The Accumulation of Abscisic Acid Increases the Innate Pool of Soluble Phenolics through Polyamine Metabolism in Rice Seedlings under Hexavalent Chromium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedlings Preparation and Experiment Design
2.2. Growth Measurement of Rice Seedling
2.3. Measurement of Cr
2.4. Measurement of ABA
2.5. Extraction and Quantification of Total Soluble Phenolics
2.6. Measurement of Polyamine-Related Compounds
2.7. RNA Extraction and RT-qPCR Analysis
2.8. Data Analysis
3. Results
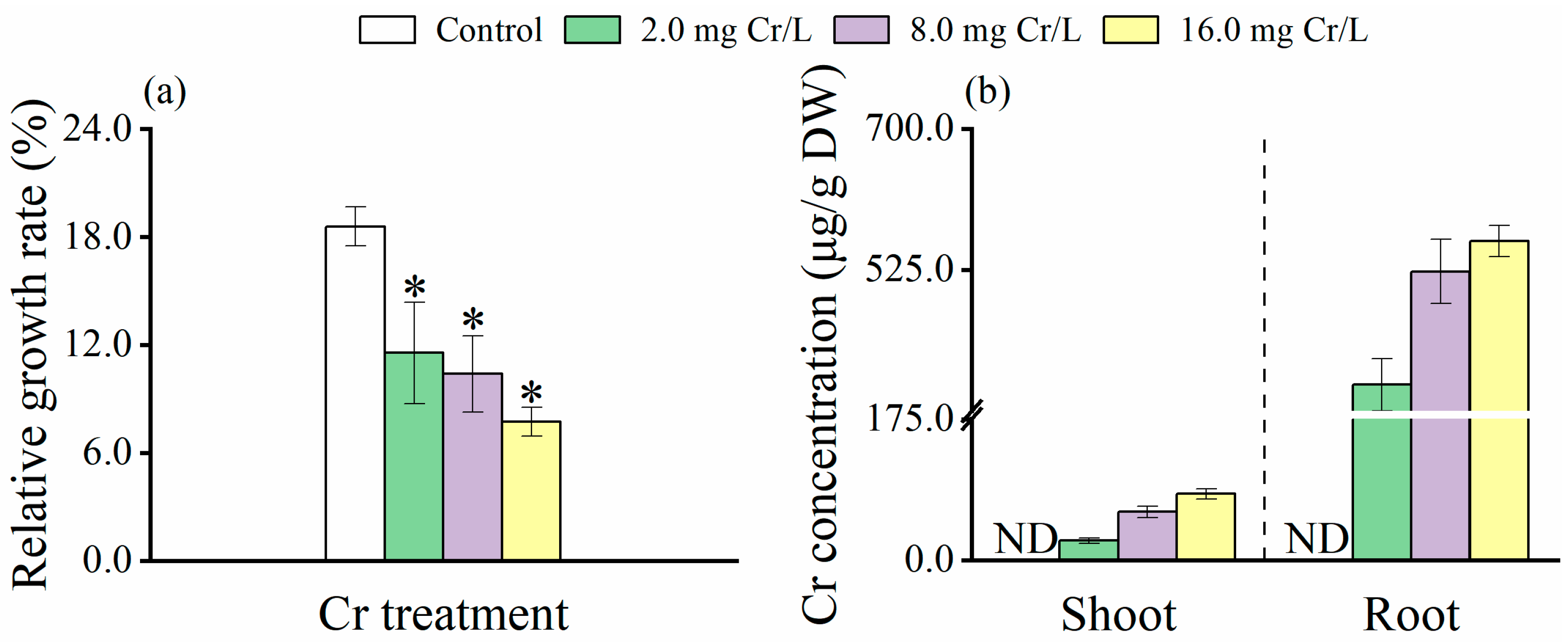
3.1. Growth Response of Rice Plants to Cr(VI)
3.2. Distribution of Cr(VI) in Rice Tissues
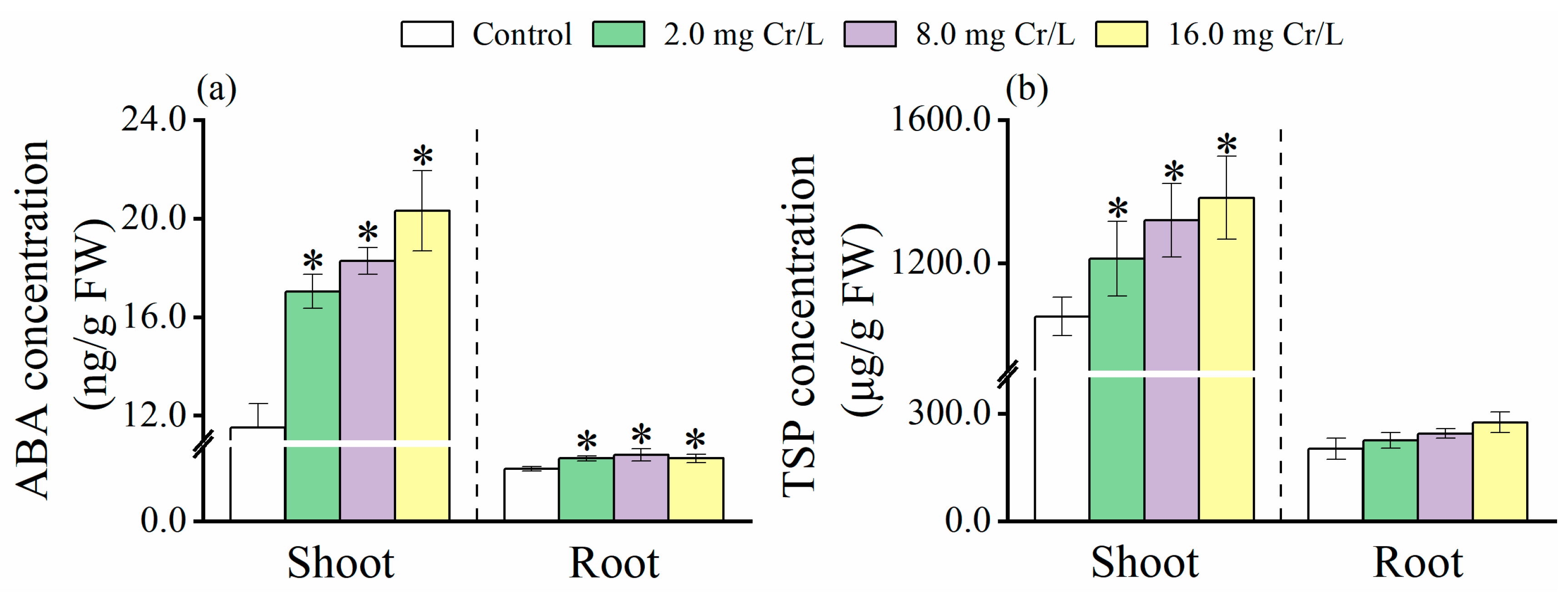
3.3. Changes in ABA Level
3.4. Changes in TSPs
3.5. Changes in Arg, Orn and Met
3.6. Changes in PAs Compounds
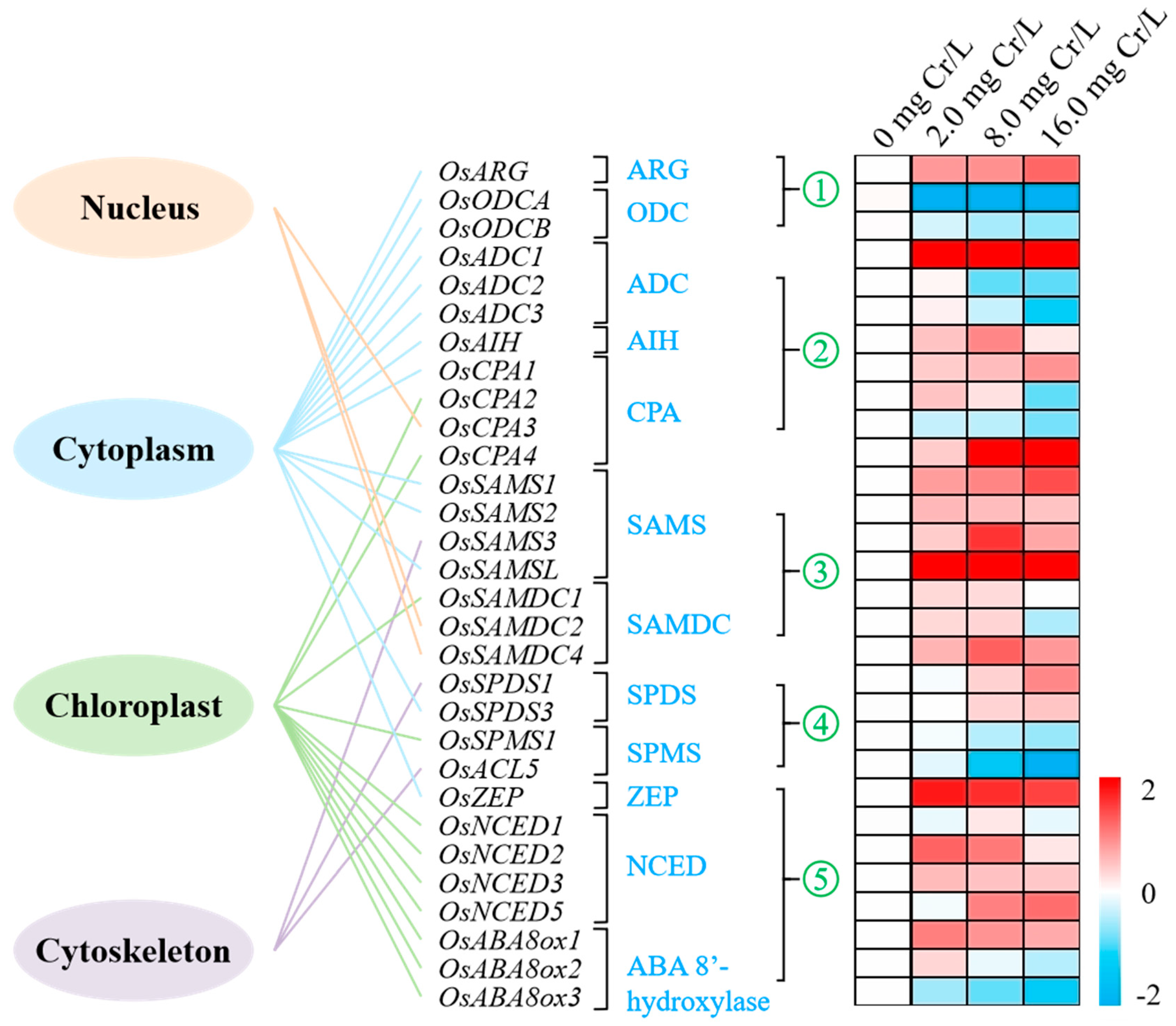
3.7. Subcellular Localization of Genes
3.8. Gene Expression Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pourret, O.; Hursthouse, A. It’s time to replace the term “heavy metals” with “potentially toxic elements” when reporting environmental research. Int. J. Environ. Res. Public Health 2010, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Z.; Lin, Y.J.; Zhang, Q. Metallothioneins enhance chromium detoxification through scavenging ROS and stimulating metal chelation in Oryza sativa. Chemosphere 2019, 220, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Espinosa, J.; Garcia-Ibañez, P.; Lopez-Zaplana, A.; Yepes-Molina, L.; Albaladejo-Marico, L.; Carvajal, M. Confronting secondary metabolites with water uptake transport in plants under abiotic stress. Int. J. Mol. Sci. 2023, 24, 2826. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Ullah, A.; Feng, Y.X.; Tian, P.; Yu, X.Z. Proline-mediated activation of glyoxalase II improve methylglyoxal detoxification in Oryza sativa L. under chromium injury: Clarification via vector analysis of enzymatic activities and gene expression. Plant Physiol. Biochem. 2023, 201, 107867. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.J.; Feng, Y.X.; Li, Y.H.; Lin, Y.J.; Yu, X.Z. Unraveling genes promoting ROS metabolism in subcellular organelles of Oryza sativa in response to trivalent and hexavalent chromium. Sci. Total Environ. 2020, 744, 140951. [Google Scholar] [CrossRef] [PubMed]
