Phthalates and Non-Phthalate Plasticizers and Thyroid Dysfunction: Current Evidence and Novel Strategies to Reduce Their Spread in Food Industry and Environment
Abstract
:1. Introduction
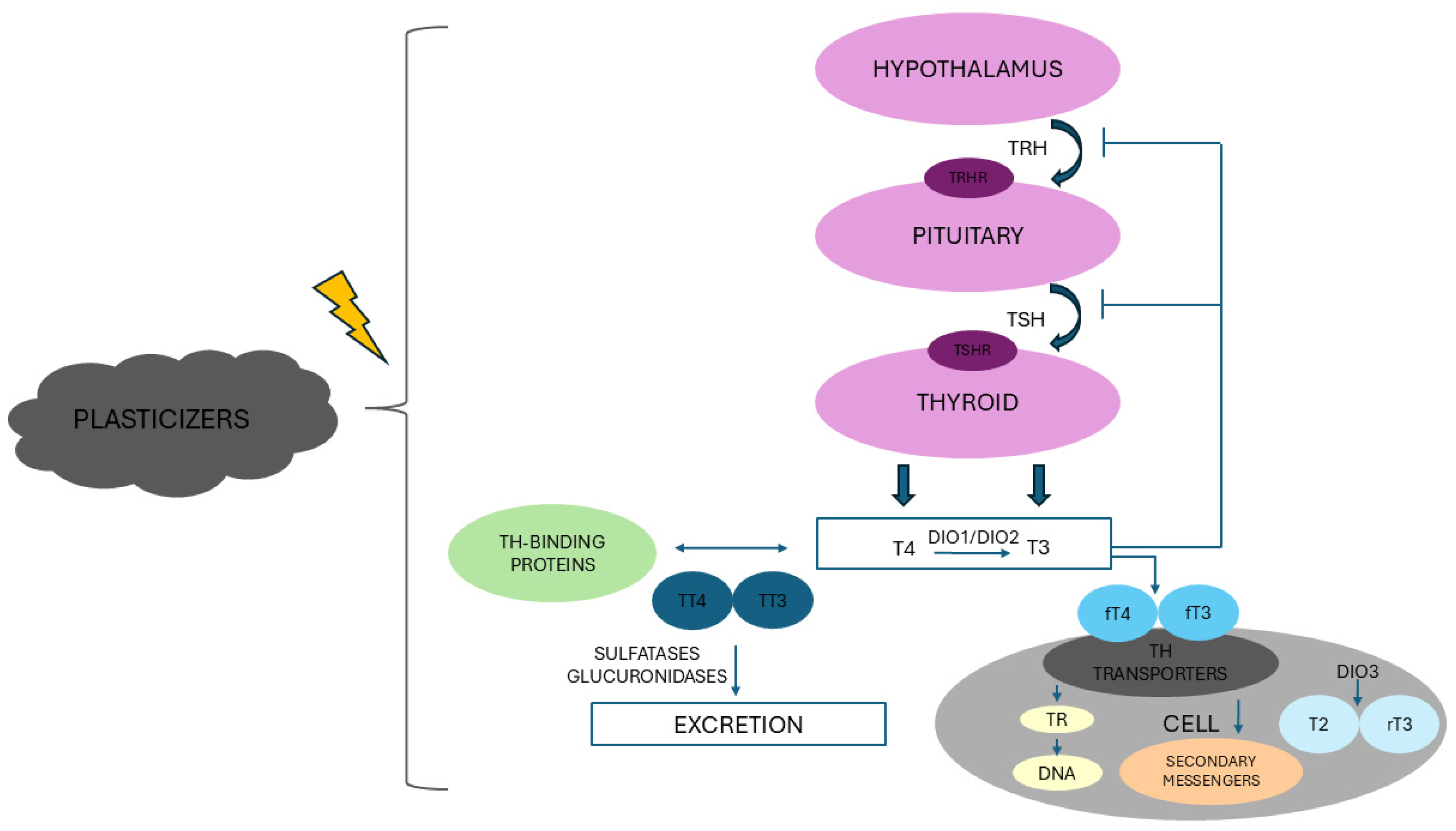
2. Thyroid Axis: An Overview
3. Plasticizers
3.1. Phthalates
3.2. Non-Phthalate Plasticizers
4. The Association Between Exposure to Phthalates and Thyroid Dysfunction
4.1. The Association Between Phthalate Exposure and Thyroid Disease
| Clues | Reference | Pitfalls | Reference |
|---|---|---|---|
| Plasma MEHP levels significantly and markedly increased in children with HT compared to controls | [102] | No significant changes in plasma MEHP levels between HT and healthy children | [102] |
| Plasma MEHP levels mildly correlated with plasma concentration of selenoprotein P | [102] | No associations revealed between plasma DEHP/MEHP levels and thyroid parameters (fT4, fT3, TSH, TPO antibodies) | [102] |
| Urinary levels of MECPP, MEP, MEHOP, and molar sum of DEHP metabolites significantly associated with a higher risk of SCH during pregnancy | [99] | Small sample size | [99,102,109,111,114,115] |
| Urinary concentrations of MEHP, MEHHP, and MCiNP significantly higher in adolescents with TCC than in the control group | [109] | No significant differences in urinary levels of phthalate metabolites between pregnant women with SCH and controls except for MEP | [99] |
| Higher ORs for adolescents with TCC in the highest tertile of MEHP compared to controls | [109] | Urinary concentration of MiBP and MMP significantly higher in healthy adolescents compared to those with TCC | [109] |
| Significantly strong association of serum DEHP with the rate of DTC in patients with benign thyroid nodules | [111] | Frequency of TCC adolescents in the third group of MCiNP, MCPP, and MiBP lower than that of healthy controls | [109] |
