Rapid Response of Daphnia magna Motor Behavior to Mercury Chloride Toxicity Based on Target Tracking
Abstract
:1. Introduction
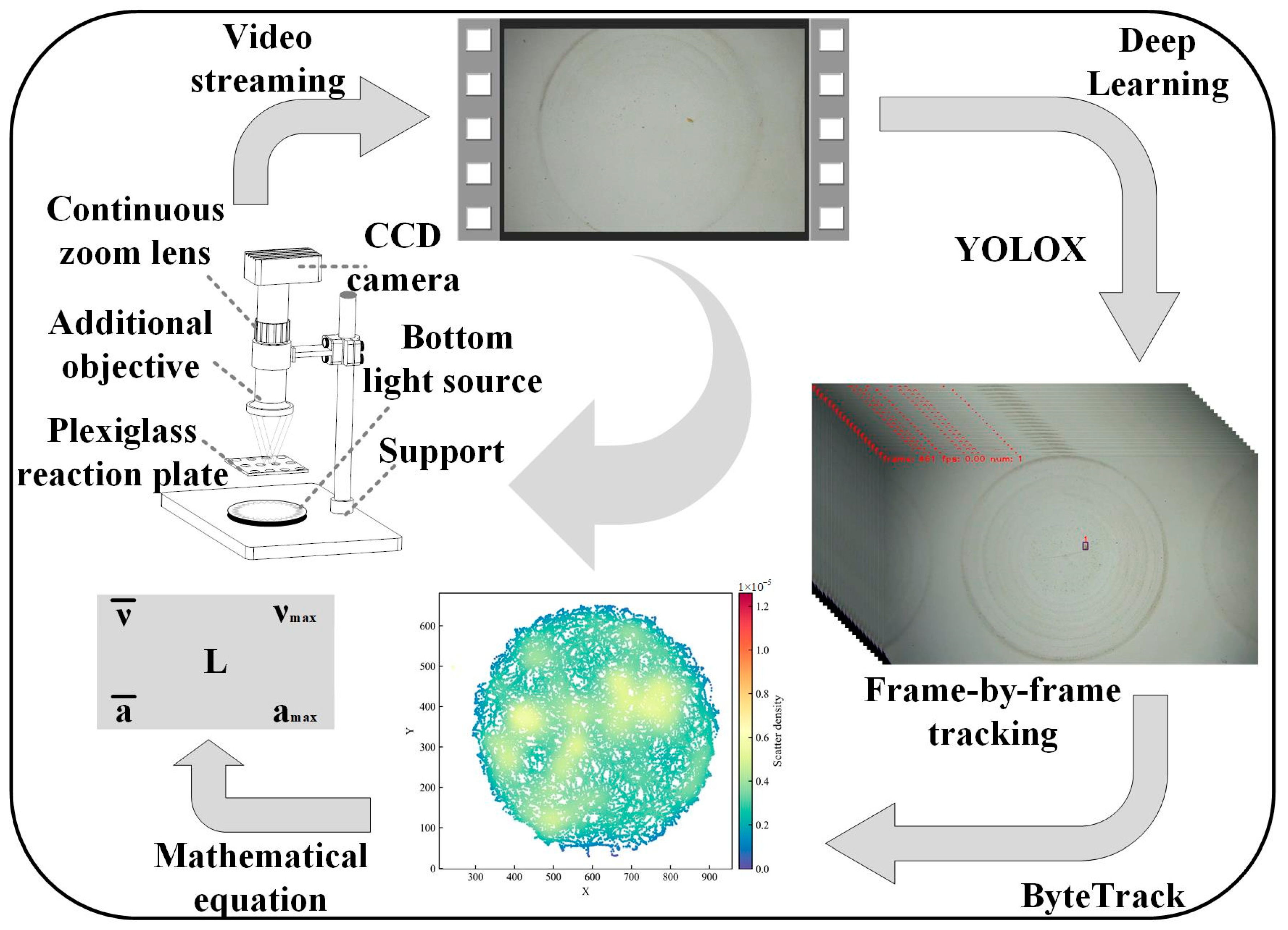
2. Materials and Methods
2.1. Materials and Study Species
2.2. Experimental Design
2.3. Data Processing
3. Results and Discussion
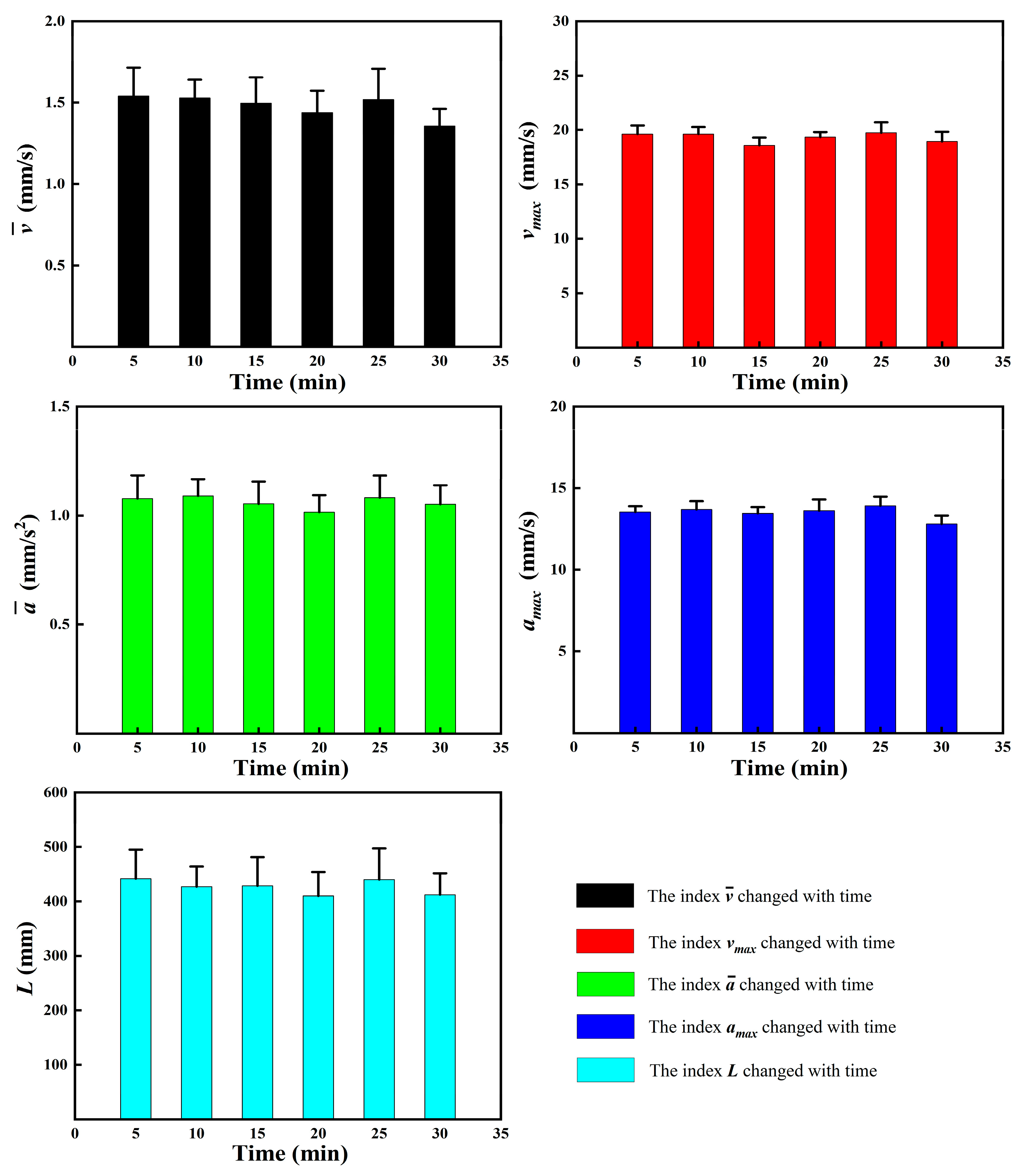
3.1. Observation Results under Controlled Conditions
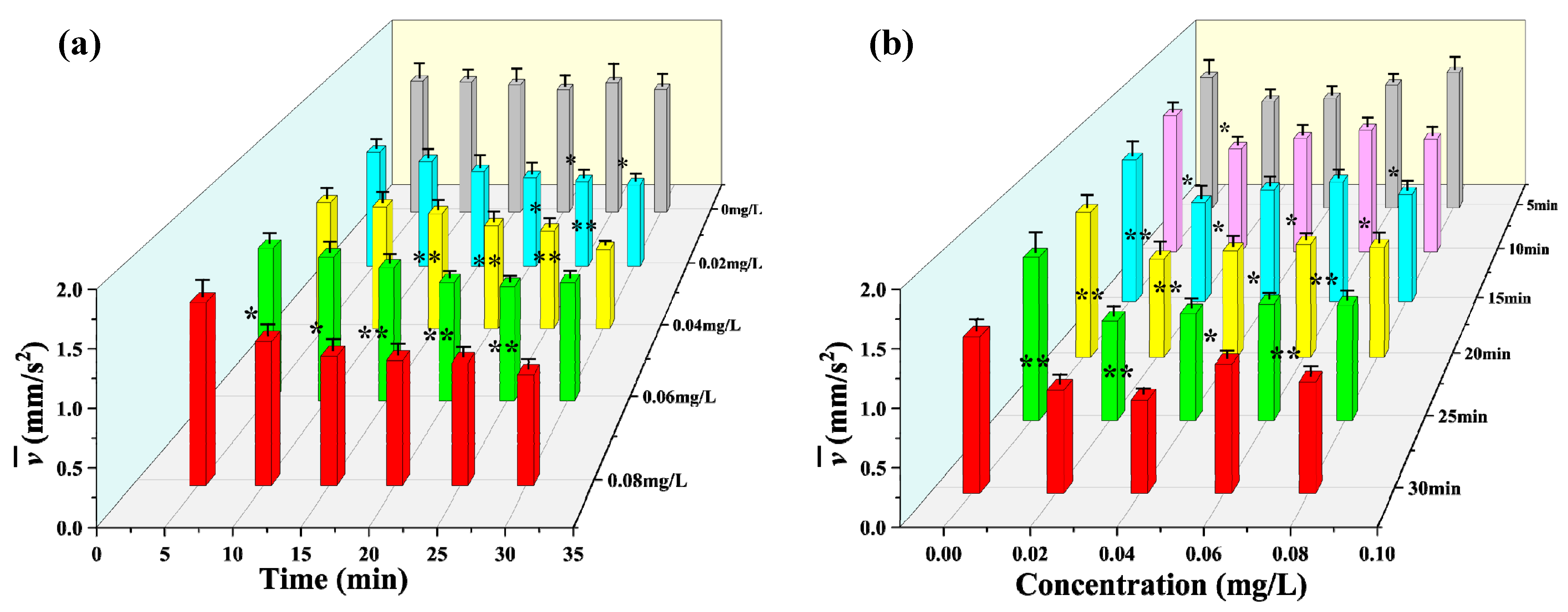
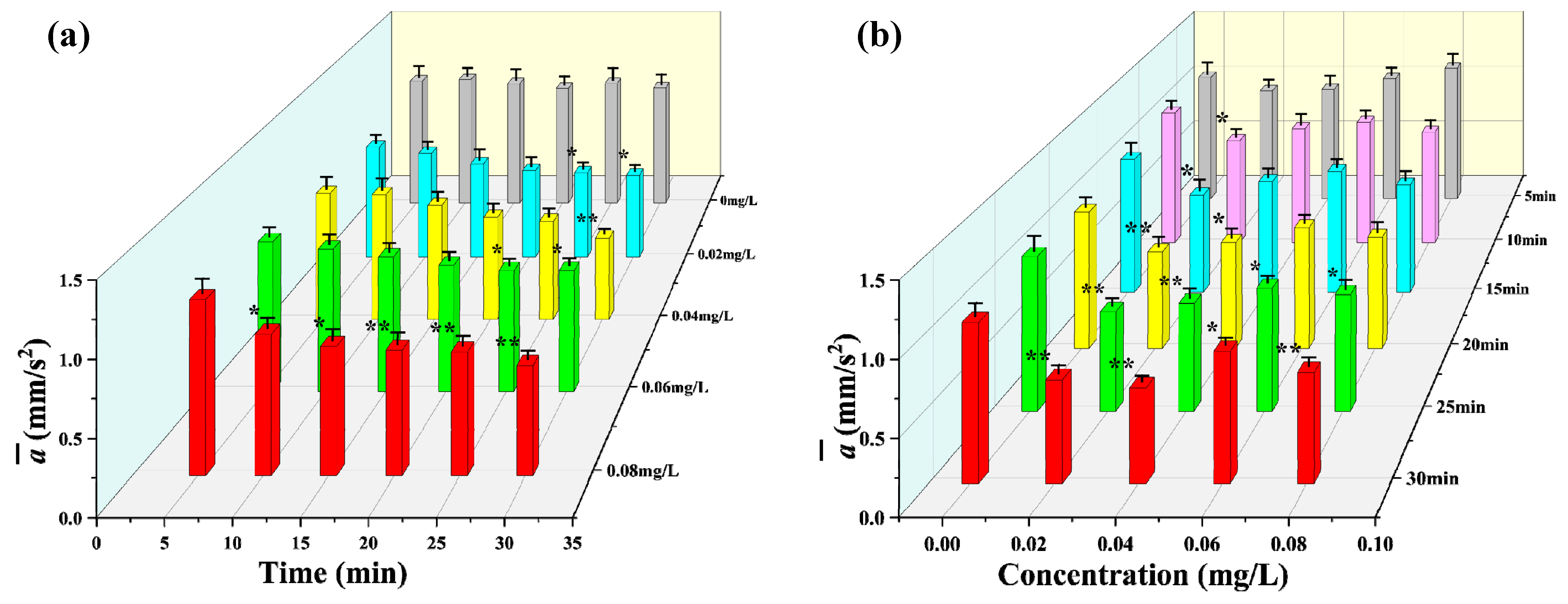
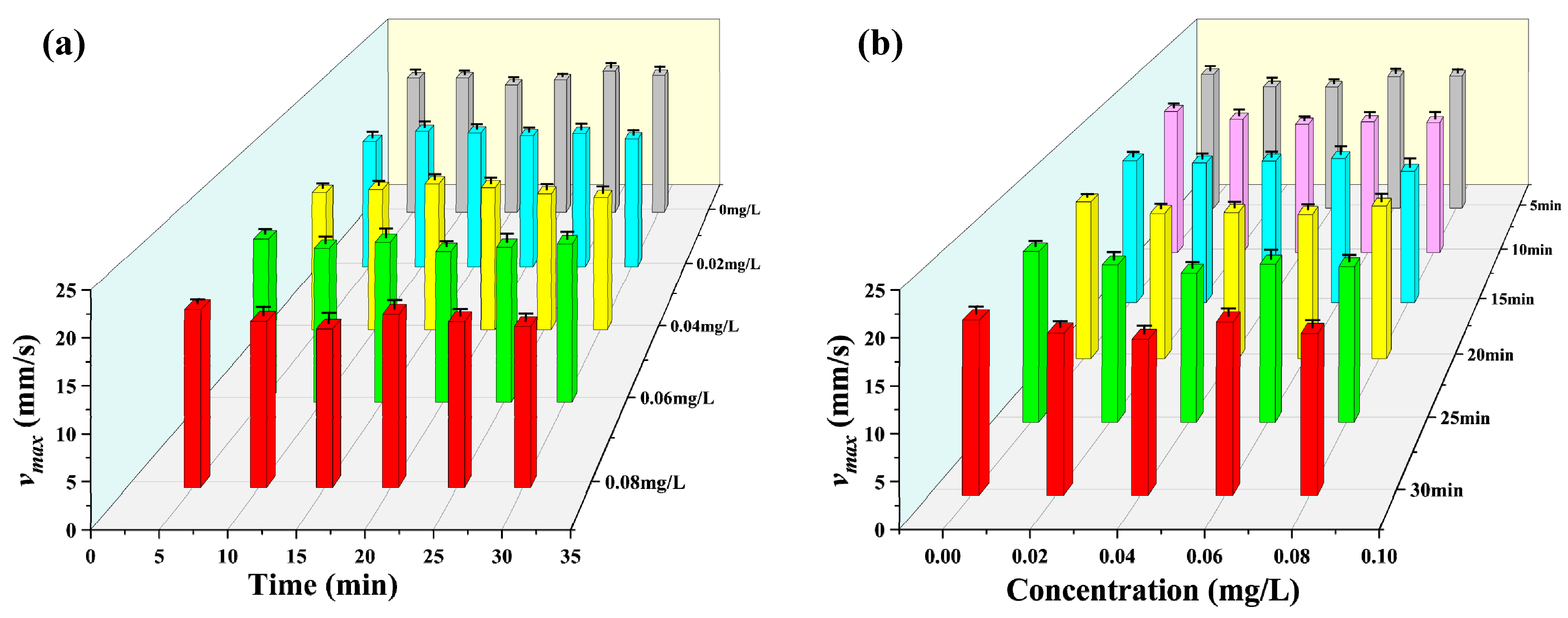
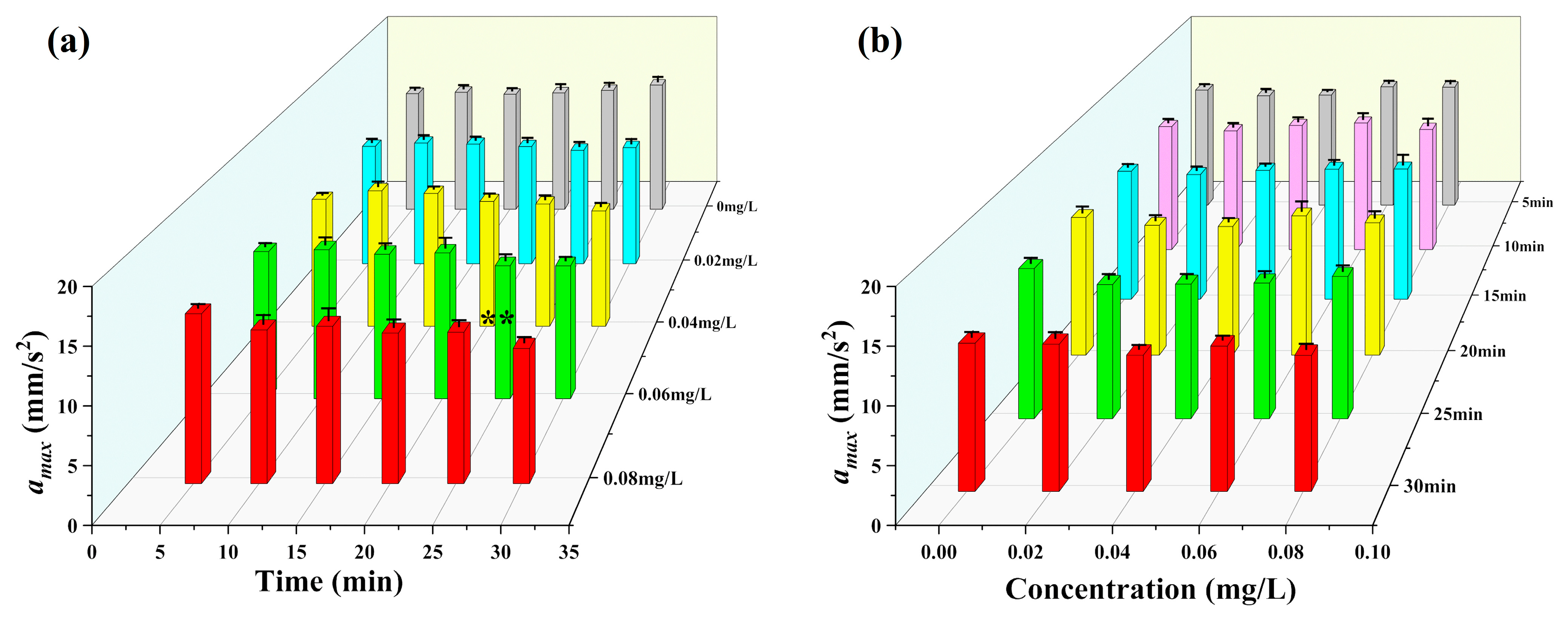
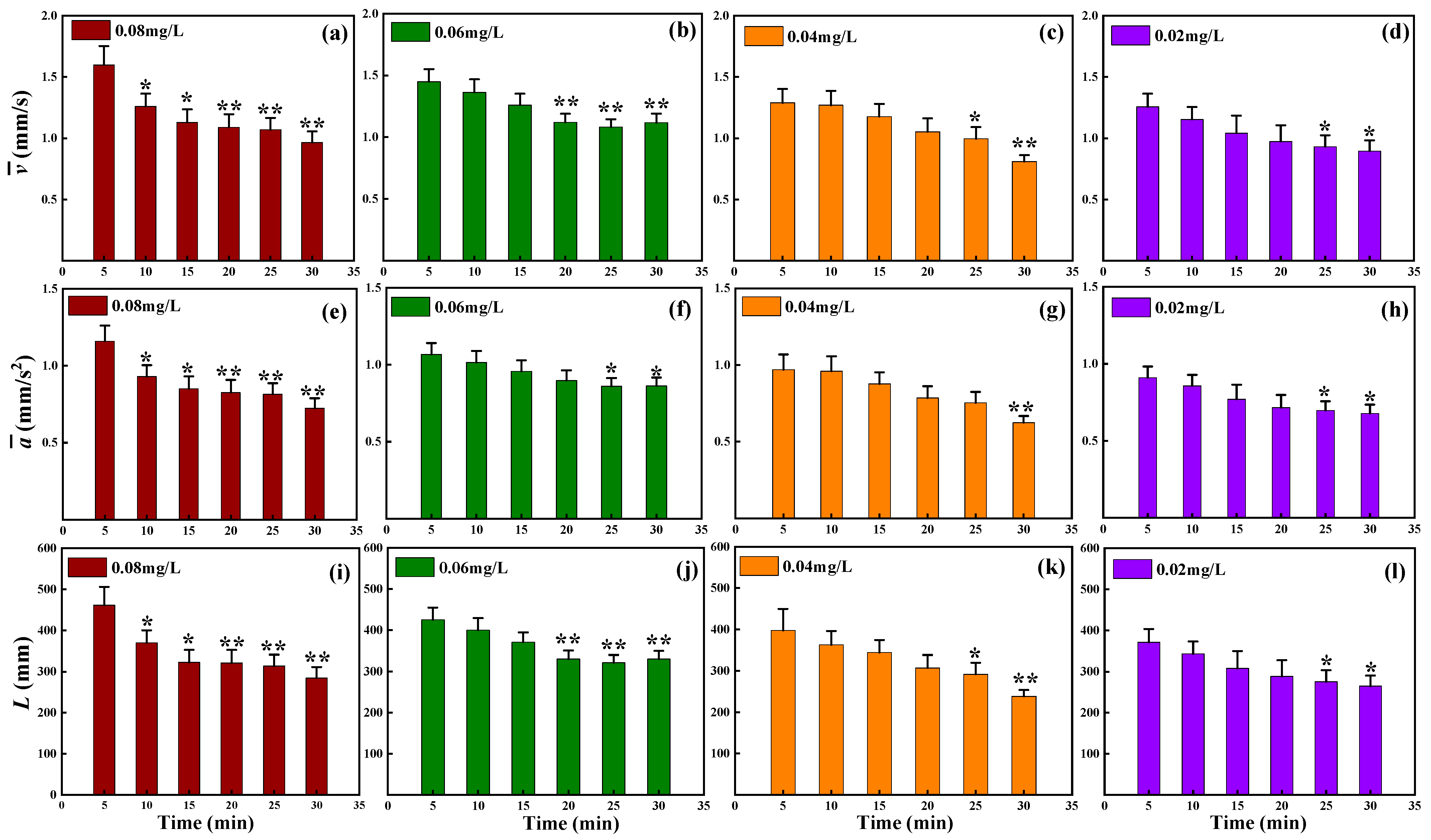
3.2. Observation Results under Toxic Stress Conditions
3.3. Comparison of Different Indicators with Respect to Toxicity Response
