Impact of a Nanoscale Iron–Chlorobenzene Mixture on Pulmonary Injury in Rat Pups: Extending Exposure Knowledge Using Network Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pollutants
2.2. Experimental Animals
2.3. Network Toxicology Prediction
2.3.1. Identification of TCDD Target Proteins
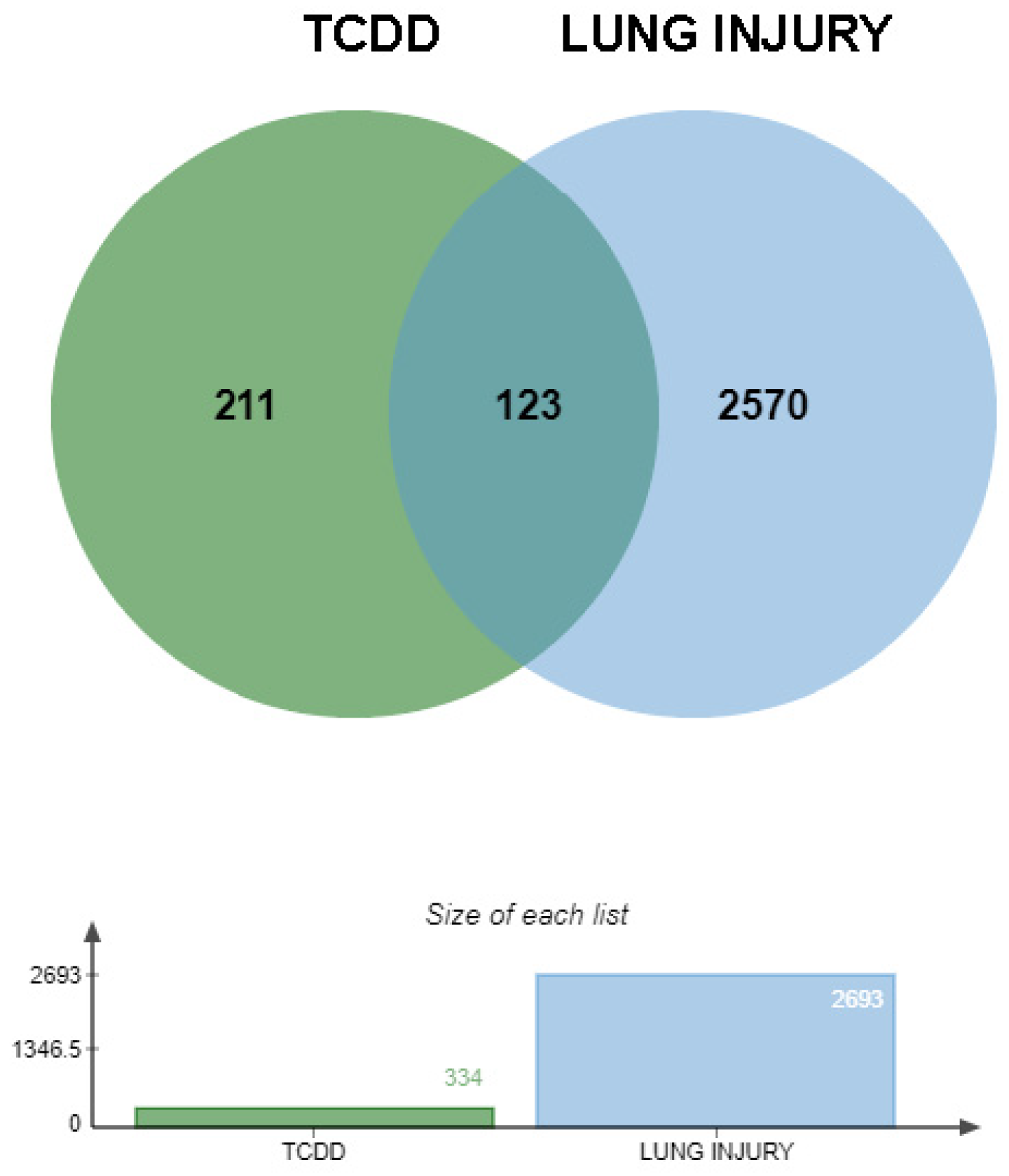
