Perinatal Exposure to Glyphosate or a Commercial Formulation Alters Uterine Mechanistic Pathways Associated with Implantation Failure in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
2.3. Experimental Design
2.4. Histomorphological Analysis
2.5. Immunohistochemistry Assays
2.6. Quantification of Cell Proliferation and Protein Expression
2.7. Dual Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
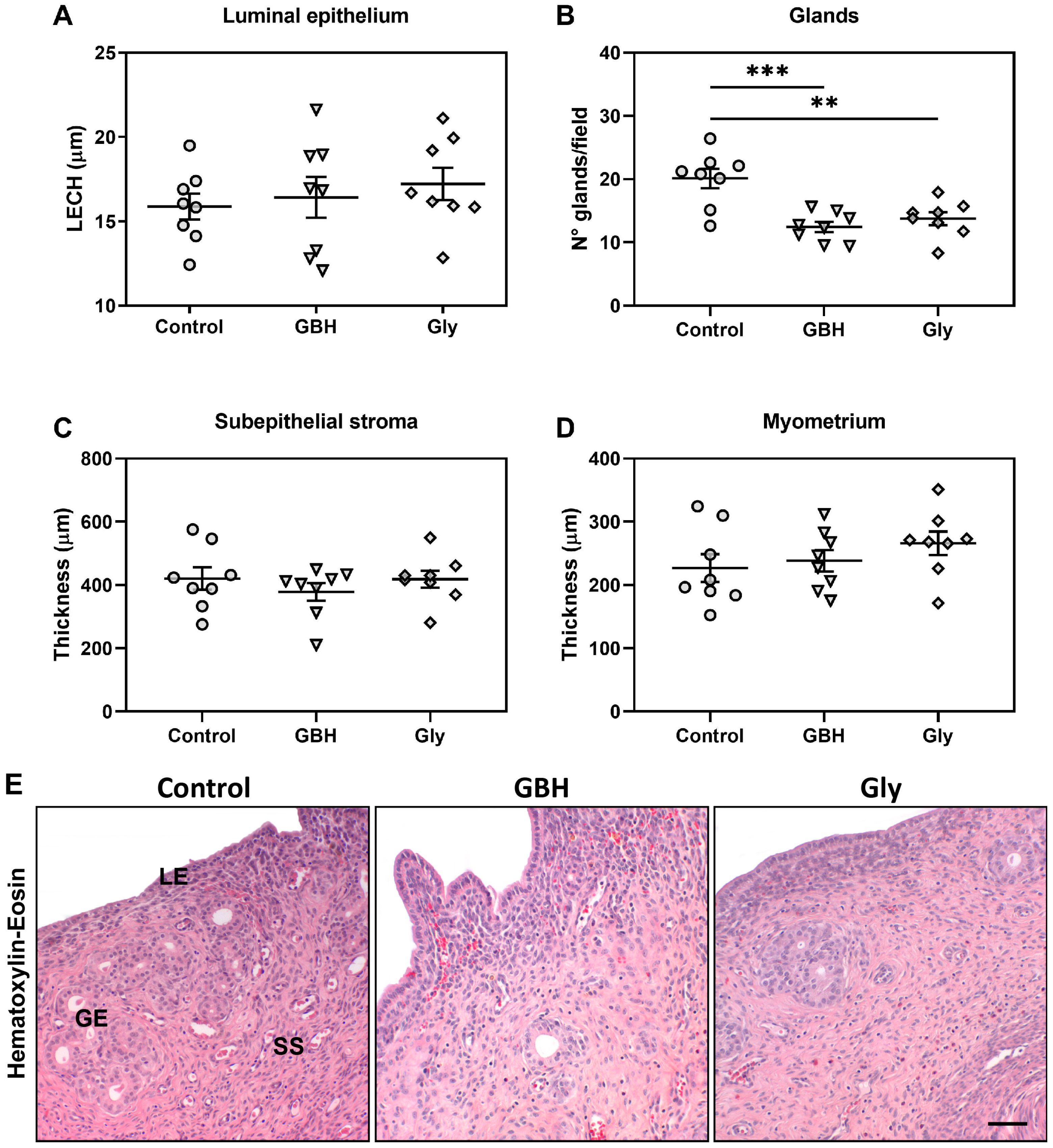
3.1. Uterine Histomorphology on GD5
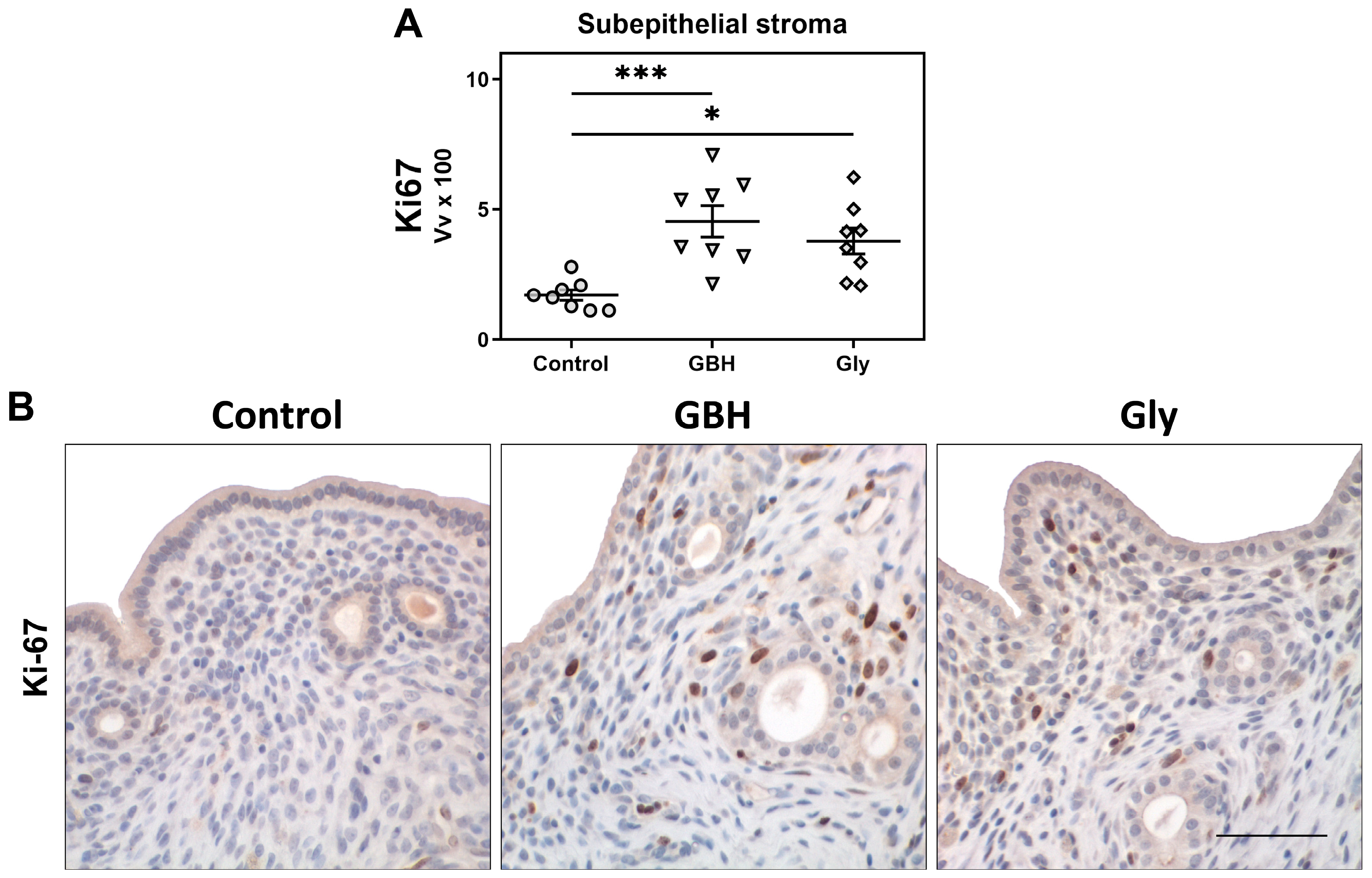
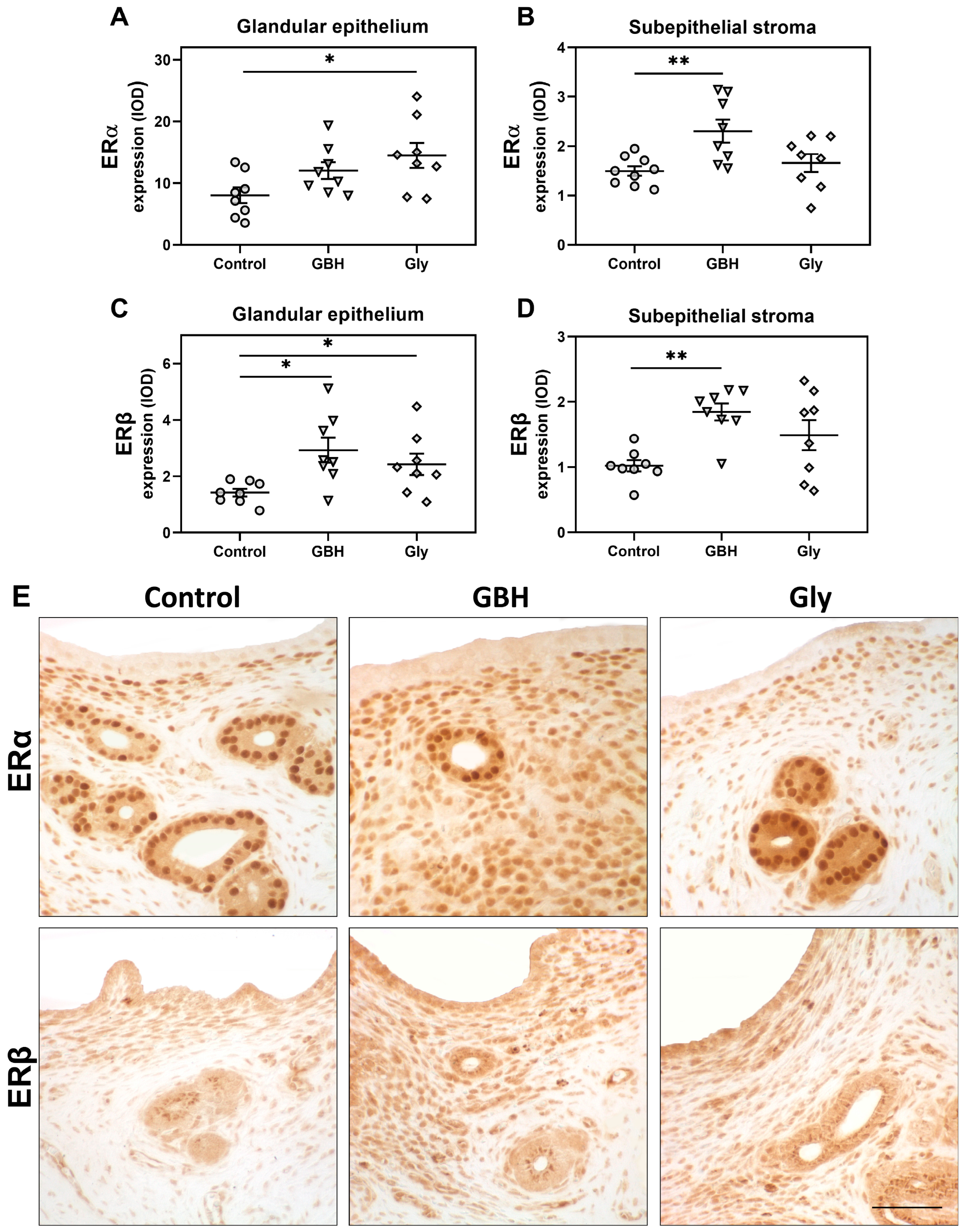
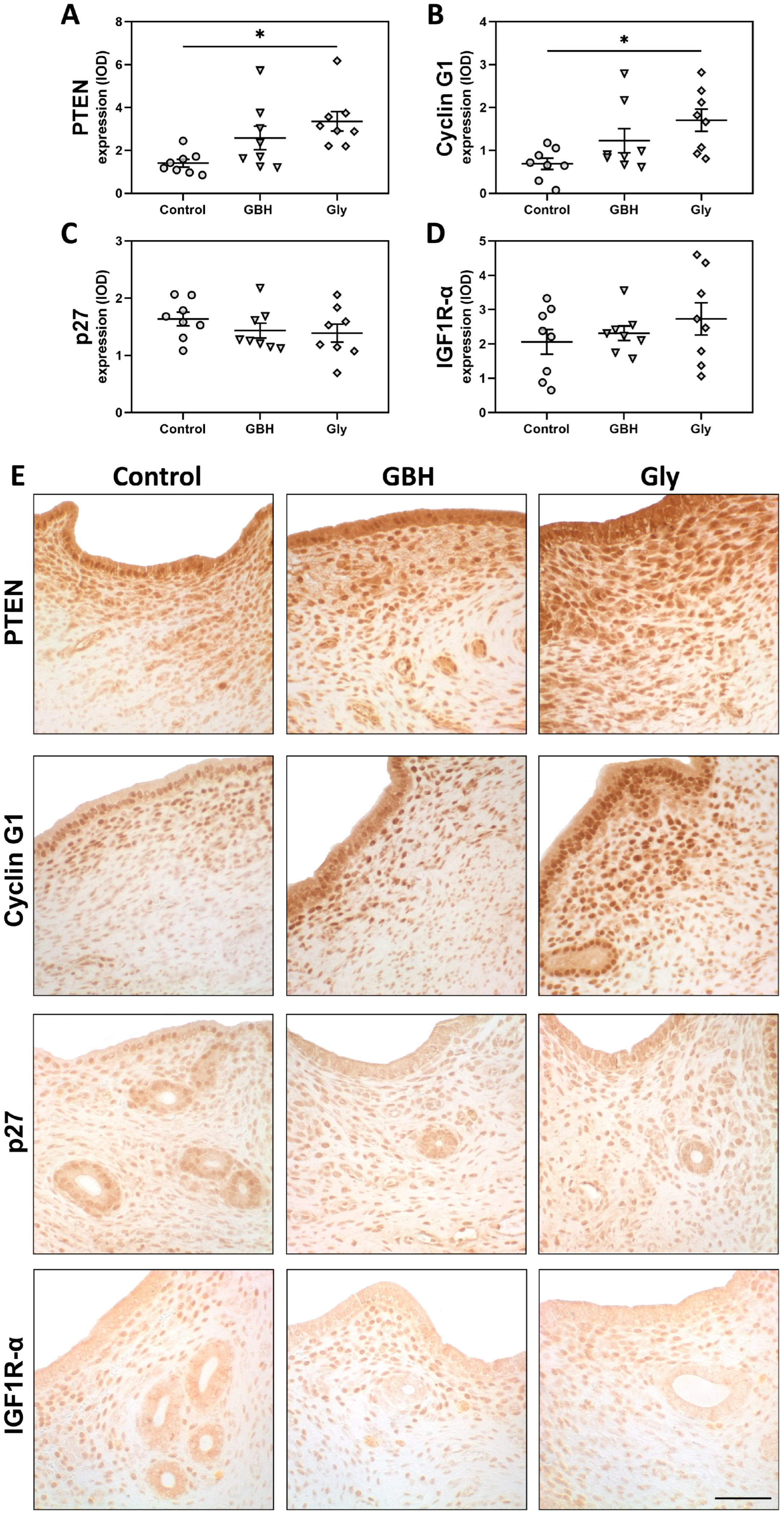
3.2. Stromal Cell Proliferation and Protein Expression of Estrogen Receptors and Cell Cycle Regulators
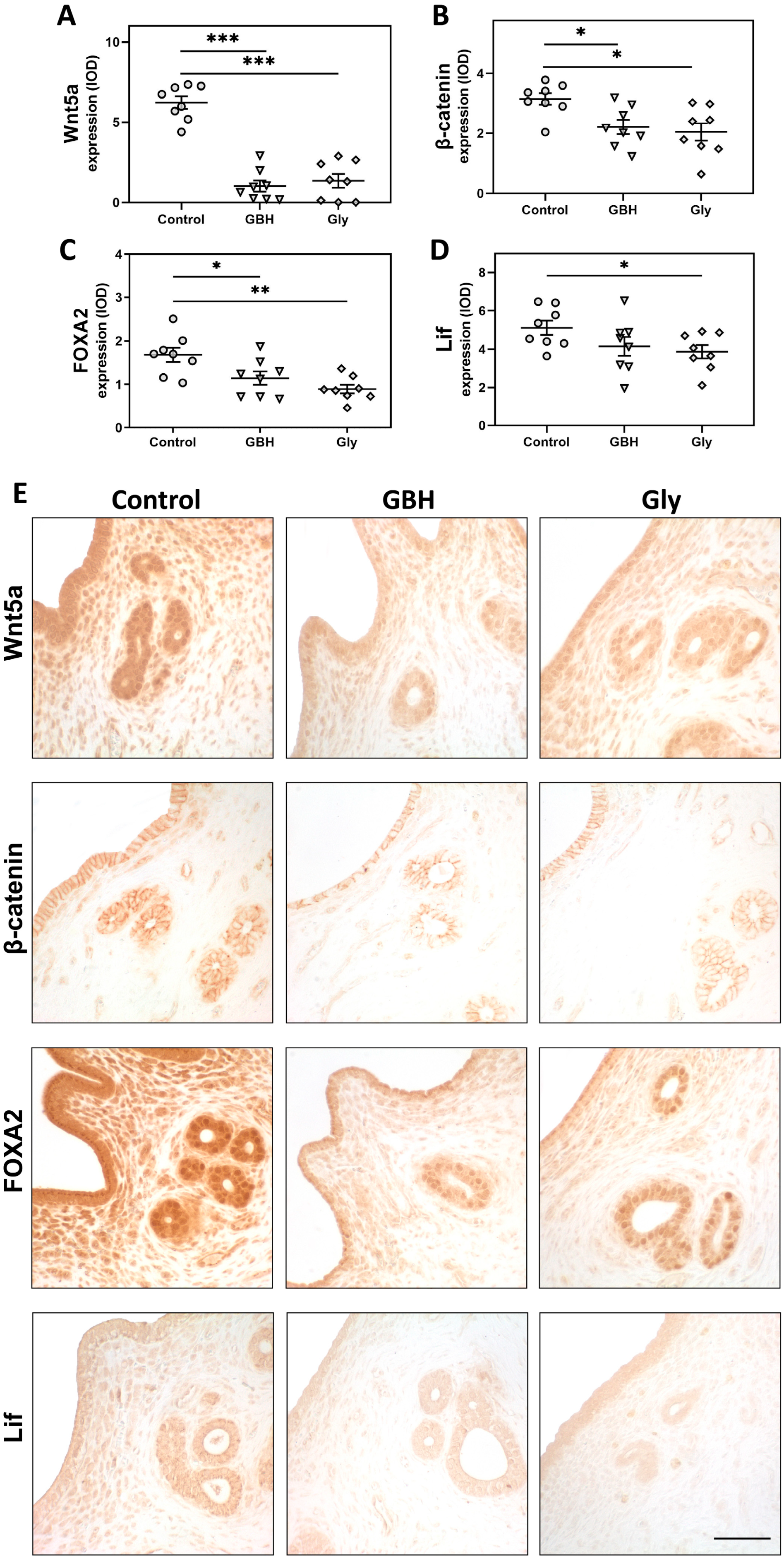
3.3. Protein Expression of a Key Endocrine Pathway for Endometrial Receptivity
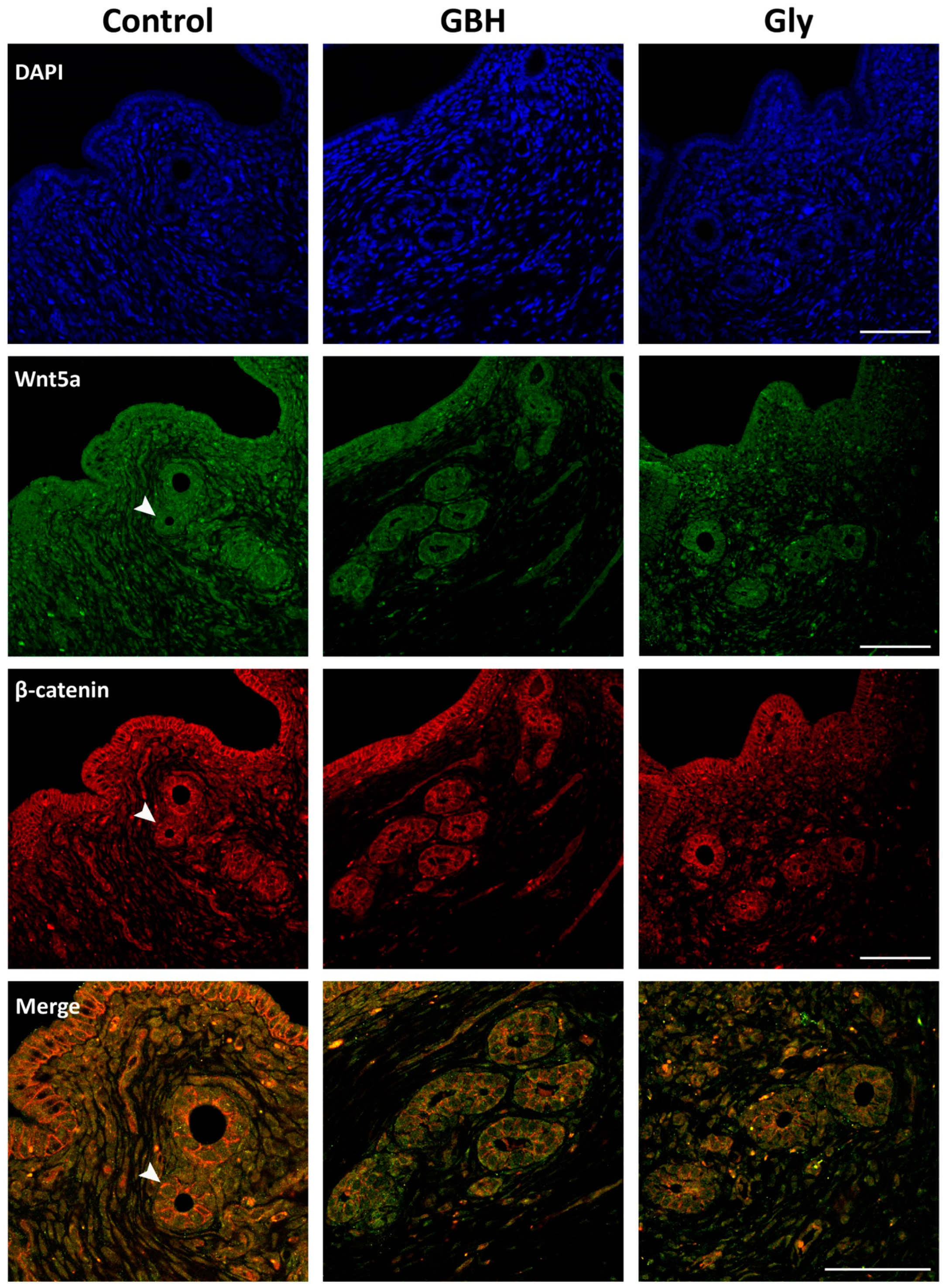
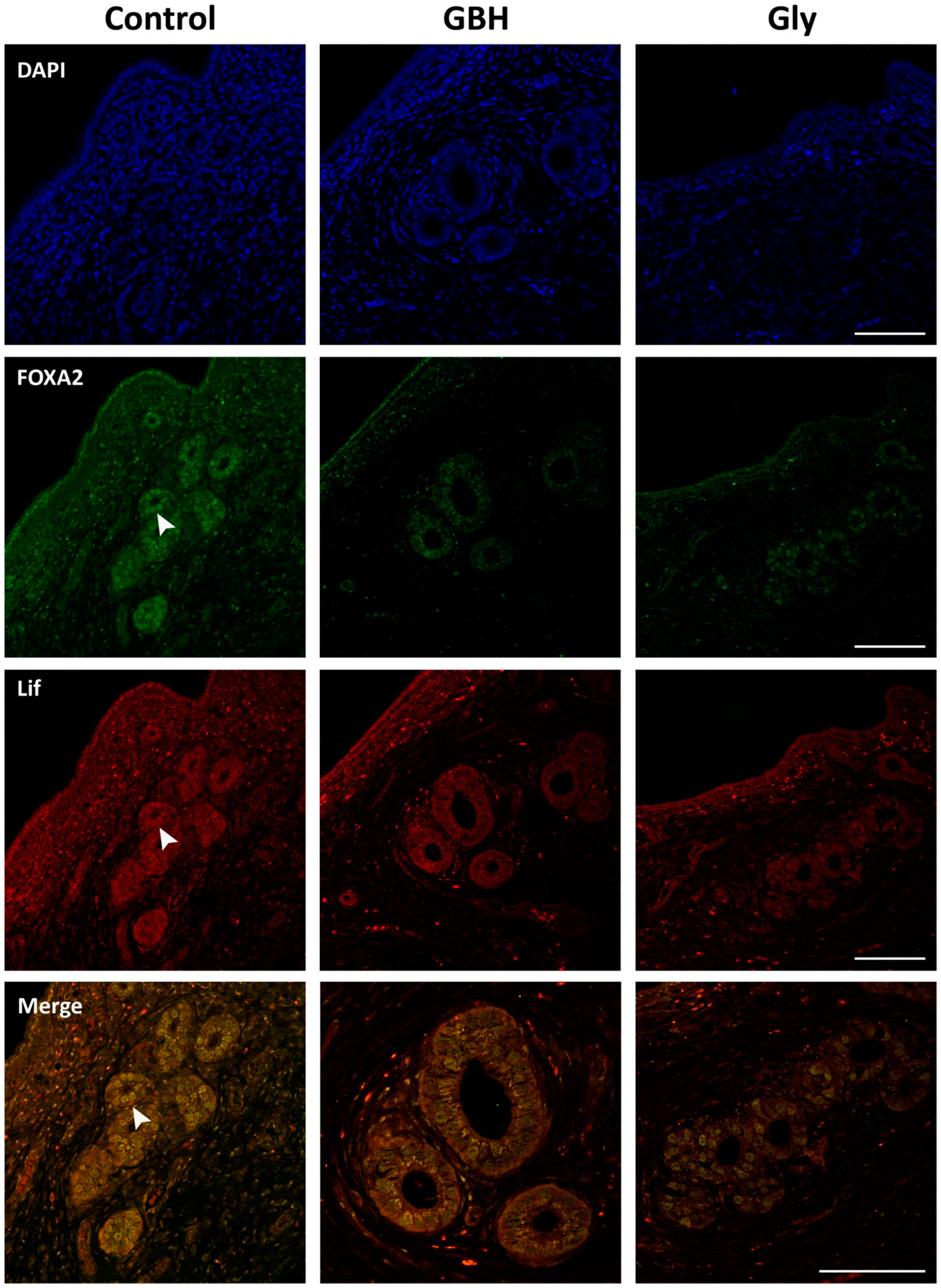
3.4. Colocalization of Wnt5a/β-Catenin and FOXA2/Lif
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | aminomethylphosphonic acid |
| DAPI | 4′6-diamidino-2-phenylindole dihydrochloride |
| E2 | 17β-estradiol |
| EDC | endocrine-disrupting chemical |
| EFSA | European Food Safety Authority |
| EPA | Environmental Protection Agency |
| ER | estrogen receptor |
| FOXA2 | forkhead box A2 |
| GBH | glyphosate-based herbicide |
| GD | gestational day |
| Gly | glyphosate |
| IGF1R-α | insulin-like growth factor 1 receptor alpha |
| IOD | integrated optical density |
| KO | knockout |
