Perfluoroalkyl and Polyfluoroalkyl Substances in Relation to the Participant-Reported Total Pregnancy and Live Birth Numbers among Reproductive-Aged Women in the United States
Abstract
:1. Introduction
2. Materials and Methods
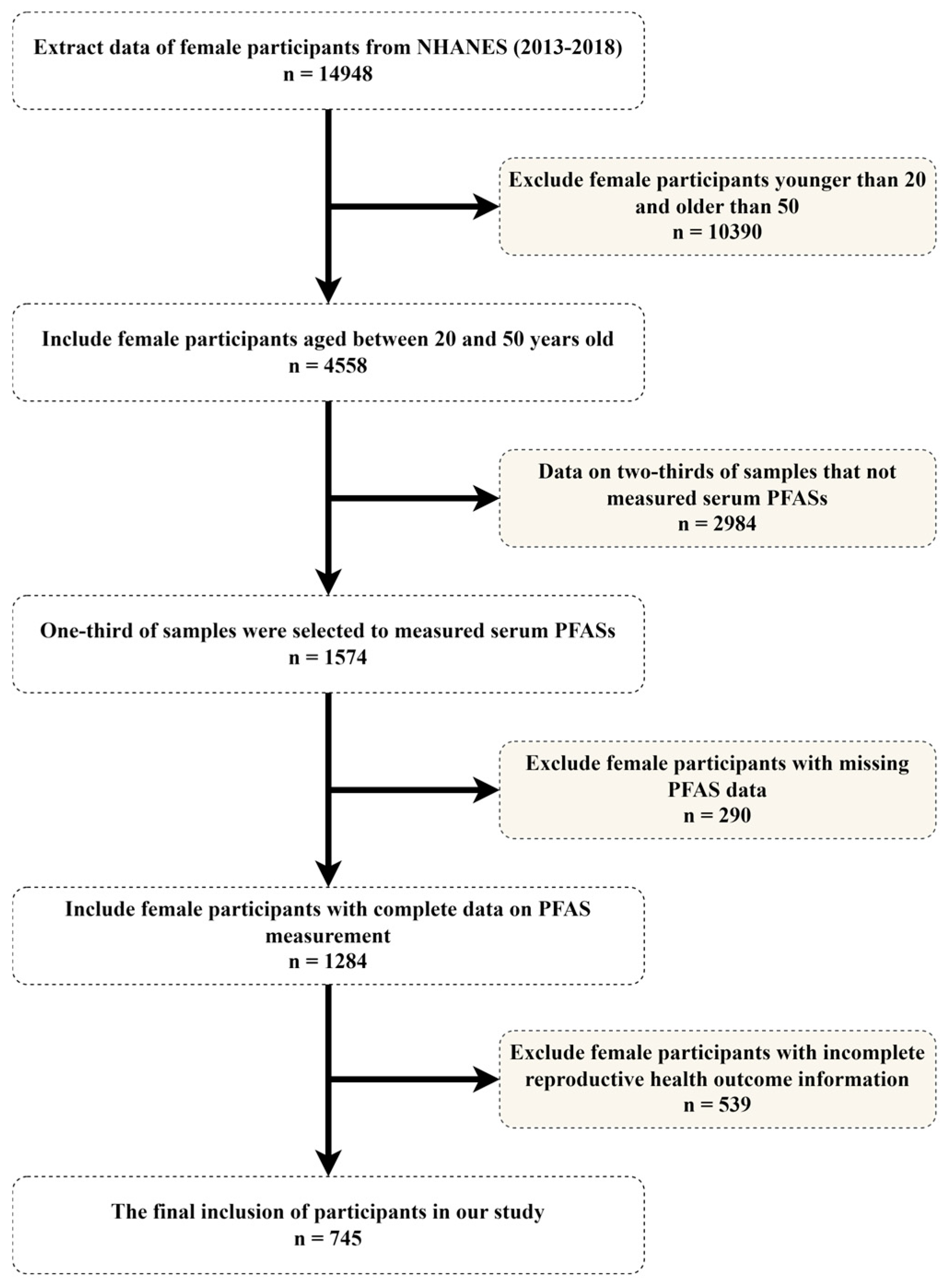
2.1. Research Participants
2.2. PFAS Determinations
2.3. Reproductive Data
2.4. Other Variables
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Participants
3.2. Distribution and Correlations of PFAS Concentrations
3.3. Association between Individual PFASs and the Total Pregnancy and Live Birth Numbers
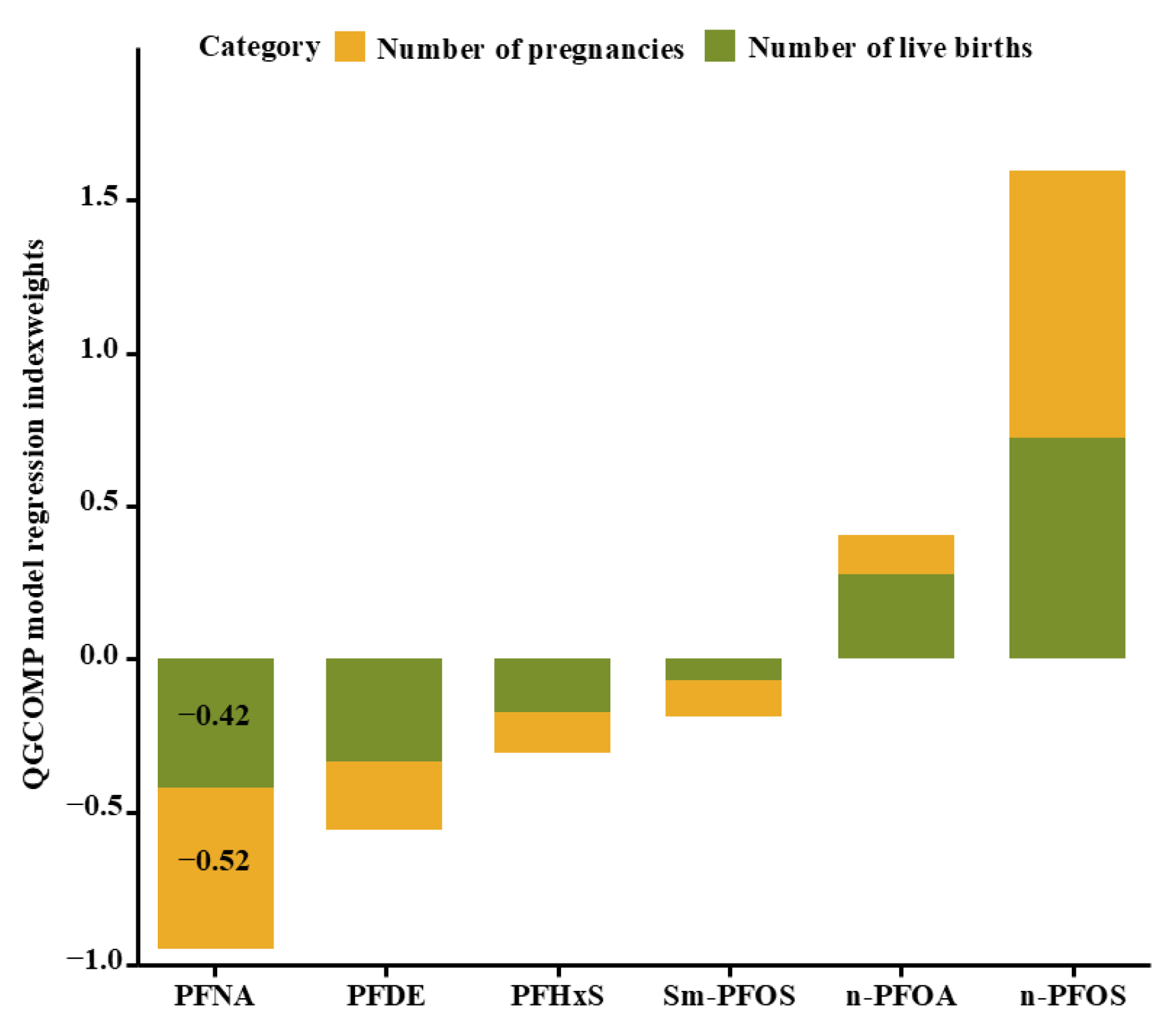
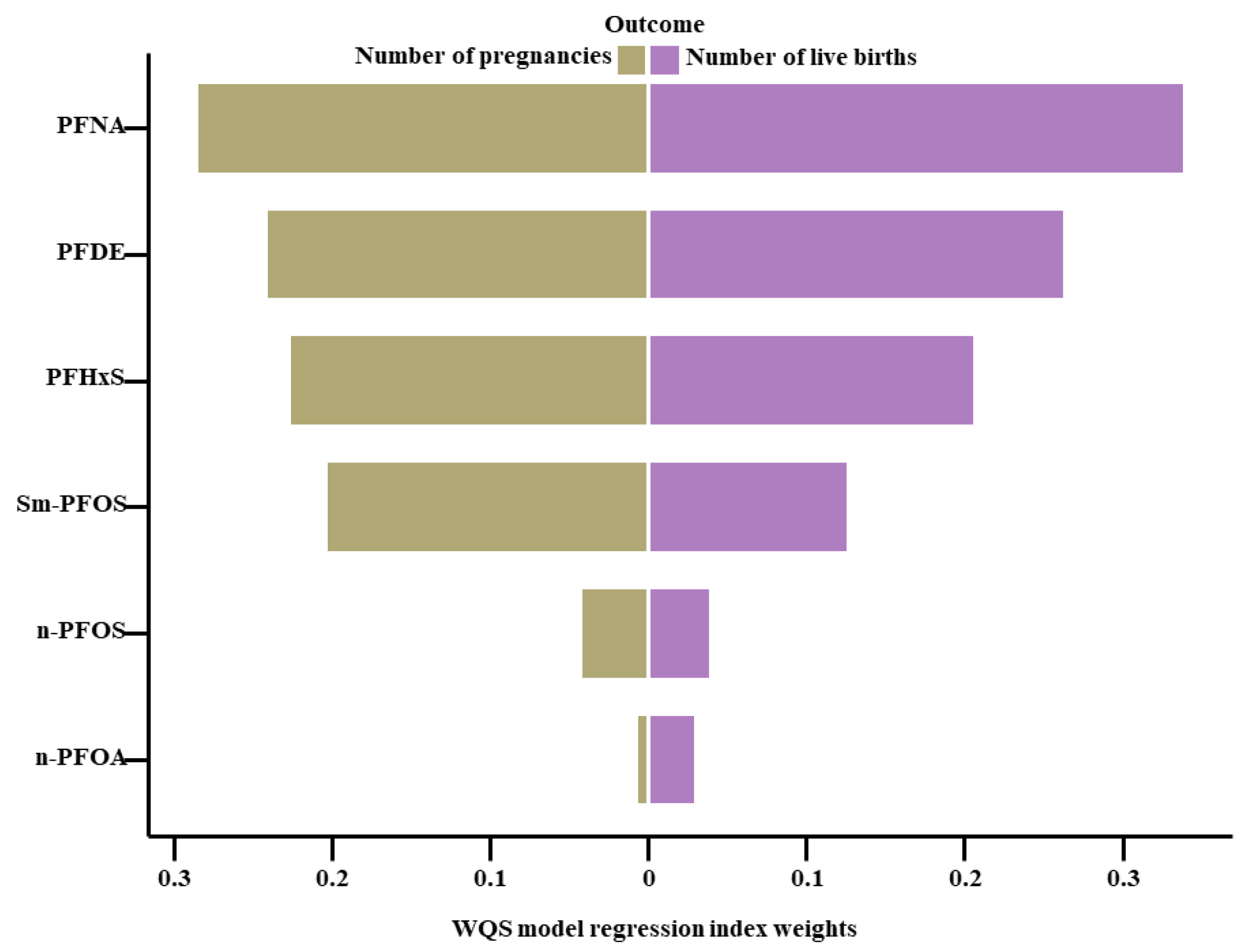
3.4. Relationship between Exposure to PFAS Mixtures and the Total Pregnancy and Live Birth Numbers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility prevalence and the methods of estimation from 1990 to 2021: A systematic review and meta-analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef] [PubMed]
- Eskew, A.M.; Jungheim, E.S. A History of Developments to Improve in vitro Fertilization. Mo. Med. 2017, 114, 156–159. [Google Scholar]
- Maconochie, N.; Doyle, P.; Prior, S. The National Women’s Health Study: Assembly and description of a population-based reproductive cohort. BMC Public Health 2004, 4, 35. [Google Scholar] [CrossRef]
