Comparison of Transgenerational Neurotoxicity between Pristine and Amino-Modified Nanoplastics in C. elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoplastic Properties
2.2. Animal Maintenance
2.3. Exposure
2.4. Neurotoxicity Assessment
2.5. Gene Expression
2.6. RNA Interference (RNAi)
2.7. Data Analysis
3. Results
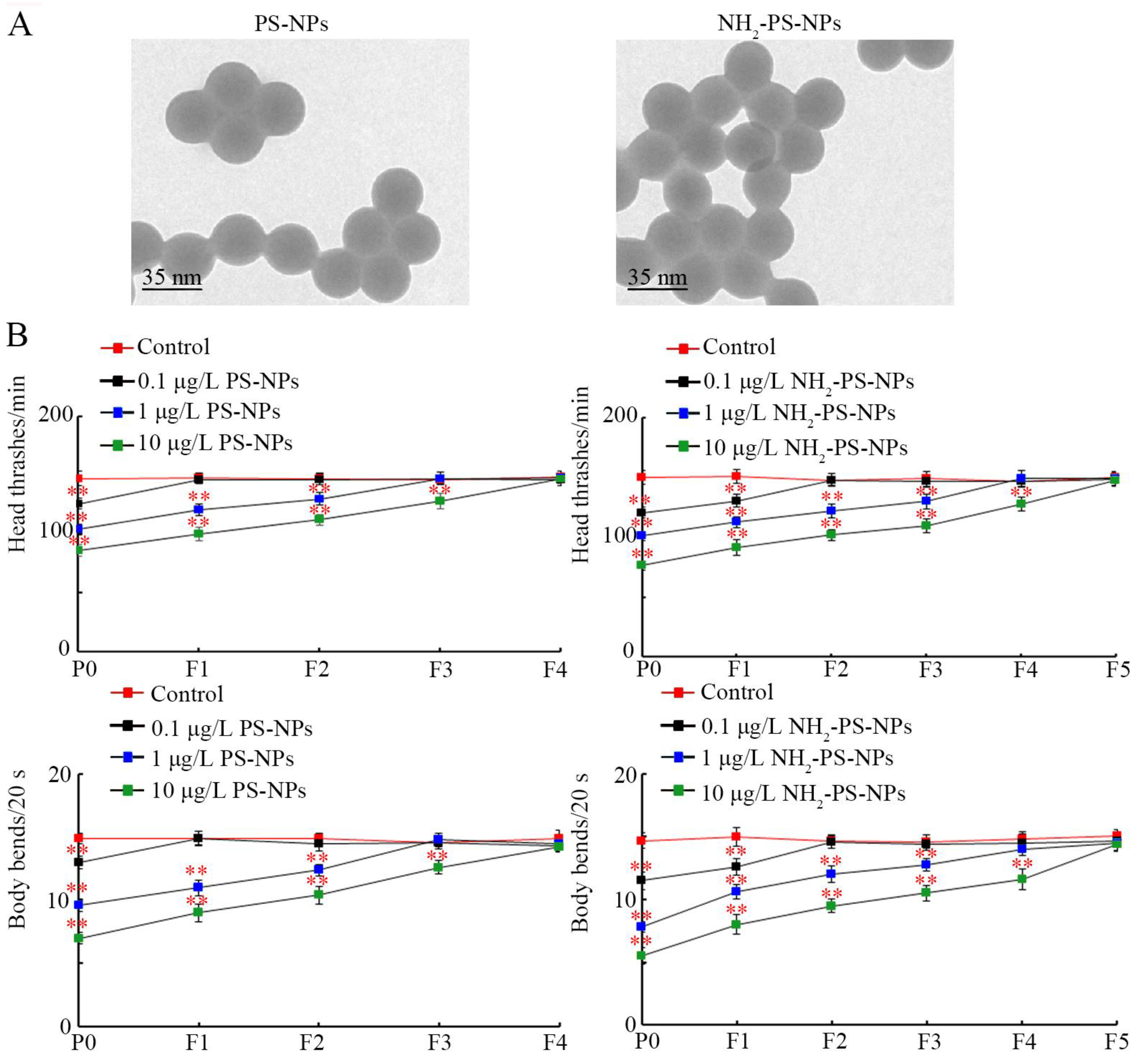
3.1. Amino Modification Increased Transgenerational Toxicity of PS-NPs on Locomotion
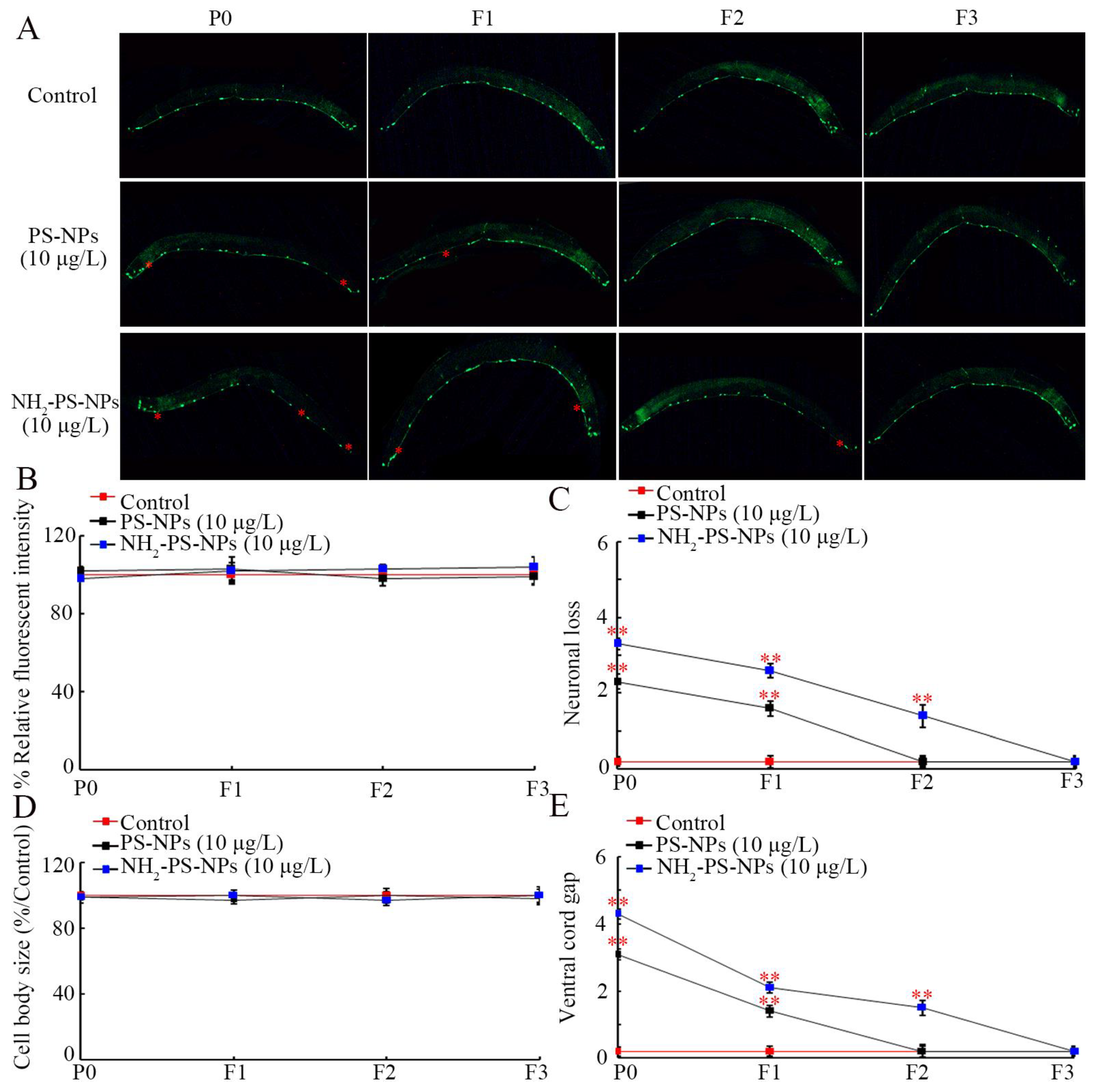
3.2. Amino Modification Increased Transgenerational Toxicity of PS-NPs on Neuronal Development of D-Type Motor Neurons
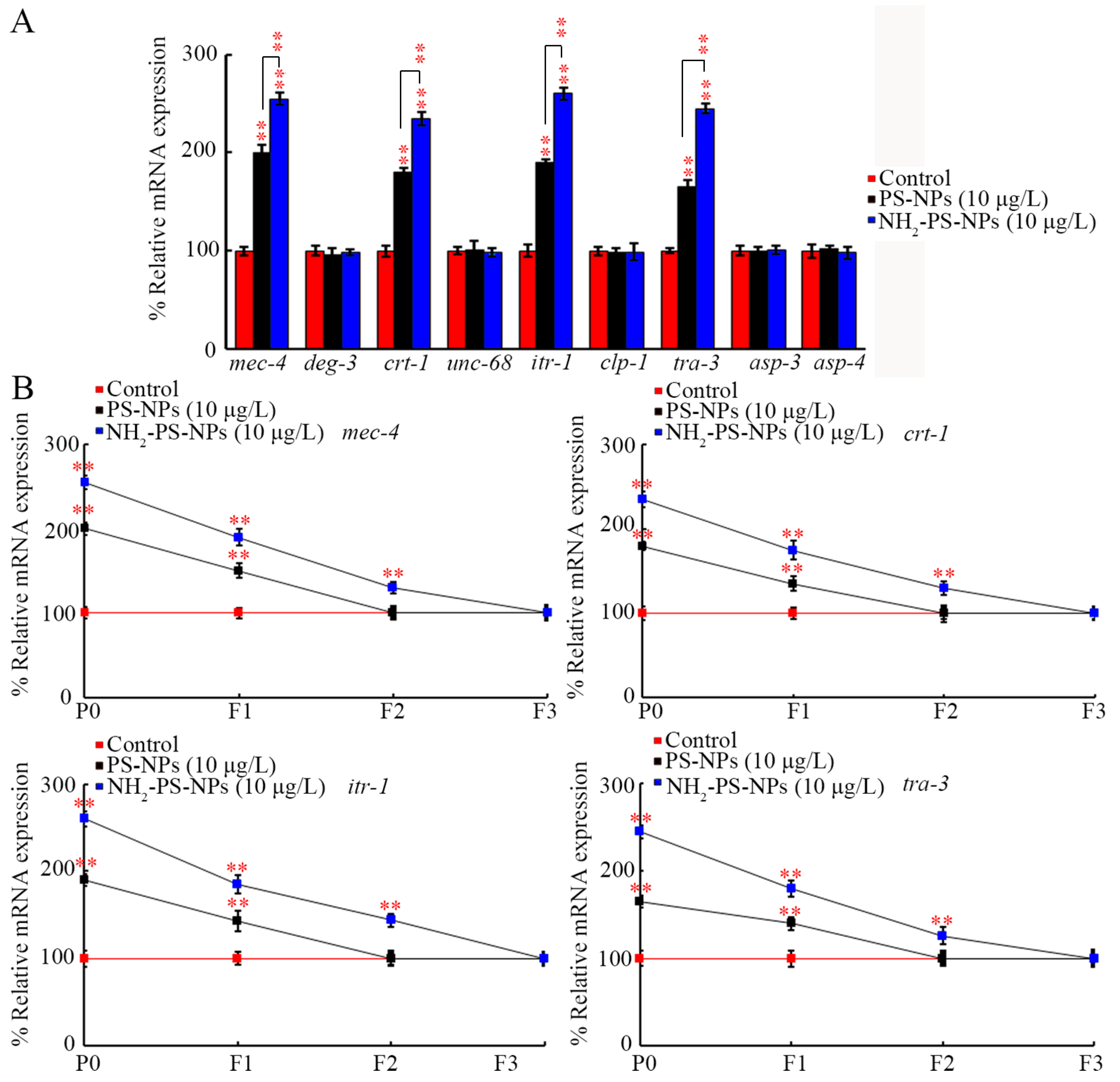
3.3. Amino Modification Strengthened Transgenerational Effect of PS-NPs on Expressions of Genes Governing Neurodegeneration
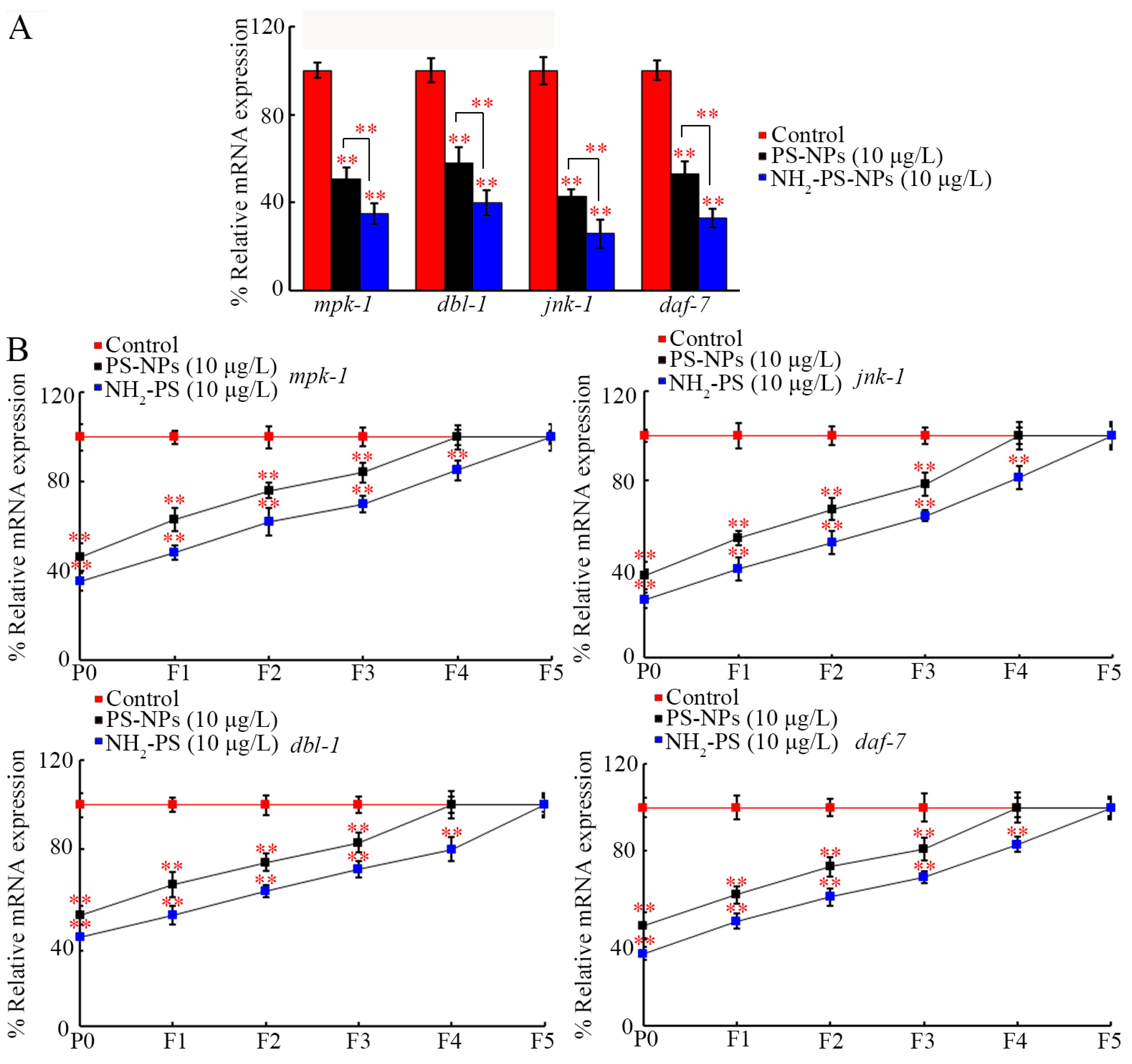
3.4. Amino Modification Strengthened Transgenerational Inhibition of jnk-1, mpk-1, daf-7, and dbl-1 by PS-NP Exposure
3.5. RNAi of jnk-1, mpk-1, daf-7, and dbl-1 Increased Neurotoxicity of Both Pristine and Amino-Modified PS-NPs
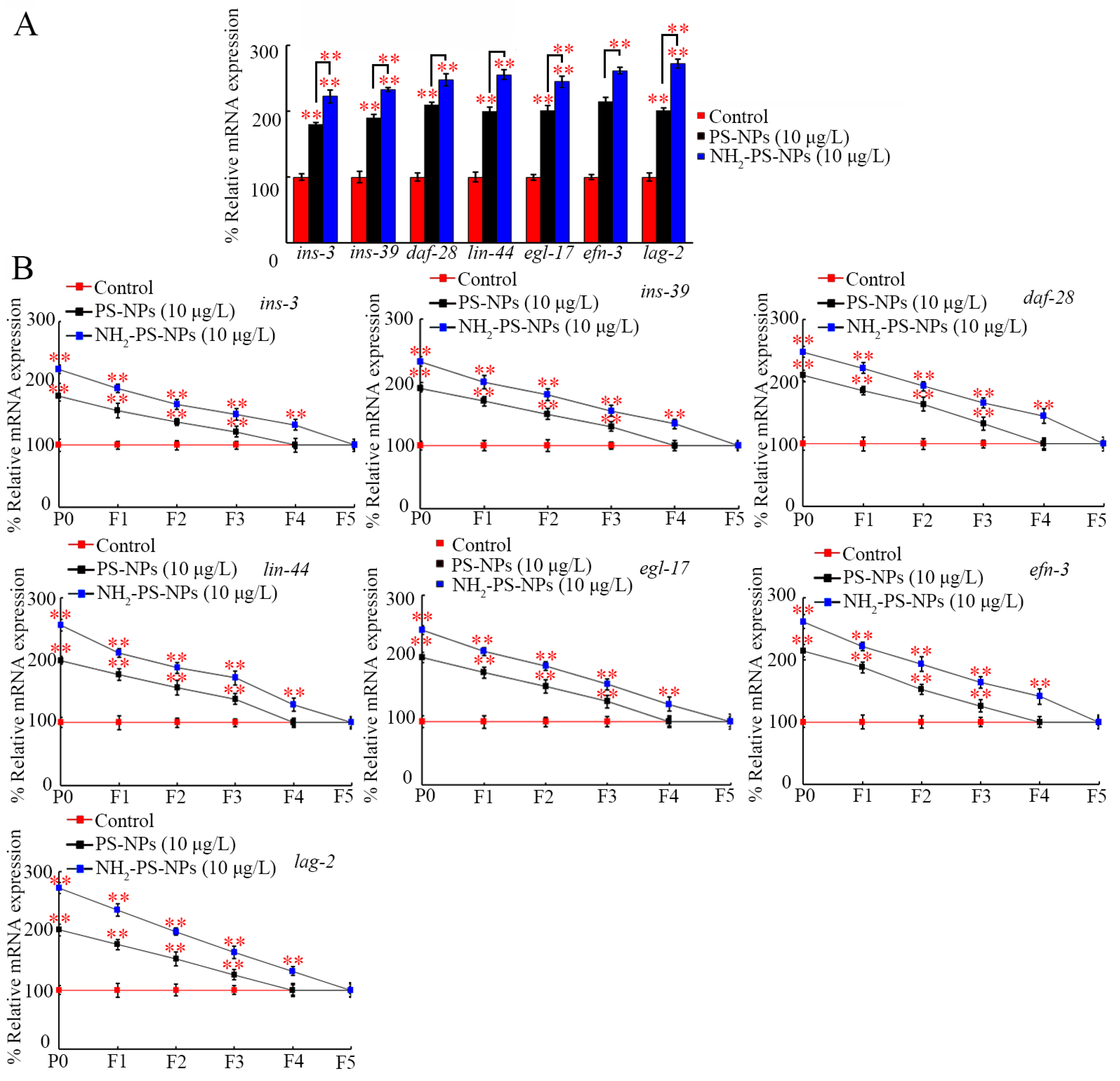
3.6. Amino Modification Strengthened Transgenerational Activation of Germline Ligand Genes by PS-NP Exposure
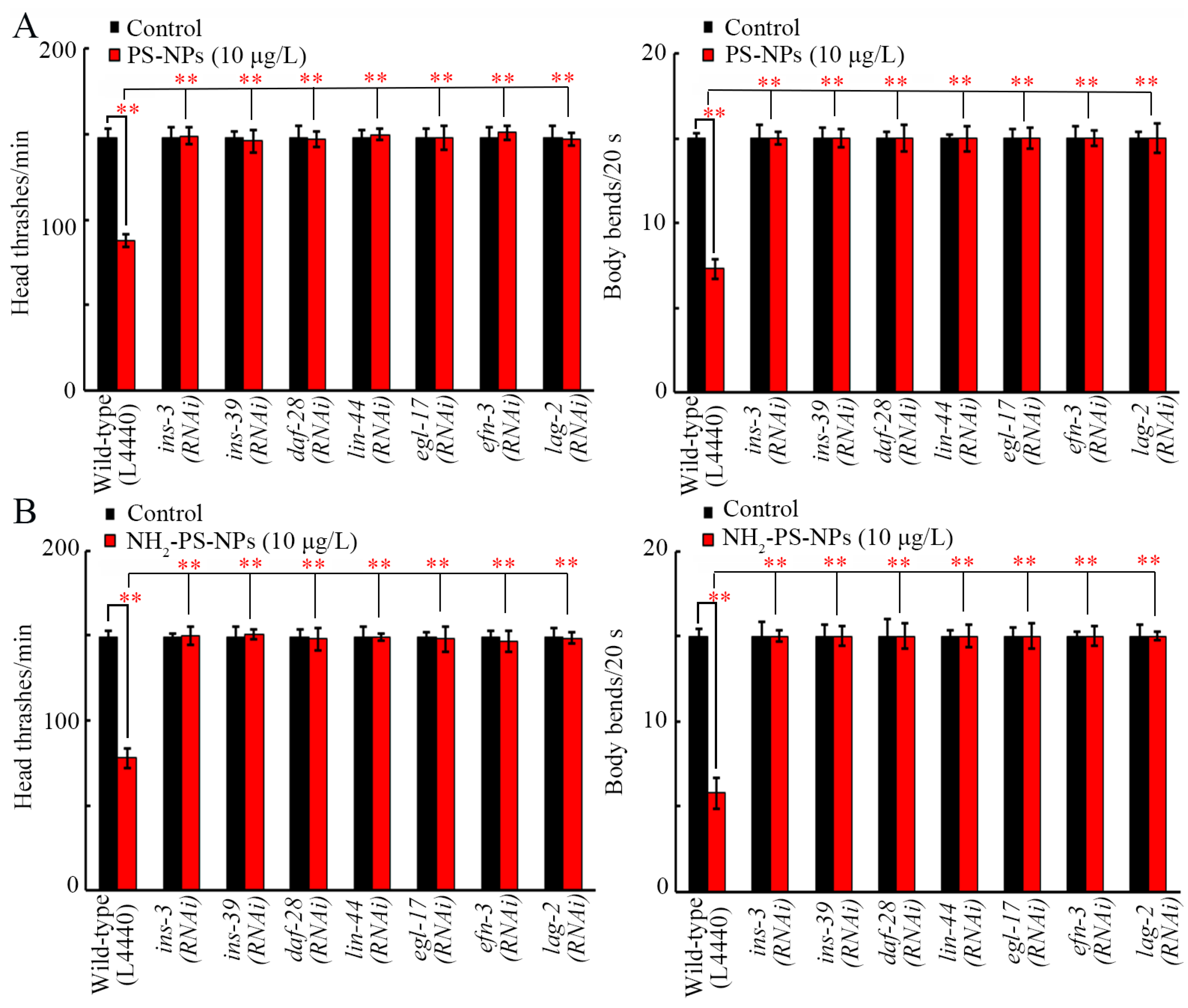
3.7. RNAi of ins-3, ins-39, daf-28, lin-44, egl-17, efn-3, and lag-2 Inhibited Neurotoxicity of Both Pristine and Amino-Modified PS-NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Leistenschneider, D.; Wolinski, A.; Cheng, J.; Ter Halle, A.; Duflos, G.; Huvet, A.; Paul-Pont, I.; Lartaud, F.; Galgani, F.; Lavergne, É.; et al. A critical review on the evaluation of toxicity and ecological risk assessment of plastics in the marine environment. Sci. Total Environ. 2023, 896, 164955. [Google Scholar] [CrossRef]
- Andrady, A.L. Weathering and fragmentation of plastic debris in the ocean environment. Mar. Pollut. Bull. 2022, 180, 113761. [Google Scholar] [CrossRef] [PubMed]
- Colwell, J.; Pratt, S.; Lant, P.; Laycock, B. Hazardous state lifetimes of biodegradable plastics in natural environments. Sci. Total Environ. 2023, 894, 165025. [Google Scholar] [CrossRef] [PubMed]
- Jebashalomi, V.; Charles, P.E.; Rajaram, R.; Sadayan, P. A critical review on nanoplastics and its future perspectives in the marine environment. Environ. Monit. Assess. 2023, 195, 1186. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Haider, A.; Ahmad, H.M.; Mohyuddin, A.; Umer Aslam, H.M.; Nadeem, S.; Javed, M.; Othman, M.H.D.; Goh, H.H.; Chew, K.W. Source, occurrence, distribution, fate, and implications of microplastic pollutants in freshwater on environment: A critical review and way forward. Chemosphere 2023, 325, 138367. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D.-Y. Cellular uptake, transport, and organelle response after exposure to microplastics and nanoplastics: Current knowledge and perspectives for environmental and health risks. Rev. Environ. Contam. Toxicol. 2022, 260, 12. [Google Scholar] [CrossRef]