| LECH | luminal epithelial cell height |
| Lif | leukemia inhibitory factor |
| P4 | progesterone |
| PND | postnatal day |
| PTEN | phosphatase and tensin homolog |
| Wnt5a | wingless-type MMTV integration site 5a. |
References
- Busnelli, A.; Reschini, M.; Cardellicchio, L.; Vegetti, W.; Somigliana, E.; Vercellini, P. How Common Is Real Repeated Implantation Failure? An Indirect Estimate of the Prevalence. Reprod. Biomed. Online 2020, 40, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B.A.; Young, S.L. What Exactly Is Endometrial Receptivity? Fertil. Steril. 2019, 111, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular Cues to Implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, J. Update of Wnt Signaling in Implantation and Decidualization. Reprod. Med. Biol. 2016, 15, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rosario, G.X.; Stewart, C.L. The Multifaceted Actions of Leukaemia Inhibitory Factor in Mediating Uterine Receptivity and Embryo Implantation. Am. J. Reprod. Immunol. 2016, 75, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.M.; Peng, W.; Pru, J.K.; Pru, C.A.; Demayo, F.J.; Spencer, T.E. Forkhead Box A2 (FOXA2) Is Essential for Uterine Function and Fertility. Proc. Natl. Acad. Sci. USA 2017, 114, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Villacorte, M.; Suzuki, K.; Hirasawa, A.; Ohkawa, Y.; Suyama, M.; Maruyama, T.; Aoki, D.; Ogino, Y.; Miyagawa, S.; Terabayashi, T.; et al. β-Catenin Signaling Regulates Foxa2 Expression during Endometrial Hyperplasia Formation. Oncogene 2013, 32, 3477–3482. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Bartos, A.; Park, C.; Sun, X.; Li, Y.; Cha, S.W.; Ajima, R.; Ho, H.Y.H.; Yamaguchi, T.P.; Dey, S.K. Appropriate Crypt Formation in the Uterus for Embryo Homing and Implantation Requires Wnt5a-ROR Signaling. Cell Rep. 2014, 8, 382–392. [Google Scholar] [CrossRef]
- Dhakal, P.; Kelleher, A.M.; Behura, S.K.; Spencer, T.E. Sexually Dimorphic Effects of Forkhead Box A2 (FOXA2) and Uterine Glands on Decidualization and Fetoplacental Development. Proc. Natl. Acad. Sci. USA 2020, 117, 23952–23959. [Google Scholar] [CrossRef]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Köntgen, F.; Abbondanzo, S.J. Blastocyst Implantation Depends on Maternal Expression of Leukaemia Inhibitory Factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef]
- Bala, R.; Singh, V.; Rajender, S.; Singh, K. Environment, Lifestyle, and Female Infertility. Reprod. Sci. 2021, 28, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.P.; Bleak, T.C.; Calaf, G.M. Glyphosate and the Key Characteristics of an Endocrine Disruptor: A Review. Chemosphere 2021, 270, 128619. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Najmi, A.; Mogus, J.P. Agrochemicals with Estrogenic Endocrine Disrupting Properties: Lessons Learned? Estrogenic Agrochemicals: Lessons Learned. Mol. Cell. Endocrinol. 2020, 518, 110860. [Google Scholar] [CrossRef] [PubMed]
