Cadmium Exposure and Noncommunicable Diseases in Environmentally Exposed Brazilian Population: Cross-Sectional Study without Association of GSTP1 Polymorphism
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Evaluation
2.2. Cadmium Determination
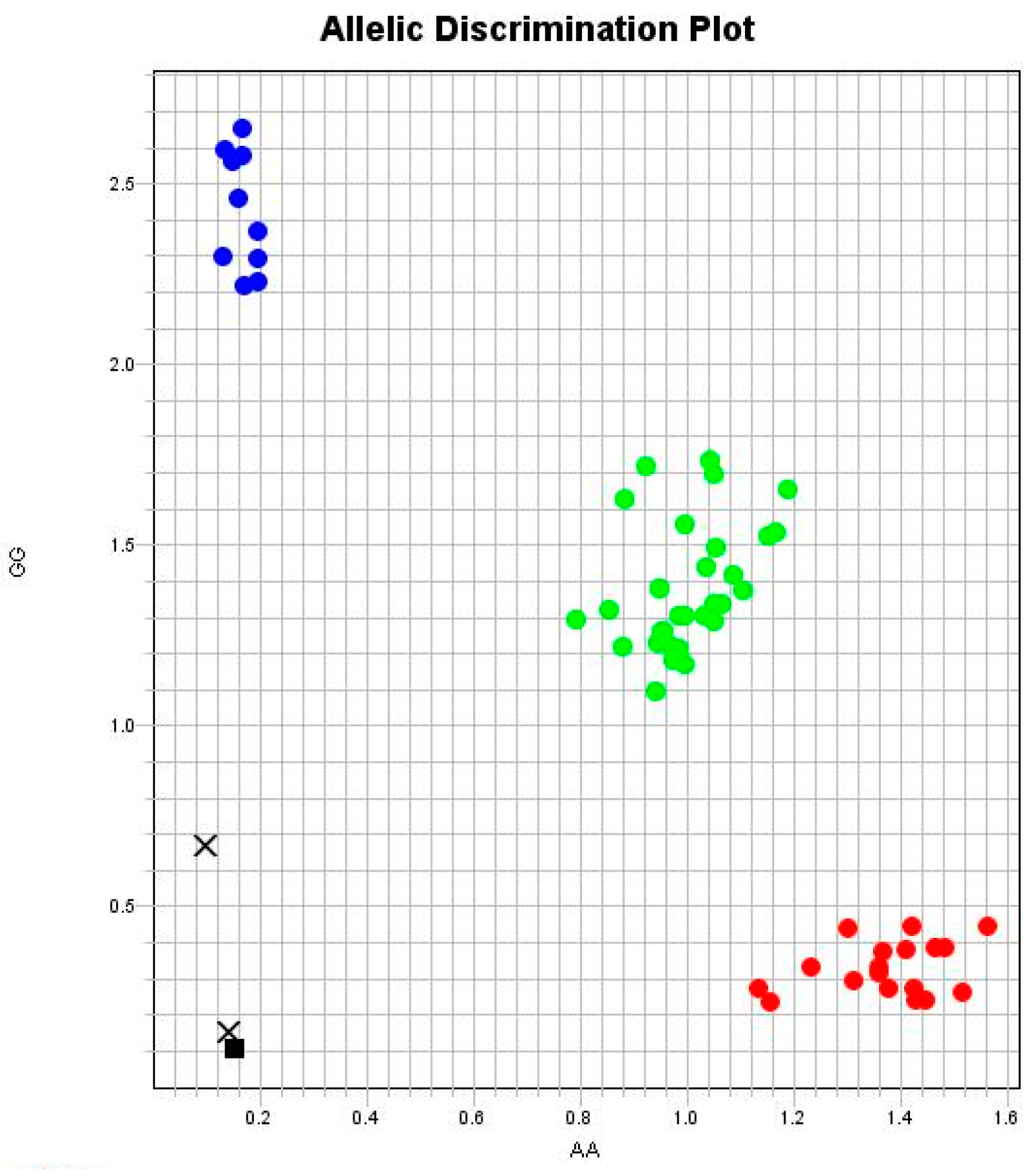
2.3. Polymorphism Genotyping
2.4. Statistical Analysis
3. Results
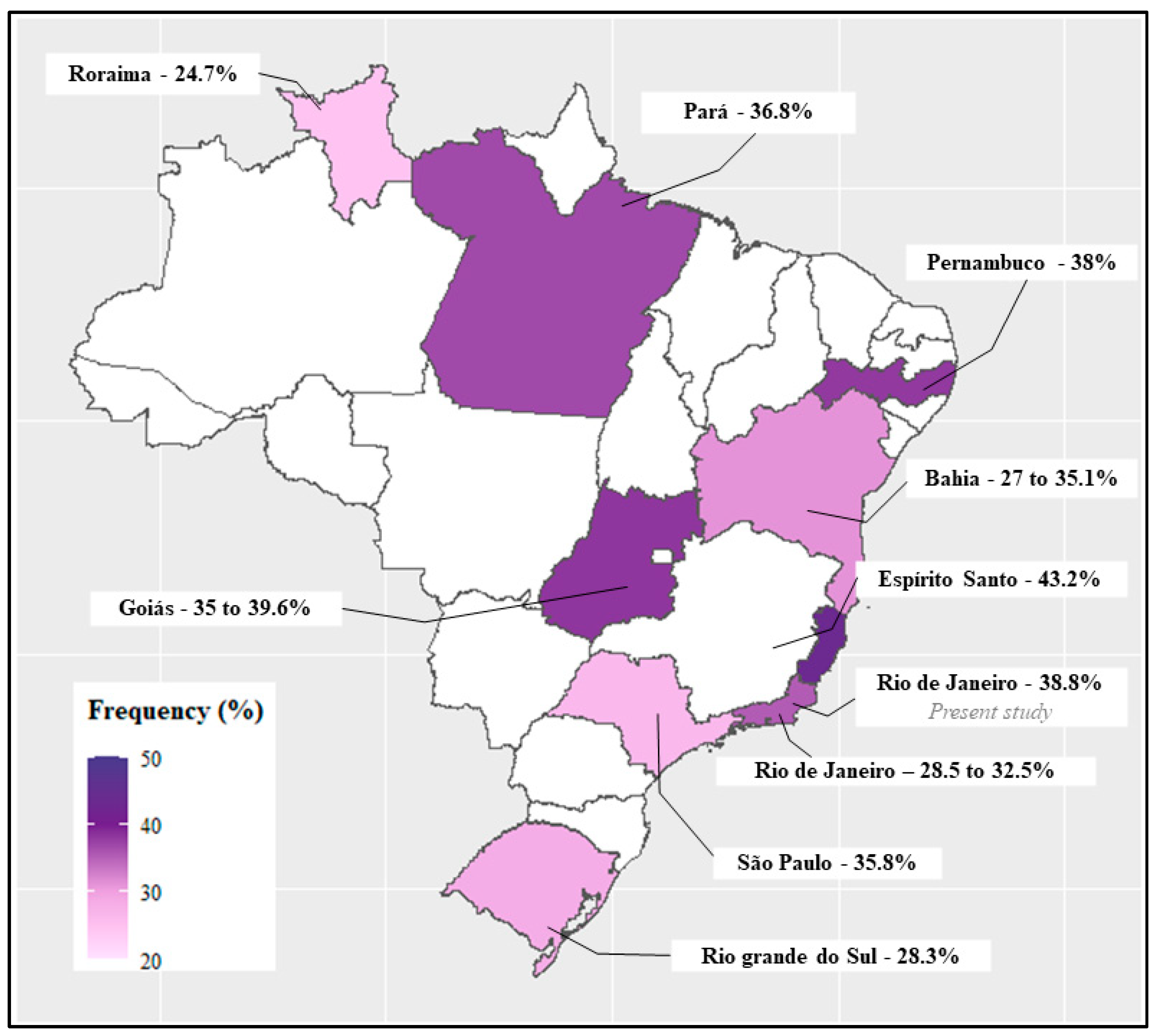
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagliari, B.G.; Moreira, M.D.F.R.; Mannarino, C.F.; Santos, G.B.D. Risk of Exposure to Metals in Soil Contaminated by Steel Industry Waste for a Population in Volta Redonda, RJ. Rev. Ambiente Água 2021, 16, 1. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A Review of Toxicity and Mechanisms of Individual and Mixtures of Heavy Metals in the Environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Chen, Y.; Qu, J.; Sun, S.; Shi, Q.; Feng, H.; Zhang, Y.; Cao, S. Health Risk Assessment of Total Exposure from Cadmium in South China. Chemosphere 2021, 269, 128673. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-L.; Fei, J.; Cao, P.; Zhang, C.; Tang, M.-M.; Cheng, J.-Y.; Zhao, H.; Fu, L. Serum Cadmium Positively Correlates with Inflammatory Cytokines in Patients with Chronic Obstructive Pulmonary Disease. Environ. Toxicol. 2022, 37, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Marzec, J.M.; Nadadur, S.S. Inflammation Resolution in Environmental Pulmonary Health and Morbidity. Toxicol. Appl. Pharmacol. 2022, 449, 116070. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Dalkiran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef]
- Rana, M.N.; Tangpong, J.; Rahman, M.M. Toxicodynamics of Lead, Cadmium, Mercury and Arsenic- Induced Kidney Toxicity and Treatment Strategy: A Mini Review. Toxicol. Rep. 2018, 5, 704–713. [Google Scholar] [CrossRef]
- Riederer, A.M.; Belova, A.; George, B.J.; Anastas, P.T. Urinary Cadmium in the 1999–2008 U.S. National Health and Nutrition Examination Survey (NHANES). Environ. Sci. Technol. 2013, 47, 1137–1147. [Google Scholar] [CrossRef]
- Yang, J.; Lo, K.; Yang, A. Trends in Urinary and Blood Cadmium Levels in U.S. Adults with or without Comorbidities, 1999–2018. Nutrients 2022, 14, 802. [Google Scholar] [CrossRef] [PubMed]
