Association between Serum 6:2 Chlorinated Polyfluorinated Ether Sulfonate Concentrations and Lung Cancer
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, M.; Chen, W. Epidemiology of lung cancer in China. Thorac Cancer 2019, 10, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung Cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- The World Health Organization. International Agency for Research on Cancer. Global Cancer. Observatory: Cancer Today; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Martin-Sanchez, J.C.; Lunet, N.; Gonzalez-Marron, A.; Lidon-Moyano, C.; Matilla-Santander, N.; Cleries, R.; Malvezzi, M.; Negri, E.; Morais, S.; Costa, A.R.; et al. Projections in Breast and Lung Cancer Mortality among Women: A Bayesian Analysis of 52 Countries Worldwide. Cancer Res. 2018, 78, 4436–4442. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, L.; Zhou, C. Lung cancer in China: Current and prospect. Curr. Opin. Oncol. 2021, 33, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Kubik, M.; et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA-J. Am. Med. Assoc. 2021, 325, 962–970. [Google Scholar]
- Gharibvand, L.; Shavlik, D.; Ghamsary, M.; Beeson, W.L.; Soret, S.; Knutsen, R.; Knutsen, S.F. The Association between Ambient Fine Particulate Air Pollution and Lung Cancer Incidence: Results from the AHSMOG-2 Study. Environ. Health Perspect. 2017, 125, 378–384. [Google Scholar] [CrossRef]
- Milosevic, N.; Milanovic, M.; Sazdanic Velikic, D.; Sudji, J.; Jovicic-Bata, J.; Spanovic, M.; Sevo, M.; Lukic Sarkanovic, M.; Torovic, L.; Bijelovic, S.; et al. Biomonitoring Study of Toxic Metal(loid)s: Levels in Lung Adenocarcinoma Patients. Toxics 2024, 12, 490. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Mao, W.; Qu, J.; Zhong, S.; Wu, X.; Mao, K.; Liao, K.; Jin, H. Associations between urinary parabens and lung cancer. Environ. Sci. Pollut. Res. Int. 2023, 30, 66186–66194. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Mao, W.; Liao, K.; Zhang, Y.; Jin, H. Association between urinary bisphenol analogue concentrations and lung cancer in adults: A case-control study. Environ. Pollut. 2022, 315, 120323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Wei, X.; Dai, J. Temporal Trends in Prenatal Exposure (1998–2018) to Emerging and Legacy Per- and Polyfluoroalkyl Substances (PFASs) in Cord Plasma from the Beijing Cord Blood Bank, China. Environ. Sci. Technol. 2020, 54, 12850–12859. [Google Scholar] [CrossRef]
- Shi, Y.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, C.; Liang, Y.; Cai, Y. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environ. Sci. Technol. 2016, 50, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- European Union Environment Programme. Chemicals Listed in Annex A. 2019. Available online: http://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/tabid/5837/Default.aspx (accessed on 17 August 2023).