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, Q.; Wang, L.; Pan, B.; Guan, W.; Sun, X.; Cai, A. Distribution features and controls of heavy metals in surface sediments from the riverbed of the Ningxia-Inner Mongolian reaches, Yellow River, China. Chemosphere 2016, 144, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Chen, J.; Yuan, L.; Zhang, Y.; Xue, J.; Yang, B.; Wu, H. Risk assessment of trace metal(loid) pollution in surface water of industrial areas along the Huangpu River and Yangtze River Estuary in Shanghai, China. Reg. Stud. Mar. Sci. 2023, 57, 102746. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Cui, L.; Gao, X.; Wang, Y.; Zhang, H.; Lv, X.; Lei, K. Salinity-dependent aquatic life criteria of inorganic mercury in coastal water and its ecological risk assessment. Environ. Res. 2023, 217, 114957. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO 2007, 36, 12–18. [Google Scholar] [CrossRef]
- Sandborgh-englund, G.; Einarsson, C.; Sandström, M.; Ekstrand, J. Gastrointestinal Absorption of Metallic Mercury. Arch. Environ. Health Int. J. 2004, 59, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The Toxicology of Mercury—Current Exposures and Clinical Manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, J.-Z.; Yu, L.-M.; Goyer, R.A.; Waalkes, M.P. Mercury in Traditional Medicines: Is Cinnabar Toxicologically Similar to Common Mercurials? Exp. Biol. Med. 2008, 233, 810–817. [Google Scholar] [CrossRef]
- Wang, M.; Tong, Y.; Chen, C.; Liu, X.; Lu, Y.; Zhang, W.; He, W.; Wang, X.; Zhao, S.; Lin, Y. Ecological risk assessment to marine organisms induced by heavy metals in China’s coastal waters. Mar. Pollut. Bull. 2018, 126, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D. Daphnia