| Geometric mean of urinary levels of MMP, MEHHP, and MEHP higher in patients with TC and benign nodules than in heathy subjects | [114] | No significant changes in serum DEHP concentration (only exposed subjects) between patients with thyroid nodules and those with DTC | [111] |
| Highest tertiles of MMP, MEHHP, and MEHP significantly associated with increased risk of TC | [114] | No significant correlation between serum DEHP concentration and TSH levels (only subjects not under levothyroxine treatment) | [111] |
| MMP, MEOHP, MEHHP, and MEHP levels significantly and positively correlated with risk of benign thyroid nodules in both categorical and continuous analyses | [114] | Geometric mean of urinary levels of MBP and MBzP in patients with TC and benign nodules lower than that in healthy subjects | [114] |
| Effect modification by sex between MEP and risk of benign nodules and between MEOHP and risk of TC | [114] | The second and the third tertiles of MBP significantly and inversely correlated with TC risk and with the risk of benign thyroid nodules as both categorical and continuous variable | [114] |
| Higher concentration of urinary molar sum of DEHP metabolites in PTC subjects | [115] | At high iodine levels, urinary concentrations of MBP and MEP not significantly associated with increased risk of PTC | [115] |
| Both the urinary molar sum of DEHP metabolites and concentration of single DEHP metabolites significantly correlated with increased risk of PTC | [115] | Single measurement of urinary phthalate metabolites or differences in methods of measurements of urinary metabolites | [99,102,109,111,114] |
| Signals of positive association between urinary MBP concentration and TC risk in the multivariable analysis | [115] | Lack of evaluation of exposure to other EDCs, nutrient/iodine intake, sex hormones, and pre-existing thyroid dysfunction | [99,115] |