- Khansakorn, N.; Wongwit, W.; Tharnpoophasiam, P.; Hengprasith, B.; Suwannathon, L.; Chanprasertyothin, S.; Sura, T.; Kaojarern, S.; Sritara, P.; Sirivarasai, J. Genetic Variations of Glutathione S-Transferase Influence on Blood Cadmium Concentration. J. Toxicol. 2012, 2012, 356126. [Google Scholar] [CrossRef] [PubMed]
- Rossini, A.; Rapozo, D.C.M.; Amorim, L.M.F.; Macedo, J.M.B.; Medina, R.; Neto, J.F.N.; Gallo, C.V.M.; Pinto, L.F.R. Frequencies of GSTM1, GSTT1, and GSTP1 Polymorphisms in a Brazilian Population. Genet. Mol. Res. 2002, 1, 233–240. [Google Scholar] [PubMed]
- Da Silva, J.; Moraes, C.R.; Heuser, V.D.; Andrade, V.M.; Silva, F.R.; Kvitko, K.; Emmel, V.; Rohr, P.; Bordin, D.L.; Andreazza, A.C.; et al. Evaluation of Genetic Damage in a Brazilian Population Occupationally Exposed to Pesticides and Its Correlation with Polymorphisms in Metabolizing Genes. Mutagenesis 2008, 23, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.C.; Jones, P.W.; Fryer, A.A. Glutathione S-Transferase: Genetics and Role in Toxicology. Toxicol. Lett. 2000, 112–113, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, A.; Behjati, M.; Karimian, A. Association Analysis of GSTP1-Rs1695 Polymorphism with the Risk of Oral Cancer: A Literature Review, an Updated Meta- Analysis, and a Structural Assessment. Asian Pac. J. Cancer Prev. 2022, 23, 3859–3868. [Google Scholar] [CrossRef]
- Perini, J.A.; Silva, M.C.D.; Correa, L.V.; Silva, Y.M.; Borges, R.M.; Moreira, M.D.F.R. Chronic Cadmium Exposure and Genetic Polymorphisms of MMP-2 and MMP-9 in a Population Exposed to Steel Slag in the State of Rio de Janeiro, Brazil: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 15304. [Google Scholar] [CrossRef]
- Silva, M.C.D.; Oliveira, R.A.A.D.; Vasconcellos, A.C.S.D.; Rebouças, B.H.; Pinto, B.D.; Lima, M.D.O.; Jesus, I.M.D.; Machado, D.E.; Hacon, S.S.; Basta, P.C.; et al. Chronic Mercury Exposure and GSTP1 Polymorphism in Munduruku Indigenous from Brazilian Amazon. Toxics 2023, 11, 138. [Google Scholar] [CrossRef]
- Da Silva, M.C.; Basta, P.C.; Hofer, C.B.; de Oliveira, M.A.F.; Kempton, J.W.; de Oliveira, R.A.A.; de Vasconcellos, A.C.S.; Perini, J.A. The GSTP1 Rs1695 Polymorphism Is Associated with Mercury Levels and Neurodevelopmental Delay in Indigenous Munduruku Children from the Brazilian Amazon. Toxics 2024, 12, 441. [Google Scholar] [CrossRef]
- Rossini, A.; Rapozo, D.C.M.; Soares Lima, S.C.; Guimarães, D.P.; Ferreira, M.A.; Teixeira, R.; Kruel, C.D.P.; Barros, S.G.S.; Andreollo, N.A.; Acatauassú, R.; et al. Polymorphisms of GSTP1 and GSTT1, but Not of CYP2A6, CYP2E1 or GSTM1, Modify the Risk for Esophageal Cancer in a Western Population. Carcinogenesis 2007, 28, 2537–2542. [Google Scholar] [CrossRef]