- Moriello, C.; Costabile, M.; Spinelli, M.; Amoresano, A.; Palumbo, G.; Febbraio, F.; Piscopo, M. Altered expression of protamine-like and their DNA binding induced by Cr(VI): A possible risk to spermatogenesis? Biomolecules 2022, 12, 700. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, A.; Adhikari, S.; Ghosh, S.; Azahar, I.; Arun, K.; Shaw, A.K.; Roy, D.; Roy, C.; Saha, S.; Zahed Hossain, Z. Imbalance of redox homeostasis and antioxidant defense status in maize under chromium (VI) stress. Environ. Exp. Bot. 2020, 169, 103873. [Google Scholar] [CrossRef]
- Ao, M.; Chen, X.; Deng, T.; Sun, S.; Tang, Y.; Morel, J.L.; Qiu, R.; Wang, S. Chromium biogeochemical behaviour in soil-plant systems remediation strategies: A critical review. J. Hazard. Mater. 2022, 424, 127233. [Google Scholar] [CrossRef]
- Perin, E.C.; Messias, R.S.; Borowski, J.M.; Crizel, R.L.; Schott, I.B.; Carvalho, I.R.; Rombaldi, C.V.; Galli, V. ABA-dependent salt and drought stress improve strawberry fruit quality. Food Chem. 2019, 271, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Podlešáková, K.; Ugena, L.; Spíchal, L.; Doležal, K.; De Diego, N. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019, 48, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Ramazan, S.; Nazir, I.; Waseem Yousuf, W.; John, R. Environmental stress tolerance in maize (Zea mays): Role of polyamine metabolism. Funct. Plant Biol. 2022, 50, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Du, K.; Xie, J.; Sun, G.; Wang, P.; Chen, X.; Cao, Z.; Wang, B.; Chao, Q.; Li, X.; et al. Activated malate circulation contributes to the manifestation of light-dependent mosaic symptoms. Cell Rep. 2023, 42, 112333. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, C.; Lettieri, G.; Verrillo, M.; Morelli, M.; Carraturo, F.; Guida, M.; Piscopo, M. Possible molecular mechanisms underlying the decrease in the antibacterial activity of protamine-like proteins after exposure of Mytilus galloprovincialis to chromium and mercury. Int. J. Mol. Sci. 2023, 24, 9345. [Google Scholar] [CrossRef] [PubMed]