- Zhu, Y.; Che, R.; Zong, X.; Wang, J.; Li, J.; Zhang, C.; Wang, F. A comprehensive review on the source, ingestion route, attachment and toxicity of microplastics/nanoplastics in human systems. J. Environ. Manag. 2024, 352, 120039. [Google Scholar] [CrossRef]
- Yang, S.; Li, M.; Kong, R.Y.C.; Li, L.; Li, R.; Chen, J.; Lai, K.P. Reproductive toxicity of micro- and nanoplastics. Environ. Int. 2023, 177, 108002. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Tang, M.; Ding, G.; Lu, X.; Huang, Q.; Du, H.; Xiao, G.; Wang, D. Exposure to nanoplastic particles enhances Acinetobacter survival, biofilm formation, and serum resistance. Nanomaterials 2022, 12, 4222. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Nielsen, T.G. Microplastic exposure studies should be environmentally realistic. Proc. Natl. Acad. Sci. USA 2016, 113, E4121–E4122. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Estrela, F.N.; Rodrigues, A.S.L.; Chagas, T.Q.; Pereira, P.S.; Silva, F.G.; Malafaia, G. Nanopolystyrene particles at environmentally relevant concentrations causes behavioral and biochemical changes in juvenile grass carp (Ctenopharyngodon idella). J. Hazard. Mater. 2021, 403, 123864. [Google Scholar] [CrossRef]
- Materic, D.; Peacock, M.; Dean, J.; Futter, M.; Maximov, T.; Moldan, F.; Rockmann, T.; Holzinger, R. Presence of nanoplastics in rural and remote surface waters. Environ. Res. Lett. 2022, 17, e054036. [Google Scholar] [CrossRef]
- Xiao, F.; Feng, L.J.; Sun, X.D.; Wang, Y.; Wang, Z.W.; Zhu, F.P.; Yuan, X.Z. Do polystyrene nanoplastics have similar effects on duckweed (Lemna minor L.) at environmentally relevant and observed-effect concentrations? Environ. Sci. Technol. 2022, 56, 4071–4079. [Google Scholar] [CrossRef]
- Qiu, W.; Ye, J.; Su, Y.; Zhang, X.; Pang, X.; Liao, J.; Wang, R.; Zhao, C.; Zhang, H.; Hu, L.; et al. Co-exposure to environmentally relevant concentrations of cadmium and polystyrene nanoplastics induced oxidative stress, ferroptosis and excessive mitophagy in mice kidney. Environ. Pollut. 2023, 333, 121947. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Hua, X.; Zhao, Y.; Bian, Q.; Wang, D.-Y. Polyethylene nanoplastics cause reproductive toxicity associated with activation of both estrogenic hormone receptor NHR-14 and DNA damage checkpoints in C. elegans. Sci. Total Environ. 2024, 906, 167471. [Google Scholar] [CrossRef]
- Zhuang, Z.-H.; Liu, T.-W.; Liu, Z.-Y.; Wang, D.-Y. Polystyrene nanoparticles strengthen high glucose toxicity associated with alteration in insulin signaling pathway in C. elegans. Ecotoxicol. Environ. Saf. 2024, 272, 116056. [Google Scholar] [CrossRef]