| Urinary levels of DEHP metabolites significantly and strongly correlated with a higher risk of PTC regardless of iodine status | [115] | Single-center study | [99,102,109,114,115] |
| Lack of correction for urinary creatinine | [99] | ||
| Lack of assessment of the association between phthalate exposure and thyroid function | [109] |
4.2. Thyrotoxicity of Phthalates: The Underlying Mechanisms
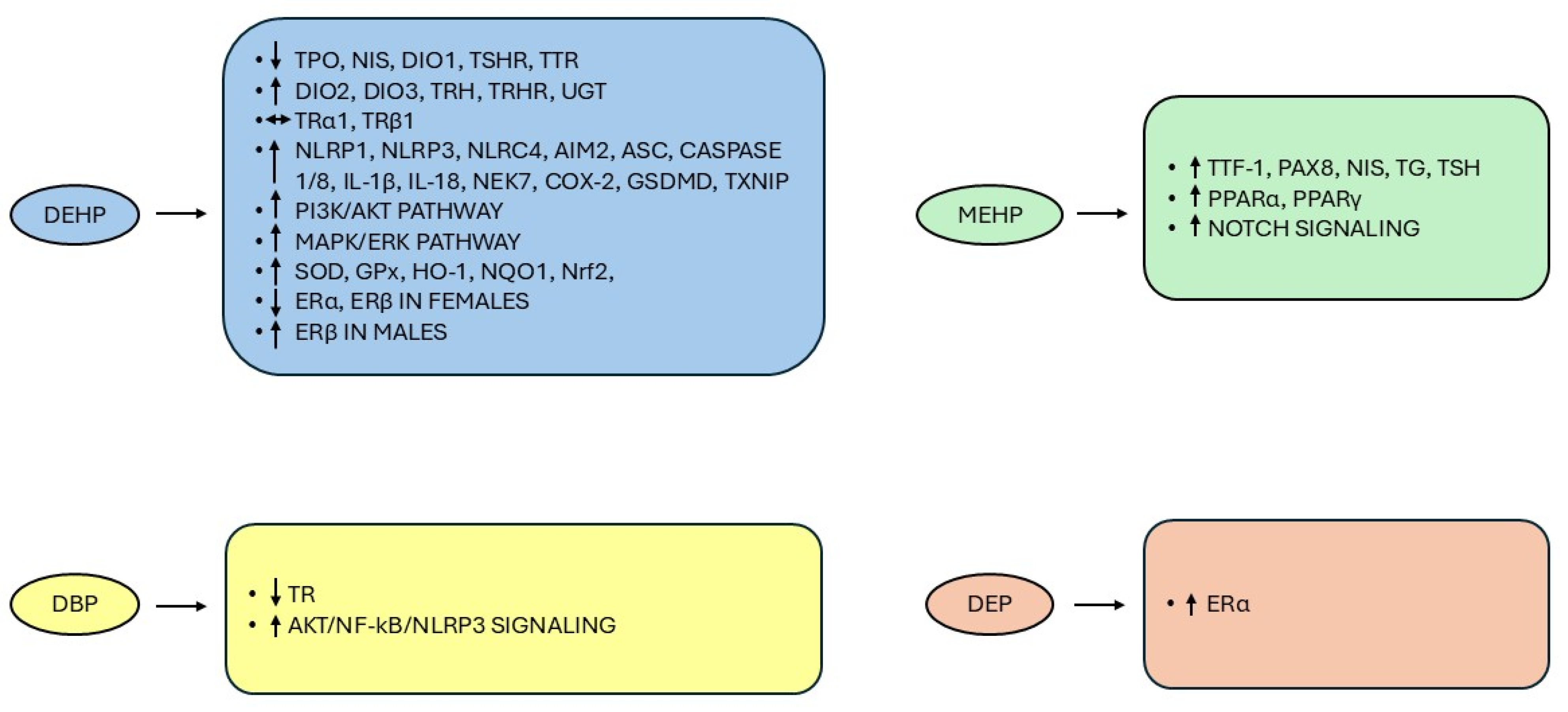
- Exposure to phthalates may interfere with the HPT axis at various biological levels. DEHP can affect TH biosynthesis by reducing serum levels of TPO, NIS, and hepatic DIO1 mRNA level (with consequent decrease in T3 production) and upregulating DIO2 and DIO3 in rats [116]. DEHP also inhibited gene and protein expression of TTR, reducing the biological effects of THs on target tissues [121,122]. Upon DEHP exposure in rats, mRNA expression of TSH receptors (TSHR) was downregulated, with concomitant upregulation of TRH and TRH receptors (TRHR) and no changes in TRα1 and TRβ1 mRNA expression, which collectively reduced the secretion of pituitary TSH and the consequent production of THs [23,121,123]. Conversely, prior studies based on reporter gene assays showed that high concentrations of DEHP, DBP, and DnBP exerted TR antagonistic activities [124,125]. Furthermore, in contrast with [121], zebrafish larvae exposed to MEHP were characterized by induced transcription of genes involved in thyroid development (thyroid transcription factor-1—TTF-1 and paired box 8—PAX8; the main transcription factors involved in the expression of thyroid differentiation marker genes such as NIS, TSHR, and TG [126]) and TH synthesis (NIS, TG, and TSH) [122]. DEHP can further induce the gene expression of uridine 5′-diphospho-glucuronosyltransferases, the liver enzymes that catalyze conjugation of THs with glucuronic acid, in both rats and zebrafish embryos/larvae, thereby promoting catabolism and excretion of THs [121,122].
