Comparative Study of the Effects of Drugs Targeting Adrenergic Receptors on the Early Life Stages of Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Maintenance and Embryo Production
2.3. Exposure Conditions for Embryos and Larvae
2.4. The Measurement of Heartbeat, Blood Flow and Vessel Diameter
2.5. Locomotor Behavior Assay
2.6. Gene Transcription Analysis
2.7. Acetylcholinesterase (AChE) Activity Measurement
2.8. Data Analysis
3. Results
3.1. Effects on the Embryonic Development of Zebrafish
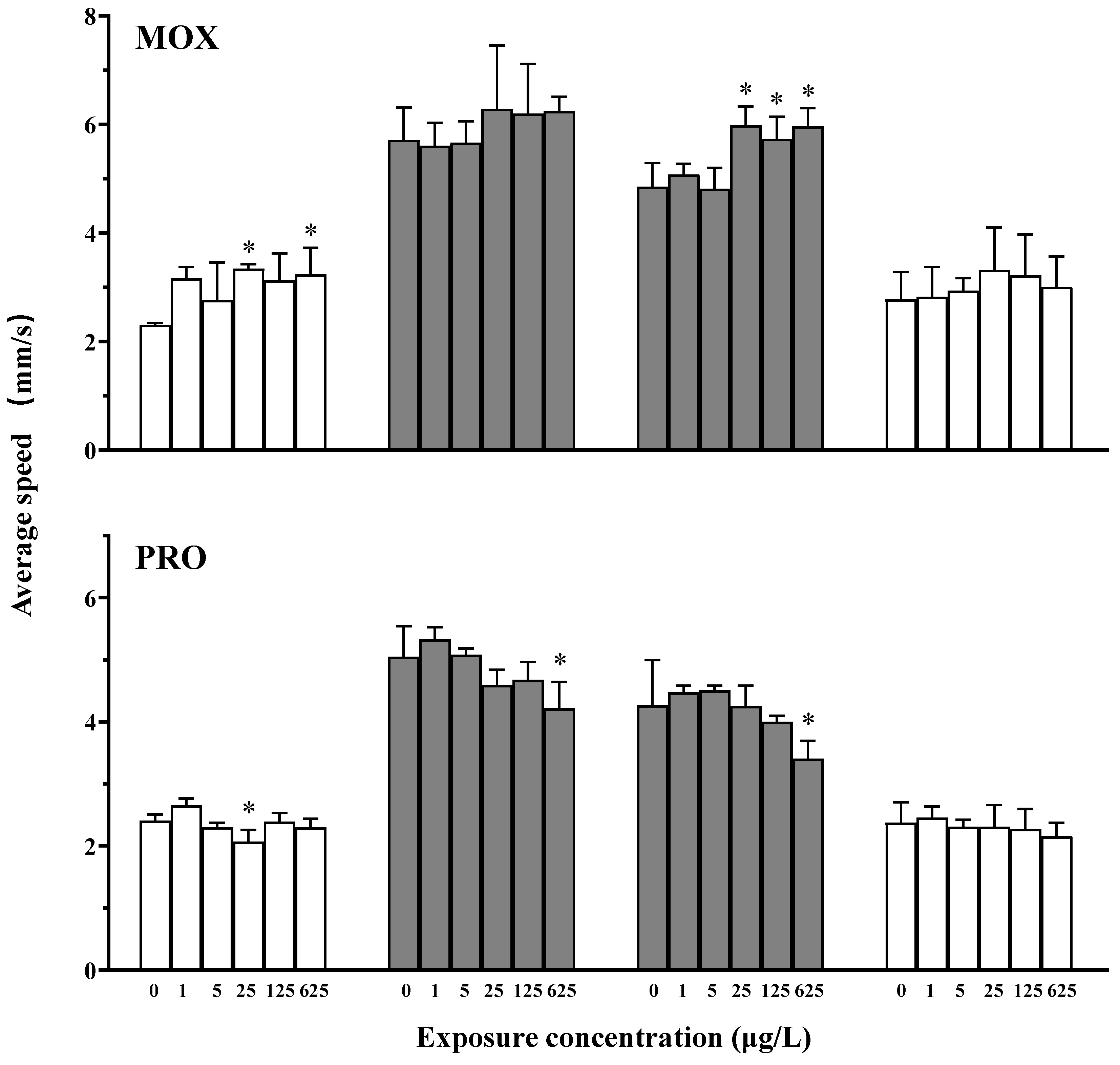
3.2. Effects on the Locomotor Behavior of Zebrafish
3.3. Effects on the Heartbeat, Blood Flow and Vessel Diameter of Zebrafish
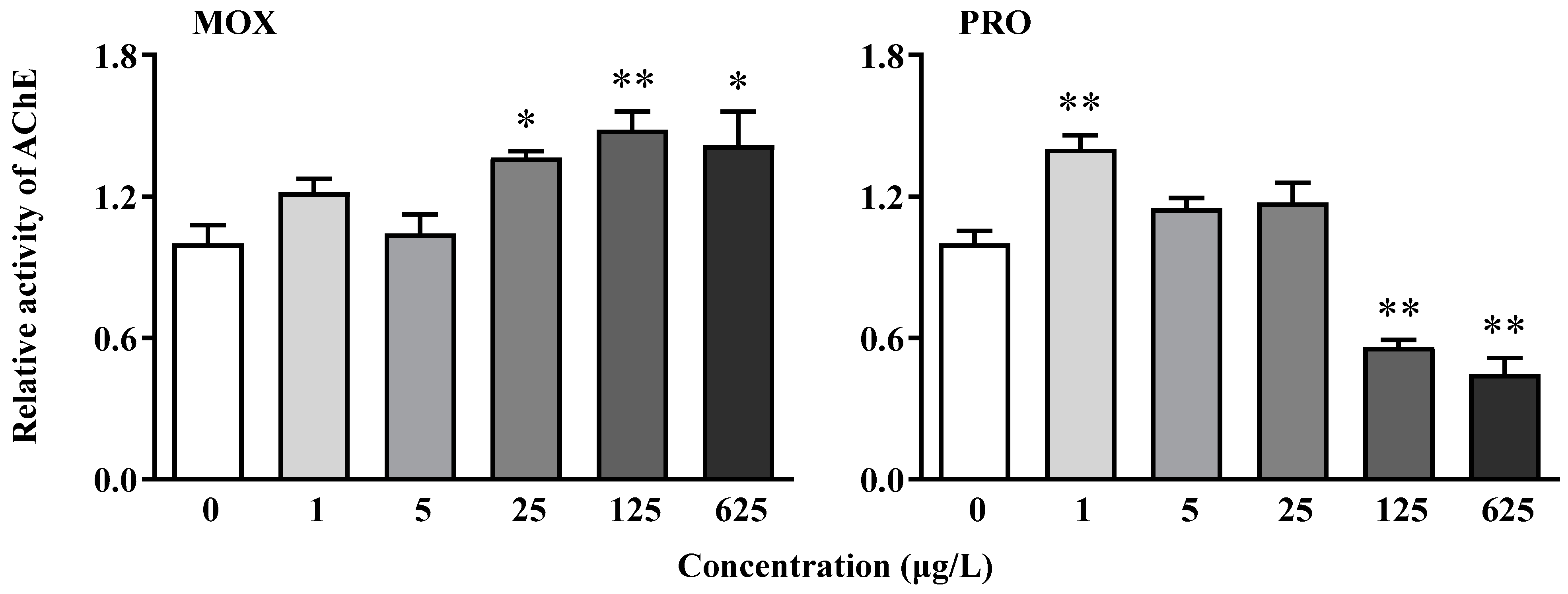
3.4. Effects on AChE Activity of Zebrafish
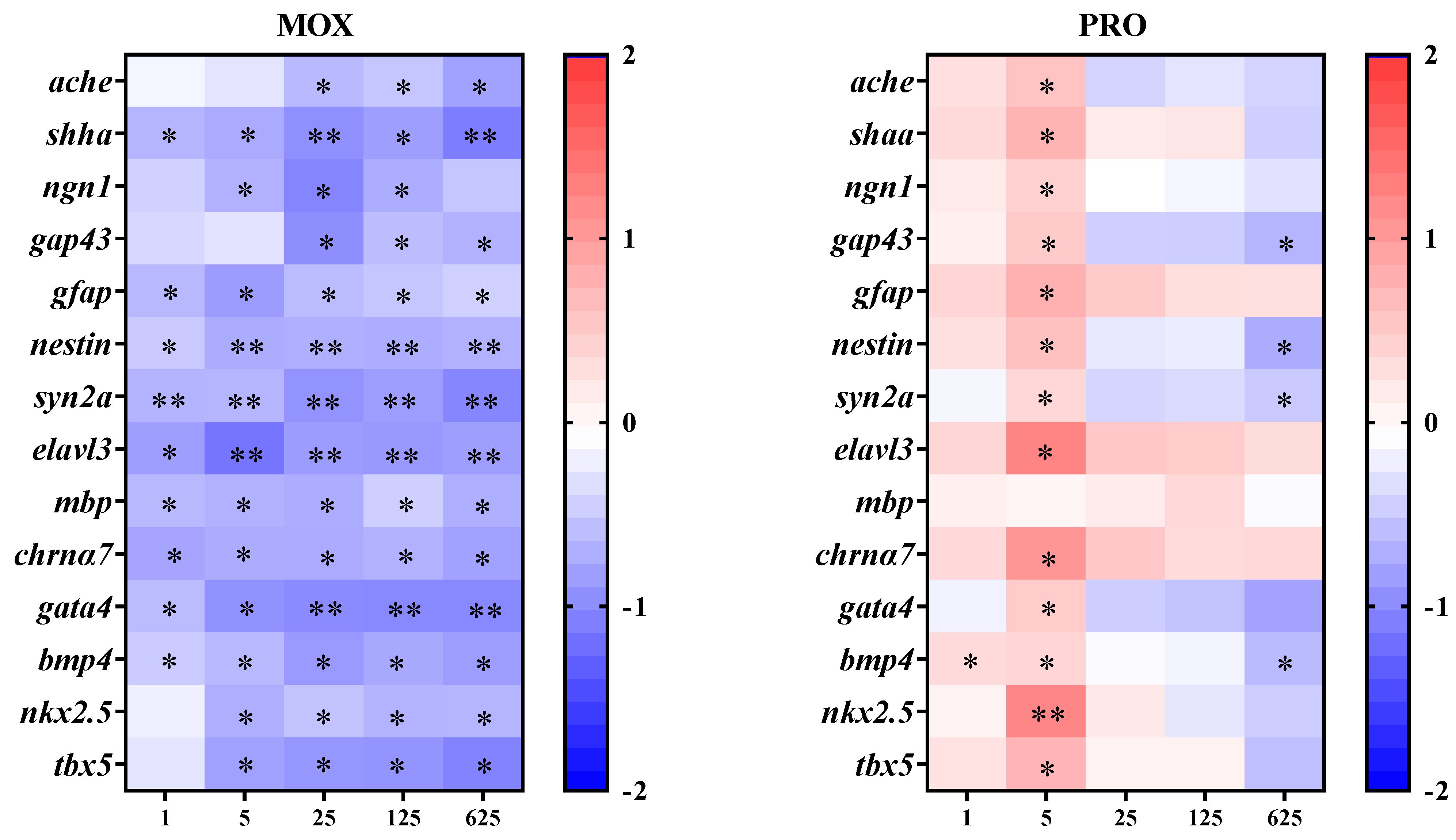
3.5. Effects on Transcriptional Response of Genes Related to the Cardiovascular System in Zebrafish Larvae
3.6. Effects on Transcriptional Responses of Genes Related to the Nervous System in the Zebrafish Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ciccarelli, M.; Sorriento, D.; Coscioni, E.; Iaccarino, G.; Santulli, G. Chapter 11—Adrenergic Receptors. In Endocrinology of the Heart in Health and Disease; Schisler, J.C., Lang, C.H., Willis, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 285–315. [Google Scholar]
- Engelhardt, S.; Hein, L. Adrenergic System. In Transgenic Models in Pharmacology; Offermanns, S., Hein, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 33–63. [Google Scholar]
- Vardanyan, R.; Hruby, V. Chapter 12—Adrenoblockers. In Synthesis of Best-Seller Drugs; Vardanyan, R., Hruby, V., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 201–214. [Google Scholar]
