Removal of the Highly Toxic Anticoccidial Monensin Using Six Different Low-Cost Bio-Adsorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bio-Adsorbent Materials
2.3. Characterization of the Bio-Adsorbents
2.4. Experimental Design
2.4.1. Influence of Environmental Factors
2.4.2. Experiments on Adsorption and Desorption (Batch Tests)
2.4.3. Quantification of MON
2.5. Calculation and Statistical Treatment
3. Results
3.1. Bio-Adsorbents Characteristics
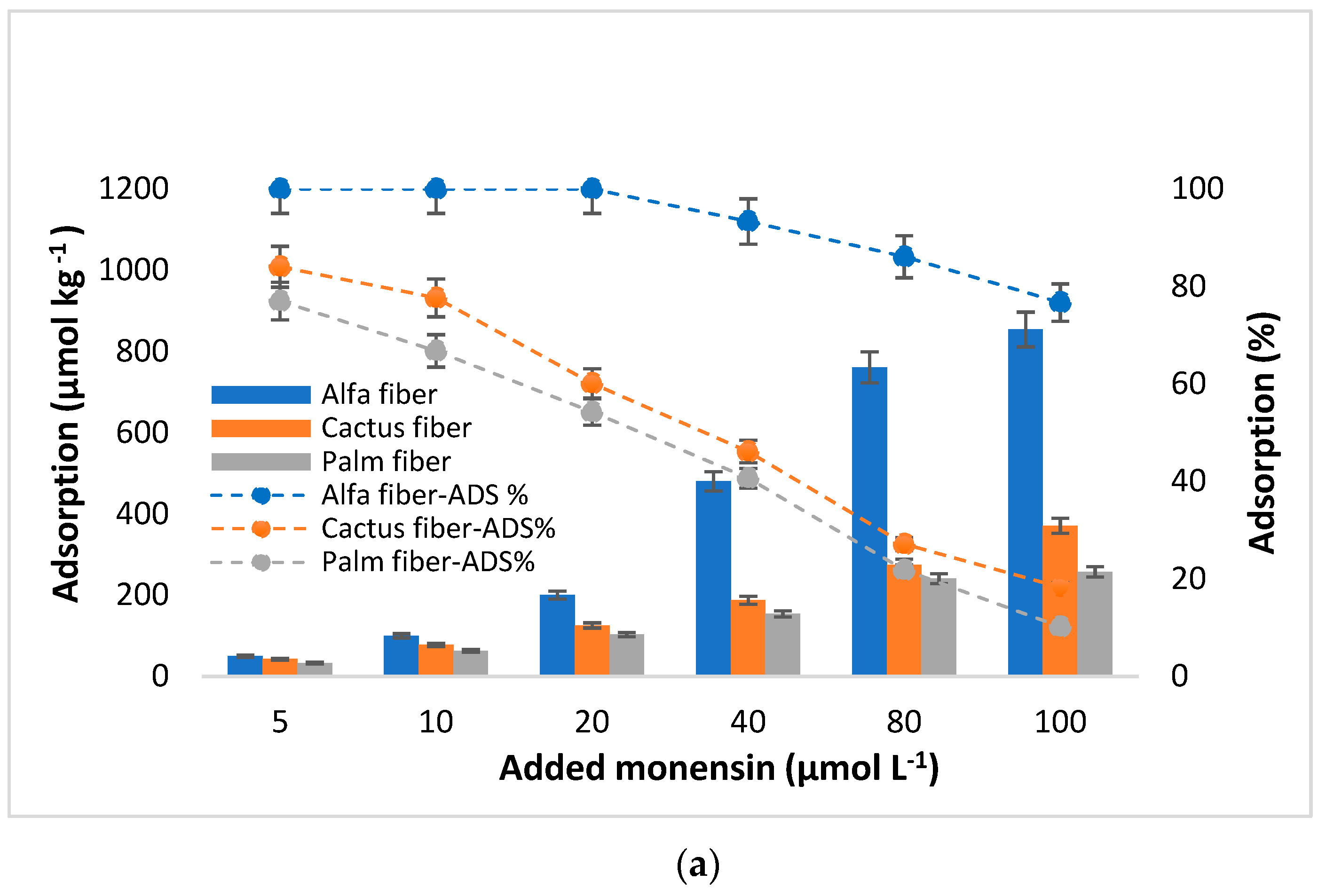
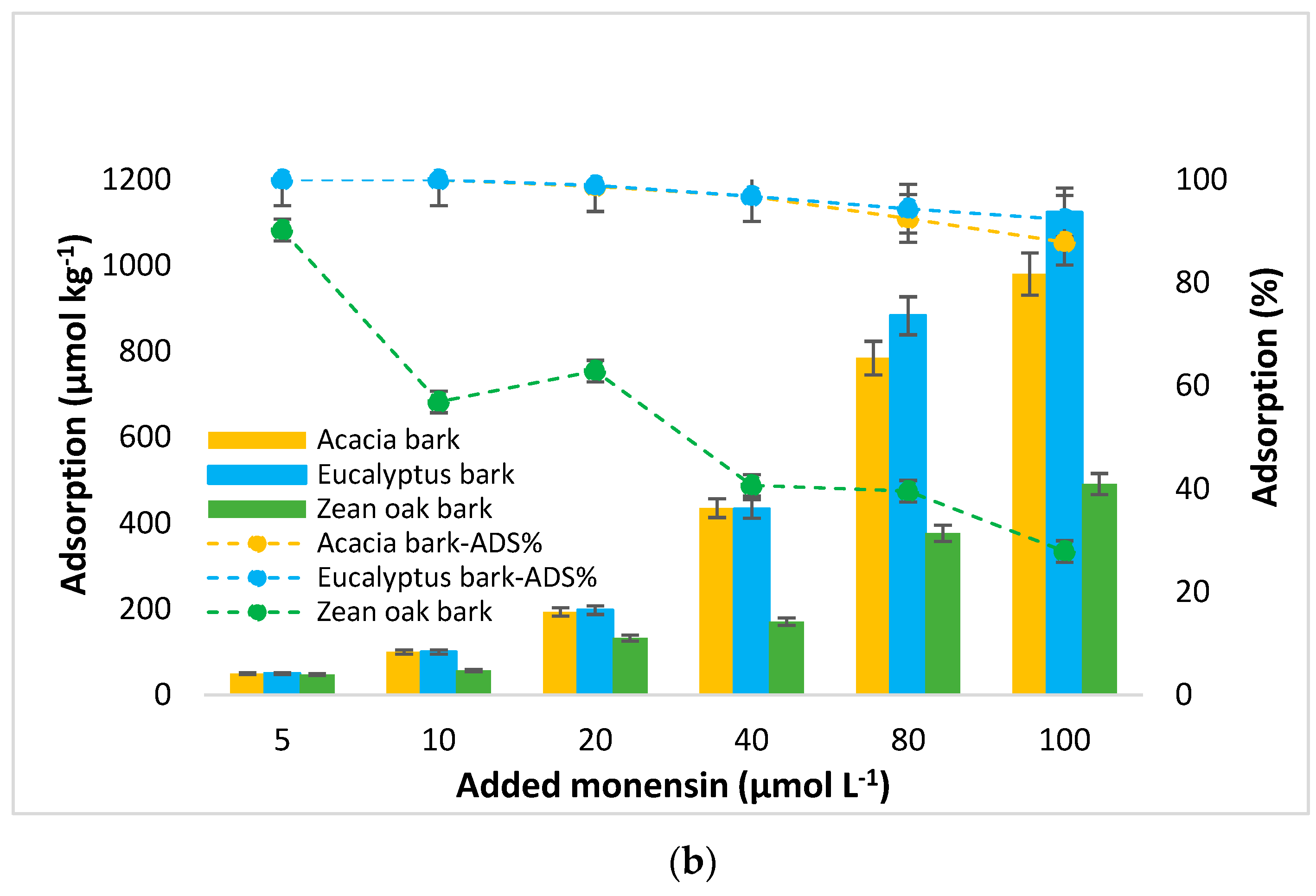
3.2. MON Adsorption
3.3. Fitting of Experimental Data to Adsorption Models
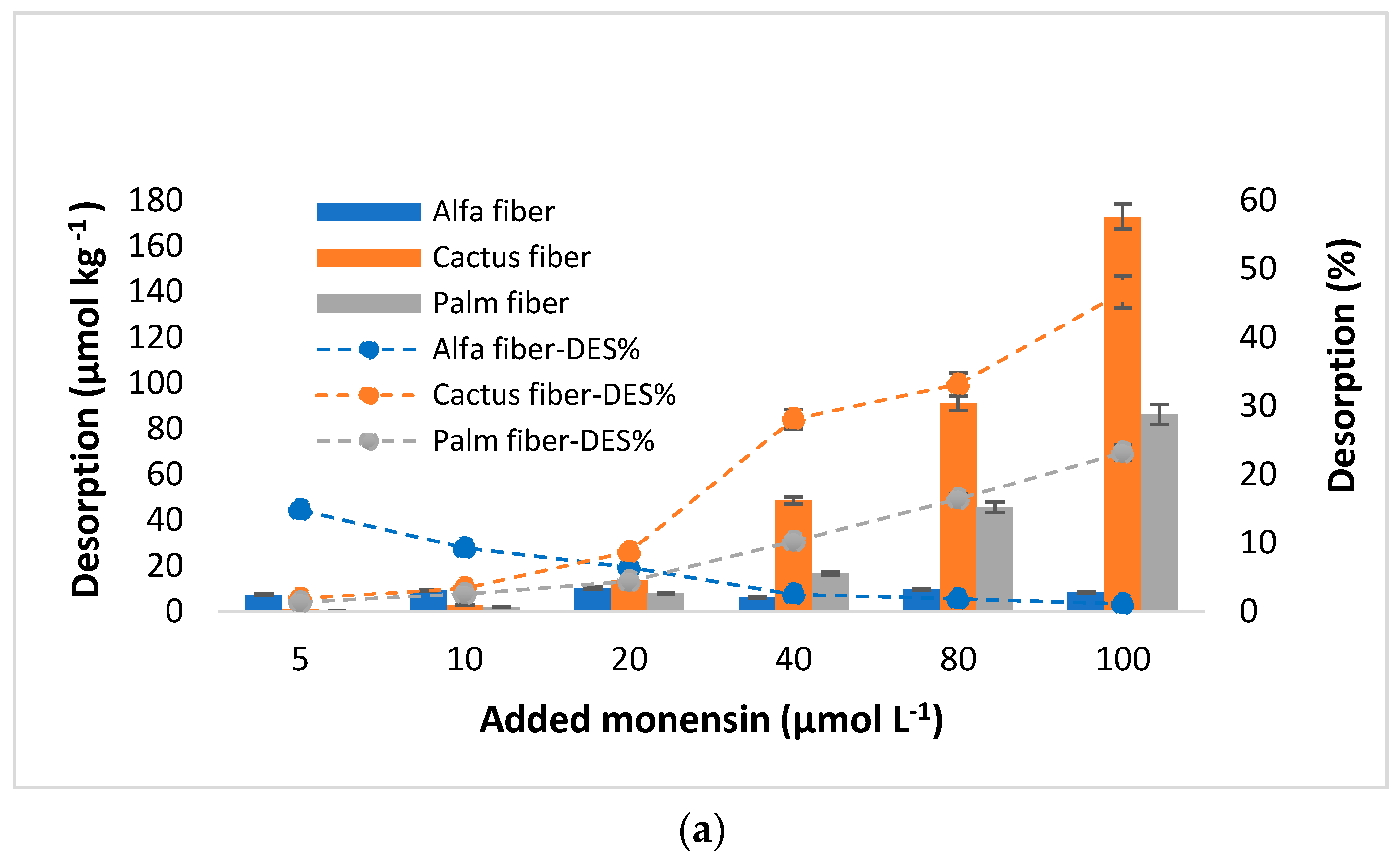
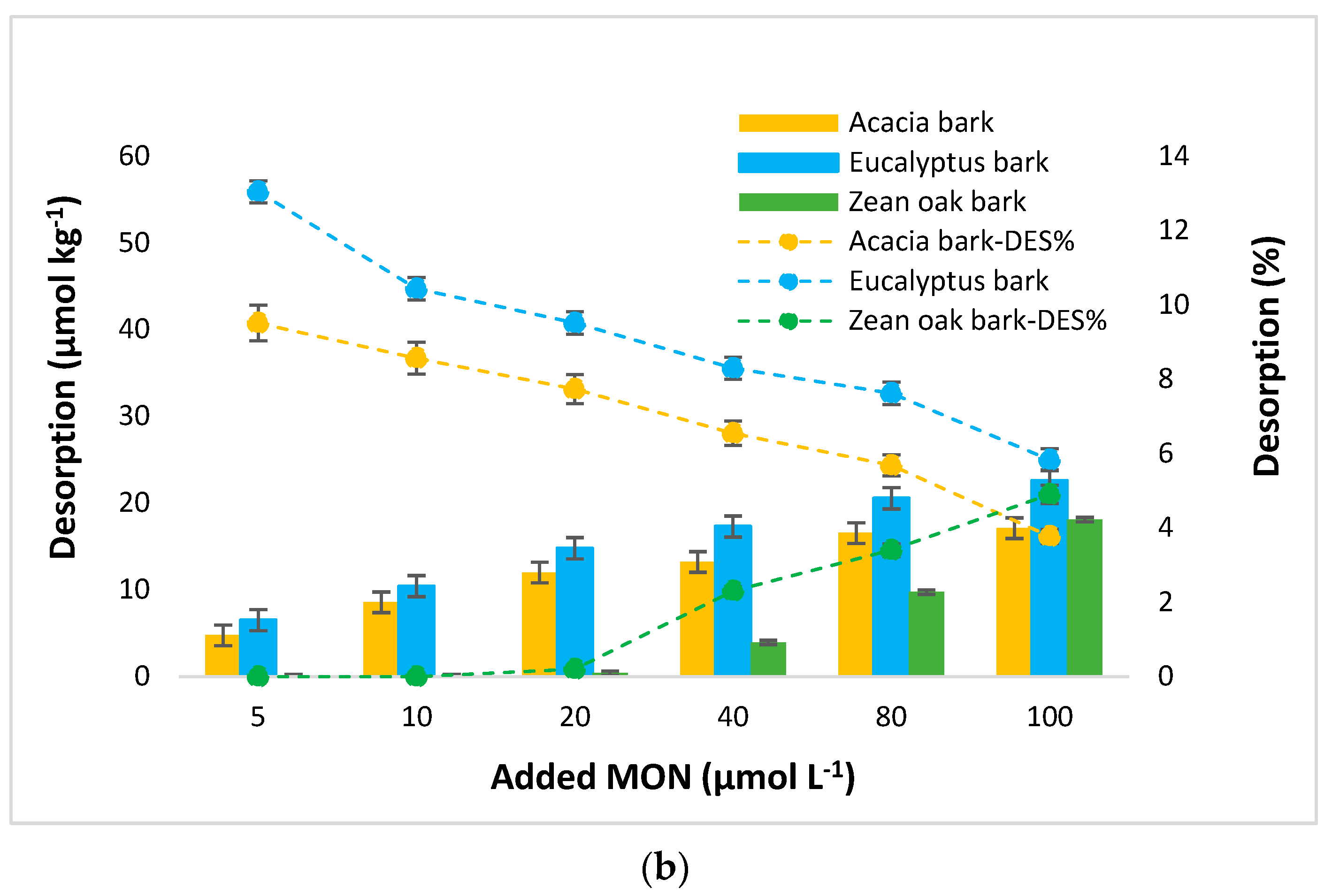
3.4. MON Desorption
4. Discussion
4.1. MON Adsorption
4.2. Fitting to Adsorption Models
4.3. Scatchard Plots Analysis
4.4. MON Desorption
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chee-Sanford, J.C.; Mackie, R.I.; Koike, S.; Krapac, I.G.; Lin, Y.F.; Yannarell, A.C.; Maxwell, S.; Aminov, R.I. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J. Environ. Qual. 2009, 38, 1086–1108. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid. Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.R.; Gales, A.C.; Laxminarayan, R.; Dodd, P.C. Antimicrobial Resistance: Addressing a global threat to humanity. PLoS Med. 2023, 20, e1004264. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, S.C.; Chander, Y.; Singh, A.K. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar] [CrossRef]
- Ghasemi-Sadabadi, M.; Ebrahimnezhad, Y.; Shaddel-Tili, A.; Bannapour-Ghaffari, V.; Saemi Peste-Bigelow, S. Comparison of using ionophore and non-ionophore coccidiostats on performance, carcass characteristics, blood biochemical parameters and gut microbial flora in broiler chickens. Iran. J. Appl. Anim. Sci. 2020, 10, 693–704. [Google Scholar]
- Sassman, S.A.; Lee, L.S. Sorption and degradation in soils of veterinary ionophore antibiotics: Monensin and lasalocid. Environ. Toxicol. Chem. 2007, 26, 1614–1621. [Google Scholar] [CrossRef]
- Ekinci, İ.B.; Chłodowska, A.; Olejnik, M. Ionophore toxicity in animals: A review of clinical and molecular aspects. Int. J. Mol. Sci. 2023, 24, 1696–1710. [Google Scholar] [CrossRef]
- Food and Drug Administration. New Animal Drugs; Monensin. Federal Register 69, 68783–68784. Available online: http://www.fda.gov/ohrms/dockets/98fr/04-26091.pdf (accessed on 1 June 2024).
- Furtula, V.; Huang, L.; Chambers, P.A. Determination of veterinary pharmaceuticals in poultry litter and soil by methanol extraction and liquid chromatography-tandem mass spectrometry. J. Environ. Sci. Health 2009, 44, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Barmaz, D.; Cabrera, M.L.; Pavlostathis, S.G.; Huang, C.H. Detection and quantification of ionophore antibiotics in runoff, soil and poultry litter. J. Chromatogr. A 2013, 1312, 10–17. [Google Scholar] [CrossRef]
- Hafner, S.C.; Harter, T.; Parikh, S.J. Evaluation of monensin transport to shallow groundwater after irrigation with dairy lagoon water. J. Environ. Qual. 2016, 45, 480–487. [Google Scholar] [CrossRef]
- Capleton, A.C.; Courage, C.; Rumsby, P.; Holmes, P.; Stutt, E.; Boxall, A.B.A.; Levy, L.S. Prioritizing veterinary medicines according to their potential indirect human exposure and toxicity profile. Toxicol. Lett. 2006, 163, 213–223. [Google Scholar] [CrossRef]
- Rokka, M.; Jestoi, M.; Peltonen, K. Trace level determination of polyther ionophores in feed. BioMed Res. Int. J. 2013, 2013, 151363. [Google Scholar] [CrossRef]
- El Sayed, E.M.; Prasher, S.O. Fate and transport of monensin in the presence of nonionic surfactant Brij35 in soil. Sci. Total Environ. 2014, 490, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Prasher, S.O.; Patel, R.M. Removal of ionophoric antibiotics in free water surface constructed wetlands. Ecol. Eng. 2012, 41, 13–21. [Google Scholar] [CrossRef]
