Unveiling the Mysteries of Contrast-Induced Acute Kidney Injury: New Horizons in Pathogenesis and Prevention
Abstract
1. Introduction
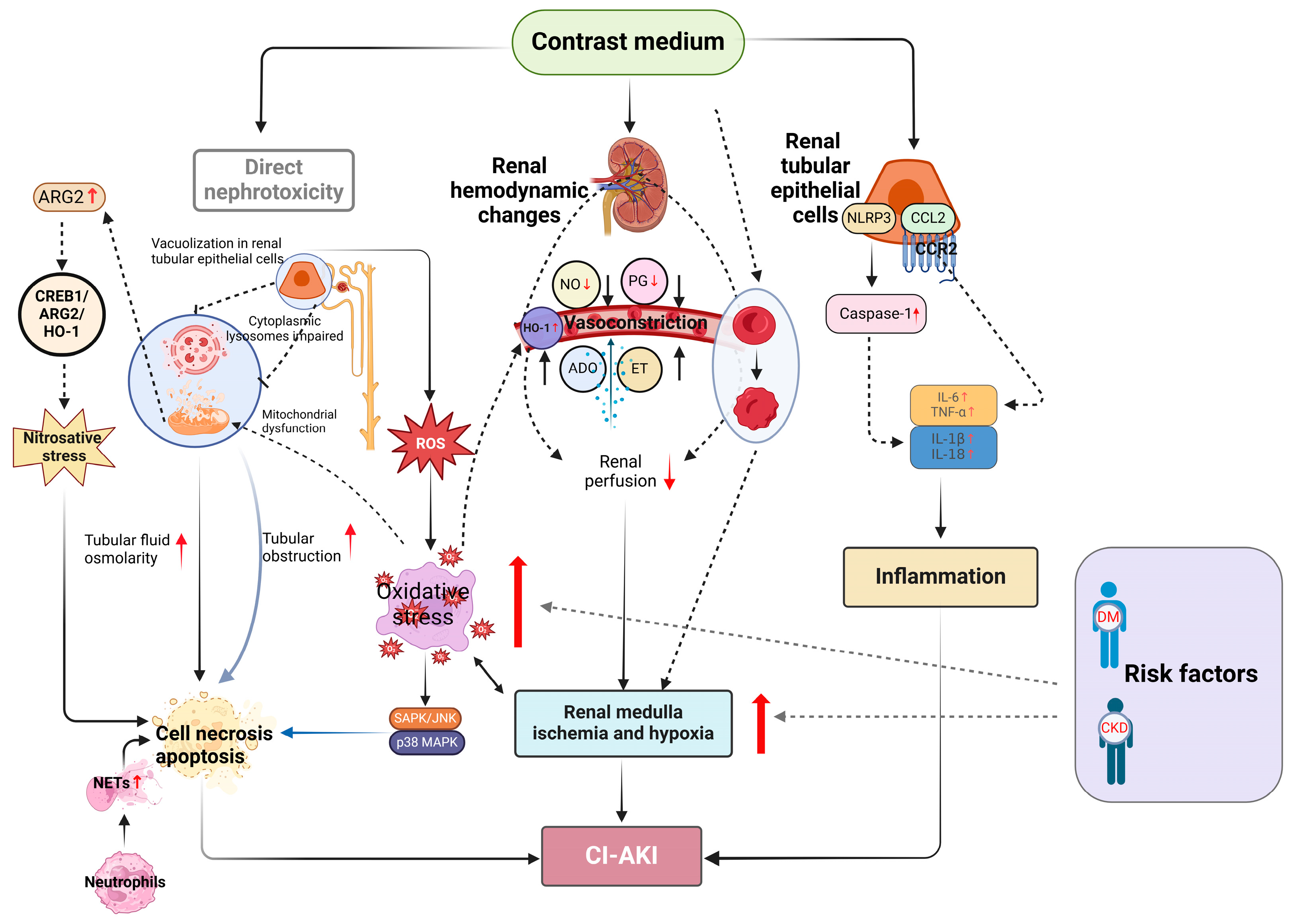
2. Pathogenesis of CI-AKI
2.1. The Direct Nephrotoxicity of CM
2.2. Renal Medulla Ischemia and Hypoxia
2.3. Oxidative Stress and Apoptosis
2.4. Inflammation and Immunity
2.5. Ferroptosis
2.6. Neutrophil Extracellular Traps
2.7. Nitrosative Stress
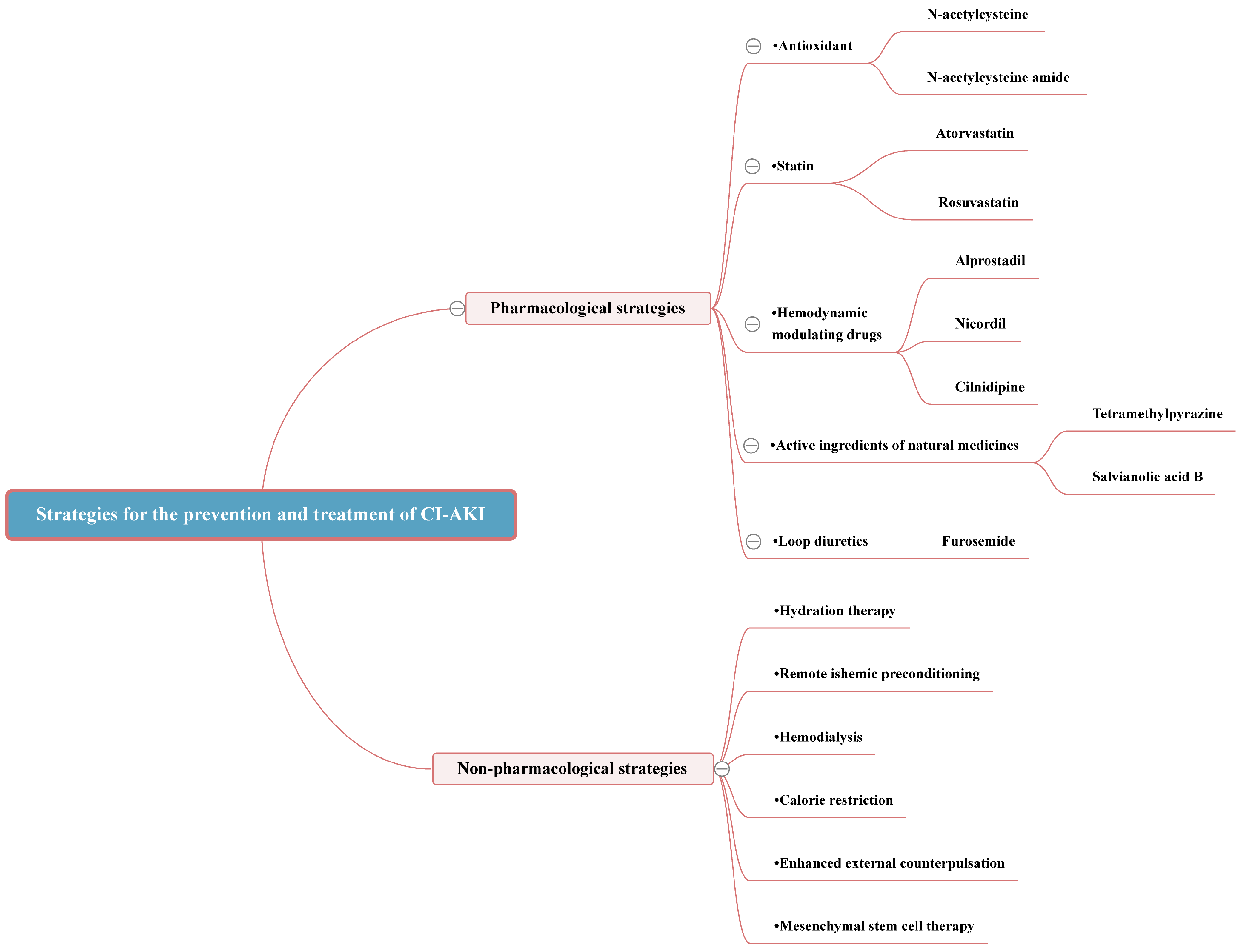
3. Strategies for the Prevention and Treatment of CI-AKI
3.1. An Integrated Strategy for Early Prevention of CI-AKI
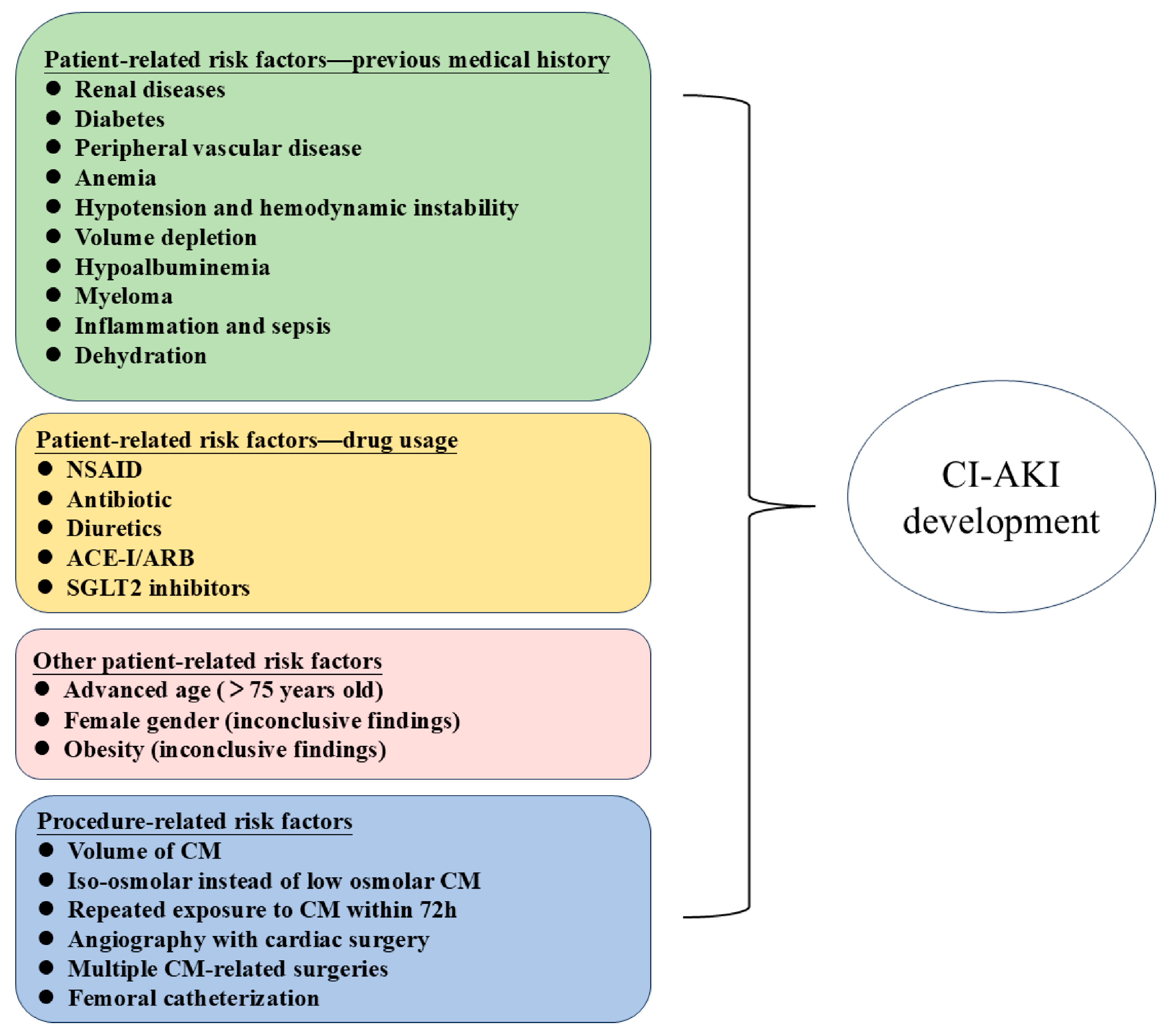
3.1.1. Identifying High-Risk Groups
3.1.2. Finding Sensitive Biomarkers
3.1.3. Risk Stratification through Predictive Modeling
3.2. Pharmacological Strategies
3.2.1. Antioxidant
3.2.2. Statin
3.2.3. Hemodynamic Modulating Drugs
3.2.4. Active Ingredients of Natural Medicines
3.3. Non-Pharmacological Strategies
3.3.1. Hydration Therapy
3.3.2. Remote Ischemic Preconditioning
3.3.3. Hemodialysis
3.3.4. Calorie Restriction
3.3.5. Enhanced External Counterpulsation
3.3.6. Mesenchymal Stem Cell Therapy
| Type of Strategy | Intervention | Study Type | Study Design | Study Results | Ref. |
|---|---|---|---|---|---|
| Pharmacological strategies | NAC | Meta-analysis | Multiple RCTs | The risk of CI-AKI and Scr levels are significantly decreased with NAC use. | [76] |
| NACA | Experimental | In Vivo Animal Model | NACA may protect against CI-AKI through modulating Trx1 and ASK1/p38 MAPK pathway to result in the inhibition of apoptosis among renal cells. | [81] | |
| Trimetazidine | Clinical | RCT | Trimetazidine along with isotonic saline infusion is more effective than isotonic saline alone in reducing the risk of CI-AKI in patients with pre-existing renal dysfunction. | [122] | |
| Edaravone | Experimental | In Vivo Animal Model | Edaravone may prevent and alleviate renal impairment and oxidative stress in rat models of CI-AKI by improving renal antioxidant capacity. | [83] | |
| Febuxostat | Clinical | RCT | Febuxostat has a renoprotective effect, and it can help to reduce the incidence of CI-AKI in CKD stage 3 patients undergoing PCI. | [84] | |
| CoQ10 | Experimental | In Vivo Animal Model | CoQ10 presented an antioxidant effect on the CI-AKI in male diabetic rats by improving renal function and renal hemodynamics, preserving morphology and reducing oxidative stress. | [123] | |
| Atorvastatin | Experimental | In Vivo Animal Model | Atorvastatin can inhibit the TLR4/MyD88/NF-κB signaling pathway, help improve renal tubular epithelial cell function, and reduce injury, inflammation, and burn death. | [86] | |
| Rosuvastatin | Meta-analysis | Multiple RCTs | Preoperative rosuvastatin therapy significantly reduced the risk of CI-AKI in patients at high risk for chronic kidney disease or diabetes. | [87] | |
| Alprostadil | Clinical | RCT | For renal insufficiency patients undergoing PCI, the associative usage of alpromazil with routine treatment can effectively prevent CI-AKI. | [90] | |
| Nicordil | Clinical | RCT | Oral administration of nicorandil could reduce the incidence of CI-AKI without increasing the incidence of postoperative adverse events. | [92] | |
| Cilnidipine | Experimental | In Vivo Animal Model | Cilnidipine markedly improved kidney function and alleviated tubular cell apoptosis, oxidative stress, and mitochondrial damage induced by iohexol in vitro and in vivo. | [93] | |
| TMP | Experimental | In Vivo Animal Model | TMP’s capacity to reverse CM-induced activation of the CCL2/CCR2 pathway ameliorate renal oxidative stress and abnormal mitochondrial dynamics and regulate mitophagy in tubular cells. | [10] | |
| Sal B | Clinical | RCT | Sal B reduces the incidence of CI-AKI and protects renal function after PPCI, and the effects were superior to those of NS hydration. | [100] | |
| Non-pharmacological strategies | Hydration therapy | Meta-analysis | Multiple RCTs | Furosemide with matched hydration by the RenalGuard System may reduce the incidence of CI-AKI in high-risk patients undergoing PCI or TAVR. | [104] |
| RIPC | Meta-analysis | Multiple RCTs | The incidence of CI-AKI in patients undergoing RIPC prior to surgery was significantly reduced compared to those who did not undergo RIPC. | [108] | |
| Hemodialysis | Clinical | RCT | For patients with chronic renal failure who are undergoing PCI, periprocedural hemofiltration given in an ICU setting appears to be effective in preventing the deterioration of renal function due to CI-AKI. | [113] | |
| CR | Experimental | In Vivo Animal Model | CR protected CI-AKI via SIRT1/GPX4 activation. CR may be used to mitigate CI-AKI. | [124] | |
| EECP | Clinical | RCT | EECP increases the contrast clearance and may have an effect in reducing the risk of CI-AKI. | [119] | |
| MSC therapy | Experimental | In Vivo Animal Model and In Vitro Cellular Model | SF-MSCs might improve CI-AKI by exerting anti-apoptotic effects in a paracrine manner. | [121] |
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, Z.A.; Escaned, J.; Dudek, D.; Radhakrishnan, J.; Karimi Galougahi, K. Strategies for Renal Protection in Cardiovascular Interventions. Korean Circ. J. 2022, 52, 485–495. [Google Scholar] [CrossRef]
- de Laforcade, L.; Bobot, M.; Bellin, M.F.; Clément, O.; Grangé, S.; Grenier, N.; Wynckel, A.; Guerrot, D. Kidney and contrast media: Common viewpoint of the French Nephrology societies (SFNDT, FIRN, CJN) and the French Radiological Society (SFR) following ESUR guidelines. Diagn. Interv. Imaging 2021, 102, 131–139. [Google Scholar] [CrossRef]