- Yeo, I.C.; Shim, K.Y.; Kim, K.; Jeong, C.B. Maternal exposure to nanoplastic induces transgenerational toxicity in the offspring of rotifer Brachionus koreanus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 269, 109635. [Google Scholar] [CrossRef]
- Yi, J.; Ma, Y.; Ruan, J.; You, S.; Ma, J.; Yu, H.; Zhao, J.; Zhang, K.; Yang, Q.; Jin, L.; et al. The invisible Threat: Assessing the reproductive and transgenerational impacts of micro- and nanoplastics on fish. Environ. Int. 2024, 183, 108432. [Google Scholar] [CrossRef]
- Hua, X.; Zhang, L.; Wang, D.-Y. Nanoplastic at environmentally relevant concentrations activates germline mir-240-rab-5 signaling cascade to affect secreted ligands associated with transgenerational toxicity induction in C. elegans. Environ. Sci. Nano 2024. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, Y.-X.; Bian, Q.; Wang, D.-Y. Transgenerational response of germline nuclear hormone receptor genes to nanoplastics at predicted Environmental doses in Caenorhabditis elegans. Toxics 2024, 12, 420. [Google Scholar] [CrossRef]
- Wang, D.-Y. Exposure Toxicology in Caenorhabditis elegans; Springer Nature Singapore Pte Ltd.: Singapore, 2020. [Google Scholar]
- Li, H.; Zeng, L.; Wang, C.; Shi, C.; Li, Y.; Peng, Y.; Chen, H.; Zhang, J.; Cheng, B.; Chen, C.; et al. Review of the toxicity and potential molecular mechanisms of parental or successive exposure to environmental pollutants in the model organism Caenorhabditis elegans. Environ. Pollut. 2022, 311, 119927. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Liang, G.-Y.; Chao, J.; Wang, D.-Y. Comparison of intestinal toxicity in enhancing intestinal permeability and in causing ROS production of six PPD quinones in Caenorhabditis elegans. Sci. Total Environ. 2024, 927, 172306. [Google Scholar] [CrossRef]
- Shao, Y.-T.; Hua, X.; Li, Y.-H.; Wang, D.-Y. Comparison of reproductive toxicity between pristine and aged polylactic acid microplastics in Caenorhabditis elegans. J. Hazard. Mater. 2024, 466, 133545. [Google Scholar] [CrossRef]
- Wang, D.-Y. Toxicology at Environmentally Relevant Concentrations in Caenorhabditis elegans; Springer Nature: Dordrecht, The Netherlands, 2022. [Google Scholar]
- Yang, Y.; Li, M.; Zheng, J.; Zhang, D.; Ding, Y.; Yu, H.Q. Environmentally relevant exposure to tetrabromobisphenol A induces reproductive toxicity via regulating glucose-6-phosphate 1-dehydrogenase and sperm activation in Caenorhabditis elegans. Sci. Total Environ. 2024, 907, 167820. [Google Scholar] [CrossRef]