- Gerald, W.; Dorn, I. Adrenergic signaling polymorphisms and their impact on cardiovascular disease. Physiol. Rev. 2010, 90, 1013–1062. [Google Scholar]
- Massarsky, A.; Trudeau, V.L.; Moon, T.W. β-blockers as endocrine disruptors: The potential effects of human β-blockers on aquatic organisms. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Lukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Bialk-Bielinska, A. Beta-blockers in the environment: Part I. Mobility and hydrolysis study. Sci. Total. Environ. 2014, 493, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.F.; Giltrow, E.; Huggett, D.B.; Hutchinson, T.H.; Saye, J.; Winter, M.J.; Sumpter, J.P. Comparative physiology, pharmacology and toxicology of β-blockers: Mammals versus fish. Aquat. Toxicol. 2007, 82, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Küster, A.; Adler, N. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130587. [Google Scholar] [CrossRef] [PubMed]
- Kuster, A.; Alder, A.C.; Escher, B.I.; Duis, K.; Fenner, K.; Garric, J.; Hutchinson, T.H.; Lapen, D.R.; Pery, A.; Rombke, J.; et al. Environmental risk assessment of human pharmaceuticals in the European Union: A case study with the beta-blocker atenolol. Integr. Environ. Assess. Manag. 2010, 6 (Suppl. 1), 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Sheng, Q.; Sui, Q.; Lu, H.J. Beta-blockers in the environment: Distribution, transformation, and ecotoxicity. Environ. Pollut. 2020, 266, 115269. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef]
- Sun, L.; Xin, L.; Peng, Z.; Jin, R.; Jin, Y.; Qian, H.; Fu, Z. Toxicity and enantiospecific differences of two beta-blockers, propranolol and metoprolol, in the embryos and larvae of zebrafish (Danio rerio). Environ. Toxicol. 2014, 29, 1367–1378. [Google Scholar] [CrossRef]