- Dai, C.; Peng, L.; Li, Y.; Li, Z.; Chen, D.; Wang, F.; Lin, N. Distribution of per- and polyfluoroalkyl substances in blood, serum, and urine of patients with liver cancer and associations with liver function biomarkers. J. Environ. Sci. 2024, 139, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cui, Q.; Wang, J.; Sheng, N.; Jing, J.; Yao, B.; Dai, J. Profiles of Emerging and Legacy Per-/Polyfluoroalkyl Substances in Matched Serum and Semen Samples: New Implications for Human Semen Quality. Environ. Health Perspect. 2019, 127, 127005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, W.; Qu, J.; Hu, S.; Zhang, L.; Zhao, M.; Wu, P.; Xue, J.; Hangbiao, J. Per-/polyfluoroalkyl substance concentrations in human serum and their associations with immune markers of rheumatoid arthritis. Chemosphere 2022, 298, 134338. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Fang, Q.-L.; Cui, X.-X.; Dong, P.-X.; Qian, Z.; McMillin, S.E.; Howard, S.W.; Ou, Y.-Q.; Li, Q.-Q.; Wu, L.-Y. Non-monotonic association between chlorinated polyfluorinated ether sulfonic acids exposure and the risk of overweight/obesity status in adults. Expo. Health 2023, 15, 539–549. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Liang, L.-X.; Zhou, Y.; Mohammed, Z.; Qian, Z.M.; McMillin, S.E.; Tabet, M.; Chu, C.; Fan, Y.-Y.; Zhou, J.-X. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs) Are Associated with Eye Diseases in Humans and Eye Toxicity in Zebrafish. Environ. Health 2024, 2, 390–400. [Google Scholar] [CrossRef]
- Lin, N.; Zhang, Y.; Su, S.; Feng, Y.; Wang, B.; Li, Z. Exposure characteristics of legacy and novel per- and polyfluoroalkyl substances in blood and association with hypertension among low-exposure population. J. Hazard. Mater. 2023, 459, 132185. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wan, B.; Yu, B.; Fan, Y.; Chen, D.; Guo, L.H. Chlorinated Polyfluoroalkylether Sulfonic Acids Exhibit Stronger Estrogenic Effects than Perfluorooctane Sulfonate by Activating Nuclear Estrogen Receptor Pathways. Environ. Sci. Technol. 2020, 54, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Stabile, L.P.; Siegfried, J.M. Estrogen receptor pathways in lung cancer. Curr. Oncol. Rep. 2004, 6, 259–267. [Google Scholar] [CrossRef]