- Hua, X.; Liang, G.-Y.; Chao, J.; Wang, D.-Y. Exposure to 6-PPD quinone causes damage on mitochondrial complex I/II associated with lifespan reduction in Caenorhabditis elegans. J. Hazard. Mater. 2024, 472, 134598. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Bian, Q.; Wang, D.-Y. Exposure to 6-PPD quinone causes ferroptosis activation associated with induction of reproductive toxicity in Caenorhabditis elegans. J. Hazard. Mater. 2024, 471, 134356. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D.-Y. Disruption of dopamine metabolism by exposure to 6-PPD quinone in Caenorhabditis elegans. Environ. Pollut. 2023, 337, 122649. [Google Scholar] [CrossRef]
- Qiao, R.; Mortimer, M.; Richter, J.; Rani-Borges, B.; Yu, Z.; Heinlaan, M.; Lin, S.; Ivask, A. Hazard of polystyrene micro-and nanospheres to selected aquatic and terrestrial organisms. Sci. Total Environ. 2022, 853, 158560. [Google Scholar] [CrossRef]
- Jewett, E.; Arnott, G.; Connolly, L.; Vasudevan, N.; Kevei, E. Microplastics and their impact on reproduction-can we learn from the C. elegans model? Front. Toxicol. 2022, 4, 748912. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Wang, R.; Pu, X.; Wang, D. A review of transgenerational and multigenerational toxicology in the in vivo model animal Caenorhabditis elegans. J. Appl. Toxicol. 2023, 43, 122–145. [Google Scholar] [CrossRef]
- Li, J.; Dai, L.; Feng, Y.; Cao, Z.; Ding, Y.; Xu, H.; Xu, A.; Du, H. Multigenerational effects and mutagenicity of three flame retardants on germ cells in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2024, 269, 115815. [Google Scholar] [CrossRef]
- Shao, Y.-T.; Li, Y.-H.; Wang, D.-Y. Polylactic acid microplastics cause transgenerational reproductive toxicity associated with activation of insulin and hedgehog ligands in C. elegans. Sci. Total Environ. 2024, 942, 173746. [Google Scholar] [CrossRef]
- Chen, H.; Gu, Y.; Jiang, Y.; Yu, J.; Chen, C.; Shi, C.; Li, H. Photoaged polystyrene nanoplastics result in transgenerational reproductive toxicity associated with the methylation of histone H3K4 and H3K9 in Caenorhabditis elegans. Environ. Sci. Technol. 2023, 57, 19341–19351. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Wang, Z. Long-term exposure to polystyrene nanoparticles at environmentally relevant concentration causes suppression in heme homeostasis signal associated with transgenerational toxicity induction in Caenorhabditis elegans. J. Hazard. Mater. 2023, 459, 132124. [Google Scholar] [CrossRef]
- He, W.; Gu, A.; Wang, D. Sulfonate-modified polystyrene nanoparticle at precited environmental concentrations induces transgenerational toxicity associated with increase in germline Notch signal of Caenorhabditis elegans. Toxics 2023, 11, 511. [Google Scholar] [CrossRef]
- Hua, X.; Cao, C.; Zhang, L.; Wang, D. Activation of FGF signal in germline mediates transgenerational toxicity of polystyrene nanoparticles at predicted environmental concentrations in Caenorhabditis elegans. J. Hazard. Mater. 2023, 451, 131174. [Google Scholar] [CrossRef]