- In the thyroid follicular epithelial cell line, DBP exposure can play a central role in thyroid inflammatory damage through the activation of protein kinase B (AKT)/nuclear factor kappa B (NF-κB)/NOD-like receptors (NLRs) family pyrin domain-containing protein 3 (NLRP3) signaling [127]. In particular, the canonical NF-κB pathway, which controls several aspects of cell growth and survival, inflammation, and immune response, is implicated in the regulation of thyroid physiology, participating in the expression of various thyroid-specific genes, including NIS, PAX8, TG, TPO, and TTF-1, and also contributes to the development of several neoplasms, including TC, enhancing the proliferation and viability of thyroid neoplastic cells and their potential to migrate and colonize distant sites [128,129,130]. The canonical NF-κB signaling can be activated by a wide range of stimuli such as ROS, inflammatory cytokines (e.g., tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-1β), growth factors, radiations, infections, and oncogenic stresses and is in turn responsible for the activation of the NLRP3 inflammasome and the transcriptional induction of pro-inflammatory cytokines (pro-IL-1β and pro-IL-18, converted to mature forms by caspase-1, one of the NLRP3 components) and chemokines (TNF-α) [130]. Wu et al. [131] documented that DEHP exposure induced thyroid injury through the inflammasome pathway, with increased mRNA and protein levels in rat thyrocytes of: NLRP1, NLRP3, NLR family caspase activation and recruitment domain domain-containing protein 4 and absent in melanoma 2, the last two inflammasomes functioning as innate immune machines against bacterial and viral infections and in response to cell stress [132,133]; apoptosis-associated speck-like protein containing a CARD, which recruits and activates caspase-1 [134]; caspase-1 and -8 (the latter activated within NLRP3 and having a fundamental role in controlling apoptosis, pyroptosis, and necroptosis in the non-canonical inflammasome pathway [135,136]); IL-1β and IL-18; NIMA-related kinase 7 (a serine/threonine kinase promoting the mitotic cell cycle and involved in NLRP3 assembly and activation upon sensing mitochondrial and cytosolic ROS [137,138]); cyclooxygenase-2, which mediates increase in NLRP3 inflammasome activation [139]; gasdermin D, another component of inflammasomes required for pyroptosis, a type of programmed cell death involving an early destruction of cell membrane, and secretion of IL-1β [136]; thioredoxin-interacting protein (TXNIP), a master regulator of cellular redox that exerts an inhibitory action on thioredoxin, one of the major thiol antioxidants, with consequent increase in ROS concentration, and activates NLRP3 [138,140]. Importantly, N-acetyl cysteine, a scavenger of intracellular ROS, inhibited the increase in protein expression of TXNIP, NLRP3, caspase-1, and IL-1β, the activation of DEHP-induced inflammasome activation and pyroptosis, and the TXNIP-NLRP3 inflammasome pathway [131].
- The association between exposure to certain phthalates and TC could involve the TSH/TSHR pathway, which plays a pivotal role in the proliferation and differentiation of thyroid cells, being involved in the expression of TG, TPO, and NIS via the upregulation of TTF-1 and PAX8 [121,141]. Dong et al. [142] demonstrated that a 6-month treatment with DEHP resulted in increased mRNA and protein expression levels of NIS, TPO, TG, and TSHR with a concurrent downward trend of hypothalamus TRHR mRNA and serum TRH levels and an overall decrease in circulating TH levels. This finding is probably attributable to DEHP-induced imbalance of TSH-TSHR-cAMP-protein kinase. A signaling pathway that promotes an increase in TTF-1 and TSHR expression and activity and subsequently increased expression of NIS and TPO in thyroid tissue and secretion of THs generates a negative feedback regulation leading to inhibition of TRH and TSH production [142,143]. Furthermore, DEHP may increase TSHR expression with the subsequent activation of downstream effectors of the phosphatidylinositol-3 kinase (PI3K)/AKT and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathways, which regulate cell growth, differentiation, and apoptosis and whose genetic alterations have been associated with the onset and progression of TC [144,145,146]. DEHP may directly activate the two pathways in both rat thyroid and human thyroid follicular epithelial cell lines, likely by inducing increased production of ROS, which is accompanied by reduced activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD) and increased levels of malondialdehyde [23].