- Bagade, T.; Mersha, A.G.; Majeed, T. The social determinants of mental health disorders among women with infertility: A systematic review. BMC Women’s Health 2023, 23, 668. [Google Scholar] [CrossRef]
- Mendes, D.C.G.; Fonseca, A.; Cameirão, M.S. The psychological impact of Early Pregnancy Loss in Portugal: Incidence and the effect on psychological morbidity. Front. Public Health 2023, 11, 1188060. [Google Scholar] [CrossRef]
- Voit, F.A.C.; Kajantie, E.; Lemola, S.; Räikkönen, K.; Wolke, D.; Schnitzlein, D.D. Maternal mental health and adverse birth outcomes. PLoS ONE 2022, 17, e0272210. [Google Scholar] [CrossRef] [PubMed]
- Riestenberg, C.; Jagasia, A.; Markovic, D.; Buyalos, R.P.; Azziz, R. Health Care-Related Economic Burden of Polycystic Ovary Syndrome in the United States: Pregnancy-Related and Long-Term Health Consequences. J. Clin. Endocrinol. Metab. 2022, 107, 575–585. [Google Scholar] [CrossRef]
- Braverman, A.M.; Davoudian, T.; Levin, I.K.; Bocage, A.; Wodoslawsky, S. Depression, anxiety, quality of life, and infertility: A global lens on the last decade of research. Fertil. Steril. 2024, 121, 379–383. [Google Scholar] [CrossRef]