- Sun, L.; Liu, F.; Chen, H.; Wang, S.; Lin, X.; Chi, J.; Zhu, Q.; Fu, Z. Transcriptional responses in adult zebrafish (Danio rerio) exposed to propranolol and metoprolol. Ecotoxicology 2015, 24, 1352–1361. [Google Scholar] [CrossRef]
- Wang, H.; Lin, X.; He, Z.; Qian, B.; Sun, L. Effects of adrenergic alpha-antagonists on the early life stages of Japanese medaka (Oryzias latipes). Ecotoxicology 2022, 31, 1485–1491. [Google Scholar] [CrossRef]
- Sun, L.; Gu, L.; Tan, H.; Liu, P.; Gao, G.; Tian, L.; Chen, H.; Lu, T.; Qian, H.; Fu, Z.; et al. Effects of 17α-ethinylestradiol on caudal fin regeneration in zebrafish larvae. Sci. Total. Environ. 2019, 653, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Finn, J.; Hui, M.; Li, V.; Lorenzi, V.; de la Paz, N.; Cheng, S.H.; Lai-Chan, L.; Schlenk, D. Effects of propranolol on heart rate and development in Japanese medaka (Oryzias latipes) and zebrafish (Danio rerio). Aquat. Toxicol. 2012, 122, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Marquer, C.; Bressolle, F. Moxisylyte: A review of its pharma codynamic and pharmacokinetic properties, and its therapeutic use in impotence. Fundam. Clin. Pharmacol. 1998, 12, 377–387. [Google Scholar] [CrossRef]
- Sun, L.; Xu, W.; Peng, T.; Chen, H.; Ren, L.; Tan, H.; Xiao, D.; Qian, H.; Fu, Z. Developmental exposure of zebrafish larvae to organophosphate flame retardants causes neurotoxicity. Neurotoxicol. Teratol. 2016, 55, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Noyes, P.D.; Haggard, D.E.; Gonnerman, G.D.; Tanguay, R.L. Advanced morphological—Behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 2015, 145, 177–195. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Cowden, J.; Simmons, S.O.; Padilla, S.; Ramabhadran, R. Gene expression changes in developing zebrafish as potential markers for rapid developmental neurotoxicity screening. Neurotoxicol. Teratol. 