as a versatile model system in ecology and evolution. EvoDevo 2022, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, S. Is Daphnia magna an ecologically representative zooplankton species in toxicity tests? Environ. Pollut. 1995, 90, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, K.E.; Christensen, G.M. Effects of Various Metals on Survival, Growth, Reproduction, and Metabolism of Daphnia magna. J. Fish. Res. Board Can. 1972, 29, 1691–1700. [Google Scholar] [CrossRef]
- He, Q.; Wang, X.; Sun, P.; Wang, Z.; Wang, L. Acute and chronic toxicity of tetrabromobisphenol A to three aquatic species under different pH conditions. Aquat. Toxicol. 2015, 164, 145–154. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef]
- Wang, X.; Qu, R.; Liu, J.; Wei, Z.; Wang, L.; Yang, S.; Huang, Q.; Wang, Z. Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity. Environ. Pollut. 2016, 208, 732–738. [Google Scholar] [CrossRef]
- Altshuler, I.; Demiri, B.; Xu, S.; Constantin, A.; Yan, N.D.; Cristescu, M.E. An Integrated Multi-Disciplinary Approach for Studying Multiple Stressors in Freshwater Ecosystems: Daphnia as a Model Organism. Integr. Comp. Biol. 2011, 51, 623–633. [Google Scholar] [CrossRef]
- Tsui, M.T.K.; Wang, W.-X. Uptake and Elimination Routes of Inorganic Mercury and Methylmercury in Daphnia magna. Environ. Sci. Technol. 2004, 38, 808–816. [Google Scholar] [CrossRef]
- Bownik, A. Daphnia swimming behaviour as a biomarker in toxicity assessment: A review. Sci. Total Environ. 2017, 601–602, 194–205. [Google Scholar] [CrossRef]