2.3.2. Identification of Intersecting Genes Between TCDD and Lung Injury
2.3.3. Construction of Protein–Protein Interaction Network
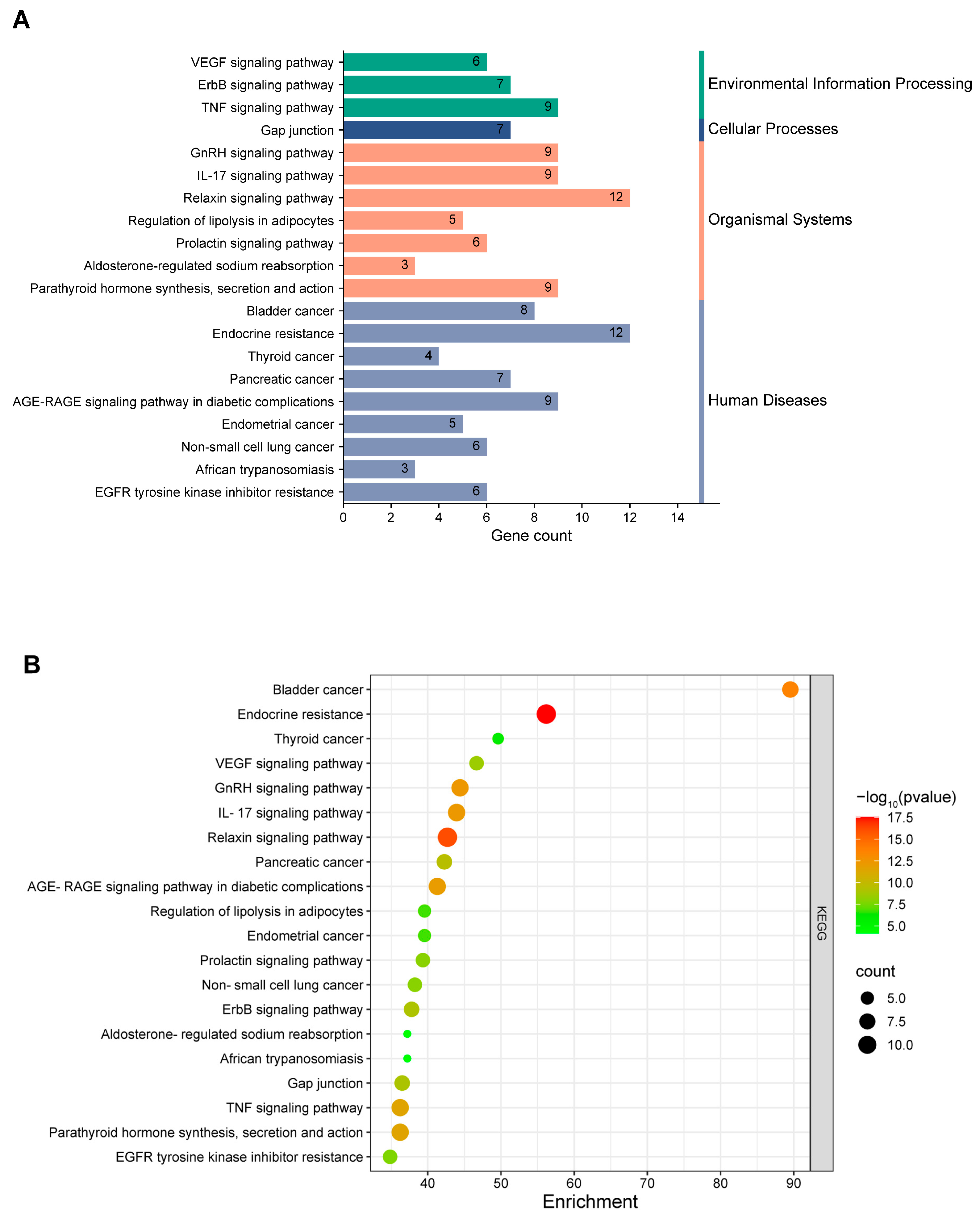
2.3.4. Enrichment Analysis of Intersection Genes
2.4. Animal Experimental Validation
2.4.1. Animal Grouping and Establishment of the Air Exposure Model
- 1.
- Animal Grouping
- 2.