- Li, C.H.; Ren, X.M.; Ruan, T.; Cao, L.Y.; Xin, Y.; Guo, L.H.; Jiang, G. Chlorinated Polyfluorinated Ether Sulfonates Exhibit Higher Activity toward Peroxisome Proliferator-Activated Receptors Signaling Pathways than Perfluorooctanesulfonate. Environ. Sci. Technol. 2018, 52, 3232–3239. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, R.; Ren, F.; Jiang, S.; Jin, H. Occurrence and partitioning of p-phenylenediamine antioxidants and their quinone derivatives in water and sediment. Sci. Total Environ. 2024, 914, 170046. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, R.; Jiang, S.; Wu, P.; Jin, H. Occurrence of p-phenylenediamine antioxidants (PPDs) and PPDs-derived quinones in indoor dust. Sci. Total Environ. 2024, 912, 169325. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lim, W.Y.; Eng, P.; Leong, S.S.; Lim, T.K.; Ng, A.W.; Tee, A.; Seow, A. Lung cancer in Chinese women: Evidence for an interaction between tobacco smoking and exposure to inhalants in the indoor environment. Environ. Health Perspect. 2010, 118, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Ma, J.; Shi, Y.; Chen, C.; Wang, Y.; Zhao, E.; Cai, Y.; Qu, G. Biomonitoring of chlorinated polyfluoroalkyl ether sulfonic acid in the general population in central and eastern China: Occurrence and associations with age/sex. Environ. Int. 2020, 144, 106043. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, H.; Cui, Q.; Sheng, N.; Yeung, L.W.Y.; Guo, Y.; Sun, Y.; Dai, J. First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ. Sci. Technol. 2017, 51, 9553–9560. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zheng, Z.; Shi, Y.; Cai, Y. Chlorinated polyfluoroalkyl ether sulfonic acids in fish, dust, drinking water and human serum: From external exposure to internal doses. Environ. Int. 2021, 157, 106820. [Google Scholar] [CrossRef]
- Liu, H.; Pan, Y.; Jin, S.; Li, Y.; Zhao, L.; Sun, X.; Cui, Q.; Zhang, B.; Zheng, T.; Xia, W.; et al. Associations of per-/polyfluoroalkyl substances with glucocorticoids and progestogens in newborns. Environ. Int. 2020, 140, 105636. [Google Scholar] [CrossRef]
- Zhan, W.; Qiu, W.; Ao, Y.; Zhou, W.; Sun, Y.; Zhao, H.; Zhang, J. Environmental Exposure to Emerging Alternatives of Per- and Polyfluoroalkyl Substances and Polycystic Ovarian Syndrome in Women Diagnosed with Infertility: A Mixture Analysis. Environ. Health Perspect. 2023, 131, 57001. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Chen, R.R.; Gao, Y.; Qu, J.L.; Wang, Z.Z.; Zhao, M.R.; Bai, X.X.; Jin, H.B. Human serum poly- and perfluoroalkyl substance concentrations and their associations with gestational diabetes mellitus. Environ. Pollut. 2023, 317, 9. [Google Scholar] [CrossRef]
- Zhao, N.; Kong, Y.; Yuan, Q.; Wei, Z.; Gu, J.; Ji, C.; Jin, H.; Zhao, M. The toxic mechanism of 6:2 Cl-PFESA in adolescent male rats: Endocrine disorders and liver inflammation regulated by the gut microbiota-gut-testis/liver axis. J. Hazard. Mater. 2023, 459, 132155. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.M.; Evans, H.J. Cigarette smoke-induced DNA damage and lung cancer risks. Nature 1980, 283, 388–390. [Google Scholar] [CrossRef]