- Hatagima, A.; Costa, E.C.B.; Marques, C.F.S.; Koifman, R.J.; Boffetta, P.; Koifman, S. Glutathione S-Transferase Polymorphisms and Oral Cancer: A Case-Control Study in Rio de Janeiro, Brazil. Oral. Oncol. 2008, 44, 200–207. [Google Scholar] [CrossRef]
- Honma, H.N.; De Capitani, E.M.; Perroud, M.W.; Barbeiro, A.S.; Toro, I.F.C.; Costa, D.B.; Lima, C.S.P.; Zambon, L. Influence of P53 Codon 72 Exon 4, GSTM1, GSTT1 and GSTP1*B Polymorphisms in Lung Cancer Risk in a Brazilian Population. Lung Cancer 2008, 61, 152–162. [Google Scholar] [CrossRef]
- Magno, L.A.V.; Talbot, J.; Talbot, T.; Borges Santos, A.M.; Souza, R.P.; Marin, L.J.; Moreli, M.L.; De Melo, P.R.S.; Corrêa, R.X.; Rios Santos, F.; et al. Glutathione S-Transferase Variants in a Brazilian Population. Pharmacology 2009, 83, 231–236. [Google Scholar] [CrossRef]
- Rocha, A.V.; Talbot, T.; Magalhães da Silva, T.; Almeida, M.C.; Menezes, C.A.; Di Pietro, G.; Rios-Santos, F. Is the GSTM1 Null Polymorphism a Risk Factor in Primary Open Angle Glaucoma? Mol. Vis. 2011, 17, 1679–1686. [Google Scholar]
- Lima Junior, M.M.D.; Reis, L.O.; Ferreira, U.; Cardoso, U.O.; Barbieri, R.B.; Mendonça, G.B.D.; Ward, L.S. Unraveling Brazilian Indian Population Prostate Good Health: Clinical, Anthropometric and Genetic Features. Int. Braz. J. Urol. 2015, 41, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Chagas, B.S.; Gurgel, A.P.A.D.; Júnior, S.S.L.P.; Lima, R.C.P.; Cordeiro, M.N.; Moura, R.R.; Coelho, A.V.C.; Nascimento, K.C.G.; Neto, J.C.S.; Crovella, S.; et al. Research Article Synergic Effect of Oral Contraceptives, GSTP1 Polymorphisms, and High-Risk HPV Infection in Development of Cervical Lesions. Genet. Mol. Res. 2017, 16, gmr16039742. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.V.W.; Alves, L.N.R.; Louro, I.D. Steroid Metabolism Gene Polymorphisms and Their Implications on Breast and Ovarian Cancer Prognosis. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.M.; Dos Anjos, L.R.B.; Alves, T.B.; Coelho, A.S.G.; Pedrino, G.R.; da Silva Santos, R.; da Silva Cruz, A.H.; da Silva Reis, A.A. Do GST Polymorphisms Influence in the Pathogenesis of Diabetic Nephropathy? Mol. Cell Endocrinol. 2018, 478, 10–16. [Google Scholar] [CrossRef]