- Air Exposure Model
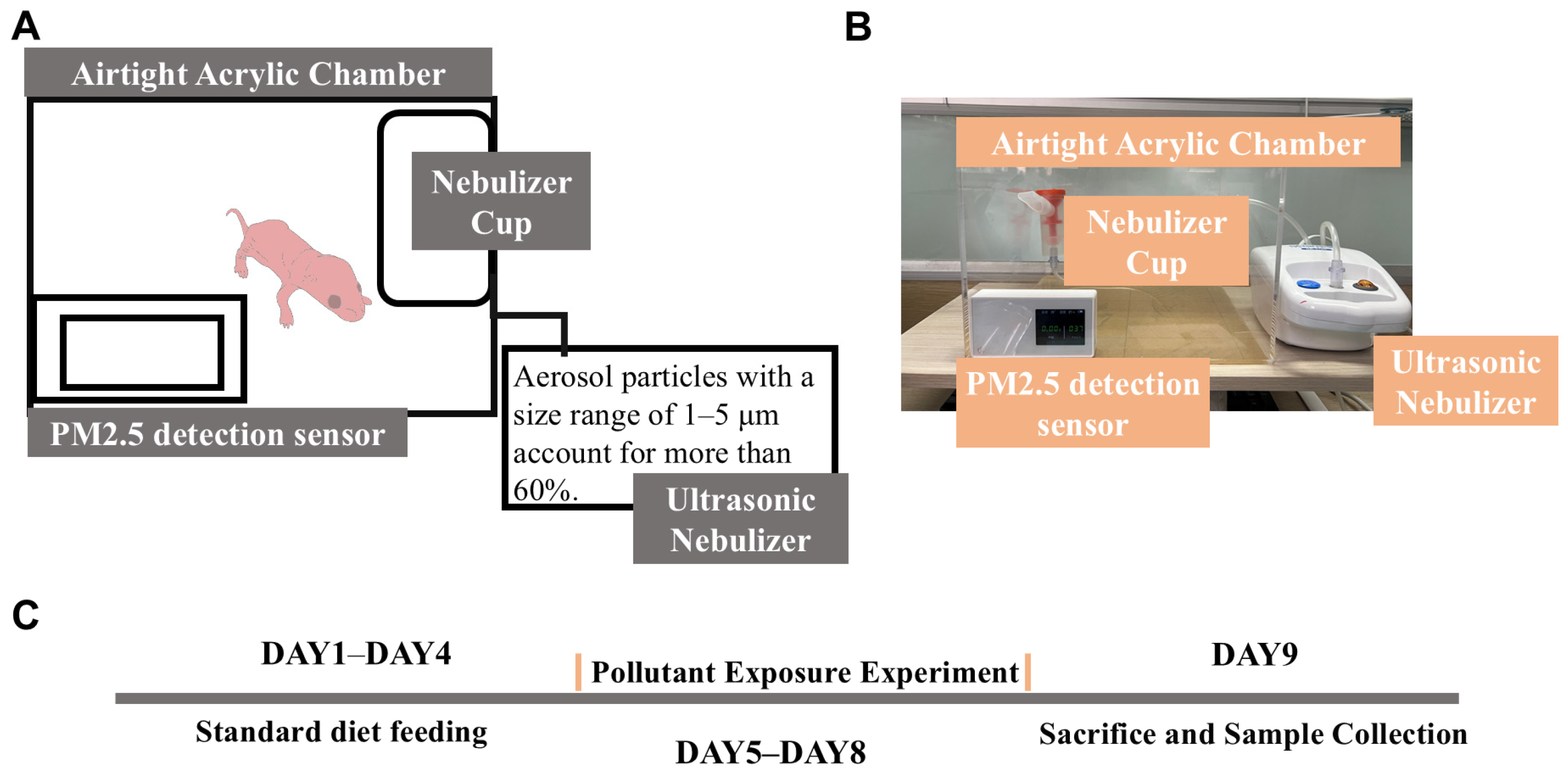
- Construction of the exposure chamber: a large transparent acrylic board was used to construct the main chamber.
- Generation of pollutant aerosols: a compressed nebulizer (NB-212C; Ludei Medical Devices, Shanghai, China) was employed to generate aerosols containing pollutants mixed with pure water, which were then introduced into the exposure chamber.
- Monitoring of the PM2.5 concentration: a PM2.5 sensor was used to continuously monitor the concentration of PM2.5 inside the sealed chamber to ensure consistent exposure conditions (Figure 1A).