- McDonald, J.S.; McDonald, R.J. Risk of Acute Kidney Injury Following IV Iodinated Contrast Media Exposure: 2023 Update, From the AJR Special Series on Contrast Media. AJR Am. J. Roentgenol. 2023, 223, e2330037. [Google Scholar] [CrossRef]
- Rancic, Z.S. Commentary on ‘Contrast Induced Nephropathy and Long-term Renal Decline After Percutaneous Transluminal Angioplasty for Symptomatic Peripheral Arterial Disease’. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 394. [Google Scholar] [CrossRef]
- Lameire, N.; Kellum, J.A. Contrast-induced acute kidney injury and renal support for acute kidney injury: A KDIGO summary (Part 2). Crit. Care 2013, 17, 205. [Google Scholar] [CrossRef]
- Marenzi, G.; Assanelli, E.; Campodonico, J.; Lauri, G.; Marana, I.; De Metrio, M.; Moltrasio, M.; Grazi, M.; Rubino, M.; Veglia, F.; et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann. Intern. Med. 2009, 150, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Rihal, C.S.; Textor, S.C.; Grill, D.E.; Berger, P.B.; Ting, H.H.; Best, P.J.; Singh, M.; Bell, M.R.; Barsness, G.W.; Mathew, V.; et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002, 105, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, R.M.; Suarez-Cuervo, C.; Wilson, R.F.; Turban, S.; Zhang, A.; Sherrod, C.; Aboagye, J.; Eng, J.; Choi, M.J.; Hutfless, S.; et al. Effectiveness of Prevention Strategies for Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.B.; Valentovic, M.A. Contrast Induced Acute Kidney Injury and Direct Cytotoxicity of Iodinated Radiocontrast Media on Renal Proximal Tubule Cells. J. Pharmacol. Exp. Ther. 2019, 370, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Duan, Y.; Zheng, J.; Ye, Z.; Hei, T.K. Tetramethylpyrazine Prevents Contrast-Induced Nephropathy via Modulating Tubular Cell Mitophagy and Suppressing Mitochondrial Fragmentation, CCL2/CCR2-Mediated Inflammation, and Intestinal Injury. Oxid. Med. Cell. Longev. 2019, 2019, 7096912. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, S.B.; Wang, L.; Luo, X.Q.; Wang, H.S.; Deng, Y.H.; Wu, X.; Wu, T.; Yan; Kang, Y.X. Apelin-13 alleviates contrast-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Ren. Fail. 2023, 45, 2179852. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Choi, J.P.; Feghali, G.A.; Schussler, J.M.; Stoler, R.M.; Vallabahn, R.C.; Mehta, A. Contrast-Induced Acute Kidney Injury. J. Am. Coll. Cardiol. 2016, 68, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.S.; Perazella, M.A.; Yee, J.; Dillman, J.R.; Fine, D.; McDonald, R.J.; Rodby, R.A.; Wang, C.L.; Weinreb, J.C. Use of Intravenous Iodinated Contrast Media in Patients with Kidney Disease: Consensus Statements from the American College of Radiology and the National Kidney Foundation. Radiology 2020, 294, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Rosen, S.; Rosenberger, C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin. J. Am. Soc. Nephrol. 2008, 3, 288–296. [Google Scholar] [CrossRef]
- Heyman, S.N.; Rosenberger, C.; Rosen, S. Regional alterations in renal haemodynamics and oxygenation: A role in contrast medium-induced nephropathy. Nephrol. Dial. Transplant. 2005, 20 (Suppl. S1), i6–i11. [Google Scholar] [CrossRef]
- Wong, P.C.; Li, Z.; Guo, J.; Zhang, A. Pathophysiology of contrast-induced nephropathy. Int. J. Cardiol. 2012, 158, 186–192. [Google Scholar] [CrossRef]
- Persson, P.B.; Hansell, P.; Liss, P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005, 68, 14–22. [Google Scholar] [CrossRef]
- Franke, R.P.; Scharnweber, T.; Fuhrmann, R.; Mrowietz, C.; Jung, F. Effect of radiographic contrast media (Iodixanol, Iopromide) on the spectrin/actin-network of the membranous cytoskeleton of erythrocytes. Clin. Hemorheol. Microcirc. 2013, 54, 273–285. [Google Scholar] [CrossRef]
- Lee, C.C.; Chan, Y.L.; Wong, Y.C.; Ng, C.J.; Chang, C.H.; Hung, C.C.; Su, T.H. Contrast-enhanced CT and Acute Kidney Injury: Risk Stratification by Diabetic Status and Kidney Function. Radiology 2023, 307, e222321. [Google Scholar] [CrossRef]
- Khaleel, S.A.; Alzokaky, A.A.; Raslan, N.A.; Alwakeel, A.I.; Abd El-Aziz, H.G.; Abd-Allah, A.R. Lansoprazole halts contrast induced nephropathy through activation of Nrf2 pathway in rats. Chem. Biol. Interact. 2017, 270, 33–40. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Z.; Gu, D.; Li, Y.; Zhang, K.; Dong, X.; Liu, L.; Zhang, J.; Chen, J.; Wu, D.; et al. Hemin mitigates contrast-induced nephropathy by inhibiting ferroptosis via HO-1/Nrf2/GPX4 pathway. Clin. Exp. Pharmacol. Physiol. 2022, 49, 858–870. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Huang, D.; Yu, C. Baicalin Alleviates Contrast-Induced Acute Kidney Injury Through ROS/NLRP3/Caspase-1/GSDMD Pathway-Mediated Proptosis in vitro. Drug Des. Dev. Ther. 2022, 16, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nie, Y.; Si, B.; Wang, T.; Hei, T.K.; Du, H.; Zhao, G.; Chen, S.; Xu, A.; Liu, Y. Silver nanoparticles protect against arsenic induced genotoxicity via attenuating arsenic bioaccumulation and elevating antioxidation in mammalian cells. J. Hazard. Mater. 2021, 413, 125287. [Google Scholar] [CrossRef]
- Zhao, K.; Gao, Q.; Zong, C.; Ge, L.; Liu, J. Cordyceps sinensis prevents contrast-induced nephropathy in diabetic rats: Its underlying mechanism. Int. J. Clin. Exp. Pathol. 2018, 11, 5571–5580. [Google Scholar]