- MEHP can induce tumorigenesis through activation of the nuclear transcription factor peroxisome proliferator-activated receptor-alpha (PPARα), a ligand-activated transcription factor belonging to the nuclear receptor superfamily and able to bind the retinoid X receptor to activate the transcription of selected genes [147]. The same phthalate metabolite can also activate PPARγ, which, in addition to regulating adipocyte differentiation and metabolism of lipids and glucose [148,149], when overexpressed, has been associated with more aggressive features in TC, i.e., increased cell growth and tendency to generate metastasis, and appears to promote the transition from differentiated to TC undifferentiated histological types with a consequent worse prognosis [150]. Conversely, depletion of PPARγ inhibited in vitro cell growth and reduced tumor growth in vivo xenograft models [148]. Indeed, PPARγ overexpression suppresses the PTEN-inhibitory action on AKT, resulting in activation of the PI3K signaling pathway, while PAX8-PPARγ rearrangement, a chromosomal translocation between the 5′ portion of PAX8 and the coding exons of PPARγ and detected in up to 60% of cases of follicular thyroid cancer (FTC), may directly induce the MAPK pathway [142,151].
- In neuroblastoma cells, MEHP also increased the protein expression of the Notch signaling cascade, thereby inducing cell proliferation and inhibiting apoptosis [152]. Notch signaling, initially identified in a mutant strain of Drosophila melanogaster and highly conserved throughout evolution, consists of four transmembrane receptor isoforms (Notch1–4) capable of binding to five different ligands (Delta-like-1, -2, -4, Jagged1, Jagged2) and participating in a variety of biological processes like cell fate (proliferation, differentiation, survival) and tissue homeostasis [153,154]. Notch signaling is also considered a key player in carcinogenesis, acting as either an oncogene or a tumor suppressor in various malignancies, including different TC histotypes, thus suggesting that its apparent dual role is cell type and context-dependent [152,153]. Among DTC, while PTC is generally characterized by high levels of Notch signaling, which is involved in promoting cell differentiation, in other less differentiated TC subtypes, namely FTC, medullary thyroid cancer, and anaplastic thyroid cancer, the low levels of Notch receptors are associated with more aggressive properties of neoplasms [153]. As recently demonstrated by Mosteiro and co-authors [155], the thyroid is strictly dependent on Notch for its homeostasis, and Notch inhibition promoted the destruction of thyroid function, with upregulation and downregulation of selected genes, including a decrease in PAX8 expression in both mouse and human thyroids. Furthermore, Notch suppression affected mitochondrial activity, thereby resulting in decreased levels of ROS and, given their essential role in TH synthesis, hypothyroidism [155]. Of note, DEHP exposure induced testicular toxicity in vivo and in vitro by inducing oxidative stress as revealed by the upregulation of SOD, GPx, heme oxygenase, NAD(P)H quinone dehydrogenase 1 and the transcription factor nuclear factor-erythroid 2 related factor (Nrf2), which overall regulate the redox balance in human cells [156]. Importantly, although the relationship between Notch and Nrf2 is controversial, the authors suggested that the toxic effects exerted by DEHP in the testis were due to a pro-oxidant mechanism that activates the Nrf2-mediated Notch1 pathway [156].