- Asare, M.O.; Száková, J.; Tlustoš, P. The fate of secondary metabolites in plants growing on Cd-, As-, and Pb-contaminated soils-a comprehensive review. Environ. Sci. Pollut. Res. 2023, 30, 11378–11398. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Llorca, M.; Pollmann, S.; Müller, M. Ethylene jasmonates signaling network mediating secondary metabolites under abiotic stress. Int. J. Mol. Sci. 2023, 24, 5990. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, X.; Wang, Q.; Zhao, Z.; Xie, B.; Xu, L.; Zhang, R. Plant defense mechanisms against ozone stress: Insights from secondary metabolism. Environ. Exp. Bot. 2024, 217, 105553. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Bačkor, M. Phenolic metabolism of Matricaria chamomilla plants exposed to nickel. J. Plant Physiol. 2009, 166, 1460–1464. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Stork, F.; Hedbavny, J. Nitrate deficiency reduces cadmium nickel accumulation in chamomile plants. J. Agric. Food Chem. 2011, 59, 5139–5149. [Google Scholar] [CrossRef]
- Toumi, I.; Moschou, P.N.; Paschalidis, K.A.; Bouamama, B.; Salem-fnayou, A.B.; Ghorbel, A.W.; Mliki, A.; Roubelakis-Angelakis, K.A. Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Physiol. 2010, 167, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Moschou, P.N.; Wu, J.; Cona, A.; Tavladoraki, P.; Angelini, R.; Roubelakis-Angelakis, K.A. The polyamines their catabolic products are significant players in the turnover of nitrogen molecules in plants. J. Exp. Bot. 2012, 63, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Degenkolbe, T.; Erban, A.; Heyer, A.G.; Kopka, J.; Kohl, K.I.; Hincha, D.K.; Zuther, E. Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS ONE 2013, 8, e60325. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chan, Z. Improvement of plant abiotic stress tolerance through modulation of the polyamine pathway. J. Inter. Plant Biol. 2014, 56, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Csávás, G.; Szalai, G.; Oláh, T.; Khalil, R.; Yordanova, R.; Gell, G.; Birinyi, Z.; Németh, E.; Janda, T. Polyamines may influence phytochelatin synthesis during Cd stress in rice. J. Hazard. Mater. 