- Bak, S.A.; Björklund, E. Occurrence of ionophores in the Danish environment. Antibiotics 2014, 3, 564–571. [Google Scholar] [CrossRef]
- Nath, K. Membrane Separation Processes, 2nd ed.; Raj Press: New Delhi, India, 2017. [Google Scholar]
- Omuferen, L.O.; Maseko, R.; Olowoyo, J.O. Occurrence of antibiotics in wastewater from hospital and conventional wastewater treatment plants and their impact on the effluent receiving rivers: Current knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 306–320. [Google Scholar] [CrossRef]
- Ajala, O.A.; Akinnawo, S.O.; Bamisaye, A.; Adedipe, D.T.; Adesina, M.O.; Okon-Akan, O.A.; Adebusuyi, T.A.; Ojedokun, A.T.; Adegoke, K.A.; Bello, O.S. Adsorptive removal of antibiotic pollutants from wastewater using biomass/biochar-based adsorbents. Royal Soc. Chem. Adv. 2023, 13, 4678–4712. [Google Scholar] [CrossRef]
- Ding, H.; Wu, Y.; Zou, B.; Lou, Q.; Zhang, W.; Zhong, J.; Lu, L.; Dai, G. Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J. Hazar Mat. 2016, 307, 350–358. [Google Scholar] [CrossRef]
- Russell, J.N.; Yost, C.K. Alternative, environmentally conscious approaches for removing antibiotics from wastewater treatment systems. Chemosphere 2021, 263, 128177–128189. [Google Scholar] [CrossRef]
- Juela, D.M. Promising adsorptive materials derived from agricultural and industrial wastes for antibiotic removal: A comprehensive review. Separ Purif. Technol. 2022, 284, 120286–120296. [Google Scholar] [CrossRef]
- Errais, E.; Duplay, J.; Darragi, F. Textile dye removal by natural clay—Case study of Fouchana Tunisian clay. Environ. Technol. 2010, 31, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.S.; Nguyen, K.M.; Nguyen, H.T.; Stefanakis, A.I.; Nguyen, P.M. Food processing wastes as a potential source of adsorbent for toxicant removal from water. Circul Econ. Sustain. 2022, 2, 491–507. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Influence of mussel shell, oak ash, and pine bark on the adsorption and desorption of sulfonamides in agricultural soils. J. Environ. Manag. 2020, 261, 110221–110233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, J.; Tian, Y.; Liu, C.; Zhang, L.; Cao, L.; Zhou, Y.; Zhang, S. Effective removal of tetracycline antibiotics from water by magnetic functionalized biochar derived from rice waste. Environ. Pollut. 2023, 330, 121681–121693. [Google Scholar] [CrossRef]
- Míguez-González, A.; Cela-Dablanca, R.; Barreiro, A.; Castillo-Ramos, V.; Sánchez-Polo, M.; López-Ramón, M.V.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Current Data on Environmental Problems due to Ionophore Antibiotics Used as Anticoccidial Drugs in Animal Production, and Proposal of New Research to Control Pollution by Means of Bio-Adsorbents and Nanotechnology. In Planet Earth: Scientific Proposals to Solve Urgent Issues, 1st ed.; Núñez-Delgado, A., Ed.; Springer: Cham, Switzerland, 2024; pp. 241–261. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Siddeeg, S.M. Cactus an eco-friendly material for wastewater treatment: A review. J. Mater. Environ. Sci. 2017, 8, 1770–1782. [Google Scholar]
- Hadjittofi, L.; Prodromou, M.; Pashalidis, I. Activated biochar derived from cactus fibres—Preparation, characterization and application on Cu(II) removal from aqueous solutions. J. Bioresour. Technol. 2014, 159, 460–464. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; El Makhfouk, M.; Qourzal, S. Biosorption characteristics of cadmium and lead onto eco-friendly dried cactus (Opuntia ficus indica) clado des. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Barka, N.; Ouzaouit, K.; Abdennouri, M.; El Makhfouk, M. Dried prickly pear cactus (Opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2013, 44, 52–60. [Google Scholar] [CrossRef]
- Prodromou, M.; Pashalidis, I. Copper(II) removal from aqueous solutions by adsorption on non-treated and chemically modified cactus fibres. Water Sci. Technol. 2013, 68, 2497–2504. [Google Scholar] [CrossRef]