2.4.2. Histopathological Examination
2.4.3. Measurement of Oxidative-Stress-Related Markers in Tissue
2.4.4. Determination of Inflammatory Cytokine RNA Expression in Tissue
2.4.5. Statistical Analysis
3. Results
3.1. Validation Results of Network Toxicology
3.1.1. Prediction of TCDD-Induced Lung Injury Targets
3.1.2. Interaction Network of Potential Targets
3.1.3. Enrichment Analysis of Intersecting Targets
3.1.4. Functional Insights from GO and KEGG Enrichment Analyses
3.2. Animal Experiment Results
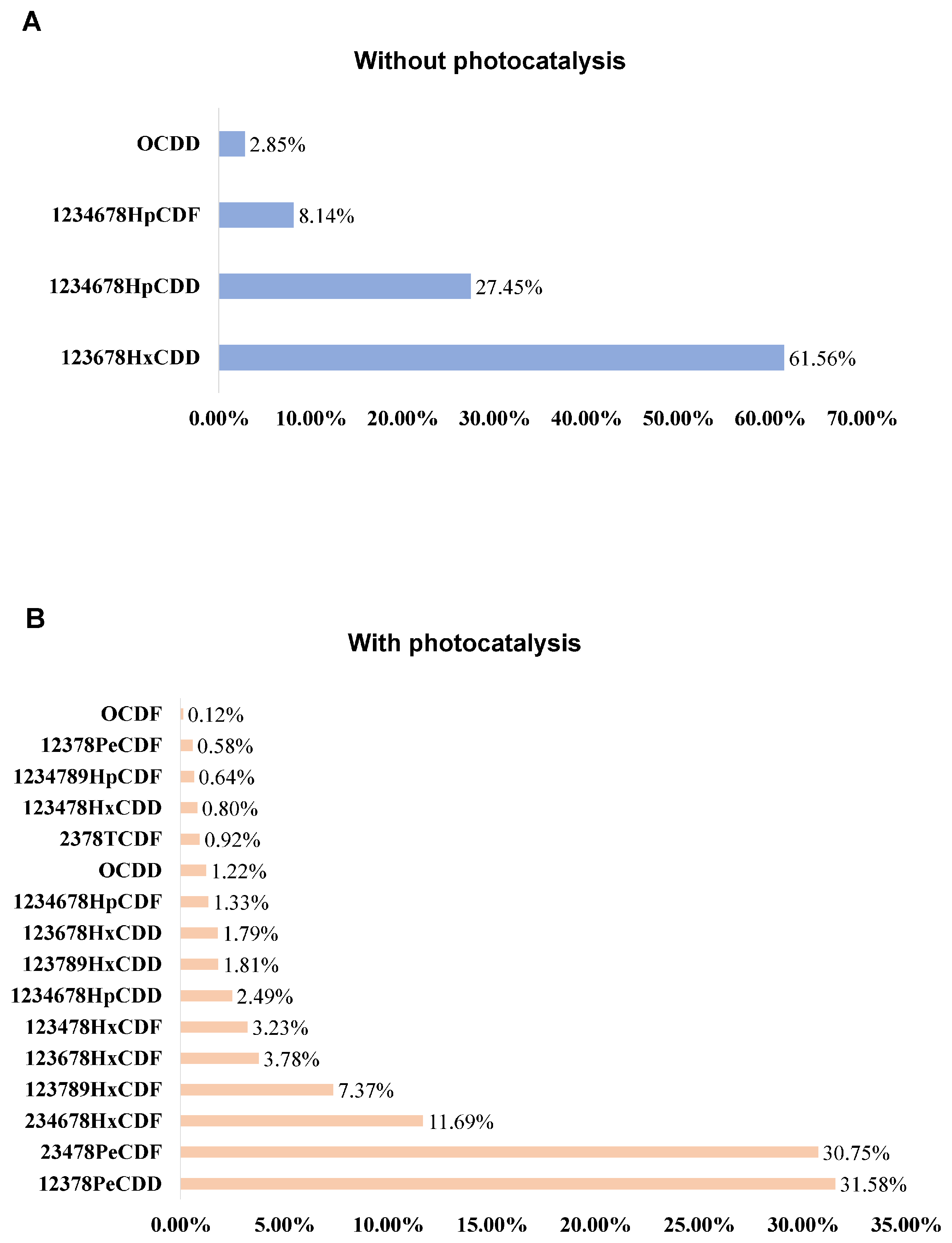
3.2.1. Characterization of Pollutants Before and After Light Induction
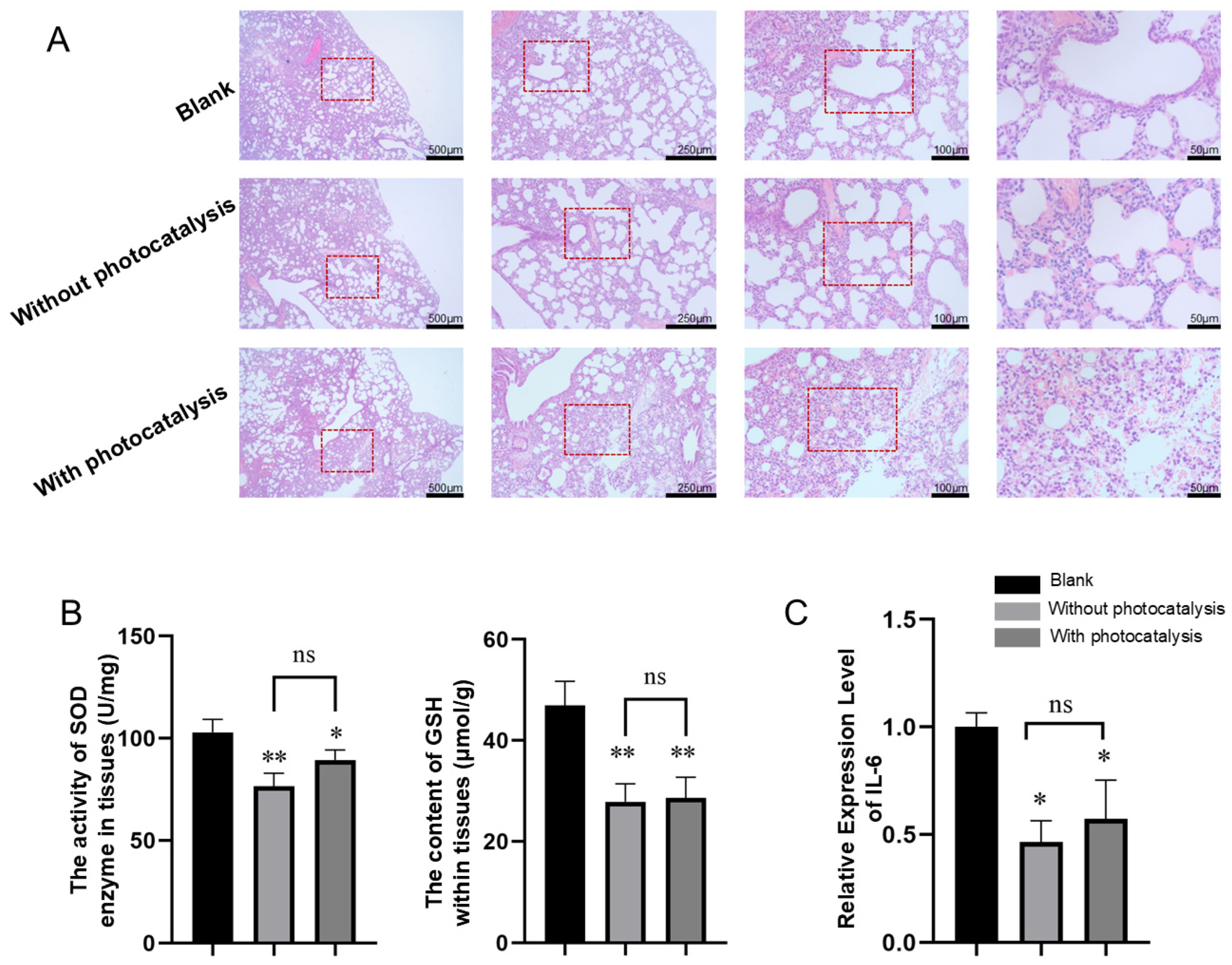
3.2.2. Histopathological Results of Lung Injury Caused by Pollutants with and Without Photocatalysis Exposure
3.2.3. Effects of Pollutants with and Without Photocatalysis on the Activity or Content of SOD and GSH in Lung Tissue
3.2.4. Effects of Pollutants with and Without Photocatalysis on the Expression of IL-6 in Lung Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| POPs | Persistent organic pollutants |
| PCDD/Fs | Polychlorinated dibenzo-p-dioxins and dibenzofurans |
| PCBs | Polychlorinated biphenyls |
| CB | Chlorobenzene |
| TCDD | 2,3,7,8-tetrachlorodibenzo-p-dioxin |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| BP | Biological process |
| CC | Cellular component |
| MF | Molecular function |
| FDR | False discovery rate |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TNF | Tumor necrosis factor |
| IL-17 | Interleukin-17 |
| TLR | Toll-like receptor |
| IL-1β | Interleukin-1β |
| IL-8 | Interleukin-8 |
| IL-6 | Interleukin-6 |
References
- Xing, Y.; Zhang, H.; Su, W.; Wang, Q.; Yu, H.; Wang, J.; Li, R.; Cai, C.; Ma, Z. The bibliometric analysis and review of dioxin in waste incineration and steel sintering. Environ. Sci. 2019, 26, 35687–35703. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.-X.; Xu, S.; Cai, P.; Chen, T.; Lin, X.; Buekens, A.; Li, X.J.C. Parameters affecting the formation mechanisms of dioxins in the steel manufacture process. Chemosphere 2019, 222, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Gouin, T.; Mackay, D.; Jones, K.C.; Harner, T.; Meijer, S.N. Evidence for the “grasshopper” effect and fractionation during long-range atmospheric transport of organic contaminants. Environ. Pollut. 2004, 128, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chen, T.; Buekens, A.; Li, X.J.I.I. De novo formation of PCDD/F during sintering: Effect of temperature, granule size and oxygen content. ISIJ Int. 2018, 58, 566–572. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Huang, Q.; Liu, X.; Zhao, J.; Zhang, Z.; Zhu, Y.; Huang, L.; Yang, K. Enhancing synergistic catalytic combustion for the co-removal of PCDD/Fs and additional pollutants from sintering flue gases: A review on catalyst development. Sep. Purif. Technol. 2025, 363, 132132. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Qin, S.; Zhu, T.; Song, J.; Xu, W. Emission reduction of PCDD/Fs by flue gas recirculation and activated carbon in the iron ore sintering. Environ. Pollut. 2023, 327, 121520. [Google Scholar] [CrossRef]