- Pirie, K.; Peto, R.; Reeves, G.K.; Green, J.; Beral, V.; Million Women Study, C. The 21st century hazards of smoking and benefits of stopping: A prospective study of one million women in the UK. Lancet 2013, 381, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, C.A.; Raaschou-Nielsen, O.; Loft, S.; Moller, P.; Vermeulen, R.; Palli, D.; Chadeau-Hyam, M.; Xun, W.W.; Vineis, P. Biomarkers of ambient air pollution and lung cancer: A systematic review. Occup. Environ. Med. 2012, 69, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Kashima, S. Air pollution: Another cause of lung cancer. Lancet Oncol. 2013, 14, 788–789. [Google Scholar] [CrossRef]
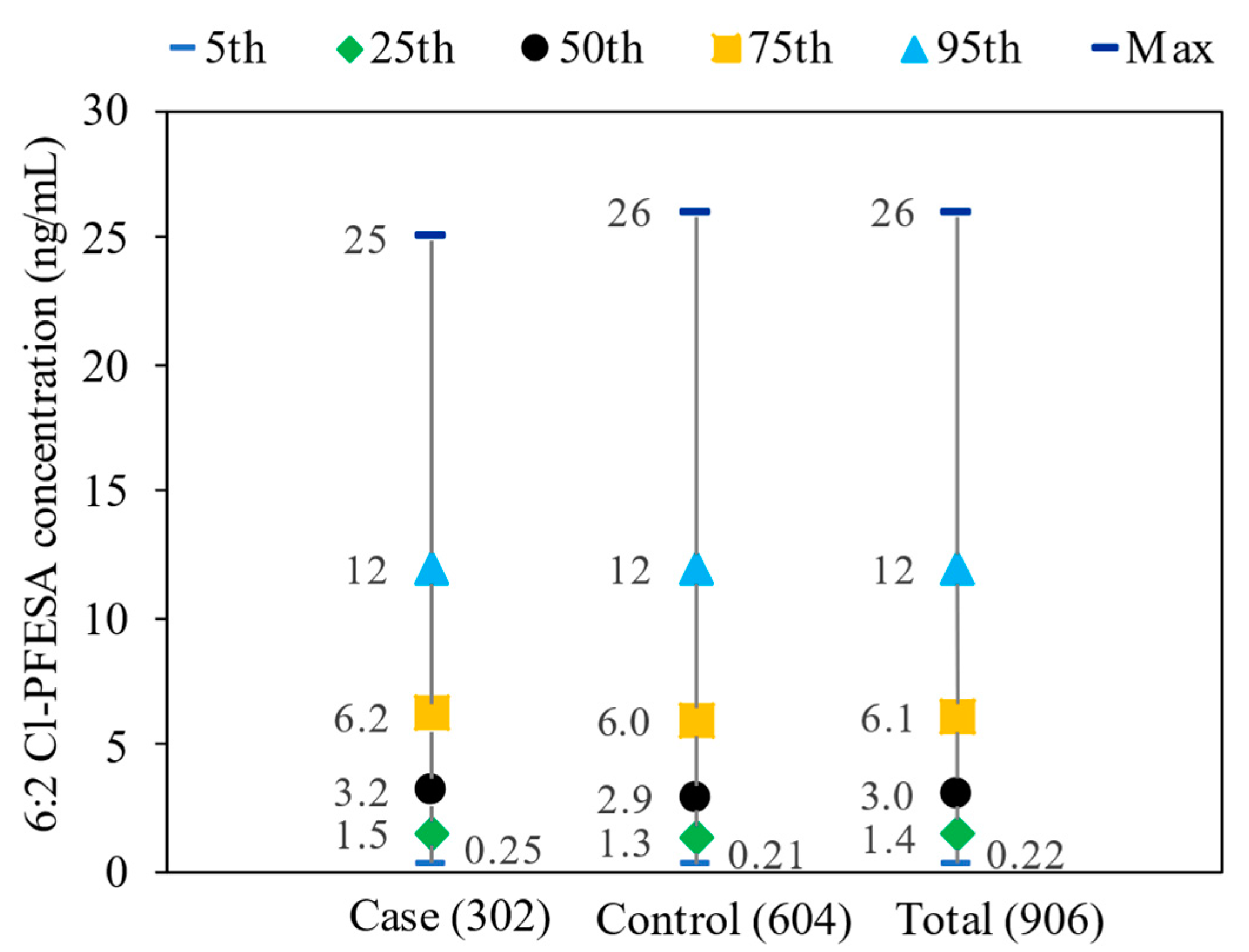
| Characteristics | Control (n = 604) | Case (n = 302) | p-Value a | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number (%) | Mean | Median | Range | Number (%) | Mean | Median | Range | ||
| Gender | N/A | ||||||||
| Male | 356 (58.9) | 4.0 | 2.9 | <LOD-26 | 178 (58.9) | 4.6 | 3.3 | <LOD-25 | |
| Female | 248 (41.1) | 4.0 | 2.9 | <LOD-25 | 124 (41.1) | 3.7 | 3.1 | <LOD-13 | |
| Age (years) | N/A | ||||||||
| <51 | 110 (18.2) | 3.7 | 2.8 | <LOD-26 | 55 (18.2) | 4.8 | 3.6 | <LOD-25 | |
| 51–60 | 120 (19.9) | 4.2 | 3.0 | 0.031–21 | 60 (19.9) | 4.4 | 3.4 | 0.11–12 | |
| 61–70 | 200 (33.1) | 4.3 | 2.9 | <LOD-25 | 100 (33.1) | 3.9 | 3.2 | 0.10–15 | |
| >70 | 174 (28.8) | 3.7 | 2.9 | <LOD-20 | 87 (28.8) | 4.1 | 3.1 | <LOD-20 | |
| Body mass index (kg/m2) | 0.842 | ||||||||
| <18.5 | 38 (6.3) | 3.5 | 2.8 | 0.033–20 | 22 (7.3) | 3.6 | 3.1 | <LOD-13 | |
| 18.5–24.9 | 375 (62.1) | 4.0 | 2.9 | <LOD-26 | 187 (61.9) | 4.4 | 3.3 | <LOD-20 | |
| >24.9 | 191 (31.6) | 4.0 | 2.8 | <LOD-25 | 93 (30.8) | 4.0 | 3.2 | <LOD-25 | |
| Educational level | 0.681 | ||||||||
| ≤9 | 494 (81.8) | 4.1 | 2.9 | <LOD-25 | 242 (80.1) | 4.2 | 3.2 | <LOD-22 | |
| >9 | 108 (17.9) | 3.8 | 2.8 | <LOD-26 | 57 (18.9) | 4.1 | 3.4 | <LOD-25 | |
| Annual household income (CNY) | 0.626 | ||||||||
| <50,000 | 216 (35.8) | 3.8 | 2.8 | <LOD-25 | 113 (37.4) | 4.1 | 3.2 | <LOD-15 | |
| 50,000–100,000 | 248 (41.0) | 4.1 | 2.9 | <LOD-26 | 114 (37.8) | 4.5 | 3.2 | <LOD-25 | |
| >100,000 | 140 (23.2) | 4.2 | 3.1 | <LOD-23 | 75 (24.8) | 4.0 | 3.6 | <LOD-22 | |
| Occupational status | 0.330 | ||||||||
| Employed | 213 (35.3) | 4.1 | 2.9 | <LOD-23 | 97 (32.1) | 4.9 | 3.3 | 0.10–25 | |
| Unemployed | 389 (64.4) | 4.0 | 2.9 | <LOD-26 | 205 (67.9) | 3.9 | 3.2 | <LOD-14 | |
| Marital status | 0.542 | ||||||||
| Married | 498 (82.5) | 4.0 | 2.8 | <LOD-25 | 244 (80.8) | 4.2 | 3.3 | <LOD-25 | |