- Bennacer, L.; Benmammar, D.; Ahfir, N.D.; Alem, A.; Mignot, M.; Pantet, M. Potential of using Alfa grass fibers (Stipa Tenacissima L.) to remove Pb2+, Cu2+, and Zn2+ from an aqueous solution. Environ. Technol. 2022, 104, 1. [Google Scholar] [CrossRef]
- Melliti, A.; Srivastava, V.; Kheriji, J.; Sillanpää, M.; Hamrouni, B. Date Palm Fiber as a novel precursor for porous activated carbon: Optimization, characterization and its application as Tylosin antibiotic scavenger from aqueous solution. Surf. Interfaces 2021, 24, 101047. [Google Scholar] [CrossRef]
- Zafar, L.; Khan, A.; Kamran, U.; Park, S.J.; Bhatti, H.N. Eucalyptus (camaldulensis) bark-based composites for efficient Basic Blue 41 dye biosorption from aqueous stream: Kinetics, isothermal, and thermodynamic studies. Surf. Interfaces 2022, 31, 101897. [Google Scholar] [CrossRef]
- Talhi, M.F.; Cheriti, A.; Belboukhari, N.; Agha, L.; Roussel, C. Biosorption of copper ions from aqueous solutions using the desert tree Acacia raddiana. Desalination Water Treat. 2010, 21, 323–327. [Google Scholar] [CrossRef]
- Fox, R.L.; Kamprath, E.J. Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil. Sci. Soc. Am. J. 1970, 34, 902–907. [Google Scholar] [CrossRef]
- Lopes, N.P.; Stark, C.B.; Gates, P.J.; Staunton, J. Fragmentation studies on monensin A by sequential electrospray mass spectrometry. Analyst 2002, 127, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Nebot, C.; Iglesias, A.; Regal, P.; Miranda, J.M.; Fente, C.; Cepeda, A. A sensitive and validated HPLC–MS/MS method for simultaneous determination of seven coccidiostats in bovine whole milk. Food Control 2011, 27, 29–36. [Google Scholar] [CrossRef]
- Peech, M. Methods of soil analysis for soil-fertility investigations. US Dept Agr. Circ. 1947, 757, 7–11. [Google Scholar]
- Rodríguez-López, L.; Santás-Miguel, V.; Cela-Dablanca, R.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Pérez-Rodríguez, P.; Arias-Estévez, M. Ciprofloxacin and Trimethoprim Adsorption/Desorption in Agricultural Soils. Int. J. Environ. Res. Public Health 2022, 19, 8426. [Google Scholar] [CrossRef] [PubMed]
- Cela-Dablanca, R.; Barreiro, A.; Rodríguez-López, L.; Santás-Miguel, V.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Amoxicillin retention/release in agricultural soils amended with different bio-adsorbent materials. Materials 2022, 15, 3200. [Google Scholar] [CrossRef]
- Hamdi, S.; Gharbi-Khelifi, H.; Barreiro, A.; Mosbahi, M.; Cela-Dablanca, R.; Brahmi, J.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Issaoui, M.; Álvarez-Rodríguez, E. Tetracycline adsorption/desorption by raw and activated Tunisian clays. Environ. Res. 2024, 242, 117536–117548. [Google Scholar] [CrossRef]
- Hamdi, S.; Mosbahi, M.; Issaoui, M.; Barreiro, A.; Cela-Dablanca, R.; Brahmi, J.; Tlili, A.; Jamoussi, F.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; et al. Experimental data and modeling of sulfadiazine adsorption onto raw and modified clays from Tunisia. Environ. Res. 2024, 248, 118309–118321. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Ferreira-Coelho, G.; Arias-Estévez, M.; Álvarez-Esmorís, C.; Carlos Nóvoa- Muñoz, J.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Competitive adsorption/desorption of tetracycline, oxytetracycline, and chlortetracycline on pine bark, oak ash, and mussel shell. J. Environ. Manag. 2019, 250, 109509–109519. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhang, Y.; Meng, D.; Liu, X.; Zhang, Z.; Gao, P.; Lin, A.; Hou, L. Removal of sulfadiazine from aqueous solution by in-situ activated biochar derived from cotton shell. Environ. Res. 2020, 191, 110104–110111. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Ferreira-Coelho, G.; Fernández-Calviño, D.; Núnez-Delgado, A.; Fernàndez-Sanjurjo, M.J.; Arias-Estévez, M.; Alvarez-Rodríguez, E. Experimental data and model prediction of tetracycline adsorption and desorption in agricultural soils. Environ. Res. 2019, 177, 108607–108620. [Google Scholar] [CrossRef]