- Oliveira De Araújo Melo, C.; Cidália Vieira, T.; Duarte Gigonzac, M.A.; Soares Fortes, J.; Moreira Duarte, S.S.; Da Cruz, A.D.; Silva, D.D.M.E. Evaluation of Polymorphisms in Repair and Detoxification Genes in Alcohol Drinkers and Non-drinkers Using Capillary Electrophoresis. Electrophoresis 2020, 41, 254–258. [Google Scholar] [CrossRef]
- Barros, J.B.d.S.; Santos, K.d.F.; Azevedo, R.M.; de Oliveira, R.P.D.; Leobas, A.C.D.; Bento, D.d.C.P.; Santos, R.d.S.; Reis, A.A.d.S. No Association of GSTP1 Rs1695 Polymorphism with Amyotrophic Lateral Sclerosis: A Case-Control Study in the Brazilian Population. PLoS ONE 2021, 16, e0247024. [Google Scholar] [CrossRef]
- de Sousa Barros, J.B.; de Faria Santos, K.; da Cruz Pereira Bento, D.; do Prado Assunção, L.; da Silva Santos, R.; da Silva Reis, A.A. Influence of GSTP1 Rs1695 Polymorphism on Survival in Male Patients’ Amyotrophic Lateral Sclerosis: A Genetic Association Study in Brazilian Population. Mol. Biol. Rep. 2022, 49, 1655–1659. [Google Scholar] [CrossRef]
- Sigel, R.K.O.; Skilandat, M.; Sigel, A.; Operschall, B.P.; Sigel, H. Complex Formation of Cadmium with Sugar Residues, Nucleobases, Phosphates, Nucleotides, and Nucleic Acids. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K., Eds.; Metal Ions in Life Sciences; Springer Netherlands: Dordrecht, The Netherlands, 2013; Volume 11, pp. 191–274. ISBN 978-94-007-5178-1. [Google Scholar]
- Bernhoft, R.A. Cadmium Toxicity and Treatment. Sci. World J. 2013, 2013, 394652. [Google Scholar] [CrossRef]
- Kim, J.; Song, H.; Lee, J.; Kim, Y.J.; Chung, H.S.; Yu, J.M.; Jang, G.; Park, R.; Chung, W.; Oh, C.-M.; et al. Smoking and Passive Smoking Increases Mortality through Mediation Effect of Cadmium Exposure in the United States. Sci. Rep. 2023, 13, 3878. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.P.K.; Michalke, B.; Geoghegan, G.; Heverin, M.; Bose-O’Reilly, S.; Hardiman, O.; Rakete, S. Urine Concentrations of Selected Trace Metals in a Cohort of Irish Adults. Env. Sci. Pollut. Res. 2022, 29, 75356–75364. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-Y.; Eom, S.-Y.; Seo, J.-W.; Lee, J.-E.; Choi, B.-S.; Kim, H.; Hong, Y.-S.; Chang, J.Y.; Jeon, M.-J.; Park, W.-J.; et al. Effects of Exposure to Lead and Cadmium on Health of Inhabitants of Abandoned Metal Mine Area in Korea. Arch. Env. Contam. Toxicol. 