The Association between Metals and Thyroid Cancer in Puerto Rico—A National Health and Nutrition Examination Survey Analysis and Ecological Study
Abstract
1. Introduction
2. Materials and Methods
2.1. NHANES Analysis
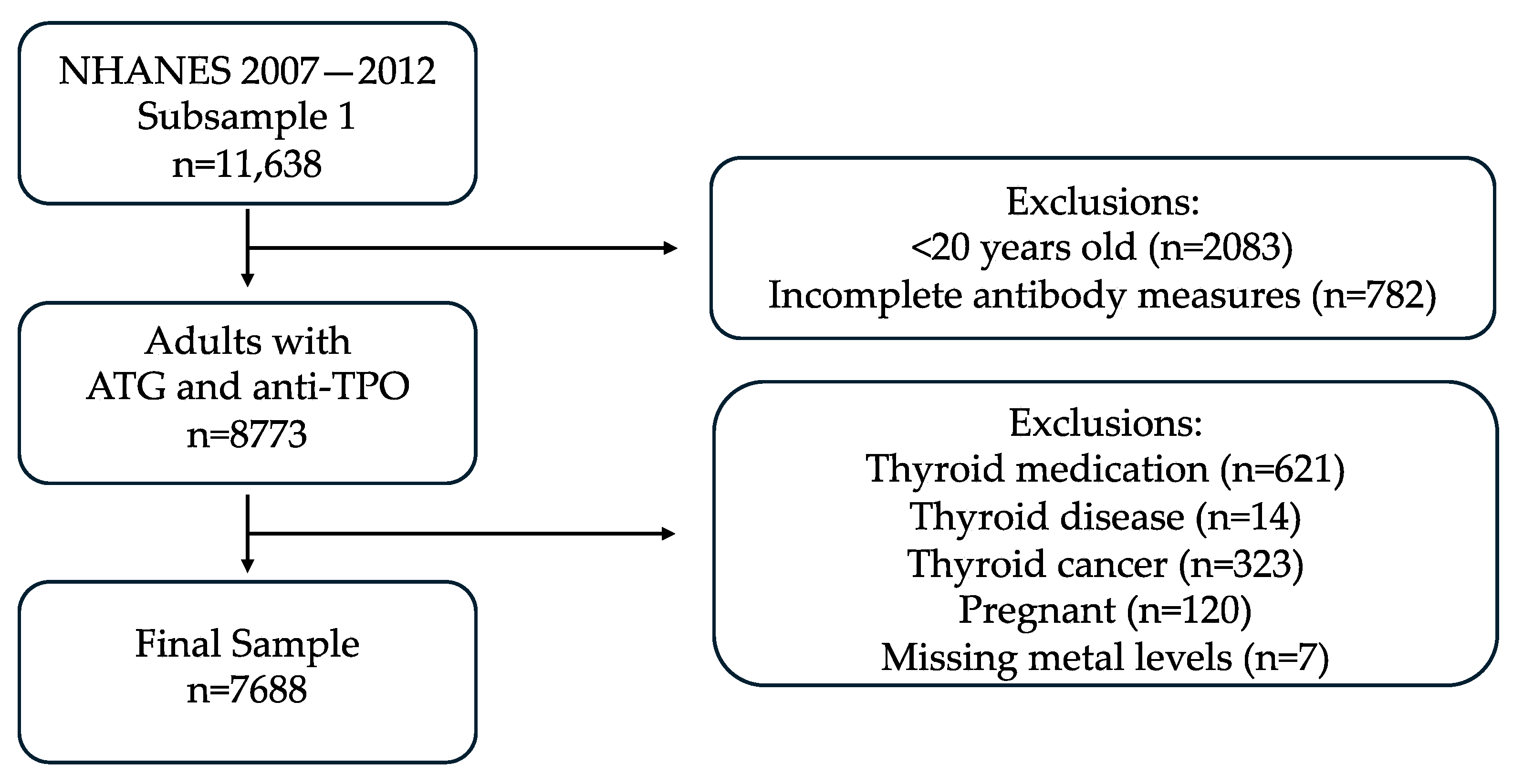
2.1.1. Data Source and Study Population
2.1.2. Outcomes and Covariates
2.1.3. Statistical Analysis
2.2. Ecological Study
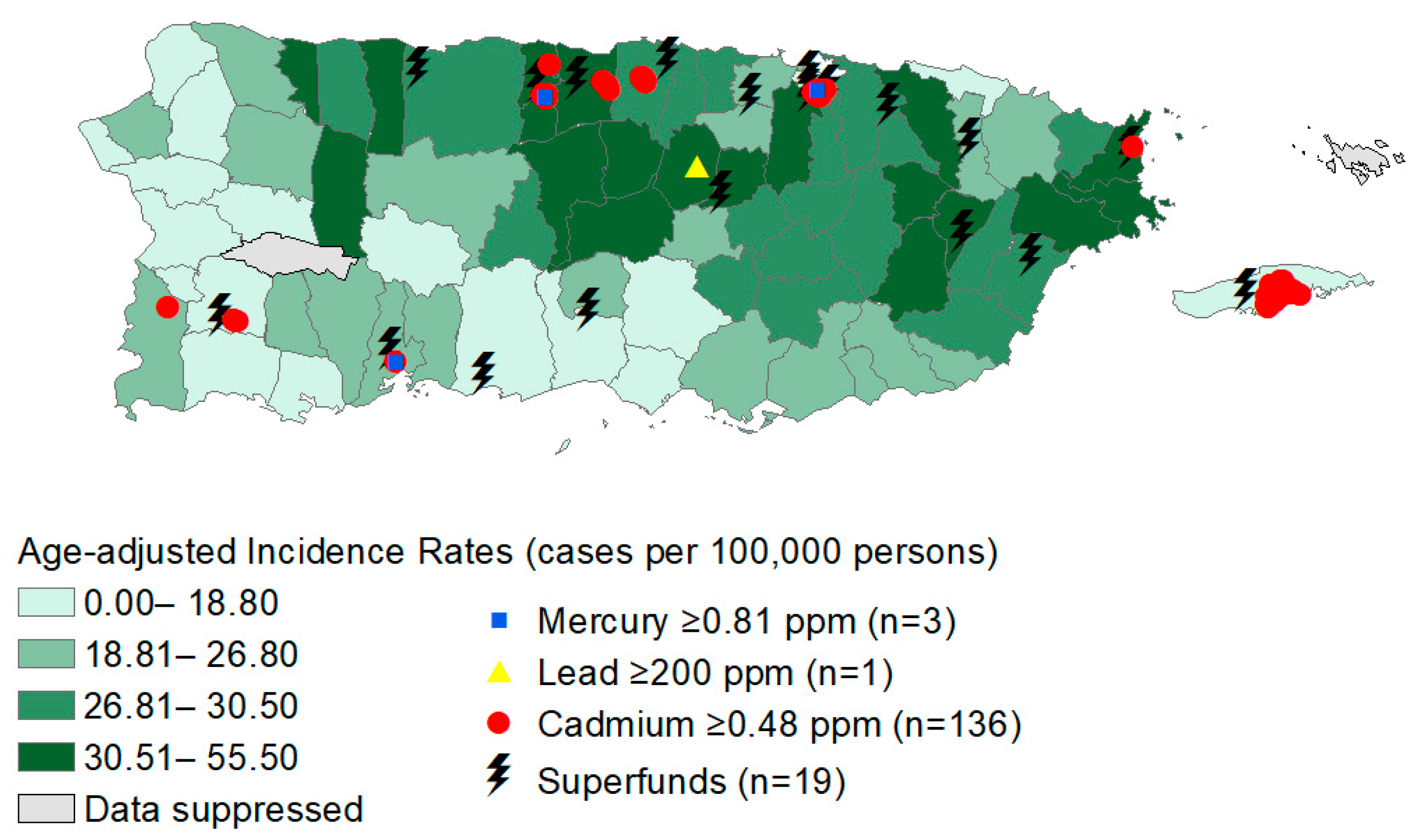
2.2.1. Thyroid Cancer Incidence
2.2.2. Heavy Metal Measurements
2.2.3. Statistical Analysis
3. Results
3.1. NHANES Analysis
3.2. Ecological Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitahara, C.M.; Schneider, A.B. Cancer Progress and Priorities: Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- SEER*Explorer Application. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=80&data_type=1&graph_type=1&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0 (accessed on 10 February 2024).