2010, 32, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yan, W.; Liu, C.; Li, L.; Yu, L.; Zhao, S.; Li, G. Microcystin-LR exposure induces developmental neurotoxicity in zebrafish embryo. Environ. Pollut. 2016, 213, 793–800. [Google Scholar] [CrossRef]
- Du, Z.; Wang, G.; Gao, S.; Wang, Z. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: By disturbing expression of the transcriptional regulators. Aquat. Toxicol. 2015, 161, 25–32. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fraysse, B.; Mons, R.; Garric, J. Development of a zebrafish 4-day toxicity of embryo-larval bioassay to assess chemicals. Ecotox. Environ. Saf. 2006, 63, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Fredriksson, S.; Sandblom, E.; Paxeus, N.; Axelsson, M. Is heart rate in fish a sensitive indicator to evaluate acute effects of β-blockers in surface water? Environ. Toxicol. Pharmacol. 2006, 22, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Dzialowski, E.; Turner, P.; Brooks, B. Physiological and reproductive effects of beta adrenergic receptor antagonists in Daphnia magna. Arch. Environ. Contam. Toxicol. 2006, 50, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.K.; Ramirez, A.J.; Mottaleb, M.; Chambliss, C.K.; Brooks, B.W. Enantiospecific toxicity of the beta-blocker propranolol to Daphnia magna and Pimephales promelas. Environ. Toxicol. Chem. 2006, 25, 1780–1786. [Google Scholar] [CrossRef]
- Blader, P.; Lam, C.S.; Rastegar, S.; Scardigli, R.; Nicod, J.C.; Simplicio, N.; Plessy, C.; Fischer, N.; Schuurmans, C.; Guillemot, F.; et al. Conserved and acquired features of neurogenin1 regulation. Development 2004, 131, 5627–5637. [Google Scholar] [CrossRef]