2021, 80, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, J.; Kim, B.; Park, E.Y. Association between Environmental Exposure to Cadmium and Risk of Suspected Non-Alcoholic Fatty Liver Disease. Chemosphere 2021, 266, 128947. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Gobe, G.C.; Ujjin, P.; Vesey, D.A. A Comparison of the Nephrotoxicity of Low Doses of Cadmium and Lead. Toxics 2020, 8, 18. [Google Scholar] [CrossRef]
- Eom, S.-Y.; Seo, M.-N.; Lee, Y.-S.; Park, K.-S.; Hong, Y.-S.; Sohn, S.-J.; Kim, Y.-D.; Choi, B.-S.; Lim, J.-A.; Kwon, H.-J.; et al. Low-Level Environmental Cadmium Exposure Induces Kidney Tubule Damage in the General Population of Korean Adults. Arch. Env. Contam. Toxicol. 2017, 73, 401–409. [Google Scholar] [CrossRef]
- Garner, R.E.; Levallois, P. Associations between Cadmium Levels in Blood and Urine, Blood Pressure and Hypertension among Canadian Adults. Environ. Res. 2017, 155, 64–72. [Google Scholar] [CrossRef]
- Ratelle, M.; Li, X.; Laird, B.D. Cadmium Exposure in First Nations Communities of the Northwest Territories, Canada: Smoking Is a Greater Contributor than Consumption of Cadmium-Accumulating Organ Meats. Env. Sci. Process Impacts 2018, 20, 1441–1453. [Google Scholar] [CrossRef]
- Shakeri, M.T.; Nezami, H.; Nakhaee, S.; Aaseth, J.; Mehrpour, O. Assessing Heavy Metal Burden Among Cigarette Smokers and Non-Smoking Individuals in Iran: Cluster Analysis and Principal Component Analysis. Biol. Trace Elem. Res. 2021, 199, 4036–4044. [Google Scholar] [CrossRef]
- Järup, L.; Åkesson, A. Current Status of Cadmium as an Environmental Health Problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Garner, R.; Levallois, P. Cadmium Levels and Sources of Exposure among Canadian Adults. Health Rep. 2016, 27, 10–18. [Google Scholar] [PubMed]
- Kuno, R.; Roquetti, M.H.; Becker, K.; Seiwert, M.; Gouveia, N. Reference Values for Lead, Cadmium and Mercury in the Blood of Adults from the Metropolitan Area of Sao Paulo, Brazil. Int. J. Hyg. Environ. Health 2013, 216, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Kira, C.S.; Sakuma, A.M.; De Capitani, E.M.; De Freitas, C.U.; Cardoso, M.R.A.; Gouveia, N. Associated Factors for Higher Lead and Cadmium Blood Levels, and Reference Values Derived from General Population of São Paulo, Brazil. Sci. Total Environ. 2016, 543, 628–635. [Google Scholar] [CrossRef]