- Scatchard, G. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949, 51, 660. [Google Scholar] [CrossRef]
- Roca Jalil, M.E.; Baschini, M.; Sapag, K. Removal of ciprofloxacin from aqueous solutions using pillared clays. Materials 2017, 10, 1345. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Suchithra, P.S. Equilibrium, kinetic and thermodynamic modeling for the adsorption of heavy metals onto chemically modified hydrotalcite. Indian J. Chem. Technol. 2010, 17, 247–259. [Google Scholar]
- Núñez-Delgado, A. Research on environmental aspects of retention/release of pollutants in soils and sorbents. What should be next? Environ. Res. 2024, 251, 118593. [Google Scholar] [CrossRef]
- Núñez-Delgado, A. Avoiding basic mistakes when programming the use of artificial intelligence in soil and environmental science research. Sci. Total Environ. 2024, 934, 173310. [Google Scholar] [CrossRef]
- Yaneva, Z.; Georgieva, N. Insights into Congo Red adsorption on agro-industrial materials—Spectral, equilibrium, kinetic, thermodynamic, dynamic and desorption studies. A review. Int. Rev. Chem. Eng. 2012, 4, 127–146. [Google Scholar]
- Bonetto, L.R.; Ferrarini, F.; De Marco, C.; Crespoa, S.; Guéganb, R.; Giovanelaa, M. Removal of methyl violet 2B dye from aqueous solution using a magnetic composite as an adsorbent. J. Water Process Eng. 2015, 6, 11–20. [Google Scholar] [CrossRef]
- Karoui, S.; Ben Arfi, R.; Fernández-Sanjurjo, M.J.; Nuñez-Delgado, A.; Ghorbal, A.; Álvarez-Rodríguez, E. Optimization of synergistic biosorption of oxytetracycline and cadmium from binary mixtures on reed-based beads: Modeling study using Brouers-Sotolongo models. Environ. Sci. Pollut. Res. 2021, 28, 46431–46447. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm I. Theoretical. J. Colloid. Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Nazari, G.; Abolghasemi, H.; Esmaieli, M. Batch adsorption of cephalexin antibiotic from aqueous solution by walnut shell-based activated carbon. J. Taiwan. Inst. Chem. Eng. 2016, 58, 357–365. [Google Scholar] [CrossRef]
- Sipos, P. Searching for optimum adsorption curve for metal sorption on soils: Comparison of various isotherm models fitted by different error functions. SN Appl. Sci. 2021, 3, 387–400. [Google Scholar] [CrossRef]
- Cela-Dablanca, R.; Nebot, C.; Rodríguez López, L.; Fernández-Calvino, D.; Arias-Estévez, M.; Núnez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E. Efficacy of different waste and by-products from forest and food industries in the removal/retention of the antibiotic cefuroxime. Processes 2021, 9, 1151–1163. [Google Scholar] [CrossRef]
- Sun, P.; Pavlostathis, S.G.; Huang, C.H. Estimation of environmentally relevant chemical properties of veterinary ionophore antibiotics. Environ. Sci. Pollut. Res. 2016, 23, 18353–18361. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, M.; Durso, L.M.; Miller, D.N.; Woodbury, B.; Ray, C.; Snow, D.D. Environmental fate and microbial effects of MON, lincomycin, and sulfamethazine residues in soil. Environ. Pollut. 2019, 246, 60–68. [Google Scholar] [CrossRef]
- Pérez, D.J.; Okada, E.; Iturburu, F.G.; De Gerónimo, E.; Cantón, G.; Aparicio, V.C.; Costa, J.L.; Menone, M.L. Monensin occurrence in surface water and its impact on aquatic biota in a stream of the southeast Pampas, Argentina. Environ. Sci. Pollut. Res. J. 2021, 28, 8530–8538. [Google Scholar] [CrossRef]
- Hilaire, S.S.; Bellows, B.; Brady, J.A.; Muir, J.P. Oxytetracycline and monensin uptake by tifton 85 bermudagrass from dairy manure-applied soil. Agronomy 2020, 10, 468–484. [Google Scholar] [CrossRef]