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The Epidemiological Landscape of Thyroid Cancer Worldwide: GLOBOCAN Estimates for Incidence and Mortality Rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [CrossRef]
- Tortolero-Luna, G.; Torres-Cintrón, C.R.; Alvarado-Ortiz, M.; Ortiz-Ortiz, K.J.; Zavala-Zegarra, D.E.; Mora-Piñero, E. Incidence of Thyroid Cancer in Puerto Rico and the US by Racial/Ethnic Group, 2011–2015. BMC Cancer 2019, 19, 637. [Google Scholar] [CrossRef]
- State Cancer Profiles > Quick Profiles. Available online: https://statecancerprofiles.cancer.gov/quick-profiles/index.php?statename=puertorico (accessed on 11 February 2024).
- Morris, L.G.T.; Sikora, A.G.; Tosteson, T.D.; Davies, L. The Increasing Incidence of Thyroid Cancer: The Influence of Access to Care. Thyroid 2013, 23, 885–891. [Google Scholar] [CrossRef]
- Albi, E.; Cataldi, S.; Lazzarini, A.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Radiation and Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 911. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Port, M.; Landi, S.; Gemignani, F.; Cipollini, M.; Elisei, R.; Goudeva, L.; Müller, J.A.; Nerlich, K.; Pellegrini, G.; et al. Obesity and the Risk of Papillary Thyroid Cancer: A Pooled Analysis of Three Case–Control Studies. Thyroid 2014, 24, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Oliveri Conti, G.; Caltabiano, R.; Buffone, A.; Zuccarello, P.; Cormaci, L.; Cannizzaro, M.A.; Ferrante, M. Role of Emerging Environmental Risk Factors in Thyroid Cancer: A Brief Review. Int. J. Environ. Res. Public Health 2019, 16, 1185. [Google Scholar] [CrossRef]
- Norouzi, F.; Alizadeh, I.; Faraji, M. Human Exposure to Pesticides and Thyroid Cancer: A Worldwide Systematic Review of the Literatures. Thyroid. Res. 2023, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, P.; Russo, M.; Gianì, F.; Pellegriti, G.; Vigneri, P.; Belfiore, A.; Rizzarelli, E.; Vigneri, R. Increased Thyroid Cancer Incidence in Volcanic Areas: A Role of Increased Heavy Metals in the Environment? Int. J. Mol. Sci. 2020, 21, 3425. [Google Scholar] [CrossRef]
- Kolonel, L.N.; Hankin, J.H.; Wilkens, L.R.; Fukunaga, F.H.; Hinds, M.W. An Epidemiologic Study of Thyroid Cancer in Hawaii. Cancer Causes Control 1990, 1, 223–234. [Google Scholar] [CrossRef]
- Thorvaldsson, S.E.; Tulinius, H.; Björnsson, J.; Bjarnason, O. Latent Thyroid Carcinoma in Iceland at Autopsy. Pathol. Res. Pract. 1992, 188, 747–750. [Google Scholar] [CrossRef]
- Brindel, P.; Doyon, F.; Rachédi, F.; Boissin, J.-L.; Sebbag, J.; Shan, L.; Chungue, V.; Bost-Bezeaud, F.; Petitdidier, P.; Paoaafaite, J.; et al. Anthropometric Factors in Differentiated Thyroid Cancer in French Polynesia: A Case-Control Study. Cancer Causes Control 2009, 20, 581–590. [Google Scholar] [CrossRef]
- Pellegriti, G.; De Vathaire, F.; Scollo, C.; Attard, M.; Giordano, C.; Arena, S.; Dardanoni, G.; Frasca, F.; Malandrino, P.; Vermiglio, F.; et al. Papillary Thyroid Cancer Incidence in the Volcanic Area of Sicily. JNCI J. Natl. Cancer Inst. 2009, 101, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- US Department of Commerce, NOAA. Exploring Puerto Rico’s Seamounts, Trenches, and Troughs: Background: Geology: NOAA Office of Ocean Exploration and Research. Available online: https://oceanexplorer.noaa.gov/okeanos/explorations/ex1502/background/geology/welcome.html (accessed on 19 June 2024).