- Heyman, S.N.; Rosen, S.; Khamaisi, M.; Idée, J.M.; Rosenberger, C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Investig. Radiol. 2010, 45, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, X.; Chen, S.; Lv, M.; Tan, J.; Yang, D. Ox-LDL aggravates contrast-induced injury of renal tubular epithelial cells. J. Biochem. Mol. Toxicol. 2023, 37, e23379. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Zhao, X.; Huang, Z.; Xie, Y.; Yu, S.; Tu, J.; Guo, D.; Xiu, J.; Mai, Z.; Li, Q.; et al. Elevation of Preprocedural Systemic Immune Inflammation Level Increases the Risk of Contrast-Associated Acute Kidney Injury Following Coronary Angiography: A Multicenter Cohort Study. J. Inflamm. Res. 2022, 15, 2959–2969. [Google Scholar] [CrossRef]
- Kwasa, E.A.; Vinayak, S.; Armstrong, R. The role of inflammation in contrast-induced nephropathy. Br. J. Radiol. 2014, 87, 20130738. [Google Scholar] [CrossRef]
- Yang, Z.; Qiao, Y.; Wang, D.; Yan, G.; Tang, C. Association Between Inflammatory Biomarkers and Contrast-induced Acute Kidney Injury in ACS Patients Undergoing Percutaneous Coronary Intervention: A Cross-sectional Study. Angiology 2023, 33197231185445. [Google Scholar] [CrossRef]
- Lu, Z.; Cheng, D.; Yin, J.; Wu, R.; Zhang, G.; Zhao, Q.; Wang, N.; Wang, F.; Liang, M. Antithrombin III Protects Against Contrast-Induced Nephropathy. EBioMedicine 2017, 17, 101–107. [Google Scholar] [CrossRef]
- Atkinson, S.J. Kidney surveillance in the spotlight: Contrast-induced acute kidney injury illuminated. J. Clin. Investig. 2018, 128, 2754–2756. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Chung, H.; Komada, T.; Platnich, J.M.; Sandall, C.F.; Choudhury, S.R.; Chun, J.; Naumenko, V.; Surewaard, B.G.; Nelson, M.C.; et al. Renal immune surveillance and dipeptidase-1 contribute to contrast-induced acute kidney injury. J. Clin. Investig. 2018, 128, 2894–2913. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cao, J.; Wei, X.; Ge, Y.; Su, Z.; Yu, D. Klotho alleviates contrast-induced acute kidney injury by suppressing oxidative stress, inflammation, and NF-KappaB/NLRP3-mediated pyroptosis. Int. Immunopharmacol. 2023, 118, 110105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, J.; Song, Z.; Li, T.; Li, Z.; Gong, X. Tetramethylpyrazine attenuates renal tubular epithelial cell ferroptosis in contrast-induced nephropathy by inhibiting transferrin receptor and intracellular reactive oxygen species. Clin. Sci. 2024, 138, 235–249. [Google Scholar] [CrossRef]
- Wu, X.; You, D.; Cui, J.; Yang, L.; Lin, L.; Chen, Y.; Xu, C.; Lian, G.; Wan, J. Reduced Neutrophil Extracellular Trap Formation During Ischemia Reperfusion Injury in C3 KO Mice: C3 Requirement for NETs Release. Front. Immunol. 2022, 13, 781273. [Google Scholar] [CrossRef]
- Wang, H.; Gao, T.; Zhang, R.; Hu, J.; Gao, S.; Wang, Y.; Qi, X.; Zhou, Y.; Zheng, G.; Dong, H. Neutrophil Extracellular Traps Aggravate Contrast-Induced Acute Kidney Injury by Damaging Glomeruli and Peritubular Capillaries. J. Inflamm. Res. 2023, 16, 5629–5646. [Google Scholar] [CrossRef]
- Yoon, S.; Eom, G.H.; Kang, G. Nitrosative Stress and Human Disease: Therapeutic Potential of Denitrosylation. Int. J. Mol. Sci. 2021, 22, 9794. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Liu, K.; Yin, W.J.; Xie, Y.L.; Wang, J.L.; Zuo, S.R.; Tang, Z.Y.; Wu, Y.F.; Zuo, X.C. Arginase2 mediates contrast-induced acute kidney injury via facilitating nitrosative stress in tubular cells. Redox Biol. 2023, 67, 102929. [Google Scholar] [CrossRef]
- Fähling, M.; Seeliger, E.; Patzak, A.; Persson, P.B. Understanding and preventing contrast-induced acute kidney injury. Nat. Rev. Nephrol. 2017, 13, 169–180. [Google Scholar] [CrossRef]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.P.; Messenger, J.C.; Rumsfeld, J.S.; et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the NCDR Cath-PCI registry. JACC Cardiovasc. Interv. 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- David, E.; Del Gaudio, G.; Drudi, F.M.; Dolcetti, V.; Pacini, P.; Granata, A.; Pretagostini, R.; Garofalo, M.; Basile, A.; Bellini, M.I.; et al. Contrast Enhanced Ultrasound Compared with MRI and CT in the Evaluation of Post-Renal Transplant Complications. Tomography 2022, 8, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.J.; Hiremath, S.; Myers, A.; Fraser-Hill, M.; Barrett, B.J. Canadian Association of Radiologists consensus guidelines for the prevention of contrast-induced nephropathy: Update 2012. Can. Assoc. Radiol. J. 2014, 65, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.S.; Li, X. The Potential Biotherapeutic Targets of Contrast-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 8254. [Google Scholar] [CrossRef]
- Azzalini, L.; Spagnoli, V.; Ly, H.Q. Contrast-Induced Nephropathy: From Pathophysiology to Preventive Strategies. Can. J. Cardiol. 2016, 32, 247–255. [Google Scholar] [CrossRef]
- Seibert, F.S.; Heringhaus, A.; Pagonas, N.; Rohn, B.; Bauer, F.; Trappe, H.J.; Landmesser, U.; Babel, N.; Westhoff, T.H. Dickkopf-3 in the prediction of contrast media induced acute kidney injury. J. Nephrol. 2021, 34, 821–828. [Google Scholar] [CrossRef]
- Nozue, T.; Michishita, I.; Mizuguchi, I. Predictive value of serum cystatin C, β2-microglobulin, and urinary liver-type fatty acid-binding protein on the development of contrast-induced nephropathy. Cardiovasc. Interv. Ther. 2010, 25, 85–90. [Google Scholar] [CrossRef]
- Jaroszyński, A.; Zaborowski, T.; Głuszek, S.; Zapolski, T.; Sadowski, M.; Załuska, W.; Cedro, A.; Małecka-Massalska, T.; Dąbrowski, W. Heat Shock Protein 27 Is an Emerging Predictor of Contrast-Induced Acute Kidney Injury on Patients Subjected to Percutaneous Coronary Interventions. Cells 2021, 10, 684. [Google Scholar] [CrossRef]
- Oksuz, F.; Yarlioglues, M.; Cay, S.; Celik, I.E.; Mendi, M.A.; Kurtul, A.; Cankurt, T.; Kuyumcu, S.; Canpolat, U.; Turak, O. Predictive Value of Gamma-Glutamyl Transferase Levels for Contrast-Induced Nephropathy in Patients With ST-Segment Elevation Myocardial Infarction Who Underwent Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2015, 116, 711–716. [Google Scholar] [CrossRef]