- Yu, C.W.; Luk, T.C.; Liao, V.H. Long-term nanoplastics exposure results in multi and trans-generational reproduction decline associated with germline toxicity and epigenetic regulation in Caenorhabditis elegans. J. Hazard. Mater. 2021, 412, 125173. [Google Scholar] [CrossRef]
- Hua, X.; Zhao, Y.; Yuan, Y.-J.; Zhang, L.; Bian, Q.; Wang, D.-Y. Nanoplastics cause transgenerational toxicity through inhibiting germline microRNA mir-38 in C. elegans. J. Hazard. Mater. 2022, 437, 129302. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.-T.; Zhao, Y.; Bi, K.; Wang, D.-Y. Increase in germline methyltransferases governing methylation of histone H3K9 is associated with transgenerational nanoplastic toxicity in Caenorhabditis elegans. Environ. Sci. Nano 2022, 9, 265–274. [Google Scholar] [CrossRef]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef] [PubMed]
- Pelegrini, K.; Pereira, T.C.B.; Maraschin, T.G.; Teodoro, L.S.; Basso, N.R.S.; De Galland, G.L.B.; Ligabue, R.A.; Bogo, M.R. Micro- and nanoplastic toxicity: A review on size, type, source, and test-organism implications. Sci. Total Environ. 2023, 878, 162954. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; An, Y.; Jiang, X.; Wu, Q.; Miao, L.; Zhang, X.; Wang, Y. Exposure to epoxy-modified nanoplastics in the range of mug/L causes dysregulated intestinal permeability, reproductive capacity, and mitochondrial homeostasis by affecting antioxidant system in Caenorhabditis elegans. Aquat. Toxicol. 2023, 264, 106710. [Google Scholar] [CrossRef]
- Gao, X.; Xu, K.; Du, W.; Wang, S.; Jiang, M.; Wang, Y.; Han, Q.; Chen, M. Comparing the effects and mechanisms of exposure to polystyrene nanoplastics with different functional groups on the male reproductive system. Sci. Total Environ. 2024, 922, 171299. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Qiu, Y.; Kong, Y.; Wang, D. Amino modification enhances reproductive toxicity of nanopolystyrene on gonad development and reproductive capacity in nematode Caenorhabditis elegans. Environ. Pollut. 2019, 254, 112978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Liu, R.; Li, W.; Wang, Y.; Wan, X.; Song, N.; Yu, Y.; Xu, J.; Bu, Y.; Zhang, A. The use of different sublethal endpoints to monitor atrazine toxicity in nematode Caenorhabditis elegans. Chemosphere 2021, 274, 129845. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y. Target Organ Toxicology in Caenorhabditis elegans; Springer Nature: Dordrecht, The Netherlands, 2019. [Google Scholar]
- Jin, Y.; Hoskins, R.; Horvitz, H.R. Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature 1994, 372, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Kong, Y.; Yuan, Y.; Wang, D. Neuronal damage induced by nanopolystyrene particles in nematode Caenorhabditis elegans. Environ. Sci. Nano 2019, 6, 2591–2601. [Google Scholar] [CrossRef]