- Demonte, L.D.; Michlig, N.; Gaggiotti, M.; Adam, C.G.; Beldoménico, H.R.; Repetti, M.R. Determination of Glyphosate, AMPA and Glufosinate in Dairy Farm Water from Argentina Using a Simplified UHPLC-MS/MS Method. Sci. Total Environ. 2018, 645, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mac Loughlin, T.M.; Peluso, M.L.; Marino, D.J.G. Evaluation of Pesticide Pollution in the Gualeguay Basin: An Extensive Agriculture Area in Argentina. Sci. Total Environ. 2022, 851, 158142. [Google Scholar] [CrossRef] [PubMed]
- Lajmanovich, R.C.; Repetti, M.R.; Cuzziol Boccioni, A.P.; Michlig, M.P.; Demonte, L.; Attademo, A.M.; Peltzer, P.M. Cocktails of Pesticide Residues in Prochilodus Lineatus Fish of the Salado River (South America): First Record of High Concentrations of Polar Herbicides. Sci. Total Environ. 2023, 870, 162019. [Google Scholar] [CrossRef] [PubMed]
- Saurat, D.; Raffy, G.; Bonvallot, N.; Monfort, C.; Fardel, O.; Glorennec, P.; Chevrier, C.; Le Bot, B. Determination of Glyphosate and AMPA in Indoor Settled Dust by Hydrophilic Interaction Liquid Chromatography with Tandem Mass Spectrometry and Implications for Human Exposure. J. Hazard. Mater. 2023, 446, 130654. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Martin, J.; Huang, R.; MacPherson, S.; Brion, O.; Owen, J.; Gaudreau, E.; Bienvenu, J.F.; Fisher, M.; Borghese, M.M.; Bouchard, M.F.; et al. Urinary Concentrations and Determinants of Glyphosate and Glufosinate in Pregnant Canadian Participants in the MIREC Study. Environ. Res. 2023, 217, 114842. [Google Scholar] [CrossRef] [PubMed]