- Wu, R.; Kong, Y.; Yin, J.; Liang, R.; Lu, Z.; Wang, N.; Zhao, Q.; Zhou, Y.; Yan, C.; Wang, F.; et al. Antithrombin Ⅲ is a Novel Predictor for Contrast Induced Nephropathy After Coronary Angiography. Kidney Blood Press. Res. 2018, 43, 170–180. [Google Scholar] [CrossRef]
- Briguori, C.; Visconti, G.; Rivera, N.V.; Focaccio, A.; Golia, B.; Giannone, R.; Castaldo, D.; De Micco, F.; Ricciardelli, B.; Colombo, A. Cystatin C and contrast-induced acute kidney injury. Circulation 2010, 121, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Bachorzewska-Gajewska, H.; Koc-Zorawska, E.; Malyszko, J.S.; Kobus, G.; Dobrzycki, S. Midkine: A novel and early biomarker of contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions. Biomed. Res. Int. 2015, 2015, 879509. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, W.; Qian, W.; Zhao, X.; Wang, L.; Yu, Y.; Liu, J.; Cheng, J. Urinary interleukin-18 as an early indicator to predict contrast-induced nephropathy in patients undergoing percutaneous coronary intervention. Exp. Ther. Med. 2014, 8, 1263–1266. [Google Scholar] [CrossRef]
- Akdeniz, D.; Celik, H.T.; Kazanci, F.; Yilmaz, H.; Yalcin, S.; Bilgic, M.A.; Ruzgaresen, N.; Akcay, A.; Eryonucu, B. Is Kidney Injury Molecule 1 a Valuable Tool for the Early Diagnosis of Contrast-Induced Nephropathy? J. Investig. Med. 2015, 63, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, C.; Anselmi, C.V.; De Micco, F.; Roscigno, G.; Visconti, G.; Golia, B.; Focaccio, A.; Ricciardelli, B.; Perna, E.; Papa, L.; et al. Neutrophil Gelatinase-Associated Lipocalin and Contrast-Induced Acute Kidney Injury. Circ. Cardiovasc. Interv. 2015, 8, e002673. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ji, J.; Fang, Y.; Jiang, S.H.; Lin, Y.M.; Bo, J.; Qian, J.Y.; Xu, X.H.; Ding, X.Q. Assessment of urinary N-acetyl-β-glucosaminidase as an early marker of contrast-induced nephropathy. J. Int. Med. Res. 2011, 39, 647–653. [Google Scholar] [CrossRef]
- Manabe, K.; Kamihata, H.; Motohiro, M.; Senoo, T.; Yoshida, S.; Iwasaka, T. Urinary liver-type fatty acid-binding protein level as a predictive biomarker of contrast-induced acute kidney injury. Eur. J. Clin. Investig. 2012, 42, 557–563. [Google Scholar] [CrossRef]
- Sağ, S.; Yıldız, A.; Aydin Kaderli, A.; Gül, B.C.; Bedir, Ö.; Ceğilli, E.; Özdemir, B.; Can, F.E.; Aydınlar, A. Association of monocyte to HDL cholesterol level with contrast induced nephropathy in STEMI patients treated with primary PCI. Clin. Chem. Lab. Med. 2017, 55, 132–138. [Google Scholar] [CrossRef]
- Kong, T.; Park, Y.S.; Lee, H.S.; Kim, S.; Han, S.; Eun, C.H.; Lee, J.W.; You, J.S.; Chung, H.S.; Park, I.; et al. A Delta Neutrophil Index for the Prediction of Contrast-Induced Nephropathy in Patients With St-Elevation Myocardial Infarction Followed By Percutaneous Coronary Intervention. Shock 2018, 49, 317–325. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, X.; Jiang, J.; Zang, X.; Chen, X.; Li, H.; Cao, H.; Wang, Q. Growth differentiation factor-15 levels and the risk of contrast induced nephropathy in patients with acute myocardial infarction undergoing percutaneous coronary intervention: A retrospective observation study. PLoS ONE 2018, 13, e0197609. [Google Scholar] [CrossRef]
- Kim, H.; Jo, K. Laboratory Predictors of Contrast-Induced Nephropathy After Neurointervention: A Prospective 3-Year Observational Study. World Neurosurg. 2020, 135, e77–e82. [Google Scholar] [CrossRef] [PubMed]
- Karauzum, I.; Karauzum, K.; Hanci, K.; Gokcek, D.; Kalas, B.; Ural, E. The Utility of Systemic Immune-Inflammation Index for Predicting Contrast-Induced Nephropathy in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Cardiorenal Med. 2022, 12, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Toprak, K. Effect of Serum C-Peptide Levels on the Development of Contrast-Induced Nephropathy in Diabetic Patients Undergoing Coronary Angiography. Angiology 2024, 75, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kinik, M.; Çamci, S.; Ari, S.; Ari, H.; Melek, M.; Bozat, T. The effect of whole blood viscosity on contrast-induced nephropathy development in patients undergoing percutaneous coronary intervention. Postgrad. Med. 2022, 134, 78–84. [Google Scholar] [CrossRef]
- Li, M.; Li, L.; Qin, Y.; Luo, E.; Wang, D.; Qiao, Y.; Tang, C.; Yan, G. Elevated TyG Index Predicts Incidence of Contrast-Induced Nephropathy: A Retrospective Cohort Study in NSTE-ACS Patients Implanted With DESs. Front. Endocrinol. 2022, 13, 817176. [Google Scholar] [CrossRef]
- Zengin Temel, T.; Satilmis, D.; Yavuz, B.G.; Afacan, M.A.; Colak, S. The Value of C-reactive Protein/Albumin Ratio in the Prediction of Contrast-Induced Nephropathy in Emergency Department Patients. Cureus 2023, 15, e39230. [Google Scholar] [CrossRef]
- Toprak, K. Atherogenic Index of Plasma is an Independent Risk Factor for Contrast Induced Nephropathy in Patients With Non-ST Elevation Myocardial Infarction. Angiology 2023, 74, 427–434. [Google Scholar] [CrossRef]
- Gorelik, Y.; Bloch-Isenberg, N.; Yaseen, H.; Heyman, S.N.; Khamaisi, M. Acute Kidney Injury After Radiocontrast-Enhanced Computerized Tomography in Hospitalized Patients With Advanced Renal Failure: A Propensity-Score-Matching Analysis. Investig. Radiol. 2020, 55, 677–687. [Google Scholar] [CrossRef]
- Connolly, M.; Kinnin, M.; McEneaney, D.; Menown, I.; Kurth, M.; Lamont, J.; Morgan, N.; Harbinson, M. Prediction of contrast induced acute kidney injury using novel biomarkers following contrast coronary angiography. Qjm 2018, 111, 103–110. [Google Scholar] [CrossRef]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar] [CrossRef]