- Ren, Z.; Lu, Y.; Li, Q.; Sun, Y.; Wu, C.; Ding, Q. Occurrence and characteristics of PCDD/Fs formed from Chlorobenzenes production in China. Chemosphere 2018, 205, 267–274. [Google Scholar] [CrossRef]
- Baker, J.I.; Hites, R.A. Is combustion the major source of polychlorinated dibenzo-p-dioxins and dibenzofurans to the environment? A mass balance investigation. Environ. Sci. Technol. 2000, 34, 2879–2886. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Crespo, J.G.; Afonso, C.A. Dioxins sources and current remediation technologies—A review. Environ. Int. 2008, 34, 139–153. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W. Size-segregated urban particulate matter: Mass closure, chemical composition, and primary and secondary matter content. Air Qual. Atmos. Health 2016, 9, 533–550. [Google Scholar] [CrossRef]
- Kosco, J.; Bidwell, M.; Cha, H.; Martin, T.; Howells, C.T.; Sachs, M.; Anjum, D.H.; Gonzalez Lopez, S.; Zou, L.; Wadsworth, A.J.N.M.; et al. Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles. Nat. Mater. 2020, 19, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S. The effects and pathogenesis of PM2.5 and its components on chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Kajekar, R. Environmental factors and developmental outcomes in the lung. Pharmacol. Ther. 2007, 114, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Rifas-Shiman, S.L.; Litonjua, A.A.; Oken, E.; Gillman, M.W.; Kloog, I.; Luttmann-Gibson, H.; Zanobetti, A.; Coull, B.A.; Schwartz, J.; et al. Lifetime Exposure to Ambient Pollution and Lung Function in Children. Am. J. Respir. Crit. Care Med. 2016, 193, 881–888. [Google Scholar] [CrossRef]
- Pinkerton, K.E.; Joad, J.P. The mammalian respiratory system and critical windows of exposure for children’s health. Environ. Health Perspect. 2000, 108 (Suppl. S3), 457–462. [Google Scholar] [CrossRef]
- Selevan, S.G.; Kimmel, C.A.; Mendola, P. Identifying critical windows of exposure for children’s health. Environ. Health Perspect. 2000, 108 (Suppl. S3), 451–455. [Google Scholar] [CrossRef]
- Benjamin, J.T.; Plosa, E.J.; Sucre, J.M.; van der Meer, R.; Dave, S.; Gutor, S.; Nichols, D.S.; Gulleman, P.M.; Jetter, C.S.; Han, W.; et al. Neutrophilic inflammation during lung development disrupts elastin assembly and predisposes adult mice to COPD. J. Clin. Investig. 2021, 131, e139481. [Google Scholar] [CrossRef]
- Newbury, J.B.; Arseneault, L.; Beevers, S.; Kitwiroon, N.; Roberts, S.; Pariante, C.M.; Kelly, F.J.; Fisher, H.L. Association of air pollution exposure with psychotic experiences during adolescence. JAMA Psychiatry 2019, 76, 614–623. [Google Scholar] [CrossRef]