- Omidvar-Mehrabadi, A.; Ebrahimi, F.; Shahbazi, M.; Mohammadnia-Afrouzi, M. Cytokine and chemokine profiles in women with endometriosis, polycystic ovary syndrome, and unexplained infertility. Cytokine 2024, 178, 156588. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zheng, C.; Fang, Q.; Xu, W.; Yin, Z. A case of congenital rubella syndrome and epidemiology of related cases in China, 2014–2023. Hum. Vaccines Immunother. 2024, 20, 2334917. [Google Scholar] [CrossRef]
- Gunderson, E.P.; Greenberg, M.; Nguyen-Huynh, M.N.; Tierney, C.; Roberts, J.M.; Go, A.S.; Tao, W.; Alexeeff, S.E. Early Pregnancy Blood Pressure Patterns Identify Risk of Hypertensive Disorders of Pregnancy Among Racial and Ethnic Groups. Hypertension 2022, 79, 599–613. [Google Scholar] [CrossRef]
- Garovic, V.D.; Dechend, R.; Easterling, T.; Karumanchi, S.A.; McMurtry Baird, S.; Magee, L.A.; Rana, S.; Vermunt, J.V.; August, P. Hypertension in Pregnancy: Diagnosis, Blood Pressure Goals, and Pharmacotherapy: A Scientific Statement From the American Heart Association. Hypertension 2022, 79, e21–e41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Tan, Z.; Li, C.; Han, Z.; Zhou, J.; Yin, Y. The