2017, 340, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Janda, T. Speculation: Polyamines are important in abiotic stress signaling. Plant Sci. 2015, 237, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.A.; Gori, A.; Da-Silva, C.J.; Cecilia Brunetti, C. Abscisic acid biosynthesis and signaling in plants: Key targets to improve water use efficiency and drought tolerance. Appl. Sci. 2020, 10, 6322. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Wang, X.; Luo, S.X.; Yu, P.; Luo, L.; Zhao, J.J.; Wang, Y.H.; Shen, S.X.; Chen, X.P. Advances in phenylaprapanoid metabolism and its enzyme genes in solanaceae vegetables. Acta Hortic. Sin. 2017, 44, 1738–1748. [Google Scholar]
- Singh, A.A.; Ghosh, A.; Agrawal, M.; Agrawal, S.B. Secondary metabolites responses of plants exposed to ozone: An update. Environ. Sci. Pollut. Res. 2023, 30, 88281–88312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, Y.X.; Tian, P.; Yu, X.Z. Proline-mediated regulation on jasmonate signals repressed anthocyanin accumulation through the MYB-bHLH-WDR complex in rice under chromium exposure. Front. Plant Sci. 2022, 13, 953398. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Lin, Y.J.; Zhang, H.; Yu, X.Z. Identification of the key genes involved in proline-mediated modification of cell wall components in rice seedlings under trivalent chromium exposure. Toxics 2024, 12, 4. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. J. Agr. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Feng, Y.X.; Lin, Y.J.; Yu, X.Z. Comparative effects of sodium hydrosulfide and proline on functional repair in rice chloroplast through the D1 protein and thioredoxin system under simulated thiocyanate pollution. Chemosphere 2021, 284, 131389. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Docimo, T.; Reichelt, M.; Schneider, B.; Al, E. The first step in the biosynthesis of cocaine in Erythroxylum coca: The characterization of arginine and ornithine decarboxylases. Plant Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef]
- Li, C.Z.; Feng, Y.X.; Tian, P.; Yu, X.Z. Mathematical estimation of endogenous proline as a bioindicator to regulate the stress of trivalent chromium on rice plants grown in different nitrogenous conditions. Toxics 2023, 11, 803. [Google Scholar] [CrossRef]
