts for photothermocatalysis toluene and NO with free-ammonia. J. Colloid Interface Sci. 2022, 608 Pt 3, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yu, X.; Dang, X.; Meng, X.; Zhang, Y.; Qin, C. Non-thermal plasma coupled with MOx/γ-Al2O3 (M: Fe, Co, Mn, Ce) for chlorobenzene degradation: Analysis of byproducts and the reaction mechanism. J. Environ. Chem. Eng. 2021, 9, 106562. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, X.; Hong, K.; Jiang, J.; Zhang, L.; Chen, J.; Rao, Z. Hierarchically porous carbon materials derived from MIL-88(Fe) for superior high-rate and long cycling-life sodium ions batteries. J. Electroanal. Chem. 2019, 852, 113525. [Google Scholar] [CrossRef]
- Xu, W.; Xue, W.; Huang, H.; Wang, J.; Zhong, C.; Mei, D. Morphology controlled synthesis of α-Fe2O3-x with benzimidazole-modified Fe-MOFs for enhanced photo-Fenton-like catalysis. Appl. Catal. B Environ. 2021, 291, 120129. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, H.X.; Chen, Y.; Chen, Y.J.; Zhuang, L.; Ou, H. Enhanced photocatalysis degradation of organophosphorus flame retardant using MIL-101(Fe)/persulfate: Effect of irradiation wavelength and real water matrixes. Chem. Eng. J. 2019, 368, 273–284. [Google Scholar] [CrossRef]
- Ren, Z.; Wu, Z.; Song, W.; Xiao, W.; Guo, Y.; Ding, J.; Suib, S.L.; Gao, P.-X. Low temperature propane oxidation over Co3O4 based nano-array catalysts: Ni dopant effect, reaction mechanism and structural stability. Appl. Catal. B Environ. 2016, 180, 150–160. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). J. Macromol. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Z.; Jia, J.; Wang, Y. Ag nanoparticles supported on wormhole HMS material as catalysts for CO oxidation: Effects of preparation methods. Powder Technol. 2012, 230, 212–218. [Google Scholar] [CrossRef]
- Martins, R.C.; Quinta-Ferreira, R.M. Catalytic ozonation of phenolic acids over a Mn-Ce-O catalyst. Appl. Catal. B 2009, 90, 268. [Google Scholar] [CrossRef]
- Wang, H.; Huang, S.; Liao, L.; Mo, S.; Zhou, X.; Fan, Y. Performance and mechanism analysis of sludge-based biochar loaded with Co and Mn as photothermal catalysts for simultaneous removal of acetone and NO at low temperature. Environ. Sci. Pollut. Res. 2024, 31, 2891–2906. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Low, J.; Liu, T.; Yu, J.; Cao, S. Hierarchical hollow cages of Mn-Co layered double hydroxide as supercapacitor electrode materials. Appl. Surf. Sci. 2017, 413, 35–40. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Liu, Z.-Q.; Haruta, M.; Shen, W. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 2009, 458, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.M.; Wang, Y.H.; Li, M.R.; Zhu, Y.N.; Zhu, Z.Q.; Mo, S.P.; Zhang, L.H.; Tang, S.; Zhou, X.B.; Zhang, Y.P. Introducing cerium into TiO2@MnOx hollow-sphere structure for highly active photothermocatalysis degradation of ethyl acetate and NO under H2O at low temperature. J. Rare Earths 2024, 55, 889–898. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Fan, Y.; Zhu, Z.; Liao, L.; Mo, S.; Zhang, L.; Tang, S.; Zhou, X. Highly active nanostick-assembled TiO2@MnOx hollow-sphere structure for photothermocatalysis of ethyl acetate and NO with free-ammonia at low temperature: Resistence, key reaction steps and mechanisms. Appl. Surf. Sci. 2023, 607, 154887. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, X.; Peng, R.; Zhao, M.; Ye, D. Catalytic properties of manganese oxide polyhedra with hollow and solid morphologies in toluene removal. Appl. Surf. Sci. 2017, 405, 20–28. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, Q.; Li, J.; Sun, Y.; Ren, Q.; Zou, S.; Zhang, Q.; Lu, J.; Fu, M.; Mo, D.; et al. Highly efficient mesoporous MnO2 catalysts for the total toluene oxidation: Oxygen-Vacancy defect engineering and involved intermediates using in situ DRIFTS. Appl. Catal. B Environ. 2020, 264, 118464. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Wang, D.; Wang, M.; Lin, Z.; Tang, H. Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrason. Sonochem. 2010, 17, 526–533. [Google Scholar] [CrossRef]
- Niu, C.; Wang, B.; Xing, Y.; Su, W.; He, C.; Xiao, L.; Xu, Y.; Zhao, S.; Cheng, Y.; Shi, J.-W. Thulium modified MnOx/TiO2 catalyst for the low-temperature selective catalytic reduction of NO with ammonia. J. Clean. Prod. 2021, 290, 125858. [Google Scholar] [CrossRef]
- Li, C.Q.; Sun, Z.M.; Song, A.K.; Dong, X.B.; Zheng, S.L. Flowing nitrogen atmosphere induced rich oxygen vacancies overspread the surface of TiO2/kaolinite composite for enhanced photocatalytic activity within broad radiation spectrum. Appl. Catal. B 2018, 236, 76. [Google Scholar] [CrossRef]
- Ko, S.; Tang, X.; Gao, F.; Wang, C.; Liu, H.; Liu, Y. Selective catalytic reduction of NOx with NH3 on Mn, Co-BTC-derived catalysts: Influence of thermal treatment temperature. J. Solid State Chem. 2022, 307, 122843. [Google Scholar] [CrossRef]
- Zeng, X.; Li, B.; Liu, R.; Li, X.; Zhu, T. Investigation of promotion effect of Cu doped MnO2 catalysts on ketone-type VOCs degradation in a one-stage plasma-catalysis system. Chem. Eng. J. 2020, 384, 123362. [Google Scholar] [CrossRef]
- Vikrant, K.; Weon, S.; Kim, K.-H.; Sillanpää, M. Platinized titanium dioxide (Pt/TiO2) as a multi-functional catalyst for thermocatalysis, photocatalysis, and photothermal catalysis for removing air pollutants. Appl. Mater. Today 2021, 23, 100993. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, S.; Bi, F.; Chen, J.; Wang, Y.; Cui, L.; Xu, J.; Zhang, X. Highly efficient photothermal catalysis of toluene over Co3O4/TiO2 p-n heterojunction: The crucial roles of interface defects and band structure. Appl. Catal. B Environ. 2022, 315, 121550. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, M.; Li, Y.; Yang, Y.; Mao, M.; Zhao, X. UV-Vis-Infrared Light Driven Thermocatalytic Activity of Octahedral Layered Birnessite Nanoflowers Enhanced by a Novel Photoactivation. Adv. Funct. Mater. 2016, 26, 4518–4526. [Google Scholar] [CrossRef]