- Kongtip, P.; Nankongnab, N.; Phupancharoensuk, R.; Palarach, C.; Sujirarat, D.; Sangprasert, S.; Sermsuk, M.; Sawattrakool, N.; Woskie, S.R. Glyphosate and Paraquat in Maternal and Fetal Serums in Thai Women. J. Agromedicine 2017, 22, 282–289. [Google Scholar] [CrossRef]
- Camiccia, M.; Candiotto, L.Z.P.; Gaboardi, S.C.; Panis, C.; Kottiwitz, L.B.M. Determination of Glyphosate in Breast Milk of Lactating Women in a Rural Area from Paraná State, Brazil. Braz. J. Med. Biol. Res. 2022, 55, e12194. [Google Scholar] [CrossRef]
- Akerman, G.; Rowland, J.; Trujillo, J. Weight of Evidence Conclusions on the Tier 1 Screening Assays for the List 1 Chemicals. 2015. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2012-0330-0039 (accessed on 7 August 2024).
- Álvarez, F.; Arena, M.; Auteri, D.; Binaglia, M.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F.; Egsmose, M.; Fait, G.; Ferilli, F.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Glyphosate. EFSA J. 2023, 21, e08164. [Google Scholar] [CrossRef]
- Silver, M.K.; Fernandez, J.; Tang, J.; McDade, A.; Sabino, J.; Rosario, Z.; Vega, C.V.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Prenatal Exposure to Glyphosate and Its Environmental Degradate, Aminomethylphosphonic Acid (Ampa), and Preterm Birth: A Nested Case-Control Study in the Protect Cohort (Puerto Rico). Environ. Health Perspect. 2021, 129, 057011. [Google Scholar] [CrossRef] [PubMed]