- Zhang, Q.; Feng, Y.X.; Lin, Y.J.; Yu, X.Z. Indigenous proline is a two-dimensional safety-relief valve in balancing specific amino acids in rice under hexavalent chromium stress. J. Agric. Food Chem. 2021, 69, 11185–11195. [Google Scholar] [CrossRef]
- Soudek, P.; Ursu, M.; Petrová, Š.; Vaněk, T. Improving crop tolerance to heavy metal stress by polyamine application. Food Chem. 2016, 213, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.P.; Groppa, M.D.; Recalde, L.; Verstraeten, S.V. Effects of polyamines on cadmium- and copper-mediated alterations in wheat (Triticum aestivum L.) and sunflower (Helianthus annuus L.) seedling membrane fluidity. Arch. Biochem. Biophy. 2018, 654, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Purnima Yumnam, J.; Harshavardhan, M.; Kuamr, P.S.; Jyoti, N.; Kumar, S.; Naik, M.; Misao, L. Impact of polyamines and mycorrhiza on chlorophyll substance of maize grown under cadmium toxicity. Inter. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1635–1639. [Google Scholar] [CrossRef]
- Shah, A.A.; Riaz, L.; Siddiqui, M.H.; Nazar, R.; Ahmed, S.; Yasin, N.A.; Ali, A.; Mukherjee, S.; Hussaan, M.; Javad, S.; et al. Spermine-mediated polyamine metabolism enhances arsenic-stress tolerance in Phaseolus vulgaris by expression of zinc-finger proteins related genes and modulation of mineral nutrient homeostasis and antioxidative system. Environ. Pollut. 2022, 300, 118941. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.H.; Takahashi, T.; Angelini, R. Editorial: Molecular mechanisms underlying polyamine functions in plants. Front. Plant Sci. 2017, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Pál, M.; Szalai, G.; Gondor, O.K.; Janda, T. Unfinished story of polyamines: Role of conjugation, transport and light-related regulation in the polyamine metabolism in plants. Plant Sci. 2021, 308, 110923. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Bhardwaj, R.; Gupta, B.D.; Dutt, P.; Gupta, R.K.; Kanwar, M.; Dutt, P. Changes induced by Cu2+ and Cr6+ metal stress in polyamines, auxins, abscisic acid titers and antioxidative enzymes activities of radish seedlings. Braz. J. Plant Physiol. 2010, 22, 263–270. [Google Scholar] [CrossRef][Green Version]