- Zhang, X.L.; Ye, J.H.; Yuan, J.; Cai, T.; Xiao, B.; Liu, Z.; Zhao, K.F.; Yang, L.; He, D.N. Excellent low-temperature catalytic performance of nanosheet Co-Mn oxides for total benzene oxidation. Appl. Catal. A-Gen. 2018, 566, 104. [Google Scholar] [CrossRef]
- Shan, C.P.; Zhang, Y.; Zhao, Q. Acid Etching-Induced In Situ Growth of λ-MnO2 over CoMn Spinel for Low-Temperature Volatile Organic Compound Oxidation. Environ. Sci. Technol. 2022, 56, 10381–10390. [Google Scholar] [CrossRef]
- Ding, X.; Liu, H.; Chen, J.; Wen, M.; Li, G.; An, T.; Zhao, H. In situ growth of well-aligned Ni-MOF nanosheets on nickel foam for enhanced photocatalytic degradation of typical volatile organic compounds. Nanoscale 2020, 12, 9462–9470. [Google Scholar] [CrossRef]





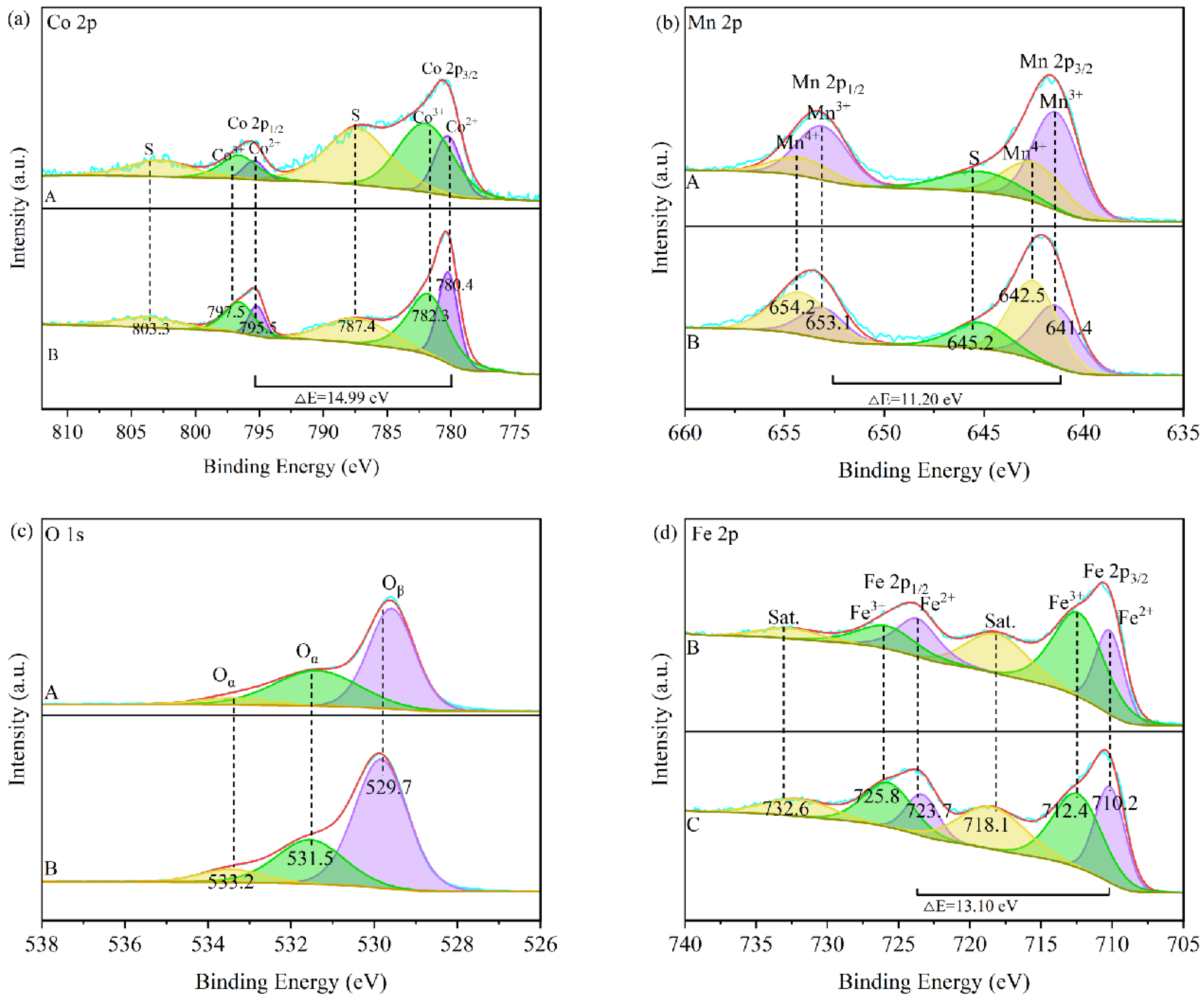

| Catalyst | SBET (m2·g−1) | Vpore (cm3·g−1) | Pore Diameter (nm) | Mn4+/Mn3+ | Co3+/Co2+ | Fe3+/Fe2+ | Oα/(Oα + Oβ) (%) |
|---|---|---|---|---|---|---|---|
| A | 71.14 | 0.24 | 13.88 | 1.43 | 1.41 | 0.65 | 43.5% |
| B | 59.15 | 0.22 | 15.51 | 0.92 | 1.04 | 0.51 | 36.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fan, Y.; Wang, Y.; Zhu, Y.; Zhu, Z.; Mo, S.; Zhou, X.; Zhang, Y. Performance and Mechanism of Co and Mn Loaded on Fe-Metal-Organic Framework Catalysts with Different Morphologies for Simultaneous Degradation of Acetone and NO by Photothermal Coupling. Toxics 2024, 12, 524. https://doi.org/10.3390/toxics12070524
Li Y, Fan Y, Wang Y, Zhu Y, Zhu Z, Mo S, Zhou X, Zhang Y. Performance and Mechanism of Co and Mn Loaded on Fe-Metal-Organic Framework Catalysts with Different Morphologies for Simultaneous Degradation of Acetone and NO by Photothermal Coupling. Toxics. 2024; 12(7):524. https://doi.org/10.3390/toxics12070524
Chicago/Turabian StyleLi, Yuanzhen, Yinming Fan, Yanhong Wang, Yinian Zhu, Zongqiang Zhu, Shengpeng Mo, Xiaobin Zhou, and Yanping Zhang. 2024. "Performance and Mechanism of Co and Mn Loaded on Fe-Metal-Organic Framework Catalysts with Different Morphologies for Simultaneous Degradation of Acetone and NO by Photothermal Coupling" Toxics 12, no. 7: 524. https://doi.org/10.3390/toxics12070524
APA StyleLi, Y., Fan, Y., Wang, Y., Zhu, Y., Zhu, Z., Mo, S., Zhou, X., & Zhang, Y. (2024). Performance and Mechanism of Co and Mn Loaded on Fe-Metal-Organic Framework Catalysts with Different Morphologies for Simultaneous Degradation of Acetone and NO by Photothermal Coupling. Toxics, 12(7), 524. https://doi.org/10.3390/toxics12070524