- Sassman, S.A.; Lee, L.S. Sorption of three tetracyclines by several soils: Assessing the role of pH and cation exchange. Environ. Sci. Technol. 2005, 39, 7452–7459. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Escario, M.; Miguel, E.; Pérez, R.A. Persistence and availability of veterinary antibiotics in soil and soil-manure systems. Sci. Total Environ. 2018, 643, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Della, K.D.; Henini, G.; Laidani, Y. A biosorbent material from brahea edulis palm leaves—Application to amoxicillin adsorption. Cellul. Chem. Technol. 2023, 57, 903–910. [Google Scholar] [CrossRef]
- Hansima, M.A.C.K.; Zvomuya, F.; Amarakoon, I. Fate of veterinary antimicrobials in Canadian prairie soils–a critical review. Sci. Total Environ. 2023, 892, 164387–164399. [Google Scholar] [CrossRef] [PubMed]
- Parolo, M.E.; Avena, M.J.; Pettinari, G.R.; Baschini, M.T. Influence of Ca2+ on tetracycline adsorption on montmorillonite. J. Colloid Interf. Sci. 2012, 368, 420–426. [Google Scholar] [CrossRef]
- Cox, B.G.; Truong, N.g.V.; Rzeszotarska, V.; Schneider, H. Rates and equilibria of alkali metal and silver ion complex formation with monensin in ethanol. J. Amer Chem. Soc. 1984, 106, 5965–5969. [Google Scholar] [CrossRef]
- Hussain, S.A.; Prasher, S.O. Understanding the sorption of ionophoric pharmaceuticals in a treatment wetland. Wetlands 2011, 31, 563–571. [Google Scholar] [CrossRef]
- Srivastava, R.; Rupainwar, D.C. Eucalyptus bark powder as an effective adsorbent: Evaluation of adsorptive characteristics for various dyes. Desalin Water Treat. 2009, 11, 302–313. [Google Scholar] [CrossRef]
- Acelas, N.; Lopera, S.M.; Porras, J.; Torres-Palma, R.A. Evaluating the removal of the antibiotic cephalexin from aqueous solutions using an adsorbent obtained from palm oil fiber. Molecules 2021, 26, 3340–3356. [Google Scholar] [CrossRef]
- Paksamut, J.; Boonsong, P. Removal of copper (II) ions in aqueous solutions using tannin-rich plants as natural bio-adsorbents. IOP Conf. Series. Mat. Sci. Eng. 2018, 317, 012058–012064. [Google Scholar] [CrossRef]
- Biswas, K.; Saha, S.K.; Ghosh, U.C. Adsorption of fluoride from aqueous solution by a synthetic iron (III)− aluminum (III) mixed oxide. Indust Eng. Chem. Res. 2007, 46, 5346–5356. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; ƠCarroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazar Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Ahmadi, S.; Igwegbe, C.A. Removal of methylene blue on zinc oxide nanoparticles: Nonlinear and linear adsorption isotherms and kinetics study. Sigma J. Eng. Nat. Sci. 2020, 38, 289–303. [Google Scholar]
- Mirizadeh, S.; Al Arni, S.; Elwaheidi, M.; Salih, A.A.M.; Converti, A.; Alberto Casazza, A. Adsorption of tetracycline and ciprofloxacin from aqueous solution on raw date palm waste. Process Eng. (MMPE) 2023, 46, 1957–1964. [Google Scholar] [CrossRef]
- Míguez-González, A.; Cela-Dablanca, R.; Barreiro, A.; Rodríguez-López, L.; Rodríguez-Seijo, A.; Arias-Estévez, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Castillo-Ramos, V.; Álvarez-Rodríguez, E. Adsorption of antibiotics on bio-adsorbents derived from the forestry and agro-food industries. Environ. Res. 2023, 233, 116360–116368. [Google Scholar] [CrossRef]
- Nwakonobi, T.U.; Onoja, S.B.; Ogbaje, H. Removal of certain heavy metals from brewery wastewater using date palm seeds activated carbon. Appl. Eng. Agric. 2018, 34, 233–238. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Agboola, O.D.; Benson, N.U. Physisorption and chemisorption mechanisms influencing micro (nano) plastics-organic chemical contaminants interactions: A review. Front. Environ. Sci. 2021, 9, 678574. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A.; Mandal, A.B. Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol. 2013, 10, 231–242. [Google Scholar] [CrossRef]