- Wang, S.-T.; Liu, H.-L.; Zhao, Y.-Y.; Rui, Q.; Wang, D.-Y. Dysregulated mir-354 enhanced the protective response to nanopolystyrene by affecting the activity of TGF-β signaling pathway in nematode Caenorhabditis elegans. NanoImpact 2020, 20, 100256. [Google Scholar] [CrossRef]
- Liu, H.-L.; Zhang, R.-J.; Wang, D.-Y. Response of DBL-1/TGF-β signaling-mediated neuron-intestine communication to nanopolystyrene in nematode Caenorhabditis elegans. Sci. Total Environ. 2020, 745, 1141047. [Google Scholar] [CrossRef]
- Qu, M.; Li, D.; Zhao, Y.-L.; Yuan, Y.-J.; Wang, D.-Y. Exposure to low-dose nanopolystyrene induces the response of neuronal JNK MAPK signaling pathway in nematode Caenorhabditis elegans. Environ. Sci. Eur. 2020, 32, 58. [Google Scholar] [CrossRef]
- Qu, M.; Li, D.; Qiu, Y.-X.; Wang, D.-Y. Neuronal ERK MAPK signaling in response to low-dose nanopolystyrene exposure by suppressing insulin peptide expression in Caenorhabditis elegans. Sci. Total Environ. 2020, 724, 138378. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, Y.; Zhang, L.; Wang, D. Procatechuic acid and protocatechuic aldehyde increase survival of Caenorhabditis elegans after fungal infection and inhibit fungal virulence. Front. Pharmacol. 2024, 15, 1396733. [Google Scholar] [CrossRef]
- Xu, R.; Hua, X.; Rui, Q.; Wang, D. Alteration in Wnt signaling mediates induction of transgenerational toxicity of polystyrene nanoplastics in C. elegans. NanoImpact 2022, 28, 100425. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wang, D.-Y. Exposure to 6-PPD quinone at environmentally relevant concentrations inhibits both lifespan and healthspan in C. elegans. Environ. Sci. Technol. 2023, 57, 19295–19303. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.-X.; Wang, D.-Y. Paeoniflorin increases the survival of Pseudomonas aeruginosa infected Caenorhabditis elegans at the immunosuppression stage by activating PMK-1, BAR-1, and EGL-1 signals. Arch. Pharm. Res. 2023, 46, 616–628. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Wang, D.-Y. Exposure to 6-PPD quinone enhances glycogen accumulation in Caenorhabditis elegans. Environ. Pollut. 2024, 359, 124600. [Google Scholar] [CrossRef]
- Wan, X.; Liang, G.-Y.; Wang, D.-Y. Neurotoxicity and accumulation of CPPD quinone at environmentally relevant concentrations in Caenorhabditis elegans. Chemosphere 2024, 361, 142499. [Google Scholar] [CrossRef]