- Ferron, M.M.; Kuno, R.; Campos, A.E.M.D.; Castro, F.J.V.D.; Gouveia, N. Cadmium, Lead and Mercury in the Blood of Workers from Recycling Sorting Facilities in São Paulo, Brazil. Cad. Saúde Pública 2020, 36, e00072119. [Google Scholar] [CrossRef] [PubMed]
- NR 7—Programa de Controle Médico de Saúde Ocupacional—PCMSO. Atualizada pela Portaria MPT nº 567, em 10/03/2022. Available online: https://www.gov.br/trabalho-e-emprego/pt-br/acesso-a-informacao/participacao-social/conselhos-e-orgaos-colegiados/comissao-tripartite-partitaria-permanente/arquivos/normas-regulamentadoras/nr-07-atualizada-2022-1.pdf (accessed on 9 July 2024).
- Zhang, H.; Reynolds, M. Cadmium Exposure in Living Organisms: A Short Review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Lei, X.; Du, L.; Yu, W.; Wang, Y.; Ma, N.; Qu, B. GSTP1 as a Novel Target in Radiation Induced Lung Injury. J. Transl. Med. 2021, 19, 297. [Google Scholar] [CrossRef]
- Michalczyk, K.; Kapczuk, P.; Witczak, G.; Bosiacki, M.; Kurzawski, M.; Chlubek, D.; Cymbaluk-Płoska, A. The Associations between Metalloestrogens, GSTP1, and SLC11A2 Polymorphism and the Risk of Endometrial Cancer. Nutrients 2022, 14, 3079. [Google Scholar] [CrossRef]
- Dusinská, M.; Ficek, A.; Horská, A.; Raslová, K.; Petrovská, H.; Vallová, B.; Drlicková, M.; Wood, S.G.; Stupáková, A.; Gasparovic, J.; et al. Glutathione S-Transferase Polymorphisms Influence the Level of Oxidative DNA Damage and Antioxidant Protection in Humans. Mutat. Res. 2001, 482, 47–55. [Google Scholar] [CrossRef]
- Simões, T.C.; Meira, K.C.; Santos, J.D.; Câmara, D.C.P. Prevalências de doenças crônicas e acesso aos serviços de saúde no Brasil: Evidências de três inquéritos domiciliares. Ciênc. Saúde Coletiva 2021, 26, 3991–4006. [Google Scholar] [CrossRef]
- Ganguly, K.; Levänen, B.; Palmberg, L.; Åkesson, A.; Lindén, A. Cadmium in Tobacco Smokers: A Neglected Link to Lung Disease? Eur. Respir. Rev. 2018, 27, 170122. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | BCd a,b (µg L−1) | UcCd c,d (µg g−1) | ||||
|---|---|---|---|---|---|---|
| ≤0.69 (n = 101) | >0.69 (n = 97) | p-Value d | ≤0.37 (n = 84) | >0.37 (n = 86) | p-Value d | |
| Sex | N (%) | N (%) | ||||
| Women | 59 (58.4) | 62 (63.9) | 0.5 | 51 (60.7) | 52 (60.5) | 1.0 |
| Men | 42 (41.6) | 35 (36.1) | 33 (39.3) | 34 (39.5) | ||
| Age (years) | ||||||
| ≤50 | 57 (56.4) | 44 (45.4) | 0.2 | 47 (56.0) | 36 (41.9) | 0.1 |
| >50 | 44 (43.6) | 53 (54.6) | 37 (44.0) | 50 (58.1) | ||
| Smoking status e | ||||||