- Toth, J. Adsorption, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Conde-Cid, M.; Ferreira-Coelho, G.; Núñez-Delgado, A.; Fernández-Calviño, D.; Arias-Estévez, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Competitive adsorption of tetracycline, oxytetracycline and chlortetracycline on soils with different pH value and organic matter content. Environ. Res. 2019, 178, 108669–108681. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, W.F.D.P.; Dognani, G.; Alencar, D.L.N.; Silva Parizi, M.P.; Boina, R.F.; Cabrera, F.C.; Job, A.E. Chemical treatment of sugarcane bagasse and its influence on glyphosate adsorption. Matéria (Rio Jan.) 2022, 27, e13142. [Google Scholar] [CrossRef]
- Sukul, P.; Lamshöft, M.; Zühlke, S.; Spiteller, M. Sorption and desorption of sulfadiazine in soil and soil-manure systems. Chemosphere 2008, 73, 1344–1350. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Fernández-Calviño, D.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Arias-Estévez, M.; Álvarez-Rodríguez, E. Estimation of adsorption/desorption Freundlich’s affinity coefficients for oxytetracycline and chlortetracycline from soil properties: Experimental data and pedotransfer functions. Ecotox Environ. Safe 2020, 196, 110584–110596. [Google Scholar] [CrossRef] [PubMed]
- Kakaei, S.; Sattarzadeh Khameneh, E.; Rezazadeh, F.; Hosseini, M.H. Heavy metal removing by modified bentonite and study of catalytic activity. J. Mol. Struct. 2020, 1199, 126989–127011. [Google Scholar] [CrossRef]
- Dahlquist, F.W. The Meaning of Scatchard and Hill Plots, Methods of Enzymology, Hirs, C.H.W., Timasheff, S.N., Eds.; Academic Press: New York, NY, USA, 1978; Volume 48, 270–299. [CrossRef]
- Gerente, C.; Couespel du Mesnil, P.; Andrès, Y.; Thibault, J.F.; Le Cloirec, P. Removal of metal ions from aqueous solution on low cost natural polysaccharides Sorption mechanism approach. React. Funct. Polym. 2000, 46, 135–144. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Santás-Miguel, V.; Cela-Dablanca, R.; Pérez-Rodríguez, P.; Núñez-Delgado, A.; Álvarez Rodríguez, E.; Rodríguez-Seijo, A.; Arias-Estévez, M. Valorization of forest by-products as bio-adsorbents for emerging contaminants. J. Environ. Chem. Eng. 2023, 11, 111437–111448. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Y.; Shen, G.; Zhang, H.; Yuan, Z.; Zhang, W. Adsorption/desorption behavior and mechanisms of sulfadiazine and sulfamethoxazole in agricultural soil systems. Soil. Til. Res. 2019, 186, 233–241. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Wang, J.J.; Dodla, S.K.; Eberhardt, T.L.; Groom, L. Effect of biochar amendment on tylosin adsorption–desorption and transport in two different soils. J. Environ. Qual. 2012, 41, 1185–1192. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Miao, D.; Chen, Z.; Tao, Y. Sorption–desorption of typical tetracyclines on different soils: Environment hazards analysis with partition coefficients and hysteresis index. Environ. Eng. Sci. 2015, 32, 865–871. [Google Scholar] [CrossRef]
- Vega, F.A.; Covelo, E.F.; Couce, M.L.A. Applying Freundlich, Langmuir and Temkim Models in Cu and Pb soil sorption experiments. Span. J. Soil. Sci. 2011, 1, 20–37. [Google Scholar] [CrossRef]
- Núñez-Delgado, A.; Otero-Pérez, X.L.; Álvarez-Rodríguez, E. Editorial: Current Research on Soil Science and Related Aspects of Environmental Sciences in Galicia. Span. J. Soil. Sci. 2023, 13, 11485. [Google Scholar] [CrossRef]
- Roy, R.; Núñez-Delgado, A.; Sultana, S.; Wang, J.; Munir, A.; Battaglia, M.L.; Sarker, T.; Seleiman, M.F.; Barmon, M.; Zhang, R. Additions of optimum water, spent mushroom compost and wood biochar to improve the growth performance of Althaea rosea in drought-prone coal-mined spoils. J. Environ. Manag. 2021, 295, 113076. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed on a request from the Commission on the safety and the efficacy of product “BIO-COX 120G” as feed additive in accordance with Council Directive 70/524/EEC. EFSA J. 2004, 75, 1–51. [Google Scholar]