- Certain phthalates, such as DEHP, DEP, DBP, DiNP, and BBP, appear to act as estrogen agonists in experimental studies [157,158,159,160]. 17β-estradiol (E2) is the most potent physiological estrogen in vertebrates whose effects are not only restricted to reproduction, being implicated in a multitude of actions including cell growth and differentiation even in non-reproductive tissues [158,161]. E2 binds to the nuclear estrogen receptors (ERs) ERα and ERβ, and the formed complexes promote the transcription of genes containing estrogen response element sequences, which also may lead to the development of several types of estrogen-responsive cancers [158,161] including breast [162], endometrial [163], and ovarian cancers [164]. Furthermore, consistent with the 3- to 4-fold prevalence of both benign and malignant thyroid tumors in females compared to males, both ER isoforms have been detected in goiter tissues and TC, and it has been hypothesized that changes in the ERα:ERβ subtype ratio expression may enhance cell proliferation (ERα dominance) or induce cell apoptosis and tumor suppression in PTC (ERβ dominance) [165,166]. Alternatively, the non-genomic signaling of E2, which occurs through its membrane receptors, stimulates the activation of MAPK and PI3K pathways, which represent the major signaling cascades involved in thyroid tumorigenesis [165,167]. DEP treatment may activate ERα without binding the receptor in human breast cancer cells and induce cell proliferation to the same extent as E2 but through AKT activation alone without any effect on the MAPK/ERK signaling cascade [158]. Besides, perinatal exposure to DEHP affected the pituitary ERα and ERβ expression pattern from prepubertal and adult female rats and reduced the number of both the endocrine cells, lactotrophs and somatotrophs, expressing ERα and ERβ, suggesting that DEHP exposure can lead to cell deregulation in the pituitary gland, promoting an imbalance in ER expression [159]. Conversely, perinatal DEHP exposure in male rats induced changes in the pattern of both ER isoforms in prepubertal rats that became relevant only in adulthood, clearly indicating a sex-dependent impact of DEHP on pituitary ERs due to compensatory mechanisms that occur in the prepubertal age but are not sufficient to balance DEHP effects in adults [160].
- The reported data therefore supports an interaction of certain parent phthalates and phthalate metabolites with the HPT axis, with the consequent possibility of thyroid dysfunction, injury, and even cancer through multiple biological processes (Figure 3), although further studies evaluating the effects of phthalate mixtures at environmental doses would provide more valuable information to translate findings from the experimental models into real-world exposure-outcome relationships in humans.
5. The Effects of Non-Phthalate Plasticizers on the Thyroid Gland
6. The Dietary Source of Phthalates and Non-Phthalates: Current Status and Future Strategies
6.1. Edible Compounds and Plasticizers: Current Status
6.2. Future Strategies to Reduce Plasticizers in Food
7. Main Issues Related to the Migration Process from FCMs to Foodstuffs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| ATBC | Tributyl acetyl citrate |
| BBP | Benzyl butyl phthalate |
| BBzP | Benzyl butyl phthalate |
| BMI | Body mass index |
| CARD | Caspase activation and recruitment domain |
| DBP | Dibutyl phthalate |
| DEHA | Di(2-ethylhexyl) adipate |
| DEHP | Di-(2-ethylhexyl) phthalate |
| DEHS | Bis(2-ethylhexyl) sebacate |
| DEHTP | Di(2-ethylhexyl) terephthalate |
| DEP | Diethyl phthalate |
| DIBA | Diisobutyl adipate |
| DiBP | Di-isobutyl phthalate |
| DiDP | Di-isodecyl phthalate |
| DINCH | Diisononyl 1,2-cyclohexanedicarboxylic acid |
| DiNP | Di-isononyl phthalate |
| DMP | Dimethyl phthalate |
| DnBP | Di-isononyl phthalate |
| DTC | Differentiated thyroid cancer |
| EDC | Endocrine disrupting chemical |
| ERK | Extracellular signal-regulated kinase |
| DIO | Deiodinase |
| E2 | Estradiol |
| ER | Estrogen receptor |
| fT3 | Free triiodothyronine |
| fT4 | Free thyroxine |