- Sangkham, S.; Phairuang, W.; Sherchan, S.P.; Pansakun, N.; Munkong, N.; Sarndhong, K.; Islam, M.A.; Sakunkoo, P.J.E.A. An update on adverse health effects from exposure to PM2.5. Environ. Adv. 2024, 18, 100603. [Google Scholar] [CrossRef]
- Huang, S. Efficient analysis of toxicity and mechanisms of environmental pollutants with network toxicology and molecular docking strategy: Acetyl tributyl citrate as an example. Sci. Total Environ. 2023, 905, 167904. [Google Scholar] [CrossRef]
- Yoshioka, W.; Tohyama, C. Mechanisms of developmental toxicity of dioxins and related compounds. Int. J. Mol. Sci. 2019, 20, 617. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Chun, D.-M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Liu, K.; Ying, M.; Chen, M.; Yan, M.; Xu, Y.; Li, Y. Photochemical conversion enhancement of the damaging effects of iron trioxide/chlorobenzene complexes on A549 cells. J. Environ. Sci. 2024, 44, 431–440. [Google Scholar] [CrossRef]
- Hulin, M.; Sirot, V.; Vasseur, P.; Mahe, A.; Leblanc, J.C.; Jean, J.; Marchand, P.; Venisseau, A.; Le Bizec, B.; Rivière, G. Health risk assessment to dioxins, furans and PCBs in young children: The first French evaluation. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 139, 111292. [Google Scholar] [CrossRef]
- Wang, L.; Pei, Y.; Li, S.; Zhang, S.; Yang, Y. Distinct molecular mechanisms analysis of three lung cancer subtypes based on gene expression profiles. J. Comput. Biol. 2019, 26, 1140–1155. [Google Scholar] [CrossRef]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef]
- Chang, Z.; Lu, M.; Kim, S.-S.; Park, J.-S.J.T.L. Potential role of HSP90 in mediating the interactions between estrogen receptor (ER) and aryl hydrocarbon receptor (AhR) signaling pathways. Toxicol. Lett. 2014, 226, 6–13. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, H.Q.; Li, Y.; Zhou, M.; Zhou, Z.; Wang, R.; Hahn, M.E.; Zhao, B. The aryl hydrocarbon receptor: A predominant mediator for the toxicity of emerging dioxin-like compounds. J. Hazard. Mater. 2022, 426, 128084. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z.J.C. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Niu, Z.; Zhao, Q.; Cao, H.; Yang, B.; Wang, S.J.S.R. Hypoxia-activated oxidative stress mediates SHP2/PI3K signaling pathway to promote hepatocellular carcinoma growth and metastasis. Sci. Rep. 2025, 15, 4847. [Google Scholar] [CrossRef]
- Wu, M.; Bian, Q.; Liu, Y.; Fernandes, A.F.; Taylor, A.; Pereira, P.; Shang, F. Sustained oxidative stress inhibits NF-κB activation partially via inactivating the proteasome. Free Radic. Biol. Med. 2009, 46, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Khattab, A.; Shata, M.; Alhasbani, A.; Khalaf, A.; Alsaeedi, S.; Thaker, M.; Said, H.; Toumi, H.R.; Alzahmi, H.J.H. Melatonin increases AKT and SOD gene and protein expressions in diabetic rats. Heliyon 2024, 10, e28639. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Guo, M.; Wang, Z.; Li, Y.; Kong, D.; Shao, J.; Tan, S.; Chen, A.; Zhang, F.; Zhang, Z.J.I.I. ROS-dependent inhibition of the PI3K/Akt/mTOR signaling is required for Oroxylin A to exert anti-inflammatory activity in liver fibrosis. Int. Immunopharmacol. 2020, 85, 106637. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J.J.S.T.; Therapy, T. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. 2024, 9, 53. [Google Scholar] [CrossRef]