- Navarro-Sempere, A.; García, M.; Rodrigues, A.S.; Garcia, P.V.; Camarinho, R.; Segovia, Y. Occurrence of Volcanogenic Inorganic Mercury in Wild Mice Spinal Cord: Potential Health Implications. Biol. Trace Elem. Res. 2022, 200, 2838–2847. [Google Scholar] [CrossRef]
- Increased Thyroid Cancer Incidence in a Basaltic Volcanic Area is Associated with Non-Anthropogenic Pollution and Biocontamination|Endocrine. Available online: https://link.springer.com/article/10.1007/s12020-015-0761-0 (accessed on 11 February 2024).
- Yang, X.; Cheng, B.; Gao, Y.; Zhang, H.; Liu, L. Heavy Metal Contamination Assessment and Probabilistic Health Risks in Soil and Maize near Coal Mines. Front. Public Health 2022, 10, 1004579. [Google Scholar] [CrossRef]
- Shen, X.; Chi, Y.; Xiong, K. The effect of heavy metal contamination on humans and animals in the vicinity of a zinc smelting facility. PLoS ONE 2019, 14, e0207423. [Google Scholar] [CrossRef]
- Nyiramigisha, P. Harmful Impacts of Heavy Metal Contamination in the Soil and Crops Grown Around Dumpsites. Rev. Agric. Sci. 2021, 9, 271–282. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (.gov). Superfund Sites in Reuse in Puerto Rico. Available online: https://www.epa.gov/superfund-redevelopment/superfund-sites-reuse-puerto-rico (accessed on 11 February 2024).
- Aviles, J.; Cuevas, D.; Cutt, D.; Maddaloni, M.; Mishkin, K.; Nace, C.; Wilkin, R.; Seda, R. Metals from Natural and Anthropogenic Sources in Puerto Rico Soils; EPA/600/F-18/382; U.S. EPA Office of Research and Development: Washington, DC, USA, 2019. [Google Scholar]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy Metals in the Volcanic Environment and Thyroid Cancer. Mol. Cell. Endocrinol. 2017, 457, 73–80. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, M.; Alerte, E.; Alsen, M.; Little, C.; Sinclair, C.; Genden, E. The Role of Heavy Metals in Thyroid Cancer: A Meta-Analysis. J. Trace Elem. Med. Biol. 2022, 69, 126900. [Google Scholar] [CrossRef]
- Jo, K.; Lim, D.-J. Clinical Implications of Anti-Thyroglobulin Antibody Measurement before Surgery in Thyroid Cancer. Korean J. Intern. Med. 2018, 33, 1050–1057. [Google Scholar] [CrossRef]
- Thyroid Peroxidase Antibody Test: What Is It? Mayo Clinic. Available online: https://www.mayoclinic.org/thyroid-disease/expert-answers/faq-20058114 (accessed on 12 February 2024).
- Fröhlich, E.; Wahl, R. Thyroid Autoimmunity: Role of Anti-Thyroid Antibodies in Thyroid and Extra-Thyroidal Diseases. Front. Immunol. 2017, 8, 521. [Google Scholar] [CrossRef]
- Spencer, C.A.; Takeuchi, M.; Kazarosyan, M.; Wang, C.C.; Guttler, R.B.; Singer, P.A.; Fatemi, S.; LoPresti, J.S.; Nicoloff, J.T. Serum Thyroglobulin Autoantibodies: Prevalence, Influence on Serum Thyroglobulin Measurement, and Prognostic Significance in Patients with Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 1998, 83, 1121–1127. [Google Scholar] [CrossRef]
- Hosseini, S.; Payne, R.J.; Zawawi, F.; Mlynarek, A.; Hier, M.P.; Tamilia, M.; Forest, V.I. Can Preoperative Thyroglobulin Antibody Levels Be Used as a Marker for Well Differentiated Thyroid Cancer? J. Otolaryngol. Head Neck Surg. 2016, 45, 31. [Google Scholar] [CrossRef] [PubMed]
- Yorita Christensen, K.L. Metals in Blood and Urine, and Thyroid Function among Adults in the United States 2007–2008. Int. J. Hyg. Environ. Health 2013, 216, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Relationships of Thyroid Hormones with Polychlorinated Biphenyls, Dioxins, Furans, and DDE in Adults|Environmental Health Perspectives|Volume 115, No. 8. Available online: https://ehp.niehs.nih.gov/doi/10.1289/ehp.10179 (accessed on 11 February 2024).
- NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/ (accessed on 9 May 2024).
- NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/AnalyticGuidelines.aspx (accessed on 9 May 2024).
- NHANES 2007–2008: Cadmium, Lead, & Total Mercury—Blood Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/PBCD_E.htm (accessed on 10 May 2024).
- NHANES 2009–2010: Cadmium, Lead, & Total Mercury—Blood Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2009-2010/PBCD_F.htm (accessed on 10 May 2024).
- NHANES 2011–2012: Cadmium, Lead, Total Mercury, Selenium, & Manganese—Blood Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/PBCD_G.htm (accessed on 10 May 2024).
- CDC. Defining Adult Overweight and Obesity. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/obesity/index.html (accessed on 10 May 2024).
- Iodine Deficiency. Available online: https://www.who.int/data/nutrition/nlis/info/iodine-deficiency (accessed on 10 May 2024).
- Benowitz, N.L.; Bernert, J.T.; Caraballo, R.S.; Holiday, D.B.; Wang, J. Optimal Serum Cotinine Levels for Distinguishing Cigarette Smokers and Nonsmokers within Different Racial/Ethnic Groups in the United States between 1999 and 2004. Am. J. Epidemiol. 2009, 169, 236–248. [Google Scholar] [CrossRef]
- Registro Central de Cancer de Puerto Rico. Tasas y Mapas, 2015–2019. Available online: https://rcpr.org/Datos-de-C%C3%A1ncer/Tasas-y-Mapas (accessed on 12 October 2022).
- Streets, D.G.; Devane, M.K.; Lu, Z.; Bond, T.C.; Sunderland, E.M.; Jacob, D.J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491. [Google Scholar] [CrossRef]
- Pamphlett, R.; Doble, P.A.; Bishop, D.P. Mercury in the Human Thyroid Gland: Potential Implications for Thyroid Cancer, Autoimmune Thyroiditis, and Hypothyroidism. PLoS ONE 2021, 16, e0246748. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, P.; Russo, M.; Ronchi, A.; Moretti, F.; Gianì, F.; Vigneri, P.; Masucci, R.; Pellegriti, G.; Belfiore, A.; Vigneri, R. Concentration of Metals and Trace Elements in the Normal Human and Rat Thyroid: Comparison with Muscle and Adipose Tissue and Volcanic versus Control Areas. Thyroid 2020, 30, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Aschner, M.; Sekacheva, M.I.; Santamaria, A.; Barbosa, F.; Ferrer, B.; Aaseth, J.; Paoliello, M.M.B.; Rocha, J.B.T.; Tinkov, A.A. Mercury and Cancer: Where Are We Now after Two Decades of Research? Food Chem. Toxicol. 2022, 164, 113001. [Google Scholar] [CrossRef]
- Zaichick, V. Content of 31 Trace Elements in Thyroid Malignant Nodules and Thyroid Tissue Adjacent to Nodules Investigated Using Neutron Activation Analysis and Inductively Coupled Plasma Mass Spectrometry. World J. Adv. Res. Rev. 2022, 13, 718–733. [Google Scholar] [CrossRef]