- Lesseur, C.; Pirrotte, P.; Pathak, K.V.; Manservisi, F.; Mandrioli, D.; Belpoggi, F.; Panzacchi, S.; Li, Q.; Barrett, E.S.; Nguyen, R.H.N.; et al. Maternal Urinary Levels of Glyphosate during Pregnancy and Anogenital Distance in Newborns in a US Multicenter Pregnancy Cohort. Environ. Pollut. 2021, 280, 117002. [Google Scholar] [CrossRef]
- Gerona, R.R.; Reiter, J.L.; Zakharevich, I.; Proctor, C.; Ying, J.; Mesnage, R.; Antoniou, M.; Winchester, P.D. Glyphosate Exposure in Early Pregnancy and Reduced Fetal Growth: A Prospective Observational Study of High-Risk Pregnancies. Environ. Health 2022, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, V.; Pacini, G.; Luque, E.H.; Varayoud, J.; Milesi, M.M. Perinatal Exposure to Glyphosate or a Glyphosate-Based Formulation Disrupts Hormonal and Uterine Milieu during the Receptive State in Rats. Food Chem. Toxicol. 2020, 143, 111560. [Google Scholar] [CrossRef] [PubMed]
- Montes, G.S.; Luque, E.H. Effects of Ovarian Steroids on Vaginal Smears in the Rat. Acta Anat. 1988, 133, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Milesi, M.M.; Lorenz, V.; Pacini, G.; Repetti, M.R.; Demonte, L.D.; Varayoud, J.; Luque, E.H. Perinatal Exposure to a Glyphosate-Based Herbicide Impairs Female Reproductive Outcomes and Induces Second-Generation Adverse Effects in Wistar Rats. Arch. Toxicol. 2018, 92, 2629–2643. [Google Scholar] [CrossRef] [PubMed]
- Bloem, T.; Kramer, G.F.; Chem, S. Glyphosate. Dietary Exposure Analysis in Support of Registration Review. 2017. Available online: https://www.regulations.gov/document/EPA-HQ-OPP-2009-0361-0071 (accessed on 7 August 2024).