correlation between serum total bile acid and adverse perinatal outcomes in pregnant women with intrahepatic cholestasis of pregnancy (ICP) and non-ICP hypercholanemia of pregnancy. Ann. Med. 2024, 56, 2331059. [Google Scholar] [CrossRef] [PubMed]
- Deji, Z.; Liu, P.; Wang, X.; Zhang, X.; Luo, Y.; Huang, Z. Association between maternal exposure to perfluoroalkyl and polyfluoroalkyl substances and risks of adverse pregnancy outcomes: A systematic review and meta-analysis. Sci. Total Environ. 2021, 783, 146984. [Google Scholar] [CrossRef]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, R.M.; Doherty, B.T.; Gallagher, L.G.; Zoeller, R.T.; Hoofnagle, A.N.; Calafat, A.M.; Karagas, M.R.; Yolton, K.; Chen, A.; Lanphear, B.P.; et al. Maternal serum perfluoroalkyl substance mixtures and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Environ. Res. 2020, 185, 109395. [Google Scholar] [CrossRef] [PubMed]
- Sulfonate, P. Technical Fact Sheet–Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) (US EPA, 2017) 2017. Available online: https://19january2021snapshot.epa.gov/sites/static/files/2017-12/documents/ffrrofactsheet_contaminants_pfos_pfoa_11-20-17_508_0.pdf (accessed on 16 August 2024).