| Treatment (μg/L) | Relative Vessel Diameter (%) | Relative Blood Flow Volume (%) | Relative Heart Rate (%) | |
|---|---|---|---|---|
| MOX | 0 | 100.0 ± 11.5 | 100.0 ± 4.0 | 100.0 ± 3.5 |
| 1 | 94.6 ± 5.1 | 99.8 ± 3.9 | 108.5 ± 15.8 | |
| 5 | 98.8 ± 3.7 | 96.4 ± 4.7 | 111.7 ± 9.1 | |
| 25 | 90.5 ± 2.6 | 93.1 ± 5.1 | 97.9 ± 6.1 | |
| 125 | 87.3 ± 7.6 | 92.1 ± 7.7 | 93.2 ± 7.4 | |
| 625 | 80.3 ± 6.1 * | 74.4 ± 11.8 ** | 85.4 ± 4.9 * | |
| PRO | 0 | 100.0 ± 5.7 | 100.0 ± 7.3 | 100.0 ± 4.3 |
| 1 | 97.0 ± 4.3 | 101.1 ± 8.6 | 97.9 ± 6.1 | |
| 5 | 95.4 ± 4.7 | 101.4 ± 5.9 | 93.2 ± 3.4 | |
| 25 | 89.2 ± 5.6 | 88.7 ± 6.7 | 90.6 ± 4.8 * | |
| 125 | 86.6 ± 3.3 * | 80.2 ± 4.0 * | 86.8 ± 2.0 ** | |
| 625 | 81.1 ± 5.2 ** | 76.5 ± 4.4 ** | 84.4 ± 3.5 ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Sun, F.; Li, Z.; Qin, Y.; Sheng, R.; Sun, L. Comparative Study of the Effects of Drugs Targeting Adrenergic Receptors on the Early Life Stages of Zebrafish. Toxics 2024, 12, 583. https://doi.org/10.3390/toxics12080583
Lv J, Sun F, Li Z, Qin Y, Sheng R, Sun L. Comparative Study of the Effects of Drugs Targeting Adrenergic Receptors on the Early Life Stages of Zebrafish. Toxics. 2024; 12(8):583. https://doi.org/10.3390/toxics12080583
Chicago/Turabian StyleLv, Junsheng, Fengzhu Sun, Zaitian Li, Yueyun Qin, Ruozhu Sheng, and Liwei Sun. 2024. "Comparative Study of the Effects of Drugs Targeting Adrenergic Receptors on the Early Life Stages of Zebrafish" Toxics 12, no. 8: 583. https://doi.org/10.3390/toxics12080583
APA StyleLv, J., Sun, F., Li, Z., Qin, Y., Sheng, R., & Sun, L. (2024). Comparative Study of the Effects of Drugs Targeting Adrenergic Receptors on the Early Life Stages of Zebrafish. Toxics, 12(8), 583. https://doi.org/10.3390/toxics12080583