- Chen, Y.L.; Fu, N.K.; Xu, J.; Yang, S.C.; Li, S.; Liu, Y.Y.; Cong, H.L. A simple preprocedural score for risk of contrast-induced acute kidney injury after percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2014, 83, E8–E16. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Yi, Y.H.; Guan, X.F.; Zhou, L.Y.; Wang, J.L.; Li, D.Y.; Zuo, X.C. Preprocedural Prediction Model for Contrast-Induced Nephropathy Patients. J. Am. Heart Assoc. 2017, 6, e004498. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, S.; Yoo, H.; Kim, K.; Kim, Y.; Park, S.; Jang, H.R.; Kim, D.K.; Huh, W.; Kim, Y.G.; et al. Risk Prediction for Contrast-Induced Nephropathy in Cancer Patients Undergoing Computed Tomography under Preventive Measures. J. Oncol. 2019, 2019, 8736163. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, B.; Han, S.; Lee, M.; Shin, G.T.; Kim, H.; Son, M.; Kim, K.H.; Kwon, J.M.; Park, R.W.; et al. Applicable Machine Learning Model for Predicting Contrast-induced Nephropathy Based on Pre-catheterization Variables. Intern. Med. 2024, 63, 773–780. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Zhu, R.; Zheng, R.; Deng, B.; Liu, P.; Wang, Y. Association of N-acetylcysteine use with contrast-induced nephropathy: An umbrella review of meta-analyses of randomized clinical trials. Front. Med. 2023, 10, 1235023. [Google Scholar] [CrossRef]
- Eligini, S.; Munno, M.; Atlas, D.; Banfi, C. N-acetylcysteine Amide AD4/NACA and Thioredoxin Mimetic Peptides Inhibit Platelet Aggregation and Protect against Oxidative Stress. Antioxidants 2023, 12, 1395. [Google Scholar] [CrossRef]
- Zhao, J.; Le, M.; Li, J.; Huang, Q.; Chen, H.; Zhang, W.; Mao, H.; Sun, Q.; Li, A.; Zhao, Y.; et al. LINC00938 alleviates hypoxia ischemia encephalopathy induced neonatal brain injury by regulating oxidative stress and inhibiting JNK/p38 MAPK signaling pathway. Exp. Neurol. 2023, 367, 114449. [Google Scholar] [CrossRef]
- Permeisari, D. Future insights of pharmacological prevention for AKI post cardiopulmonary bypass surgery (based on PK/PD approach). Front. Pharmacol. 2022, 13, 975641. [Google Scholar] [CrossRef]
- Atlas, D. Emerging therapeutic opportunities of novel thiol-amides, NAC-amide (AD4/NACA) and thioredoxin mimetics (TXM-Peptides) for neurodegenerative-related disorders. Free Radic. Biol. Med. 2021, 176, 120–141. [Google Scholar] [CrossRef]
- Gong, X.; Duan, Y.; Zheng, J.; Wang, Y.; Wang, G.; Norgren, S.; Hei, T.K. Nephroprotective Effects of N-Acetylcysteine Amide against Contrast-Induced Nephropathy through Upregulating Thioredoxin-1, Inhibiting ASK1/p38MAPK Pathway, and Suppressing Oxidative Stress and Apoptosis in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 8715185. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Pandey, S.; Bagang, N.; Mehra, K.; Singh, G. Trimetazidine an emerging paradigm in renal therapeutics: Preclinical and clinical insights. Eur. J. Pharmacol. 2021, 913, 174624. [Google Scholar] [CrossRef] [PubMed]
- Alshogran, O.Y.; Al Tahrawi, A.Y.; Nusair, S.D. Exploring the effects of edaravone in rats with contrast-induced acute kidney injury. Life Sci. 2022, 309, 121006. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, I.I.; Abdellatif, Y.A.; Saad, R.E.; Teama, N.M. Renoprotective effect of febuxostat on contrast-induced acute kidney injury in chronic kidney disease patients stage 3: Randomized controlled trial. BMC Nephrol. 2023, 24, 65. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Z.; Wang, F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. 2020, 259, 118379. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.Z.; Li, Y.J.; Su, B.H.; Li, C.J.; Zeng, R. Atorvastatin reduces contrast media-induced pyroptosis of renal tubular epithelial cells by inhibiting the TLR4/MyD88/NF-κB signaling pathway. BMC Nephrol. 2023, 24, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, Y.; Jin, Q.; Bian, L.; Lin, P. Meta-analysis of rosuvastatin efficacy in prevention of contrast-induced acute kidney injury. Drug Des. Dev. Ther. 2018, 12, 3685–3690. [Google Scholar] [CrossRef]
- Liu, L.Y.; Liu, Y.; Wu, M.Y.; Sun, Y.Y.; Ma, F.Z. Efficacy of atorvastatin on the prevention of contrast-induced acute kidney injury: A meta-analysis. Drug Des. Dev. Ther. 2018, 12, 437–444. [Google Scholar] [CrossRef]
- Dołegowska, B.; Pikuła, E.; Safranow, K.; Olszewska, M.; Jakubowska, K.; Chlubek, D.; Gutowski, P. Metabolism of eicosanoids and their action on renal function during ischaemia and reperfusion: The effect of alprostadil. Prostaglandins Leukot. Essent. Fatty Acids 2006, 75, 403–411. [Google Scholar] [CrossRef]
- Liu, D.; Gao, F.; Li, L.; Jian, X.; Xiao, B. The prophylactic effect of alprostadil on contrast-induced nephropathy in renal insufficiency patients after percutaneous coronary intervention. Am. J. Transl. Res. 2021, 13, 3766–3772. [Google Scholar]
- Liu, X.; Zhang, P.; Zhang, J.; Zhang, X.; Yang, S.; Fu, N. The Preventive Effect of Alprostadil on the Contrast-Induced Nephropathy of Coronary Heart Disease Treated by Percutaneous Coronary Intervention in Moderate and High-Risk Population Stratified by Mehran Score. Angiology 2022, 73, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, W.Y.; Yang, S.C.; Fu, N.K.; Liu, X.G.; Zhang, X.; Cong, H.L.; Lin, W.H.; Tian, F.S.; Lu, C.Z.; et al. Preventive Effects of Nicorandil Against Contrast-Induced Nephropathy in Patients With Moderate Renal Insufficiency Undergoing Percutaneous Coronary Intervention. Angiology 2020, 71, 183–188. [Google Scholar] [CrossRef]