- UNEP. Listing of perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride in Annex B of the Stockholm Convention (UNEP/POPS/COP. 4/SC-4/17). In Proceedings of the Fourth Meeting of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, Switzerland, 4–8 May 2009. [Google Scholar]
- UNEP. Listing of perfluorooctanoic acid (PFOA), its salts and PFOA-related compounds (UNEP/POPS/COP.9/SC-9/12). In Proceedings of the Conferences of the Parties to the Basel, Rotterdam and Stockholm conventions (BC COP-14, RC COP-9, SC COP-9), Geneva, Switzerland, 29 April–10 May 2019. [Google Scholar]
- Liu, Z.; Lu, Y.; Wang, P.; Wang, T.; Liu, S.; Johnson, A.C.; Sweetman, A.J.; Baninla, Y. Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in central and eastern China. Sci. Total Environ. 2017, 580, 1247–1256. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Thai, P.K.; Kaserzon, S.L.; O’Brien, J.W.; Mueller, J.F. Nationwide occurrence and discharge mass load of per- and polyfluoroalkyl substances in effluent and biosolids: A snapshot from 75 wastewater treatment plants across Australia. J. Hazard. Mater. 2024, 470, 134203. [Google Scholar] [CrossRef]
- Liu, Y.; Li, A.; Buchanan, S.; Liu, W. Exposure characteristics for congeners, isomers, and enantiomers of perfluoroalkyl substances in mothers and infants. Environ. Int. 2020, 144, 106012. [Google Scholar] [CrossRef]
- ATSDR. PFAS in the U.S. Population. Available online: https://www.atsdr.cdc.gov/pfas/health-effects/us-population.html#print (accessed on 9 August 2023).
- Rodríguez-Jorquera, I.A.; Colli-Dula, R.C.; Kroll, K.; Jayasinghe, B.S.; Parachu Marco, M.V.; Silva-Sanchez, C.; Toor, G.S.; Denslow, N.D. Blood Transcriptomics Analysis of Fish Exposed to Perfluoro Alkyls Substances: Assessment of a Non-Lethal Sampling Technique for Advancing Aquatic Toxicology Research. Environ. Sci. Technol. 2019, 53, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Salazar, Z.; Arenas, E.; Betancourt, M.; Ducolomb, Y.; González-Márquez, H.; Casas, E.; Teteltitla, M.; Bonilla, E. Effect of perfluorooctane sulfonate on viability, maturation and gap junctional intercellular communication of porcine oocytes in vitro. Toxicol. Vitr. 2016, 35, 93–99. [Google Scholar] [CrossRef] [PubMed]
- López-Arellano, P.; López-Arellano, K.; Luna, J.; Flores, D.; Jiménez-Salazar, J.; Gavia, G.; Teteltitla, M.; Rodríguez, J.J.; Domínguez, A.; Casas, E.; et al. Perfluorooctanoic acid disrupts gap junction intercellular communication and induces reactive oxygen species formation and apoptosis in mouse ovaries. Environ. Toxicol. 2019, 34, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Grønnestad, R.; Johanson, S.M.; Müller, M.H.B.; Schlenk, D.; Tanabe, P.; Krøkje, Å.; Jaspers, V.L.B.; Jenssen, B.M.; Ræder, E.M.; Lyche, J.L.; et al. Effects of an environmentally relevant PFAS mixture on dopamine and steroid hormone levels in exposed mice. Toxicol. Appl. Pharmacol. 2021, 428, 115670. [Google Scholar] [CrossRef]
- Blake, B.E.; Fenton, S.E. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, J.T.; Avula, V.; Fry, R.C. Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: A Potential Mechanistic Role for Placental Peroxisome Proliferator-Activated Receptors (PPARs). Curr. Environ. Health Rep. 2020, 7, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Liu, C.; Shen, G.; Yang, I.V.; Kechris, K.; Borengasser, S.J.; Boyle, K.E.; Zhang, W.; Smith, H.A.; Calafat, A.M.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Umbilical Cord Blood DNA Methylation, and Cardio-Metabolic Indicators in Newborns: The Healthy Start Study. Environ. Health Perspect. 2020, 128, 127014. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhang, B.; Gao, Z.; Zhang, Q.; Deng, H.; Han, L.; Shen, X.L. Perfluorooctanoic acid induces hepatocellular endoplasmic reticulum stress and mitochondrial-mediated apoptosis in vitro via endoplasmic reticulum-mitochondria communication. Chem. Biol. Interact. 