- Aluri, J.; Cooper, M.A.; Schuettpelz, L.G.J.C. Toll-like receptor signaling in the establishment and function of the immune system. Cells 2021, 10, 1374. [Google Scholar] [CrossRef]
- Huangfu, L.; Li, R.; Huang, Y.; Wang, S.J.S.T.; Therapy, T. The IL-17 family in diseases: From bench to bedside. Signal Transduct. Target. Ther. 2023, 8, 402. [Google Scholar] [CrossRef]
- Matsumura, F.; FA Vogel, C. Evidence supporting the hypothesis that one of the main functions of the aryl hydrocarbon receptor is mediation of cell stress responses. Biol. Chem. 2006, 387, 1189–1194. [Google Scholar] [CrossRef]
- Tian, Y.; Ke, S.; Denison, M.S.; Rabson, A.B.; Gallo, M.A. Ah receptor and NF-κB interactions, a potential mechanism for dioxin toxicity. J. Biol. Chem. 1999, 274, 510–515. [Google Scholar] [CrossRef]
- Pernomian, L.; Duarte-Silva, M.; de Barros Cardoso, C.R. The aryl hydrocarbon receptor (AHR) as a potential target for the control of intestinal inflammation: Insights from an immune and bacteria sensor receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef]
- Chen, M.; Yin, M.; Su, Y.; Li, R.; Liu, K.; Wu, Z.; Weng, X. Atmospheric heterogeneous reaction of chlorobenzene on mineral α-Fe2O3 particulates: A chamber experiment study. Front. Environ. Sci. Eng. 2023, 17, 134. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gu, X.; Sun, H.; Fu, C.; Zhang, Y.; Dong, P. Biomimetic Modification and In Vivo Safety Assessment of Superparamagnetic Iron Oxide Nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 4100–4107. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, Z.; Yang, B.; Zhu, X.; Song, E.; Song, Y. Long-term pulmonary iron oxide nanoparticles exposure disrupts hepatic iron-lipid homeostasis and increases plaque vulnerability in ApoE−/− mice. Environ. Pollut. 2024, 341, 122905. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A.J.A. Nanoantioxidants: Recent trends in antioxidant delivery applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N.J.A. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Chelombitko, M.A. Role of reactive oxygen species in inflammation: A minireview. Mosc. Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C.J.B. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Profumo, E.; Buttari, B.; Tinaburri, L.; D’Arcangelo, D.; Sorice, M.; Capozzi, A.; Garofalo, T.; Facchiano, A.; Businaro, R.; Kumar, P.; et al. Oxidative Stress Induces HSP90 Upregulation on the Surface of Primary Human Endothelial Cells: Role of the Antioxidant 7,8-Dihydroxy-4-methylcoumarin in Preventing HSP90 Exposure to the Immune System. Oxidative Med. Cell. Longev. 