- Melvin, S.D.; Wilson, S.P. The utility of behavioral studies for aquatic toxicology testing: A meta-analysis. Chemosphere 2013, 93, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.M.; Truong, L.; Mandrell, D.; Marvel, S.; Zhang, G.; Tanguay, R.L. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2016, 90, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.R.; Roslev, P. Behavioral responses of juvenile Daphnia magna after exposure to glyphosate and glyphosate-copper complexes. Aquat. Toxicol. 2016, 179, 36–43. [Google Scholar] [CrossRef]
- Noss, C.; Dabrunz, A.; Rosenfeldt, R.R.; Lorke, A.; Schulz, R. Three-Dimensional Analysis of the Swimming Behavior of Daphnia magna Exposed to Nanosized Titanium Dioxide. PLoS ONE 2013, 8, e80960. [Google Scholar] [CrossRef] [PubMed]
- Untersteiner, H.; Kahapka, J.; Kaiser, H. Behavioural response of the cladoceran Daphnia magna Straus to sublethal Copper stress—Validation by image analysis. Aquat. Toxicol. 2003, 65, 435–442. [Google Scholar] [CrossRef]
- Christensen, B.T.; Lauridsen, T.L.; Ravn, H.W.; Bayley, M. A comparison of feeding efficiency and swimming ability of Daphnia magna exposed to cypermethrin. Aquat. Toxicol. 2005, 73, 210–220. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia magna model in the toxicity assessment of pharmaceuticals: A review. Sci. Total Environ. 2021, 763, 143038. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Available online: https://www.iso.org/standard/40518.html (accessed on 20 April 2023).
- National Public Service Platform for Standards Information. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=CF2E75792CF341FCD45F2613140293D1 (accessed on 20 April 2023).