- Hua, X.; Wang, D.-Y. Polyethylene nanoparticles at environmentally relevant concentrations enhances neurotoxicity and accumulation of 6-PPD quinone in Caenorhabditis elegans. Sci. Total Environ. 2024, 918, 170760. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Hua, X.; Wang, D.-Y. Exposure to 6-PPD quinone enhances lipid accumulation through activating metabolic sensors of SBP-1 and MDT-15 in Caenorhabditis elegans. Environ. Pollut. 2023, 333, 121937. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, Y.; Hua, X.; Li, Y.; Wang, D. Polylactic acid microparticles in the range of μg/L reduce reproductive capacity by affecting the gonad development and the germline apoptosis in Caenorhabditis elegans. Chemosphere 2023, 336, 139193. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhuang, Z.; Wang, D. Paeoniflorin mitigates high glucose-induced lifespan reduction by inhibiting insulin signaling in Caenorhabditis elegans. Front. Pharmacol. 2023, 14, 1202379. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ding, G.; Li, L.; Xiao, G.; Wang, D. Exposure to polystyrene nanoparticles at predicted environmental concentrations enhances toxic effects of Acinetobacter johnsonii AC15 infection on Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2023, 262, 115131. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhao, Y.; Hua, X.; Wang, D. Induction of transgenerational toxicity is associated with the activated germline insulin signals in nematodes exposed to nanoplastic at predicted environmental concentrations. Ecotoxicol. Environ. Saf. 2022, 243, 114022. [Google Scholar] [CrossRef]
- Zhao, Y.; Hua, X.; Bian, Q.; Wang, D.-Y. Nanoplastic exposure at predicted environmental concentrations induces activation of germline Ephrin signal associated with toxicity formation in the Caenorhabditis elegans offspring. Toxics 2022, 10, 699. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, X.; Huang, R.; Tang, C.; Hu, C.; Ning, P.; Wang, F. Cytotoxicity and genotoxicity of polystyrene micro- and nanoplastics with different size and surface modification in A549 cells. Int. J. Nanomed. 2022, 17, 4509–4523. [Google Scholar] [CrossRef]
- Zhao, J.; Miao, L.; Yao, Y.; Adyel, T.M.; Cheng, H.; Liu, S.; Liu, Y.; Hou, J. Surface modification significantly changed the effects of nano-polystyrene on sediment microbial communities and nitrogen metabolism. J. Hazard. Mater. 2023, 460, 132409. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Wang, D. Comparison of transgenerational reproductive toxicity induced by pristine and amino modified nanoplastics in Caenorhabditis elegans. Sci. Total Environ. 2021, 768, 144362. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental hormesis in living systems: The role of hormetic trade-offs. Sci. Total Environ. 2023, 901, 166022. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spano, S.M.; Salinaro, A.T.; Calabrese, V. Hormetic nutrition and redox regulation in gut-brain axis disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Ontario, M.L.; Salinaro, A.T.; Caligiuri, I.; Rampulla, F.; Zimbone, V.; Modafferi, S.; Rizzolio, F.; Canzonieri, V.; Calabrese, E.J.; et al. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free Radic. Biol. Med. 2022, 179, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Wang, D.-Y. Intestinal mitochondrial unfolded protein response induced by nanoplastic particles in Caenorhabditis elegans. Chemosphere 2021, 267, 128917. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Zhang, R.-J.; Wang, D.-Y. Induction of protective response to polystyrene nanoparticles associated with methylation regulation in Caenorhabditis elegans. Chemosphere 2021, 271, 129589. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Tian, L.-J.; Qu, M.; Wang, D.-Y. Acetylation regulation associated with the induction of protective response to polystyrene nanoparticles in Caenorhabditis elegans. J. Hazard. Mater. 2021, 411, 125035. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Dong, W.-T.; Wu, Q.-L.; Wang, D.-Y. Induction of protective response associated with expressional alterations in neuronal G protein-coupled receptors in polystyrene nanoparticle exposed Caenorhabditis elegans. Chem. Res. Toxicol. 2021, 34, 1308–1318. [Google Scholar] [CrossRef]
- Driscoll, M.; Gerstbrein, B. Dying for a cause: Invertebrate genetics takes on human neurodegeneration. Nat. Rev. Genet. 2003, 4, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; LaFerla, F.M.; Chan, S.L.; Leissring, M.A.; Shepel, P.N.; Geiger, J.D. Calcium signaling in the ER: Its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000, 23, 222–229. [Google Scholar] [CrossRef]
- Xu, K.; Tavernarakis, N.; Driscoll, M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron 2001, 31, 957–971. [Google Scholar] [CrossRef]
- Syntichaki, P.; Xu, K.; Driscoll, M.; Tavernarakis, N. Specific aspartyl and calpain proteases are required for neurodegeneration in C. elegans. Nature 2002, 419, 939–944. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Ruan, Q.; Wang, D. Comparison of Transgenerational Neurotoxicity between Pristine and Amino-Modified Nanoplastics in C. elegans. Toxics 2024, 12, 555. https://doi.org/10.3390/toxics12080555
Song M, Ruan Q, Wang D. Comparison of Transgenerational Neurotoxicity between Pristine and Amino-Modified Nanoplastics in C. elegans. Toxics. 2024; 12(8):555. https://doi.org/10.3390/toxics12080555
Chicago/Turabian StyleSong, Mingxuan, Qinli Ruan, and Dayong Wang. 2024. "Comparison of Transgenerational Neurotoxicity between Pristine and Amino-Modified Nanoplastics in C. elegans" Toxics 12, no. 8: 555. https://doi.org/10.3390/toxics12080555
APA StyleSong, M., Ruan, Q., & Wang, D. (2024). Comparison of Transgenerational Neurotoxicity between Pristine and Amino-Modified Nanoplastics in C. elegans. Toxics, 12(8), 555. https://doi.org/10.3390/toxics12080555