- Filippi, I.; Bonansea, R.I.; Butinof, M.; Fernández, R.A.; Llorca, M.; Farré, M.; Muñoz, S.E.; Amé, M.V. First Report of the Joint Exposure to Glyphosate and Glufosinate of a Male Population in the Province of Córdoba (Argentina). Toxics 2023, 11, 1020. [Google Scholar] [CrossRef]
- Milesi, M.M.; Varayoud, J.; Bosquiazzo, V.L.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal Exposure to Low Doses of Endosulfan Disrupts the Expression of Proteins Regulating Uterine Development and Differentiation. Reprod. Toxicol. 2012, 33, 85–93. [Google Scholar] [CrossRef]
- Alarcón, R.; Rivera, O.E.; Ingaramo, P.I.; Tschopp, M.V.; Dioguardi, G.H.; Milesi, M.M.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal Exposure to a Glyphosate-Based Herbicide Alters the Uterine Differentiation of Prepubertal Ewe Lambs. Environ. Pollut. 2020, 265, 114874. [Google Scholar] [CrossRef]
- Vigezzi, L.; Ramos, J.G.; Kass, L.; Tschopp, M.V.; Muñoz-de-Toro, M.; Luque, E.H.; Bosquiazzo, V.L. A Deregulated Expression of Estrogen-Target Genes Is Associated with an Altered Response to Estradiol in Aged Rats Perinatally Exposed to Bisphenol, A. Mol. Cell. Endocrinol. 2016, 426, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bracho, G.S.; Acosta, M.V.; Altamirano, G.A.; Tschopp, M.V.; Luque, E.H.; Kass, L.; Bosquiazzo, V.L. Androgen Receptor and Uterine Histoarchitecture in a PCOS Rat Model. Mol. Cell. Endocrinol. 2020, 518, 110973. [Google Scholar] [CrossRef]
- Weibel, E.R. Stereological Principles for Morphometry in Electron Microscopic Cytology. Int. Rev. Cytol. 1969, 26, 235–302. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.G.; Varayoud, J.; Bosquiazzo, V.L.; Luque, E.H.; Muñoz-de-Toro, M. Cellular Turnover in the Rat Uterine Cervix and Its Relationship to Estrogen and Progesterone Receptor Dynamics. Biol. Reprod. 2002, 67, 735–742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Das, S.K. Cell Cycle Regulatory Control for Uterine Stromal Cell Decidualization in Implantation. Reproduction 2009, 137, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-G.; Song, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Estrogen Is a Critical Determinant That Specifies the Duration of the Window of Uterine Receptivity for Implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 2963–2968. [Google Scholar] [CrossRef]
- Robertshaw, I.; Bian, F.; Das, S.K. Mechanisms of Uterine Estrogen Signaling during Early Pregnancy in Mice: An Update. J. Mol. Endocrinol. 2016, 56, 127–138. [Google Scholar] [CrossRef]
- Kawagoe, J.; Li, Q.; Mussi, P.; Liao, L.; Lydon, J.P.; DeMayo, F.J.; Xu, J. Nuclear Receptor Coactivator-6 Attenuates Uterine Estrogen Sensitivity to Permit Embryo Implantation. Dev. Cell 2012, 23, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, D.B.; Moyer, J.S.; Golding, T.S.; Couse, J.F.; Korach, K.S.; Smithies, O. Alteration of Reproductive Function but Not Prenatal Sexual Development after Insertional Disruption of the Mouse Estrogen Receptor Gene (Homologous Recombination/Gene Targeting/Fertility). Proc. Natl. Acad. Sci. USA 1993, 90, 11162–11166. [Google Scholar] [CrossRef]
- Weihua, Z.; Saji, S.; Mäkinen, S.; Cheng, G.; Jensen, E.V.; Warner, M.; Gustafsson, J.Å. Estrogen receptor (ER) β, a modulator of ERα in the uterus. Proc. Natl. Acad. Sci. USA 2000, 97, 5936–5941. [Google Scholar] [CrossRef]
- Tan, W.; Gu, Z.; Shen, B.; Jiang, J.; Meng, Y.; Da, Z.; Liu, H.; Tao, T.; Cheng, C. PTEN/Akt-P27(Kip1) Signaling Promote the BM-MSCs Senescence and Apoptosis in SLE Patients. J. Cell. Biochem. 2015, 116, 1583–1594. [Google Scholar] [CrossRef]
- Zhao, L.; Samuels, T.; Winckler, S.; Korgaonkar, C.; Tompkins, V.; Horne, M.C.; Quelle, D.E. Cyclin G1 Has Growth Inhibitory Activity Linked to the ARF-Mdm2-P53 and PRb Tumor Suppressor Pathways. Mol. Cancer Res. 2003, 1, 195–206. [Google Scholar] [PubMed]
- Yue, L.; Daikoku, T.; Hou, X.; Li, M.; Wang, H.; Nojima, H.; Dey, S.K.; Das, S.K. Cyclin G1 and Cyclin G2 Are Expressed in the Periimplantation Mouse Uterus in a Cell-Specific and Progesterone-Dependent Manner: Evidence for Aberrant Regulation with Hoxa-10 Deficiency. Endocrinology 2005, 146, 2424–2433. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Yang, S.; Huang, K.; Dong, X.; Sui, C.; Zhang, H. P57 and Cyclin G1 Express Differentially in Proliferative Phase Endometrium and Early Pregnancy Decidua. Int. J. Clin. Exp. Med. 2015, 8, 5144–5149. [Google Scholar] [PubMed]
- Antsiferova, Y.S.; Sotnikova, N.Y.; Bogatova, I.K.; Boitsova, A.V. Changes of Apoptosis Regulation in the Endometrium of Infertile Women with Tubal Factor and Endometriosis Undergoing in Vitro Fertilization Treatment. JBRA Assist. Reprod. 2014, 18, 2–6. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yu, Q. The PI3K/Akt Signaling Pathway Exerts Effects on the Implantation of Mouse Embryos by Regulating the Expression of RhoA. Int. J. Mol. Med. 2014, 33, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Baima, K.Z.; Tian, J.M.; Yang, K.X.; Qie, M.R.; Zhang, J.H.; He, Y.P.; Yue, L.M. Temporospatial Expression of Cyclin G1 in Normal Human Endometria. Sichuan Da Xue Xue Bao Yi Xue Ban 2008, 39, 623–626. [Google Scholar]
- Makker, A.; Goel, M.M.; Nigam, D.; Mahdi, A.A.; Das, V.; Agarwal, A.; Pandey, A.; Gautam, A. Aberrant Akt Activation During Implantation Window in Infertile Women with Intramural Uterine Fibroids. Reprod. Sci. 2018, 25, 1243–1253. [Google Scholar] [CrossRef]
- Laguë, M.N.; Detmar, J.; Paquet, M.; Boyer, A.; Richards, J.A.S.; Adamson, S.L.; Boerboom, D. Decidual PTEN Expression Is Required for Trophoblast Invasion in the Mouse. Am. J. Physiol. Endocrinol. Metab. 2010, 299, 936–946. [Google Scholar] [CrossRef]
- Lindberg, K.; Helguero, L.A.; Omoto, Y.; Gustafsson, J.Å.; Haldosén, L.A. Estrogen Receptor β Represses Akt Signaling in Breast Cancer Cells via Downregulation of HER2/HER3 and Upregulation of PTEN: Implications for Tamoxifen Sensitivity. Breast Cancer Res. 2011, 13, R43. [Google Scholar] [CrossRef]