- Tsui, M.T.K.; Wang, W.-X. Acute Toxicity of Mercury to Daphnia magna under Different Conditions. Environ. Sci. Technol. 2006, 40, 4025–4030. [Google Scholar] [CrossRef]
- Kim, H.; Yim, B.; Bae, C.; Lee, Y.-M. Acute toxicity and antioxidant responses in the water flea Daphnia magna to xenobiotics (cadmium, lead, mercury, bisphenol A, and 4-nonylphenol). Toxicol. Environ. Health Sci. 2017, 9, 41–49. [Google Scholar] [CrossRef]
- Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Inorganic mercury exposure: Toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 2010, 19, 105–123. [Google Scholar] [CrossRef]
- Chen, Z.; Du, M.; Yang, X.-D.; Chen, W.; Li, Y.-S.; Qian, C.; Yu, H.-Q. Deep-Learning-Based Automated Tracking and Counting of Living Plankton in Natural Aquatic Environments. Environ. Sci. Technol. 2023, 57, 18048–18057. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Liu, S.; Wang, F.; Li, Z.; Sun, J. YOLOX: Exceeding YOLO Series in 2021. arXiv 2021, arXiv:2107.08430. [Google Scholar]
- Zhang, Y.; Sun, P.; Jiang, Y.; Yu, D.; Weng, F.; Yuan, Z.; Luo, P.; Liu, W.; Wang, X. ByteTrack: Multi-object Tracking by Associating Every Detection Box. In Proceedings of the European Conference on Computer Vision 2022, Tel Aviv, Israel, 23–27 October 2022; pp. 1–21. [Google Scholar]
- Yuan, S.; Liang, C.; Li, W.; Letcher, R.J.; Liu, C. A comprehensive system for detection of behavioral change of D. magna exposed to various chemicals. J. Hazard. Mater. 2021, 402, 123731. [Google Scholar] [CrossRef]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. NeuroToxicology 2011, 32, 116–122. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Wang, X.; Qi, P.; Zhang, B.; Zeng, Y.; Fu, R.; Miao, M. AChE inhibition: One dominant factor for swimming behavior changes of Daphnia magna under DDVP exposure. Chemosphere 2015, 120, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.; Puga, S.; Cardoso, V.; Pinto-Ribeiro, F.; Raimundo, J.; Barata, M.; Pousão-Ferreira, P.; Pacheco, M.; Almeida, A. Inorganic mercury accumulation in brain following waterborne exposure elicits a deficit on the number of brain cells and impairs swimming behavior in fish (white seabream—Diplodus sargus). Aquat. Toxicol. 2016, 170, 400–412. [Google Scholar] [CrossRef]
- Teixeira, F.B.; Fernandes, R.M.; Farias-Junior, P.M.A.; Costa, N.M.M.; Fernandes, L.M.P.; Santana, L.N.S.; Silva-Junior, A.F.; Silva, M.C.F.; Maia, C.S.F.; Lima, R.R. Evaluation of the Effects of Chronic Intoxication with Inorganic Mercury on Memory and Motor Control in Rats. Int. J. Environ. Res. Public Health 2014, 11, 9171–9185. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.S.; Soares, M.C.S.; Lima, R.S.; Magalhães, V.F. Effects of Cylindrospermopsis raciborskii (cyanobacteria) on the swimming behavior of Daphnia (cladocera). Environ. Toxicol. Chem. 2014, 33, 223–229. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Bownik, A. Cyanobacterial anabaenopeptin-B, microcystins and their mixture cause toxic effects on the behavior of the freshwater crustacean Daphnia magna (Cladocera). Toxicon 2021, 198, 1–11. [Google Scholar] [CrossRef]
- Xuereb, B.; Lefèvre, E.; Garric, J.; Geffard, O. Acetylcholinesterase activity in Gammarus fossarum (Crustacea Amphipoda): Linking AChE inhibition and behavioural alteration. Aquat. Toxicol. 2009, 94, 114–122. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Zhao, N.; Yin, G.; Wang, T.; Jv, X.; Han, S.; An, L. Rapid Response of Daphnia magna Motor Behavior to Mercury Chloride Toxicity Based on Target Tracking. Toxics 2024, 12, 621. https://doi.org/10.3390/toxics12090621
Qin F, Zhao N, Yin G, Wang T, Jv X, Han S, An L. Rapid Response of Daphnia magna Motor Behavior to Mercury Chloride Toxicity Based on Target Tracking. Toxics. 2024; 12(9):621. https://doi.org/10.3390/toxics12090621
Chicago/Turabian StyleQin, Feihu, Nanjing Zhao, Gaofang Yin, Tao Wang, Xinyue Jv, Shoulu Han, and Lisha An. 2024. "Rapid Response of Daphnia magna Motor Behavior to Mercury Chloride Toxicity Based on Target Tracking" Toxics 12, no. 9: 621. https://doi.org/10.3390/toxics12090621
APA StyleQin, F., Zhao, N., Yin, G., Wang, T., Jv, X., Han, S., & An, L. (2024). Rapid Response of Daphnia magna Motor Behavior to Mercury Chloride Toxicity Based on Target Tracking. Toxics, 12(9), 621. https://doi.org/10.3390/toxics12090621