- Hamza, S.; Saad, H.; Charrier, B.; Ayed, N.; Charrier-El Bouhtoury, F. Physico-chemicalcharacterization of Tunisian plant fibers and its utilization as reinforcement forplaster based composites. Ind. Crops Prod. 2013, 49, 357–365. [Google Scholar] [CrossRef]
- Jaishankar, M.; Mathew, B.B.; Shah, M.S.; Murthy, T.P.K.; Gowda, K.R.S. Biosorption of Few Heavy Metal Ions Using Agricultural Wastes. J. Environ. Pollut. Hum. Health 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.H. Adsorption of diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrog. Energy 2016, 41, 10380–10390. [Google Scholar] [CrossRef]
- Qlihaa, A.; Dhimni, S.; Melrhaka, F.; Hajjaji, N.; Srhiri, A. Physico chemical characterization of a Moroccan clay. J. Mater. Environ. Sci. 2016, 7, 1741–1750. [Google Scholar]
- Riyajan, S.; Maneechay, S. Preparation and Properties of Natural Rubber Latex-GModified Cationic Polyacrylamide Copolymers and its Palm Oil Absorbent. Rubber Compos. 2014, 43, 264–270. [Google Scholar] [CrossRef]
- Saramolee, P.; Lopattananon, N.; Sahakaro, K. Preparation and some properties of modified natural rubber bearing grafted poly(methyl methacrylate) and epoxide groups. Eur. Polym. J. 2014, 56, 1–10. [Google Scholar] [CrossRef]



| Alfa Fiber | Cactus Fiber | Palm Fiber | Acacia Bark | Eucalyptus Bark | Zean Oak Bark | |
|---|---|---|---|---|---|---|
| pHw | 5.1 | 7.4 | 5.5 | 4.9 | 5.4 | 5.7 |
| pHKCl | 4.7 | 7.6 | 6.9 | 4.2 | 5.1 | 4.8 |
| pHPZC | 6.6 | 6.2 | 4.3 | 7.1 | 7.4 | 5.8 |
| EC | 21 | 204 | 818 | 19.7 | 4.2 | 5.9 |
| H% | 9.3 | 6.2 | 5.4 | 10.7 | 11.5 | 7.0 |
| DM | 90.7 | 93.8 | 94.6 | 89.3 | 88.5 | 93.0 |
| P | 86.6 | 48.0 | 41.6 | 62.8 | 65.7 | 57.5 |
| As | 2.45 | 3.34 | 4.36 | 1.22 | 1.42 | 1.91 |
| VM | 97.55 | 96.66 | 95.64 | 98.58 | 98.58 | 98.09 |
| BD | 1.28 | 0.96 | 0.81 | 1.42 | 1.53 | 1.1 |
| RD | 1.65 | 1.23 | 1.14 | 1.86 | 1.97 | 1.43 |
| SI | 2.56 | 1.43 | 1.08 | 2.82 | 2.96 | 1.84 |
| OM | 40.72 | 21.76 | 18.84 | 49.21 | 50.25 | 29.85 |
| OC% | 19.54 | 10.44 | 9.04 | 23.62 | 24.12 | 14.32 |
| Ale | 0.11 | 0.05 | 0.05 | 0.18 | 0.11 | 0.09 |
| Cae | 6.74 | 2.04 | 1.97 | 7.44 | 7.98 | 4.22 |
| Ke | 2.22 | 3.04 | 2.96 | 2.02 | 2.07 | 2.79 |
| Mge | 1.57 | 3.06 | 2.99 | 1.03 | 0.86 | 2.81 |
| Nae | 2.32 | 1.05 | 0.96 | 3.77 | 4.04 | 1.12 |
| eCEC | 13.97 | 9.26 | 8.55 | 14.45 | 15.08 | 11.04 |
| Paricle size (%) | ||||||
| 0.075–0.1 mm | 86.17 | 52.28 | 66.71 | 26.14 | 31.30 | 39.70 |
| 0.05–0.075 mm | 11.68 | 30.61 | 15.77 | 68.43 | 65.45 | 53.40 |
| 0.05–0.02 mm | 2.15 | 10.73 | 13.47 | 4.16 | 3.25 | 6.78 |
| <0.02 mm | -- | 6.38 | 4.05 | 1.27 | -- | 1.20 |
| Alfa Fiber | Cactus Fiber | Palm Fiber | Acacia Bark | Eucalyptus Bark | Zean-Oak Bark | ||
|---|---|---|---|---|---|---|---|
| Freundlich model | KF | 227.7 | 116.1 | 57.4 | 331.1 | 470.4 | 195.5 |
| Error | 40.1 | 9.8 | 19.1 | 93.1 | 101.2 | 6.4 | |
| n | 1.415 | 0.212 | 0.236 | 2.484 | 2.685 | 0.458 | |
| Error | 0.05 | 0.03 | 0.01 | 0.11 | 0.28 | 0.09 | |
| R2 | 0.762 | 0.826 | 0.817 | 0.723 | 0.741 | 0.842 | |
| Langmuir model | KL | 0.14 | 0.06 | 0.04 | 0.27 | 0.31 | 0.08 |
| Error | 0.01 | 0.00 | 0.01 | 0.02 | 0.03 | 0.00 | |
| qm | 957.9 | 835.8 | 809.9 | 987.2 | 1046.1 | 527.9 | |
| Error | 97.4 | 80.7 | 60.6 | 107.5 | 140.3 | 37.48 | |
| R2 | 0.997 | 0.962 | 0.995 | 0.972 | 0.989 | 0.934 | |
| Linear model | Kd | 28.85 | 5.04 | 2.29 | 114.9 | 167.8 | 6.46 |
| Error | 5.52 | 0.49 | 0.28 | 30.4 | 34.9 | 0.3 | |
| R2 | 0.634 | 0.622 | 0.611 | 0.631 | 0.578 | 0.601 | |
| Sips model | Ks | 3.21 | 1.00 | 0.18 | 4.77 | 6.2 | 1.85 |
| Error | 0.13 | 0.01 | 0.00 | 0.93 | 1.1 | 0.05 | |
| n | 1.523 | 0.270 | 0.173 | 2.137 | 2.754 | 0.441 | |
| Error | 0.13 | 0.1 | 0.007 | 0.24 | 0.22 | 0.02 | |
| qm | 925.6 | 474.5 | 336.1 | 969.3 | 925.6 | 704.6 | |
| Error | 10.3 | 32.1 | 23.3 | 47.8 | 35.1 | 15.5 | |