- Kelleher, A.M.; Demayo, F.J.; Spencer, T.E. Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev. 2019, 40, 1424–1445. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.L.; Teixeira, Á.A.C.; Soares, A.F.; Da Cunha, F.M.; Da Silva Júnior, V.A.; Vieira Filho, L.D.; Wanderley-Teixeira, V. Effects of Melatonin in Rats in the Initial Third Stage of Pregnancy Exposed to Sub-Lethal Doses of Herbicides. Acta Histochem. 2017, 119, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Rosario, G.; Cohen, T.V.; Hu, J.; Stewart, C.L. Tissue-Specific Ablation of the LIF Receptor in the Murine Uterine Epithelium Results in Implantation Failure. Endocrinology 2017, 158, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, A.M.; Milano-Foster, J.; Behura, S.K.; Spencer, T.E. Uterine Glands Coordinate On-Time Embryo Implantation and Impact Endometrial Decidualization for Pregnancy Success. Nat. Commun. 2018, 9, 2435. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.A.; Jonnaert, M.; Labelle-Dumais, C.; Kuroda, K.; Clarke, H.J.; Dufort, D. Uterine Wnt/Beta-Catenin Signaling Is Required for Implantation. Proc. Natl. Acad. Sci. USA 2005, 102, 8579–8584. [Google Scholar] [CrossRef]
- Dhakal, P.; Fitzgerald, H.C.; Kelleher, A.M.; Liu, H.; Spencer, T.E. Uterine Glands Impact Embryo Survival and Stromal Cell Decidualization in Mice. FASEB J. 2021, 35, e21938. [Google Scholar] [CrossRef] [PubMed]
- Fouladi-Nashta, A.A.; Jones, C.J.P.; Nijjar, N.; Mohamet, L.; Smith, A.; Chambers, I.; Kimber, S.J. Characterization of the Uterine Phenotype during the Peri-Implantation Period for LIF-Null, MF1 Strain Mice. Dev. Biol. 2005, 281, 1–21. [Google Scholar] [CrossRef]
- Schimpf, M.G.; Milesi, M.M.; Luque, E.H.; Varayoud, J. Glyphosate-Based Herbicide Enhances the Uterine Sensitivity to Estradiol in Rats. J. Endocrinol. 2018, 239, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Margioula-Siarkou, C.; Prapas, Y.; Petousis, S.; Milias, S.; Ravanos, K.; Dagklis, T.; Kalogiannidis, I.; Mavromatidis, G.; Haitoglou, C.; Prapas, N.; et al. LIF Endometrial Expression Is Impaired in Women with Unexplained Infertility While LIF-R Expression in All Infertility Sub-Groups. Cytokine 2017, 96, 166–172. [Google Scholar] [CrossRef]
- Lin, A.; Yin, J.; Cheng, C.; Yang, Z.; Yang, H. Decreased Expression of FOXA2 Promotes Eutopic Endometrial Cell Proliferation and Migration in Patients with Endometriosis. Reprod. Biomed. Online 2018, 36, 181–187. [Google Scholar] [CrossRef]
- Moberg, C.; Bourlev, V.; Ilyasova, N.; Olovsson, M. Endometrial Expression of LIF and Its Receptor and Peritoneal Fluid Levels of IL-1α and IL-6 in Women with Endometriosis Are Associated with the Probability of Pregnancy. Arch. Gynecol. Obstet. 2015, 292, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Stoikos, C.; Stafford-Bell, M.; Clark, I.; Paiva, P.; Kovacs, G.; Salamonsen, L.A. Interleukin-11, IL-11 Receptor and Leukemia Inhibitory Factor Are Dysregulated in Endometrium of Infertile Women with Endometriosis during the Implantation Window. J. Reprod. Immunol. 2006, 69, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Milesi, M.M.; Lorenz, V.; Beldomenico, P.M.; Vaira, S.; Varayoud, J.; Luque, E.H. Response to Comments on: Perinatal Exposure to a Glyphosate-Based Herbicide Impairs Female Reproductive Outcomes and Induces Second-Generation Adverse Effects in Wistar Rats. Arch. Toxicol. 2019, 93, 3635–3638. [Google Scholar] [CrossRef] [PubMed]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [PubMed]
| Antibodies | Dilution | Supplier |
|---|---|---|
| Primary | ||
| Anti-Ki67 (clone MIB-5) | 1/15 | Dako Crop. (Carpinteria, CA, USA) |
| Anti-FOXA2 | 1/800 | Generated and validated in our Institute [32] |
| Anti-Wnt5a | 1/800 | Generated and validated in our Institute [33] |
| Anti-Lif (sc-515931) | 1/50 | Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) |
| Anti-β-catenin (sc-7963) | 1/800 | Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) |
| Anti-PTEN | 1/750 | Generated and validated in our Institute [34] |
| Anti-Cyclin G1 (sc-7865) | 1/25 | Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) |
| Anti-p27 (sc-528) | 1/800 | Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) |
| Anti-IGF1R-α (sc-712) | 1/100 | Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) |
| Anti-ERα (clone 6F-11) | 1/100 | Novocastra (Newcastle upon Tyne, UK) |
| Anti-ERβ (51-7900) | 1/200 | Zymed (San Francisco, CA, USA) |
| Secondary | ||
| Anti-mouse (B8774) | 1/100 | Sigma-Aldrich (St. Louis, MO, USA) |
| Anti-rabbit (B8895) | 1/200 | Sigma-Aldrich (St. Louis, MO, USA) |
| Alexa Fluor 488 goat anti-rabbit (A-11034) | 1/100 | Invitrogen Molecular Probes (Eugene, OR, USA) |
| TRITC-conjugated goat anti-mouse (115-025-003) | 1/100 | Jackson Immunoresearch (West Grove, PA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almirón, A.; Lorenz, V.; Varayoud, J.; Durando, M.; Milesi, M.M. Perinatal Exposure to Glyphosate or a Commercial Formulation Alters Uterine Mechanistic Pathways Associated with Implantation Failure in Rats. Toxics 2024, 12, 590. https://doi.org/10.3390/toxics12080590
Almirón A, Lorenz V, Varayoud J, Durando M, Milesi MM. Perinatal Exposure to Glyphosate or a Commercial Formulation Alters Uterine Mechanistic Pathways Associated with Implantation Failure in Rats. Toxics. 2024; 12(8):590. https://doi.org/10.3390/toxics12080590
Chicago/Turabian StyleAlmirón, Ailín, Virginia Lorenz, Jorgelina Varayoud, Milena Durando, and María Mercedes Milesi. 2024. "Perinatal Exposure to Glyphosate or a Commercial Formulation Alters Uterine Mechanistic Pathways Associated with Implantation Failure in Rats" Toxics 12, no. 8: 590. https://doi.org/10.3390/toxics12080590
APA StyleAlmirón, A., Lorenz, V., Varayoud, J., Durando, M., & Milesi, M. M. (2024). Perinatal Exposure to Glyphosate or a Commercial Formulation Alters Uterine Mechanistic Pathways Associated with Implantation Failure in Rats. Toxics, 12(8), 590. https://doi.org/10.3390/toxics12080590