- Liu, K.; Hu, C.; Yin, W.; Zhou, L.; Gu, X.; Zuo, X. An in vivo and in vitro model on the protective effect of cilnidipine on contrast-induced nephropathy via regulation of apoptosis and CaMKⅡ/mPTP pathway. J. Biochem. Mol. Toxicol. 2023, 37, e23238. [Google Scholar] [CrossRef]
- Naeem, M.; McEnteggart, G.E.; Murphy, T.P.; Prince, E.; Ahn, S.; Soares, G. Fenoldopam for the prevention of contrast-induced nephropathy (CIN)-do we need more trials? A meta-analysis. Clin. Imaging 2015, 39, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Holcslaw, T.; Bashore, T.M.; Freed, M.I.; Miller, D.; Rudnick, M.R.; Szerlip, H.; Thames, M.D.; Davidson, C.J.; Shusterman, N.; et al. Exacerbation of radiocontrast nephrotoxicity by endothelin receptor antagonism. Kidney Int. 2000, 57, 1675–1680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, X.; Wang, Q.; Tang, X.; Wang, Y.; Fu, D.; Lu, H.; Wang, G.; Norgren, S. Tetramethylpyrazine prevents contrast-induced nephropathy by inhibiting p38 MAPK and FoxO1 signaling pathways. Am. J. Nephrol. 2013, 37, 199–207. [Google Scholar] [CrossRef]
- Tongqiang, L.; Shaopeng, L.; Xiaofang, Y.; Nana, S.; Xialian, X.; Jiachang, H.; Ting, Z.; Xiaoqiang, D. Salvianolic Acid B Prevents Iodinated Contrast Media-Induced Acute Renal Injury in Rats via the PI3K/Akt/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2016, 2016, 7079487. [Google Scholar] [CrossRef]
- Dong, S.J.; Gao, X.Y.; Pei, M.X.; Luo, T.; Fan, D.; Chen, Y.L.; Jin, J.F.; Zhao, X.D. Effects and Mechanism of Salvianolic Acid B on the Injury of Human Renal Tubular Epithelial Cells Induced by Iopromide. Front. Pharmacol. 2021, 12, 761908. [Google Scholar] [CrossRef]
- Pei, M.X.; Dong, S.J.; Gao, X.Y.; Luo, T.; Fan, D.; Jin, J.F.; Zhao, X.D.; Chen, Y.L. Salvianolic Acid B Attenuates Iopromide-Induced Renal Tubular Epithelial Cell Injury by Inhibiting the TLR4/NF-κB/NLRP3 Signaling Pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 8400496. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, M.; Ma, J.; Liu, R.; Dong, Z.; Zhao, G.; Hang, J.; Wei, J.; Ma, S.; Wei, M.; et al. Protective Effects of Salvianolate on Contrast-Induced Nephropathy after Primary Percutaneous Coronary Intervention: A Prospective Multicenter Randomized Controlled Trial. Cardiology 2017, 138, 169–178. [Google Scholar] [CrossRef]
- Miao, S.; Xue, Z.K.; Zhang, Y.R.; Zhang, H.; Che, J.J.; Liu, T.; Tao, H.Y.; Li, G.; Chen, K.Y. Comparison of Different Hydration Strategies in Patients with Very Low-Risk Profiles of Contrast-Induced Nephropathy. Med. Sci. Monit. 2021, 27, e929115. [Google Scholar] [CrossRef] [PubMed]
- Pioli, M.R.; Couto, R.M.; Francisco, J.A.; Antoniassi, D.Q.; Souza, C.R.; Olivio, M.Y.; Anhê, G.F.; Giopatto, S.; Sposito, A.C.; Nadruz, W.; et al. Effectiveness of Oral Hydration in Preventing Contrast-Induced Nephropathy in Individuals Undergoing Elective Coronary Interventions. Arq. Bras. Cardiol. 2023, 120, e20220529. [Google Scholar] [CrossRef]
- Bolt, L.J.J.; Sigterman, T.A.; Krasznai, A.G.; Sikkink, C.J.M.; Schurink, G.H.; Bouwman, L.H. Prevention of postcontrast acute kidney injury after percutaneous transluminal angioplasty by inducing RenalGuard controlled furosemide forced diuresis with matched hydration: Study protocol for a randomised controlled trial. BMJ Open 2018, 8, e021842. [Google Scholar] [CrossRef]
- Putzu, A.; Boscolo Berto, M.; Belletti, A.; Pasotti, E.; Cassina, T.; Moccetti, T.; Pedrazzini, G. Prevention of Contrast-Induced Acute Kidney Injury by Furosemide With Matched Hydration in Patients Undergoing Interventional Procedures: A Systematic Review and Meta-Analysis of Randomized Trials. JACC Cardiovasc. Interv. 2017, 10, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Stokfisz, K.; Ledakowicz-Polak, A.; Zagorski, M.; Zielinska, M. Ischaemic preconditioning—Current knowledge and potential future applications after 30 years of experience. Adv. Med. Sci. 2017, 62, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Kim, J.; Park, K.H.; Ma, J.; Zhu, H. Remote Ischemic Pre-Conditioning (RIPC). Methods Mol. Biol. 2023, 2597, 11–18. [Google Scholar] [CrossRef]
- Kuusik, K.; Kasepalu, T.; Zilmer, M.; Eha, J.; Vähi, M.; Torop, L.A.; Lieberg, J.; Kals, J. The Role of RIPC in Preventing Organ Damage, Inflammation, and Oxidative Stress during Lower Limb DSA: A Randomised Controlled Trial. Oxid. Med. Cell. Longev. 2021, 2021, 6043550. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S.; Jia, P.; Xu, X.; Song, N.; Zhang, T.; Chen, R.; Ding, X. Protection of remote ischemic preconditioning against acute kidney injury: A systematic review and meta-analysis. Crit. Care 2016, 20, 111. [Google Scholar] [CrossRef]
- Moretti, C.; Cerrato, E.; Cavallero, E.; Lin, S.; Rossi, M.L.; Picchi, A.; Sanguineti, F.; Ugo, F.; Palazzuoli, A.; Bertaina, M.; et al. The EUROpean and Chinese cardiac and renal Remote Ischemic Preconditioning Study (EURO-CRIPS CardioGroup I): A randomized controlled trial. Int. J. Cardiol. 2018, 257, 1–6. [Google Scholar] [CrossRef]
- Morcos, S.K.; Thomsen, H.S.; Webb, J.A. Dialysis and contrast media. Eur. Radiol. 2002, 12, 3026–3030. [Google Scholar] [CrossRef]
- Cruz, D.N.; Perazella, M.A.; Ronco, C. The role of extracorporeal blood purification therapies in the prevention of radiocontrast-induced nephropathy. Int. J. Artif. Organs 2008, 31, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.N.; Perazella, M.A.; Bellomo, R.; Corradi, V.; de Cal, M.; Kuang, D.; Ocampo, C.; Nalesso, F.; Ronco, C. Extracorporeal blood purification therapies for prevention of radiocontrast-induced nephropathy: A systematic review. Am. J. Kidney Dis. 2006, 48, 361–371. [Google Scholar] [CrossRef]