| R2 | 0.944 | 0.873 | 0.869 | 0.925 | 0.932 | 0.884 | |
| Temkin model | Kt | 2.71 | 0.64 | - | 3.84 | 4.21 | - |
| Error | 0.004 | 0.00 | - | 0.34 | 0.001 | - | |
| bt | 4.772 | 0.344 | 0.231 | 5.022 | 6.765 | 2.522 | |
| Error | 0.8 | 0.05 | 0.09 | 0.00 | 1.33 | 1.53 | |
| R2 | 0.989 | 0.979 | 0.968 | 1.00 | 1.00 | 0.982 |
| MON Concentration Added (µmol L−1) | Hysteresis Index (HI) | |||||
|---|---|---|---|---|---|---|
| Alfa Fiber | Cactus Fiber | Palm Fiber | Acacia Bark | Eucalyptus Bark | Zean Oak Bark | |
| 5 | 0.852 | 0.977 | 0.982 | 0.904 | 0.869 | 1 |
| 10 | 0.907 | 0.956 | 0.961 | 0.914 | 0.895 | 1 |
| 20 | 0.935 | 0.855 | 0.918 | 0.921 | 0.903 | 0.996 |
| 40 | 0.973 | 0.390 | 0.748 | 0.932 | 0.914 | 0.943 |
| 80 | 0.979 | −0.221 | −0.248 | 0.938 | 0.919 | 0.914 |
| 100 | 0.985 | −1.546 | −1.281 | 0.957 | 0.936 | 0.824 |
| Average values | 0.938 | 0.235 | 0.429 | 0.928 | 0.906 | 0.946 |
| Alfa Fiber | Cactus Fiber | Palm Fiber | Acacia Bark | Eucalyptus Bark | Zean Oak Bark | ||
|---|---|---|---|---|---|---|---|
| Freundlich model | KF | 0.627 | - | 17.638 | 2.685 | - | - |
| Error | 0.263 | - | 2.143 | 0.519 | - | - | |
| n | 0.144 | - | 0.045 | 2.732 | 3.112 | 0.769 | |
| Error | 0.021 | - | 0.015 | 0.331 | 0.301 | 0.180 | |
| R2 | 0.737 | - | 0.892 | 0.719 | 0.741 | 0.868 | |
| Langmuir model | KL | 0.204 | 0.009 | 0.049 | 0.06 | 0.121 | 0.070 |
| Error | 0.001 | 0.00 | 0.022 | 0.03 | 0.042 | 0.001 | |
| qm | 281.36 | 443.21 | 324.65 | 270.58 | 254.36 | 178.12 | |
| Error | 32.02 | 97.46 | 65.13 | 104.70 | 123.23 | 54.82 | |
| R2 | 0.653 | 0.573 | 0.465 | 0.705 | 0.713 | 0.745 | |
| Linear model | Kd | 0.641 | 6.210 | 0.955 | 0.423 | 0.418 | 0.162 |
| Error | 0.182 | 0.521 | 0.026 | 0.072 | 0.068 | 0.029 | |
| R2 | 0.625 | 0.752 | 0.832 | 0.721 | 0.789 | 0.727 | |
| Sips model | Ks | 0.875 | - | - | - | - | 0.052 |
| Error | 0.00 | - | - | - | - | 0.0021 | |
| n | 0.872 | 0.445 | 1.972 | 0.582 | 0.673 | 0.341 | |
| Error | 0.00 | 0.012 | 0.052 | 0.00 | 0.00 | 0.001 | |
| qm | 59.60 | 64.211 | 175.73 | 25.195 | 28.012 | 6.055 | |
| Error | 11.00 | 15.022 | 23.06 | 19.540 | 10.332 | 2.013 | |
| R2 | 0.918 | 0.950 | 0.934 | 0.932 | 0.987 | 0.995 | |
| Temkin model | Kt | 0.381 | 1.292 | 2.887 | 1.022 | 1.307 | 0.077 |
| Error | 0.049 | 0.011 | 0.153 | 0.142 | 0.062 | 0.010 | |
| bt | 0.405 | 0.532 | 0.112 | 0.311 | 0.285 | 0.028 | |
| Error | 0.155 | 0.213 | 0.003 | 0.101 | 0.031 | 0.00 | |
| R2 | 0.998 | 0.989 | 0.984 | 1.00 | 1.00 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdi, S.; Issaoui, M.; Hammami, S.; Míguez-González, A.; Cela-Dablanca, R.; Barreiro, A.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Removal of the Highly Toxic Anticoccidial Monensin Using Six Different Low-Cost Bio-Adsorbents. Toxics 2024, 12, 606. https://doi.org/10.3390/toxics12080606
Hamdi S, Issaoui M, Hammami S, Míguez-González A, Cela-Dablanca R, Barreiro A, Núñez-Delgado A, Álvarez-Rodríguez E, Fernández-Sanjurjo MJ. Removal of the Highly Toxic Anticoccidial Monensin Using Six Different Low-Cost Bio-Adsorbents. Toxics. 2024; 12(8):606. https://doi.org/10.3390/toxics12080606
Chicago/Turabian StyleHamdi, Samiha, Manel Issaoui, Sonia Hammami, Ainoa Míguez-González, Raquel Cela-Dablanca, Ana Barreiro, Avelino Núñez-Delgado, Esperanza Álvarez-Rodríguez, and María J. Fernández-Sanjurjo. 2024. "Removal of the Highly Toxic Anticoccidial Monensin Using Six Different Low-Cost Bio-Adsorbents" Toxics 12, no. 8: 606. https://doi.org/10.3390/toxics12080606
APA StyleHamdi, S., Issaoui, M., Hammami, S., Míguez-González, A., Cela-Dablanca, R., Barreiro, A., Núñez-Delgado, A., Álvarez-Rodríguez, E., & Fernández-Sanjurjo, M. J. (2024). Removal of the Highly Toxic Anticoccidial Monensin Using Six Different Low-Cost Bio-Adsorbents. Toxics, 12(8), 606. https://doi.org/10.3390/toxics12080606