| FCMs | Food contact materials |
| FTC | Follicular thyroid cancer |
| GPx | Glutathione peroxidase |
| HPT | Hypothalamic-pituitary-thyroid |
| HT | Hashimoto’s thyroiditis |
| IL | Interleukin |
| MAPK | Mitogen-activated protein kinase |
| MBP | Monobutyl phthalate |
| MBzP | Monobenzyl phthalate |
| MCNP | Monocarboxy-isononyl phthalate |
| MCOP | Monocarboxyoctyl phthalate |
| MCPP | Mono-3-carboxylpropyl phthalate |
| MECPP | Mono-(2-ethylpentyl-5-carboxy) phthalate |
| MEHHP | Mono (2-ethyl-5-hydroxyhexyl) phthalate |
| MEHP | Monoethylhexyl phthalate |
| MEOHP | Mono (2-ethyl-5-oxohexyl) phthalate |
| MEP | Monoethyl phthalate |
| MiBP | Mono-isobutyl phthalate |
| MMP | Monomethyl phthalate |
| MnBP | Mono-n-butyl phthalate |
| NF-κB | Nuclear factor kappa B |
| NIS | Sodium-iodine symporter |
| NLRP | NOD-like receptors family pyrin domain containing protein |
| NPP | Non-phthalate plasticizers |
| Nrf2 | Nuclear factor-erythroid 2 related factor |
| PAX-8 | Paired box 8 |
| PET | Polyethylene terephthalate |
| PI3K | Phosphatidylinositol-3 kinase |
| PPAR | Peroxisome proliferator-activated receptor |
| PTC | Papillary thyroid cancer |
| ROS | Reactive oxygen species |
| SCH | Subclinical hypothyroidism |
| SOD | Superoxide dismutase |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TBG | Thyroxin-binding globulin |
| TC | Thyroid cancer |
| TCC | thyroid colloid cyst |
| TDI | Tolerable daily intake |
| TG | Thyroglobulin |
| TH | Thyroid hormone |
| TNF-α | Tumor necrosis factor-alpha |
| TPO | Thyroid peroxidase |
| TR | Thyroid hormone receptor |
| TRH | Thyrotropin-releasing hormone |
| TRHR | Thyrotropin-releasing hormone receptor |
| TSH | Thyroid-stimulating hormone |
| TSH-β | Thyroid-stimulating hormone beta subunit |
| TSHR | Thyroid-stimulating hormone receptor |
| TT3 | Total triiodothyronine |
| TT4 | Total thyroxine |
| TTF-1 | Thyroid transcription factor-1 |
| TTR | Transthyretin |
| TXNIP | Thioredoxin-interacting protein |
References
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid hormone regulation of metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Yen, P.M. Metabolic Messengers: Thyroid Hormones. Nat. Metab. 2024, 6, 639–650. [Google Scholar] [CrossRef]
- Xiang, S.T.; Cao, Y.; Dong, J.; Li, C.; Qiu, J.; Li, X. The association between urinary phthalate metabolites and serum thyroid function in US adolescents. Sci. Rep. 2023, 13, 11601. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lai, Y.; Yang, J.; Yao, Y.; Li, Y.; Teng, W.; Shan, Z. The Relationship Between Thyroid Function and Metabolic Syndrome and Its Components: A Cross-Sectional Study in a Chinese Population. Front. Endocrinol. 2021, 12, 661160. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Hu, H.; Qu, H.; Xu, Y.; Li, Q. Prevalence and Trends of Thyroid Disease Among Adults, 1999–2018. Endocr. Pr. 2023, 29, 875–880. [Google Scholar] [CrossRef]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Andersson, M. GLOBAL ENDOCRINOLOGY: Global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 2021, 185, R13–R21. [Google Scholar] [CrossRef]
- Pearce, E.N.; Zimmermann, M.B. The Prevention of Iodine Deficiency: A History. Thyroid. 2023, 33, 143–149. [Google Scholar] [CrossRef]
- Biban, B.G.; Lichiardopol, C. Iodine Deficiency, still a Global Problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar]
- Maniakas, A.; Davies, L.; Zafereo, M.E. Thyroid Disease Around the World. Otolaryngol. Clin. North Am. 2018, 51, 631–642. [Google Scholar] [CrossRef]
- Petranović Ovčariček, P.; Görges, R.; Giovanella, L. Autoimmune Thyroid Diseases. Semin. Nucl. Med. 2024, 54, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Uppal, N.; Collins, R.; James, B. Thyroid noduless: Global, economic, and personal burdens. Front. Endocrinol. 2023, 14, 1113977. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, M.; Zhang, K.; Zhang, H.; Lai, Y. Diagnostic strategy for malignant and benign thyroid noduless smaller than 10 mm based on surface-enhanced Raman spectroscopy and machine learning. Chem. Eng. J. 2023, 471, 144794. [Google Scholar] [CrossRef]