| Divorced and widowed | 106 (17.5) | 3.9 | 3.1 | <LOD-26 | 58 (19.2) | 4.0 | 3.1 | <LOD-22 | |
| Dietary habit | 0.909 | ||||||||
| Spicy food | 474 (78.5) | 4.2 | 2.9 | <LOD-26 | 236 (78.1) | 4.2 | 3.2 | <LOD-25 | |
| Non-spicy food | 130 (21.5) | 3.4 | 2.9 | <LOD-20 | 66 (21.9) | 4.2 | 3.7 | 0.10–14 | |
| Residence | 0.957 | ||||||||
| Urban | 455 (75.3) | 3.9 | 2.8 | <LOD-26 | 228 (75.5) | 4.2 | 3.2 | <LOD-25 | |
| Rural | 149 (24.7) | 4.3 | 3.1 | <LOD-23 | 74 (24.5) | 4.1 | 3.2 | 0.10–22 | |
| Smoking habit | 0.565 | ||||||||
| Nonsmoker | 368 (61.0) | 4.0 | 2.9 | <LOD-25 | 178 (58.9) | 4.0 | 3.1 | <LOD-25 | |
| Current smoker | 236 (39.0) | 4.1 | 2.8 | <LOD-26 | 124 (41.1) | 4.6 | 3.4 | <LOD-20 | |
| Alcohol consumption habit | 0.304 | ||||||||
| Nondrinker | 418 (69.2) | 3.9 | 2.9 | <LOD-25 | 219 (72.5) | 4.1 | 3.2 | <LOD-22 | |
| Current drinker | 186 (30.8) | 4.2 | 2.9 | <LOD-26 | 83 (27.5) | 4.6 | 3.2 | <LOD-25 | |
| Family history of lung cancer | 0.004 | ||||||||
| No | 583 (96.5) | 4.0 | 2.9 | <LOD-26 | 279 (92.4) | 4.3 | 3.2 | <LOD-25 | |
| Yes | 16 (2.7) | 3.7 | 2.5 | 0.29–10 | 20 (6.6) | 3.9 | 4.1 | 0.54–8.6 | |
| History of any lung disease b | 0.003 | ||||||||
| No | 580 (96.0) | 4.0 | 2.9 | <LOD-26 | 275 (91.1) | 4.3 | 3.3 | <LOD-25 | |
| Yes | 23 (3.9) | 3.1 | 2.3 | <LOD-13 | 26 (8.6) | 3.3 | 2.8 | 0.11–10 | |
| Histologic type | N/A | ||||||||
| Non-small cell carcinoma | N/A | N/A | N/A | N/A | 168 (55.6) | 4.3 | 3.2 | <LOD-20 | |
| Small cell carcinoma | N/A | N/A | N/A | N/A | 71 (23.5) | 3.9 | 3.5 | 0.11–11 | |
| Squamous cell carcinoma | N/A | N/A | N/A | N/A | 63 (20.9) | 4.1 | 2.9 | <LOD-25 | |
| Cases/Controls (n) | Crude | Adjusted a | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Total participants (n = 906) | |||
| Q1 (<1.3) | 56/151 | Ref. | Ref. |
| Q2 (1.3–2.9) | 68/151 | 1.21 (0.79, 1.84) | 1.20 (0.79, 1.85) |
| Q3 (2.9–6.0) | 87/151 | 1.55 (1.03, 2.32) | 1.51 (1.00, 2.28) |
| Q4 (>6.0) | 91/151 | 1.62 (1.08, 2.42) | 1.59 (1.06, 2.39) |
| p for trend * | 0.018 | 0.026 | |
| Excluding participants who have a family history of lung cancer (n = 774) | |||
| Q1 (<1.3) | 46/129 | Ref. | Ref. |
| Q2 (1.3–2.8) | 58/129 | 1.26 (0.79, 1.99) | 1.23 (0.77, 1.96) |
| Q3 (2.8–5.7) | 77/129 | 1.67 (1.07, 2.59) | 1.60 (1.02, 2.50) |
| Q4 (>5.7) | 77/129 | 1.67 (1.07, 2.59) | 1.63 (1.04, 2.55) |
| p for trend * | 0.027 | 0.036 | |
| Excluding participants who were diagnosed with any lung disease (n = 753) | |||
| Q1 (<1.3) | 46/126 | Ref. | Ref. |
| Q2 (1.3–2.9) | 58/125 | 1.27 (0.80, 2.01) | 1.20 (0.75, 1.95) |
| Q3 (2.9–6.0) | 71/126 | 1.54 (0.98, 2.41) | 1.42 (0.89, 2.25) |
| Q4 (>6.0) | 76/125 | 1.66 (1.07, 2.59) | 1.62 (1.02, 2.55) |