- Das, S.; Majumder, B.; Biswas, A.K. Comparative study on the influence of silicon and selenium to mitigate arsenic induced stress by modulating TCA cycle, GABA, and polyamine synthesis in rice seedlings. Ecotoxicology 2022, 31, 468–489. [Google Scholar] [CrossRef]
- Hatmi, S.; Villaume, S.; Trotel-Aziz, P.; Barka, E.A.; Clément, C.; Aziz, A. Osmotic stress and ABA affect immune response and susceptibility of grapevine berries to gray mold by priming polyamine accumulation. Front. Plant Sci. 2018, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Bitrián, M.; Zarza, X.; Altabella, T.; Tiburcio, A.F.; Alcázar, R. Polyamines under abiotic stress: Metabolic crossroads and hormonal crosstalks in plants. Metabolites 2012, 2, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Ali, S.; Ramakrishna, G.; Singh, A.; Park, S.; Mahmoudi, H.; Bae, H. Revisiting the role of polyamines in plant growth and abiotic stress resilience: Mechanisms, crosstalk, and future perspectives. J. Plant Growth Regul. 2023, 42, 5074–5098. [Google Scholar] [CrossRef]
- Sharma, D.; Shree, B.; Kumar, S.; Kumar, V.; Sharma, S.; Sharma, S. Stress induced production of plant secondary metabolites in vegetables: Functional approach for designing next generation super foods. Plant Physiol. Biochem. 2022, 192, 252–272. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Dardanelli, M.S.; de Córdoba, F.J.F.; Estévez, J.; Contreras, R.; Cubo, M.T.; Rodriguez-Carvajal, M.A.; Gil-Serrano, A.M.; Baena, F.J.L.; Bellogín, R.; Manyani, H.; et al. Changes in flavonoids secreted by Phaseolus vulgaris roots in the presence of salt and the plant growth-promoting rhizobacterium Chryseobacterium balustinum. Appl. Soil Ecol. 2012, 57, 31–38. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Jiang, G.; Ye, N.; Chu, Z.; Xu, X.; Zhang, J.; Zhu, G. A key ABA catabolic gene, OsABA8ox3, is involved in drought stress resistance in rice. PLoS ONE 2015, 10, e0116646. [Google Scholar] [CrossRef]
- Zhu, G.; Ye, N.; Zhang, J. Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol. 2009, 50, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef]