- Kim, S.; Song, S.-H.; Lee, C.-W.; Kwon, J.-T.; Park, E.Y.; Oh, J.-K.; Kim, H.-J.; Park, E.; Kim, B. Low-Leve Environmental Mercury Exposure and Thyroid Cancer Risk Among Residents Living Near National Industrial Complexes in South Korea: A Population-Based Cohort Study. Thyroid 2022, 32, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.M.; Pinion, D.; Pineda, E.; Aboueisha, H.; Hussein, M.H.; Fawzy, M.S.; Toraih, E.A.; Kandil, E. Elucidating the Link between Thyroid Cancer and Mercury Exposure: A Review and Meta-Analysis. Environ. Sci. Pollut. Res. 2024, 31, 12841–12855. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Unoki, T.; Abiko, Y.; Toyama, T.; Uehara, T.; Tsuboi, K.; Nishida, M.; Kaji, T.; Kumagai, Y. Methylmercury, an Environmental Electrophile Capable of Activation and Disruption of the Akt/CREB/Bcl-2 Signal Transduction Pathway in SH-SY5Y Cells. Sci. Rep. 2016, 6, 28944. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Rannug, A. Arsenic, Cadmium, Mercury and Nickel Stimulate Cell Growth via NADPH Oxidase Activation. Chem.Biol. Interact. 2014, 224, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Baralić, K.; Djukic-Cosic, D.; Bulat, Z.; Tinkov, A.; Panieri, E.; Saso, L. The Role of Toxic Metals and Metalloids in Nrf2 Signaling. Antioxidants 2021, 10, 630. [Google Scholar] [CrossRef]
- Ziros, P.G.; Manolakou, S.D.; Habeos, I.G.; Lilis, I.; Chartoumpekis, D.V.; Koika, V.; Soares, P.; Kyriazopoulou, V.E.; Scopa, C.D.; Papachristou, D.J.; et al. Nrf2 Is Commonly Activated in Papillary Thyroid Carcinoma, and It Controls Antioxidant Transcriptional Responses and Viability of Cancer Cells. J. Clin. Endocrinol. Metab. 2013, 98, E1422–E1427. [Google Scholar] [CrossRef]
- Rossi, E. Low Level Environmental Lead Exposure—A Continuing Challenge. Clin. Biochem. Rev. 2008, 29, 63–70. [Google Scholar]
- Landrigan, P.; Baloh, R.; Barthel, W.; Whitworth, R.; Staehling, N.; Rosenblum, B. Neuropsychological dysfunction in children with chronic low-level lead absorption. Lancet 1975, 305, 708–712. [Google Scholar] [CrossRef]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, L.; Qin, B.; Wang, N.; Li, X.; Shi, W. Association between Urinary Lead and Female Breast Cancer: A Population-Based Cross-Sectional Study. Discov. Med. 2023, 35, 1177–1189. [Google Scholar] [CrossRef]
- Rhee, J.; Graubard, B.I.; Purdue, M.P. Blood Lead Levels and Lung Cancer Mortality: An Updated Analysis of NHANES II and III. Cancer Med. 2021, 10, 4066–4074. [Google Scholar] [CrossRef] [PubMed]
- Dw, S. The Influence of Various Factors on the Uptake of Iodine by the Thyroid. J. Clin. Endocrinol. Metab. 1955, 15, 131–141. [Google Scholar] [CrossRef]
- Fahim, Y.A.; Sharaf, N.E.; Hasani, I.W.; Ragab, E.A.; Abdelhakim, H.K. Assessment of Thyroid Function and Oxidative Stress State in Foundry Workers Exposed to Lead. J. Health Pollut. 2020, 10, 200903. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, H.; Keten, A.; Karacaoğlu, E.; Tutkun, E.; Akçan, R. Analysis of the Hematological and Biochemical Parameters Related to Lead Intoxication. J. Forensic Leg. Med. 2012, 19, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Pekcici, R.; Kavlakoğlu, B.; Yilmaz, S.; Şahin, M.; Delibaşi, T. Effects of Lead on Thyroid Functions in Lead-Exposed Workers. Open Med. 2010, 5, 215–218. [Google Scholar] [CrossRef]
- Liu, B.; Qin, H.; Zhang, B.; Shi, T.; Cui, Y. Enhanced Oxidative Stress by Lead Toxicity Retards Cell Survival in Primary Thyroid Cells. Available online: https://www.semanticscholar.org/paper/Enhanced-oxidative-stress-by-lead-toxicity-retards-Liu-Qin/bf4d2a09d01f1f5a86618dd03fd2d2bb18dc8f9b (accessed on 8 May 2024).