| p for trend * | 0.028 | 0.037 | |
| Excluding urban participants (n = 223) | |||
| Q1 (<1.6) | 14/37 | Ref. | Ref. |
| Q2 (1.6–3.1) | 19/38 | 1.32 (0.57, 3.01) | 1.35 (0.55, 3.31) |
| Q3 (3.1–5.3) | 20/37 | 1.42 (0.63, 3.20) | 1.38 (0.57, 3.32) |
| Q4 (>5.3) | 21/37 | 1.51 (0.66, 3.44) | 1.55 (0.63, 3.77) |
| p for trend * | 0.392 | 0.375 | |
| Cases/Controls (n) | Crude | Adjusted a | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | ||
| Subjects’ gender | |||
| Male (n = 534) | |||
| Q1 (<1.3) | 30/89 | Ref. | Ref. |
| Q2 (1.3–2.9) | 38/89 | 1.26 (0.72, 2.22) | 1.27 (0.71, 2.27) |
| Q3 (2.9–5.9) | 49/89 | 1.63 (0.95, 2.80) | 1.64 (0.94, 2.87) |
| Q4 (>5.9) | 61/89 | 2.03 (1.20, 3.44) | 2.04 (1.19, 3.51) |
| p for trend * | 0.005 | 0.006 | |
| Female (n = 372) | |||
| Q1 (<1.4) | 28/62 | Ref. | Ref. |
| Q2 (1.4–2.9) | 28/62 | 1.00 (0.53, 1.88) | 0.99 (0.50, 1.94) |
| Q3 (2.9–6.1) | 41/62 | 1.46 (0.80, 2.65) | 1.43 (0.75, 2.72) |
| Q4 (>6.1) | 27/62 | 0.96 (0.51, 1.82) | 1.04 (0.53, 2.03) |
| p for trend * | 0.970 | 0.840 | |
| p for interaction | 0.239 | 0.232 | |
| Smoking habit | |||
| Smoker (n = 360) | |||
| Q1 (<1.3) | 21/59 | Ref. | Ref. |
| Q2 (1.3–2.8) | 22/59 | 1.04 (0.52, 2.10) | 1.10 (0.51, 2.34) |
| Q3 (2.8–5.7) | 36/59 | 1.71 (0.89, 3.27) | 1.86 (0.92, 3.75) |
| Q4 (>5.7) | 45/59 | 2.14 (1.14, 4.02) | 2.48 (1.25, 4.89) |
| p for trend * | 0.007 | 0.003 | |
| Nonsmoker (n = 546) | |||
| Q1 (<1.4) | 36/92 | Ref. | Ref. |
| Q2 (1.4–2.9) | 47/92 | 1.30 (0.77, 2.19) | 1.32 (0.77, 2.25) |
| Q3 (2.9–6.1) | 51/92 | 1.41 (0.84, 2.37) | 1.38 (0.81, 2.35) |
| Q4 (>6.1) | 44/92 | 1.22 (0.72, 2.07) | 1.22 (0.71, 2.09) |
| p for trend * | 0.641 | 0.662 | |
| p for interaction | 0.244 | 0.216 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, W.; Qu, J.; Guo, R.; Chen, Y.; Jin, H.; Xu, J. Association between Serum 6:2 Chlorinated Polyfluorinated Ether Sulfonate Concentrations and Lung Cancer. Toxics 2024, 12, 603. https://doi.org/10.3390/toxics12080603
Mao W, Qu J, Guo R, Chen Y, Jin H, Xu J. Association between Serum 6:2 Chlorinated Polyfluorinated Ether Sulfonate Concentrations and Lung Cancer. Toxics. 2024; 12(8):603. https://doi.org/10.3390/toxics12080603
Chicago/Turabian StyleMao, Weili, Jianli Qu, Ruyue Guo, Yuanchen Chen, Hangbiao Jin, and Jingyan Xu. 2024. "Association between Serum 6:2 Chlorinated Polyfluorinated Ether Sulfonate Concentrations and Lung Cancer" Toxics 12, no. 8: 603. https://doi.org/10.3390/toxics12080603
APA StyleMao, W., Qu, J., Guo, R., Chen, Y., Jin, H., & Xu, J. (2024). Association between Serum 6:2 Chlorinated Polyfluorinated Ether Sulfonate Concentrations and Lung Cancer. Toxics, 12(8), 603. https://doi.org/10.3390/toxics12080603