2018, 2018, 2373167. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Tong, J.; He, C.; Ling, X.; Xiang, J.; Xue, C.; Yao, G.; Sun, L.; Xie, Z. Inhibition of Cullin3 Neddylation Alleviates Diabetic Retinopathy by Activating Nrf2 Signaling to Combat ROS-Induced Oxidative Stress and Inflammation. Inflammation 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Chepurnova, D.; Samoilova, E.; Anisimov, A.; Verin, A.; Korotaeva, A.A. Compounds of IL-6 receptor complex during acute lung injury. Bull. Exp. Biol. Med. 2018, 164, 609–611. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.C. Impact of interleukin 6 levels on acute lung injury risk and disease severity in critically ill sepsis patients. World J. Clin. Cases 2024, 12, 5374. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Qi, G.H.; Peng, P.; Fang, R.J. Research progress of aryl hydrocarbon receptor in regulating intestinal inflammation. Chin. J. Anim. Nutr. 2018, 30, 4776–4785. [Google Scholar]
- Lopes, F.; Coelho, F.M.; Costa, V.V.; Vieira, É.L.; Sousa, L.P.; Silva, T.A.; Vieira, L.Q.; Teixeira, M.M.; Pinho, V. Resolution of neutrophilic inflammation by H2O2 in antigen-induced arthritis. Arthritis Rheumatism 2011, 63, 2651–2660. [Google Scholar] [CrossRef]
- Miller, M.D.; Marty, M.A. Impact of environmental chemicals on lung development. Environ. Health Perspect. 2010, 118, 1155–1164. [Google Scholar] [CrossRef]
- Negretti, N.M.; Plosa, E.J.; Benjamin, J.T.; Schuler, B.A.; Habermann, A.C.; Jetter, C.S.; Gulleman, P.; Bunn, C.; Hackett, A.N.; Ransom, M. A single-cell atlas of mouse lung development. Development 2021, 148, dev199512. [Google Scholar] [CrossRef]


| Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| β-actin | ACCCGCGAGTACAACCTTCTT | TCGTCATCCATGGCGAACTGG |
| IL-6 | GCAAGAGACTTCCAGCCAGT | TGCCATTGCACAACTCTTTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Xu, Y.; Ying, M.; Chen, M. Impact of a Nanoscale Iron–Chlorobenzene Mixture on Pulmonary Injury in Rat Pups: Extending Exposure Knowledge Using Network Technology. Toxics 2025, 13, 221. https://doi.org/10.3390/toxics13030221
Liu K, Xu Y, Ying M, Chen M. Impact of a Nanoscale Iron–Chlorobenzene Mixture on Pulmonary Injury in Rat Pups: Extending Exposure Knowledge Using Network Technology. Toxics. 2025; 13(3):221. https://doi.org/10.3390/toxics13030221
Chicago/Turabian StyleLiu, Kezhou, Ying Xu, Mengjie Ying, and Meiling Chen. 2025. "Impact of a Nanoscale Iron–Chlorobenzene Mixture on Pulmonary Injury in Rat Pups: Extending Exposure Knowledge Using Network Technology" Toxics 13, no. 3: 221. https://doi.org/10.3390/toxics13030221
APA StyleLiu, K., Xu, Y., Ying, M., & Chen, M. (2025). Impact of a Nanoscale Iron–Chlorobenzene Mixture on Pulmonary Injury in Rat Pups: Extending Exposure Knowledge Using Network Technology. Toxics, 13(3), 221. https://doi.org/10.3390/toxics13030221