- Martínez-Andújar, C.; Ordiz, M.I.; Huang, Z.; Nonogaki, M.; Beachy, R.N.; Nonogaki, H. Induction of 9-cis-epoxycarotenoid dioxygenase in Arabidopsis thaliana seeds enhances seed dormancy. Proc. Natl. Acad. Sci. USA 2011, 108, 17225–17229. [Google Scholar] [CrossRef] [PubMed]
- Saika, H.; Okamoto, M.; Miyoshi, K.; Kushiro, T.; Shinoda, S.; Jikumaru, Y.; Fujimoto, M.; Arikawa, T.; Takahashi, H.; Ando, M.; et al. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 80 -hydroxylase in rice. Plant Cell Physiol. 2007, 48, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Nayeri, S.; Zahra Dehghanian, Z.; Lajayer, B.A.; Thomson, A.; Astatkie, T.; Price, G.W. CRISPR/Cas9-Mediated genetically edited ornamental aromatic plants: A promising technology in phytoremediation of heavy metals. J. Clean. Prod. 2023, 428, 139512. [Google Scholar] [CrossRef]
- Novair, S.B.; Cheraghi, M.; Faramarzi, F.; Lajayer, B.A.; Senapathi, V.; Astatkie, T.; Price, G.W. Reviewing the role of biochar in paddy soils: An agricultural and environmental perspective. Ecotox. Environ. Safe. 2023, 263, 115228. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.; Li, C.-Z.; Ullah, A.; Zhang, Q.; Yu, X.-Z. The Accumulation of Abscisic Acid Increases the Innate Pool of Soluble Phenolics through Polyamine Metabolism in Rice Seedlings under Hexavalent Chromium Stress. Toxics 2024, 12, 577. https://doi.org/10.3390/toxics12080577
Kang Y, Li C-Z, Ullah A, Zhang Q, Yu X-Z. The Accumulation of Abscisic Acid Increases the Innate Pool of Soluble Phenolics through Polyamine Metabolism in Rice Seedlings under Hexavalent Chromium Stress. Toxics. 2024; 12(8):577. https://doi.org/10.3390/toxics12080577
Chicago/Turabian StyleKang, Yi, Cheng-Zhi Li, Abid Ullah, Qing Zhang, and Xiao-Zhang Yu. 2024. "The Accumulation of Abscisic Acid Increases the Innate Pool of Soluble Phenolics through Polyamine Metabolism in Rice Seedlings under Hexavalent Chromium Stress" Toxics 12, no. 8: 577. https://doi.org/10.3390/toxics12080577
APA StyleKang, Y., Li, C.-Z., Ullah, A., Zhang, Q., & Yu, X.-Z. (2024). The Accumulation of Abscisic Acid Increases the Innate Pool of Soluble Phenolics through Polyamine Metabolism in Rice Seedlings under Hexavalent Chromium Stress. Toxics, 12(8), 577. https://doi.org/10.3390/toxics12080577