2022, 354, 109844. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.P.; Arbuckle, T.E.; Fraser, W.D. Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Hum. Reprod. 2015, 30, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, R.; Jin, F.; Lou, H.; Mao, Y.; Zhu, W.; Zhou, W.; Zhang, P.; Zhang, J. Perfluoroalkyl substances and endometriosis-related infertility in Chinese women. Environ. Int. 2017, 102, 207–212. [Google Scholar] [CrossRef]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W.; Wu, S.; Liang, F.; Li, Y.; Zhang, J.; Cui, L.; Feng, Y.; Wang, Y. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ. Pollut. 2019, 247, 824–831. [Google Scholar] [CrossRef]
- Liu, B.; Lu, X.; Jiang, A.; Lv, Y.; Zhang, H.; Xu, B. Influence of maternal endocrine disrupting chemicals exposure on adverse pregnancy outcomes: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2024, 270, 115851. [Google Scholar] [CrossRef]
- Maisonet, M.; Terrell, M.L.; McGeehin, M.A.; Christensen, K.Y.; Holmes, A.; Calafat, A.M.; Marcus, M. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ. Health Perspect. 2012, 120, 1432–1437. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, Y.; Zheng, T.; Cui, Q.; Buka, S.L.; Zhang, B.; Guo, Y.; Xia, W.; Yeung, L.W.; Li, Y.; et al. Novel Chlorinated Polyfluorinated Ether Sulfonates and Legacy Per-/Polyfluoroalkyl Substances: Placental Transfer and Relationship with Serum Albumin and Glomerular Filtration Rate. Environ. Sci. Technol. 2017, 51, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, H.; Lin, Y.; Qin, X.; Zhang, Y.; Geng, X.; Kannan, K. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ. Sci. Technol. 2013, 47, 7974–7981. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, X.; Deng, L.; Xie, J.; Huang, G.; Zeng, C.; Wu, N.; Zhu, S.; Liu, C.; Mei, H.; et al. Exposure to per- and polyfluoroalkyl substances in women with twin pregnancies: Patterns and variability, transplacental transfer, and predictors. J. Hazard. Mater. 2023, 460, 132432. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Yang, J.; Wang, Y.; Du, H.; Han, M.; Xu, L.; Liu, S.; Yi, J.; Chen, Y.; et al. Gestational exposure to perfluoroalkyl substances is associated with placental DNA methylation and birth size. Sci. Total Environ. 2023, 858, 159747. [Google Scholar] [CrossRef]
- Lan, L.; Wei, H.; Chen, D.; Pang, L.; Xu, Y.; Tang, Q.; Li, J.; Xu, Q.; Li, H.; Lu, C.; et al. Associations between maternal exposure to perfluoroalkylated substances (PFASs) and infant birth weight: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2023, 30, 89805–89822. [Google Scholar] [CrossRef]
- Tan, Y.; Zeng, Z.; Liang, H.; Weng, X.; Yao, H.; Fu, Y.; Li, Y.; Chen, J.; Wei, X.; Jing, C. Association between Perfluoroalkyl and Polyfluoroalkyl Substances and Women’s Infertility, NHANES 2013–2016. Int. J. Environ. Res. Public Health 2022, 19, 15348. [Google Scholar] [CrossRef]
- Wang, B.; Fu, J.; Gao, K.; Liu, Q.; Zhuang, L.; Zhang, G.; Long, M.; Na, J.; Ren, M.; Wang, A.; et al. Early pregnancy loss: Do Per- and polyfluoroalkyl substances matter? Environ. Int. 2021, 157, 106837. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef] [PubMed]
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef] [PubMed]
- Dunder, L.; Lind, P.M.; Salihovic, S.; Stubleski, J.; Kärrman, A.; Lind, L. Changes in plasma levels of per- and polyfluoroalkyl substances (PFAS) are associated with changes in plasma lipids—A longitudinal study over 10 years. Environ. Res. 2022, 211, 112903. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, N.K.; Ojewole, A.E.; Ojewole, C.O.; Lucky, O.P.; Kusi, J. Trends in Serum Per- and Polyfluoroalkyl Substance (PFAS) Concentrations in Teenagers and Adults, 1999-2018 NHANES. Int. J. Environ. Res. Public Health 2023, 20, 6984. [Google Scholar] [CrossRef]