- Marenzi, G.; Marana, I.; Lauri, G.; Assanelli, E.; Grazi, M.; Campodonico, J.; Trabattoni, D.; Fabbiocchi, F.; Montorsi, P.; Bartorelli, A.L. The prevention of radiocontrast-agent-induced nephropathy by hemofiltration. N. Engl. J. Med. 2003, 349, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Kume, S.; Uzu, T.; Horiike, K.; Chin-Kanasaki, M.; Isshiki, K.; Araki, S.; Sugimoto, T.; Haneda, M.; Kashiwagi, A.; Koya, D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Investig. 2010, 120, 1043–1055. [Google Scholar] [CrossRef]
- Kim, K.M.; Chung, K.W.; Jeong, H.O.; Lee, B.; Kim, D.H.; Park, J.W.; Kim, S.M.; Yu, B.P.; Chung, H.Y. MMP2-A2M interaction increases ECM accumulation in aged rat kidney and its modulation by calorie restriction. Oncotarget 2018, 9, 5588–5599. [Google Scholar] [CrossRef] [PubMed]
- Thongsricome, T.; Kositanurit, W.; Siwamogsatham, S.; Tiranathanagul, K. Enhanced external counterpulsation, focusing on its effect on kidney function, and utilization in patients with kidney diseases: A systematic review. Asian Biomed. (Res. Rev. News) 2023, 17, 208–221. [Google Scholar] [CrossRef]
- Duan, Y.; Tang, H.X. Efficacy of enhanced extracorporeal counterpulsation combined with atorvastatin in the treatment of cognitive impairment after stroke. World J. Psychiatry 2023, 13, 1027–1036. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, C.; Xiao, Q.; Wu, J.; Wu, G. Enhanced external counterpulsation: A new method to alleviate contrast-induced acute kidney injury. Contemp. Clin. Trials 2022, 113, 106653. [Google Scholar] [CrossRef]
- Zeng, C.M.; Zhao, Y.M.; Zhong, X.J.; Wu, Z.J.; Bai, J.; Qiu, S.Y.; Li, Y.Y. Reduction in risk of contrast-induced nephropathy in patients with chronic kidney disease and diabetes mellitus by enhanced external counterpulsation. Front. Endocrinol. 2022, 13, 973452. [Google Scholar] [CrossRef]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef]
- Kadono, M.; Nakashima, A.; Ishiuchi, N.; Sasaki, K.; Miura, Y.; Maeda, S.; Fujita, A.; Sasaki, A.; Nagamatsu, S.; Masaki, T. Adipose-derived mesenchymal stem cells cultured in serum-free medium attenuate acute contrast-induced nephropathy by exerting anti-apoptotic effects. Stem Cell Res. Ther. 2023, 14, 337. [Google Scholar] [CrossRef] [PubMed]
- Onbasili, A.O.; Yeniceriglu, Y.; Agaoglu, P.; Karul, A.; Tekten, T.; Akar, H.; Discigil, G. Trimetazidine in the prevention of contrast-induced nephropathy after coronary procedures. Heart 2007, 93, 698–702. [Google Scholar] [CrossRef]
- Couto, S.M.F.; da Fonseca, C.D.; Watanabe, M.; de Fátima Fernandes Vattimo, M. Protection of coenzyme Q10 against contrast-induced acute kidney injury in male diabetic rats. Diabetol. Metab. Syndr. 2021, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Wang, Y.; Zhang, Z.; Yang, D.; Gu, D.; He, B.; Zhang, X.; He, D.; Wang, H.; Jose, P.A.; et al. Calorie Restriction Protects against Contrast-Induced Nephropathy via SIRT1/GPX4 Activation. Oxid. Med. Cell. Longev. 2021, 2021, 2999296. [Google Scholar] [CrossRef]
- Gorelik, Y.; Bloch-Isenberg, N.; Heyman, S.N.; Khamaisi, M. Renal Functional Recovery Confounding the Assessment of Contrast Nephropathy: Propensity Score Analysis. Am. J. Nephrol. 2021, 52, 76–83. [Google Scholar] [CrossRef]
- Gorelik, Y.; Abassi, Z.; Bloch-Isenberg, N.; Khamaisi, M.; Heyman, S.N. Changing serum creatinine in the detection of acute renal failure and recovery following radiocontrast studies among acutely ill inpatients: Reviewing insights regarding renal functional reserve gained by large-data analysis. Pract. Lab. Med. 2022, 30, e00276. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation 2020, 142, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Brezis, M.; Agmon, Y.; Epstein, F.H. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am. J. Physiol. 1994, 267, F1059–F1062. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Brezis, M.; Greenfeld, Z.; Rosen, S. Protective role of furosemide and saline in radiocontrast-induced acute renal failure in the rat. Am. J. Kidney Dis. 1989, 14, 377–385. [Google Scholar] [CrossRef]
- Ho, K.M.; Power, B.M. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010, 65, 283–293. [Google Scholar] [CrossRef]
- Weinstein, J.M.; Heyman, S.; Brezis, M. Potential deleterious effect of furosemide in radiocontrast nephropathy. Nephron 1992, 62, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.; Werner, C.; Mann, D.; D’Elia, J.; Silva, P. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N. Engl. J. Med. 1994, 331, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, L.; Nordberg, G.F.; Nurchi, V.M.; Aaseth, J.O. Gadolinium in Medical Imaging-Usefulness, Toxic Reactions and Possible Countermeasures-A Review. Biomolecules 2022, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Jakobi, T.; Meyborg, M.; Freisinger, E.; Gebauer, K.; Stella, J.; Engelbertz, C.; Reinecke, H.; Malyar, N.M. Feasibility and impact of carbon dioxide angiography on acute kidney injury following endovascular interventions in patients with peripheral artery disease and renal impairment. J. Nephrol. 2021, 34, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Truong, A.H.T.; Brommesson, C.; du Rietz, A.; Kokil, G.R.; Boyd, R.D.; Hu, Z.; Dang, T.T.; Persson, P.O.A.; Uvdal, K. Cerium Oxide Nanoparticles with Entrapped Gadolinium for High T (1) Relaxivity and ROS-Scavenging Purposes. ACS Omega 2022, 7, 21337–21345. [Google Scholar] [CrossRef] [PubMed]
| Category | Biomarker | Molecular Weight (kDa) | Function | Significant Change in CI-AKI | Cutoff for CI-AKI Prediction | Ref. |
|---|---|---|---|---|---|---|