- Li, H.; Li, X.; Liu, J.; Jin, L.; Yang, F.; Wang, J.; Wang, O.; Gao, Y. Correlation between Serum Lead and Thyroid Diseases: Papillary Thyroid Carcinoma, Nodular Goiter, and Thyroid Adenoma. Int. J. Environ. Health Res. 2017, 27, 409–419. [Google Scholar] [CrossRef]
- Chung, H.-K.; Nam, J.S.; Ahn, C.W.; Lee, Y.S.; Kim, K.R. SomeElements in Thyroid Tissue Are Associated with More Advanced Stage of Thyroid Cancer in Korean Women. Biol. Trace Elem. Res. 2016, 171, 54–62. [Google Scholar] [CrossRef]
- Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. [Google Scholar] [CrossRef]
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein Protection of Cadmium Toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef]
- Sa, J.; Bz, S. Cadmium Effects on the Thyroid Gland. Vitam. Horm. 2014, 94, 391–425. [Google Scholar] [CrossRef]
- Al-Assaf, A.H.; Alqahtani, A.M.; Alshatwi, A.A.; Syed, N.A.; Shafi, G.; Hasan, T.N. Mechanism of Cadmium Induced Apoptosis in Human Peripheral Blood Lymphocytes: The Role of P53, Fas and Caspase-3. Environmental toxicology and pharmacology 2013, 36, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Cadmium Triggers Mitochondrial Oxidative Stress in Human Peripheral Blood Lymphocytes and Monocytes: Analysis Using In Vitro and System Toxicology Approaches. J. Trace Elements Med. Biol. 2017, 42, 117–128. [Google Scholar] [CrossRef]
- Chen, A.; Kim, S.S.; Chung, E.; Dietrich, K.N. Thyroid Hormones in Relation to Lead, Mercury, and Cadmium Exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ. Health Perspect. 2013, 121, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.A.; Eldin, A.S.S. Effect of Occupational Cadmium Exposure on the Thyroid Gland and Associated Inflammatory Markers among Workers of the Electroplating Industry. Toxicol. Ind. Health 2022, 38, 210–220. [Google Scholar] [CrossRef]
- US EPA. Puerto Rico Southern Aquifer Water Sampling Project. Available online: https://www.epa.gov/coalash/puerto-rico-southern-aquifer-water-sampling-project (accessed on 23 March 2024).
- Duan, W.; Xu, C.; Liu, Q.; Xu, J.; Weng, Z.; Zhang, X.; Basnet, T.B.; Dahal, M.; Gu, A. Levels of a Mixture of Heavy Metals in Blood and Urine and All-Cause, Cardiovascular Disease and Cancer Mortality: A Population-Based Cohort Study. Environ. Pollut. 2020, 263, 114630. [Google Scholar] [CrossRef]
| Variables | Total Population | Lead (μg/dL) | Cadmium (μg/L) | Mercury (μg/L) |
|---|---|---|---|---|
| n (%) | GM (SE) | GM (SE) | GM (SE) | |
| Sex | ||||
| Male | 4098 (52.9) | 1.479 (0.025) | 0.328 (0.007) | 1.008 (0.042) |
| Female | 3590 (47.1) | 1.048 (0.017) | 0.391 (0.008) | 0.909 (0.034) |
| Age (years) | ||||
| <40 | 2665 (38.6) | 0.931 (0.016) | 0.302 (0.007) | 0.819 (0.038) |
| 40–59 | 2552 (39.4) | 1.406 (0.025) | 0.389 (0.009) | 1.079 (0.047) |
| ≥60 | 2471 (21.9) | 1.747 (0.034) | 0.406 (0.008) | 1.029 (0.049) |
| Race/Ethnicity | ||||