- Gan, H.; Xing, Y.; Tong, J.; Lu, M.; Yan, S.; Huang, K.; Wu, X.; Tao, S.; Gao, H.; Pan, Y.; et al. Impact of Gestational Exposure to Individual and Combined Per- and Polyfluoroalkyl Substances on a Placental Structure and Efficiency: Findings from the Ma’anshan Birth Cohort. Environ. Sci. Technol. 2024, 58, 6117–6127. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Mustieles, V.; Martin, L.; Sun, Y.; Bibi, Z.; Torres, N.; Coburn-Sanderson, A.; First, O.; Souter, I.; et al. Predictors of Serum Per- and Polyfluoroalkyl Substances Concentrations among U.S. Couples Attending a Fertility Clinic. Environ. Sci. Technol. 2024, 58, 5685–5694. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Meng, L.; Ma, D.; Cao, H.; Liang, Y.; Liu, H.; Wang, Y.; Jiang, G. The occurrence of PFAS in human placenta and their binding abilities to human serum albumin and organic anion transporter 4. Environ. Pollut. 2021, 273, 116460. [Google Scholar] [CrossRef]
- Cohen, N.J.; Yao, M.; Midya, V.; India-Aldana, S.; Mouzica, T.; Andra, S.S.; Narasimhan, S.; Meher, A.K.; Arora, M.; Chan, J.K.Y.; et al. Exposure to perfluoroalkyl substances and women’s fertility outcomes in a Singaporean population-based preconception cohort. Sci. Total Environ. 2023, 873, 162267. [Google Scholar] [CrossRef]
- Lum, K.J.; Sundaram, R.; Barr, D.B.; Louis, T.A.; Buck Louis, G.M. Perfluoroalkyl Chemicals, Menstrual Cycle Length, and Fecundity: Findings from a Prospective Pregnancy Study. Epidemiology 2017, 28, 90–98. [Google Scholar] [CrossRef]
- Wikström, S.; Hussein, G.; Lingroth Karlsson, A.; Lindh, C.H.; Bornehag, C.G. Exposure to perfluoroalkyl substances in early pregnancy and risk of sporadic first trimester miscarriage. Sci. Rep. 2021, 11, 3568. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Chen, L.; Qin, Y.; Xu, Y.; Tian, Y.; Chen, L. Exposure of female mice to perfluorooctanoic acid suppresses hypothalamic kisspeptin-reproductive endocrine system through enhanced hepatic fibroblast growth factor 21 synthesis, leading to ovulation failure and prolonged dioestrus. J. Neuroendocr. 2020, 32, e12848. [Google Scholar] [CrossRef]
- Yin, X.; Di, T.; Cao, X.; Liu, Z.; Xie, J.; Zhang, S. Chronic exposure to perfluorohexane sulfonate leads to a reproduction deficit by suppressing hypothalamic kisspeptin expression in mice. J. Ovarian Res. 2021, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, H.; Wang, J.; Zheng, F.; Dai, J. Perfluorooctanoic acid exposure induces endoplasmic reticulum stress in the liver and its effects are ameliorated by 4-phenylbutyrate. Free Radic. Biol. Med. 2015, 87, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, A.; Salazar, Z.; Betancourt, M.; Ducolomb, Y.; Casas, E.; Fernández, F.; Bahena, I.; Salomón, A.; Teteltitla, M.; Martínez, R.; et al. Effect of perfluorodecanoic acid on pig oocyte viability, intracellular calcium levels and gap junction intercellular communication during oocyte maturation in vitro. Toxicol. Vitr. 2019, 58, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. J. Soc. Toxicol. 2006, 90, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. J. Soc. Toxicol. 2007, 99, 366–394. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.S.; Li, W.J.; Liu, C.; Wang, Y.; Sun, K. Reduction of progesterone, estradiol and hCG secretion by perfluorooctane sulfonate via induction of apoptosis in human placental syncytiotrophoblasts. Placenta 2015, 36, 575–580. [Google Scholar] [CrossRef]
- Suh, C.H.; Cho, N.K.; Lee, C.K.; Lee, C.H.; Kim, D.H.; Kim, J.H.; Son, B.C.; Lee, J.T. Perfluorooctanoic acid-induced inhibition of placental prolactin-family hormone and fetal growth retardation in mice. Mol. Cell. Endocrinol. 2011, 337, 7–15. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | |
|---|---|
| Age (years), mean ± SD | 37.1 ± 8.28 |
| BMI (kg/m2), mean ± SD | 30.5 ± 8.08 |
| Menarche age (years), mean ± SD | 12.5 ± 1.79 |
| Race/Ethnic origin, n (%) | |
| Mexican American | 131 (17.6) |
| Other Hispanic | 66 (8.86) |
| Non-Hispanic White | 262 (35.2) |
| Non-Hispanic Black | 165 (22.1) |
| Others | 121 (16.2) |
| Education, n (%) | |
| Less than middle school | 45 (6.04) |
| Middle school | 108 (14.5) |
| High school graduate | 152 (20.4) |
| College degree | 274 (36.8) |
| College graduate or above | 166 (22.3) |
| Marital status, n (%) | |
| Married | 403 (54.1) |
| Widowed | 12 (1.61) |
| Divorced | 72 (9.66) |
| Separated | 52 (6.98) |
| Never married | 126 (16.9) |
| Living with partner | 80 (10.7) |
| Ratio of family income to poverty, n (%) | |
| <1 | 222 (29.8) |
| 1–2 | 200 (26.8) |
| >2 | 323 (43.4) |
| Ever taken birth control pills, n (%) | |
| Yes | 529 (71.0) |
| No | 216 (29.0) |
| Ever use female hormones, n (%) | |
| Yes | 33 (4.43) |
| No | 712 (95.6) |
| Total pregnancy numbers (n), median (IQR) | 3 (2, 4) |
| Total live birth numbers (n), median (IQR) | 2 (1, 3) |
| PFASs | LOD, μg/L | Cycle 2013–2014, n = 256 | Cycle 2015–2016, n = 271 | Cycle 2017–2018, n = 218 | Cycles 2013–2018, n = 745 |
|---|---|---|---|---|---|
| PFNA | 0.10 | ||||
| n > LOD | 252 (98.44%) | 264 (97.42%) | 184 (84.40%) | 700 (93.96%) | |
| Median (IOR) | 0.50 (0.30, 0.70) | 0.40 (0.20, 0.70) | 0.30 (0.15, 0.50) | 0.40 (0.20, 0.70) | |
| PFDE | 0.10 | ||||
| n > LOD | 179 (69.92%) | 142 (52.40%) | 176 (80.73%) | 497 (66.71%) | |
| Median (IOR) | 0.20 (0.07, 0.30) | 0.10 (0.07, 0.20) | 0.20 (0.10, 0.30) | 0.10 (0.07, 0.20) | |
| PFHxS | 0.10 | ||||
| n > LOD | 250 (97.66%) | 262 (96.68%) | 216 (99.08%) | 728 (97.72%) | |
| Median (IOR) | 0.60 (0.40, 1.00) | 0.50 (0.30, 1.00) | 0.50 (0.30, 0.80) | 0.50 (0.30, 0.90) | |
| n-PFOA | 0.10 | ||||
| n > LOD | 254 (99.73%) | 267 (98.52%) | 218 (100%) | 739 (99.19%) | |
| Median (IOR) | 1.20 (0.70, 1.80) | 0.80 (0.50, 1.40) | 0.70 (0.50, 1.20) | 0.90 (0.60, 1.40) | |
| n-PFOS | 0.10 | ||||
| n > LOD | 254 (99.73%) | 268 (98.89%) | 218 (100%) | 740 (99.33%) | |
| Median (IOR) | 2.30 (1.30, 3.60) | 1.80 (1.20, 2.92) | 1.60 (1.00, 2.60) | 1.90 (1.20, 3.00) | |
| Sm-PFOS | 0.10 | ||||
| n > LOD | 250 (97.66%) | 264 (97.42%) | 217 (99.54%) | 731 (98.12%) | |
| Median (IOR) | 0.60 (0.40, 1.10) | 0.60 (0.40, 1.10) | 0.60 (0.40, 0.90) | 0.60 (0.40, 1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Li, J.; Zhou, L.; Duan, T.; Deng, L.; Yang, P.; Gong, Y. Perfluoroalkyl and Polyfluoroalkyl Substances in Relation to the Participant-Reported Total Pregnancy and Live Birth Numbers among Reproductive-Aged Women in the United States. Toxics 2024, 12, 613. https://doi.org/10.3390/toxics12080613
Huang G, Li J, Zhou L, Duan T, Deng L, Yang P, Gong Y. Perfluoroalkyl and Polyfluoroalkyl Substances in Relation to the Participant-Reported Total Pregnancy and Live Birth Numbers among Reproductive-Aged Women in the United States. Toxics. 2024; 12(8):613. https://doi.org/10.3390/toxics12080613
Chicago/Turabian StyleHuang, Guangtong, Jiehao Li, Lixin Zhou, Tiantian Duan, Langjing Deng, Pan Yang, and Yajie Gong. 2024. "Perfluoroalkyl and Polyfluoroalkyl Substances in Relation to the Participant-Reported Total Pregnancy and Live Birth Numbers among Reproductive-Aged Women in the United States" Toxics 12, no. 8: 613. https://doi.org/10.3390/toxics12080613
APA StyleHuang, G., Li, J., Zhou, L., Duan, T., Deng, L., Yang, P., & Gong, Y. (2024). Perfluoroalkyl and Polyfluoroalkyl Substances in Relation to the Participant-Reported Total Pregnancy and Live Birth Numbers among Reproductive-Aged Women in the United States. Toxics, 12(8), 613. https://doi.org/10.3390/toxics12080613