| Pre-injury phase biomarkers | DKK3(U) | 30 | Promote renal tubulointerstitial fibrosis through modulation of the canonical Wnt/b-catenin signaling pathway | NA | DKK3/creatinine ratio > 1.7 pg/mg | [46] |
| β2M(S) | 11.8 | Indicator of renal tubular injury | NA | >2.8 mg/L at baseline | [47] | |
| HSP27(S) | 22 | Protect cells against different forms of cellular stress, including oxidative stress, as well as apoptosis | NA | <19.67 μg/L | [48] | |
| GGT(S) | 68 | Responsible for the extracellular catabolism of glutathione, a major component of intracellular antioxidant protective mechanisms | NA | >26.5 U/L | [49] | |
| Antithrombin III(S) | 58 | ATIII shows anti-inflammatory properties and also enhances renal blood flow. | NA | ATⅢ activity < 75% | [50] | |
| Damage biomarkers | Cys-C(S) | 13 | Indicator of reduced kidney function | 8 h | rise ≥ 10% at 24 h | [51] |
| MK(S) | 13 | Regulates cell growth, cell survival, migration and anti-apoptotic activities in nephrogenesis and development. | 2 h | NA | [52] | |
| IL-18(U) | 18 | Indicator of renal tubular injury | 6 h | 815.61 pg/mL at 12 h | [53] | |
| KIM-1(U) | 85 | A potential biomarker of proximal tubular injury | 6 h | >366 ng/mL | [54] | |
| NGAL(U) | 25 | A potential biomarker of distal tubular injury | 6 h | >20 ng/mL | [55] | |
| NAG(U) | >130 | Indicator of renal tubular injury | 24 h | NA | [56] | |
| L-FABP(U) | 14 | L-FABP can detect renal hemodynamic change following administration of CM. | 24 h | ≥24.5 μg/g Cr | [57] | |
| Other biomarkers | MHR(S) | NA | Correlated with inflammation and has a prognostic value in patients with renal diseases. | NA | >0.95 × 109/mmol | [58] |
| DIN(S) | NA | A novel biomarker for severity of systemic and local inflammatory states | NA | >1.8% on ED admission | [59] | |
| GDF-15(S) | 40 | In response to oxidative stress, endothelial dysfunction, inflammation, and tissue injury, GDF-15 expression was increased. | NA | NA | [60] | |
| hs-CRP(S) | 22.5 | hsCRP is associated with an increased risk of CI-AKI. | NA | hs-CRP > 5 mg/DL | [61] | |
| SII(S) | NA | A relatively novel inflammatory marker combining platelet, neutrophil, and lymphocyte counts | NA | SII > 1282 | [62] | |
| C-Peptide(S) | NA | C-Peptide has a renoprotective effect in diabetic nephropathy. | NA | ≤2.39 ng/mL | [63] | |
| WBV(S) | NA | WBV is related to shear stress, atherosclerosis, and adverse cardiac events. WBV may affect renal function. | NA | WBV < 14.90 | [64] | |
| TyG index(S) | NA | A reliable and specific biomarker for insulin resistance and is associated with renal dysfunction | NA | TyG index > 9.043 | [65] | |
| CAR(S) | NA | An acute-phase reactant and is known to be associated with poor outcomes in predicting the development of CI-AKI | NA | NA | [66] | |
| AIP(S) | NA | Demonstrates plasma atherogenicity by combining TG and HDL-C in a single logarithmic fraction | NA | API > 0.62 | [67] |
| Population Characteristics | Development Dataset | Variables Included in Models | No. of Risk Factors | Risk Stratification | C Statistic | Reference |
|---|---|---|---|---|---|---|
| PCI at one hospital | 5571 | Hypotension | 8 | Low (≤4) Moderate (5–8) High (9–12) Very-high (≥13) | 0.67 | [70] |
| IABP | ||||||
| Heart failure | ||||||
| CKD | ||||||
| Diabetes | ||||||
| Age > 75 years | ||||||
| Anemia | ||||||
| Contrast volume | ||||||
| PCI at one hospital | 1500 | Age ≥ 70 | 9 | Low (≤7) Moderate (8–12) High (13–16) Very-high (≥17) | 0.82 | [71] |
| Prior MI | ||||||
| Diabetes | ||||||
| Hypotension | ||||||
| LVEF ≤ 45% | ||||||
| Anemia | ||||||
| eGFR ≤ 45 (mL/min/1.73 m2) | ||||||
| HDL < 1 mmol/L | ||||||
| Urgent PCI | ||||||
| CAG, PCI, or CECT at one hospital | 7040 | Baseline eGFR | 13 | NA | 0.91 | [72] |
| RDW | ||||||
| Triglycerides | ||||||
| The most recent SCr Before the procedure | ||||||
| HDL | ||||||
| Total cholesterol | ||||||
| LDL | ||||||
| BU | ||||||
| P-LCR | ||||||
| Serum sodium | ||||||
| PCT | ||||||
| INR | ||||||
| BG | ||||||
| CECT at one hospital | 2240 | DM | 4 | Low (0–2) Intermediate (3–4) High (5–6) | 0.73 | [73] |
| Serum albumin level < 4.3 mg/dL | ||||||
| CKD stage 5 | ||||||
| CKD stage 4 | ||||||
| PCI at three hospitals | 931 | Hematocrit | 15 | Low (<10) Moderate (10–16) High (>16) | 0.84 | [67] |
| WBC | ||||||
| Platelet count | ||||||
| MCV | ||||||
| MCHC | ||||||
| RDW | ||||||
| MPV | ||||||
| Sodium | ||||||
| Potassium | ||||||
| Bicarbonate | ||||||
| Calcium | ||||||
| Glucose | ||||||
| Creatinine | ||||||
| Age | ||||||
| Gender | ||||||
| PCI at one hospital | 23,703 | Age | 7 | NA | 0.8 | [74] |
| CKD | ||||||
| Hematocrit | ||||||
| Troponin I | ||||||
| Blood urea nitrogen | ||||||
| Base excess | ||||||
| NT-proBNP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Q.; Gong, X. Unveiling the Mysteries of Contrast-Induced Acute Kidney Injury: New Horizons in Pathogenesis and Prevention. Toxics 2024, 12, 620. https://doi.org/10.3390/toxics12080620
Wang Z, Wang Q, Gong X. Unveiling the Mysteries of Contrast-Induced Acute Kidney Injury: New Horizons in Pathogenesis and Prevention. Toxics. 2024; 12(8):620. https://doi.org/10.3390/toxics12080620
Chicago/Turabian StyleWang, Zhong, Qiuhan Wang, and Xuezhong Gong. 2024. "Unveiling the Mysteries of Contrast-Induced Acute Kidney Injury: New Horizons in Pathogenesis and Prevention" Toxics 12, no. 8: 620. https://doi.org/10.3390/toxics12080620
APA StyleWang, Z., Wang, Q., & Gong, X. (2024). Unveiling the Mysteries of Contrast-Induced Acute Kidney Injury: New Horizons in Pathogenesis and Prevention. Toxics, 12(8), 620. https://doi.org/10.3390/toxics12080620