| Non-Hispanic White | 3422 (67.8) | 1.250 (0.024) | 0.352 (0.007) | 0.912 (0.039) |
| Non-Hispanic Black | 1592 (11.2) | 1.303 (0.038) | 0.406 (0.009) | 0.988 (0.045) |
| Other | 2674 (20.9) | 1.258 (0.034) | 0.344 (0.009) | 1.115 (0.067) |
| Body Mass Index (kg/m2) | ||||
| <25 | 2369 (33.2) | 1.292 (0.032) | 0.403 (0.013) | 1.023 (0.049) |
| 25–30 | 2613 (34.1) | 1.325 (0.030) | 0.350 (0.012) | 1.018 (0.043) |
| ≥30 | 2706 (32.7) | 1.158 (0.021) | 0.319 (0.005) | 0.846 (0.031) |
| Smoking Status | ||||
| Non-smoker | 4418 (62.3) | 1.158 (0.019) | 0.255 (0.004) | 0.969 (0.035) |
| Smoker | 3270 (37.7) | 1.440 (0.029) | 0.617 (0.016) | 0.944 (0.045) |
| Ln TgAb (IU/mL) | Ln anti-TPO (IU/mL) | |
|---|---|---|
| Badj * (95% CI) | Badj * (95% CI) | |
| Ln Lead | 0.034 (−0.022, 0.089) | 0.074 (−0.014, 0.162) |
| Ln Cadmium | −0.014 (−0.063, 0.036) | 0.0003 (−0.071, 0.072) |
| Ln Mercury | −0.034 (−0.064, −0.005) | 0.006 (−0.049, 0.060) |
| Ln TgAb (IU/mL) | Ln Anti-TPO (IU/mL) | |
|---|---|---|
| Badj * (95% CI) | Badj * (95% CI) | |
| Ln Lead | ||
| Quartile 1 | Ref | Ref |
| Quartile 2 | 0.005 (−0.072, 0.082) | 0.006 (−0.122, 0.134) |
| Quartile 3 | 0.006 (−0.101, 0.114) | 0.008 (−0.136, 0.151) |
| Quartile 4 | 0.027 (−0.081, 0.136) | 0.100 (−0.054, 0.253) |
| Ln Cadmium | ||
| Quartile 1 | Ref | Ref |
| Quartile 2 | 0.040 (−0.067, 0.147) | 0.140 (−0.061, 0.341) |
| Quartile 3 | 0.018 (−0.088, 0.123) | 0.114 (−0.040, 0.268) |
| Quartile 4 | 0.013 (−0.100, 0.126) | 0.110 (−0.090, 0.310) |
| Ln Mercury | ||
| Quartile 1 | Ref | Ref |
| Quartile 2 | −0.052 (−0.147, 0.042) | −0.065 (−0.210, 0.080) |
| Quartile 3 | −0.051 (−0.135, 0.033) | 0.005 (−0.169, 0.180) |
| Quartile 4 | −0.094 (−0.185, −0.003) | −0.050 (−0.206, 0.107) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaked, Y.; Yang, J.; Monaghan, M.; van Gerwen, M. The Association between Metals and Thyroid Cancer in Puerto Rico—A National Health and Nutrition Examination Survey Analysis and Ecological Study. Toxics 2024, 12, 632. https://doi.org/10.3390/toxics12090632
Shaked Y, Yang J, Monaghan M, van Gerwen M. The Association between Metals and Thyroid Cancer in Puerto Rico—A National Health and Nutrition Examination Survey Analysis and Ecological Study. Toxics. 2024; 12(9):632. https://doi.org/10.3390/toxics12090632
Chicago/Turabian StyleShaked, Yaelle, Jessica Yang, Mathilda Monaghan, and Maaike van Gerwen. 2024. "The Association between Metals and Thyroid Cancer in Puerto Rico—A National Health and Nutrition Examination Survey Analysis and Ecological Study" Toxics 12, no. 9: 632. https://doi.org/10.3390/toxics12090632
APA StyleShaked, Y., Yang, J., Monaghan, M., & van Gerwen, M. (2024). The Association between Metals and Thyroid Cancer in Puerto Rico—A National Health and Nutrition Examination Survey Analysis and Ecological Study. Toxics, 12(9), 632. https://doi.org/10.3390/toxics12090632