| Non-smoker | 60 (76.9) | 51 (64.6) | 0.1 | 53 (75.7) | 49 (66.2) | 0.3 |
| Smoker f | 18 (23.1) | 28 (35.4) | 17 (24.3) | 25 (33.8) | ||
| Drinking status g | ||||||
| Non-drinker | 37 (46.2) | 45 (57.0) | 0.2 | 34 (47.9) | 42 (56.0) | 0.4 |
| Drinker h | 43 (53.8) | 34 (43.0) | 37 (52.1) | 33 (44.0) | ||
| Residence time (years) i | ||||||
| ≤17 | 56 (58.3) | 47 (58.0) | 1.0 | 46 (60.5) | 43 (56.6) | 0.7 |
| >17 | 40 (41.7) | 34 (42.0) | 30 (39.5) | 33 (43.4) | ||
| NCDs j | ||||||
| Cardiovascular | 34 (33.7) | 31 (32.0) | 0.9 | 34 (40.5) | 23 (26.7) | 0.1 |
| Neurological | 8 (7.9) | 3 (3.1) | 0.2 | 4 (4.8) | 4 (4.7) | 1.0 |
| Renal | 7 (6.9) | 7 (7.2) | 1.0 | 7 (8.3) | 4 (4.7) | 0.5 |
| Respiratory | 15 (14.9) | 29 (29.9) | 0.02 | 20 (23.8) | 19 (22.1) | 0.9 |
| Neoplastic | 5 (5.0) | 1 (1.0) | 0.2 | 5 (6.0) | 1 (1.2) | 0.2 |
| NCDs j | ||||||
| No | 49 (48.5) | 43 (44.3) | 0.6 | 36 (42.9) | 44 (51.2) | 0.4 |
| Yes k | 52 (51.5) | 54 (55.7) | 48 (57.1) | 42 (48.8) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perini, J.A.; Silva, Y.M.H.d.; Silva, M.C.d.; Silva, B.P.; Machado, D.E.; Moreira, M.d.F.R. Cadmium Exposure and Noncommunicable Diseases in Environmentally Exposed Brazilian Population: Cross-Sectional Study without Association of GSTP1 Polymorphism. Toxics 2024, 12, 640. https://doi.org/10.3390/toxics12090640
Perini JA, Silva YMHd, Silva MCd, Silva BP, Machado DE, Moreira MdFR. Cadmium Exposure and Noncommunicable Diseases in Environmentally Exposed Brazilian Population: Cross-Sectional Study without Association of GSTP1 Polymorphism. Toxics. 2024; 12(9):640. https://doi.org/10.3390/toxics12090640
Chicago/Turabian StylePerini, Jamila Alessandra, Yasmin Marinho Henriques da Silva, Mayara Calixto da Silva, Beatriz Pegado Silva, Daniel Escorsim Machado, and Maria de Fátima Ramos Moreira. 2024. "Cadmium Exposure and Noncommunicable Diseases in Environmentally Exposed Brazilian Population: Cross-Sectional Study without Association of GSTP1 Polymorphism" Toxics 12, no. 9: 640. https://doi.org/10.3390/toxics12090640
APA StylePerini, J. A., Silva, Y. M. H. d., Silva, M. C. d., Silva, B. P., Machado, D. E., & Moreira, M. d. F. R. (2024). Cadmium Exposure and Noncommunicable Diseases in Environmentally Exposed Brazilian Population: Cross-Sectional Study without Association of GSTP1 Polymorphism. Toxics, 12(9), 640. https://doi.org/10.3390/toxics12090640