- Pizzato, M.; Li, M.; Vignat, J.; Laversanne, M.; Singh, D.; La Vecchia, C.; Vaccarella, S. The epidemiological landscape of TC worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022, 10, 264–272. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of TC. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Diab, N.; Daya, N.R.; Juraschek, S.P.; Martin, S.S.; McEvoy, J.W.; Schultheiß, U.T.; Köttgen, A.; Selvin, E. Prevalence and Risk Factors of Thyroid Dysfunction in Older Adults in the Community. Sci. Rep. 2019, 9, 13156. [Google Scholar] [CrossRef]
- Pearce, E.N. Endocrine Disruptors and Thyroid Health. Endocr. Pr 2024, 30, 172–176. [Google Scholar] [CrossRef]
- Rodrigues, V.G.; Henrique, G.; Sousa-Vidal, É.K.; de Souza, R.M.M.; Tavares, E.F.C.; Mezzalira, N.; Marques, T.d.O.; Alves, B.M.; Pinto, J.A.A.; Irikura, L.N.N.; et al. Thyroid under Attack: The Adverse Impact of Plasticizers, Pesticides, and PFASs on Thyroid Function. Endocrines 2024, 5, 430–453. [Google Scholar] [CrossRef]
- Kalofiri, P.; Balias, G.; Tekos, F. The EU endocrine disruptors’ regulation and the glyphosate controversy. Toxicol. Rep. 2021, 8, 1193–1199. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen. Comp. Endocrinol. 2015, 219, 74–88. [Google Scholar] [CrossRef]
- Bereketoglu, C.; Pradhan, A. Plasticizers: Negative impacts on the thyroid hormone system. Environ. Sci. Pollut. Res. Int. 2022, 29, 38912–38927. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Phthalates in Cosmetics. 2022. Available online: https://www.fda.gov/cosmetics/cosmetic-ingredients/phthalates-cosmetics#:~:text=Phthalates%20are%20chemical%20compounds%20developed,to%20which%20they%20are%20applied (accessed on 3 November 2024).
- Ye, H.; Ha, M.; Yang, M.; Yue, P.; Xie, Z.; Liu, C. Di2-ethylhexyl phthalate disrupts thyroid hormone homeostasis through activating the Ras/Akt/TRHr pathway and inducing hepatic enzymes. Sci. Rep. 2017, 7, 40153. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Moon, H.B. Contamination and historical trends of legacy and emerging plasticizers in sediment from highly industrialized bays of Korea. Sci. Total Environ. 2021, 765, 142751. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Oguri, T.; Takagi, M.; Ueyama, J.; Isobe, T. Evaluating the risk of phthalate and non-phthalate plasticizers in dust samples from 100 Japanese houses. Environ. Int. 2024, 83, 108399. [Google Scholar] [CrossRef]
- Di Bella, G.; Porretti, M.; Cafarelli, M.; Litrenta, F.; Potortì, A.G.; Turco, V.L.; Albergamo, A.; Xhilari, M.; Faggio, C. Screening of phthalate and non-phthalate plasticizers and bisphenols in Sicilian women’s blood. Environ. Toxicol. Pharmacol. 2023, 100, 104166. [Google Scholar] [CrossRef] [PubMed]
- Sanmartin, C.; Venturi, F.; Taglieri, I.; Nari, A.; Andrichi, G.; Zinna, A. Study of the migration of chemicals from conventional food contact materials into food and environment: Hurdles and limits for a whole hazard identification and risk assessment. Agrochimica 2018, 62, 179–200. [Google Scholar]
- Godlewska, M.; Banga, B.J. Thyroid peroxidase as a dual active site enzyme: Focus on biosynthesis, hormonogenesis and thyroid disorders of autoimmunity and cancer. Biochimie 2019, 160, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by stealth—Interference of endocrine disrupting chemicals on hormonal crosstalk with thyroid axis function in humans and other animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef]
- Welsh, K.J.; Soldin, S.J. Diagnosis of Endocrine Disease: How reliable are free thyroid and total T3 hormone assays? Eur. J. Endocrinol. 2016, 175, R255–R263. [Google Scholar] [CrossRef]
- Fröhlich, E.; Wahl, R. Physiological Role and Use of Thyroid Hormone Metabolites—Potential Utility in COVID-19 Patients. Front. Endocrinol. 2021, 12, 587518. [Google Scholar] [CrossRef]
- Bikle, D.D. The Free Hormone Hypothesis: When, Why, and How to Measure the Free Hormone Levels to Assess Vitamin D, Thyroid, Sex Hormone, and Cortisol Status. JBMR Plus 2020, 5, e10418. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.L.; Obeng-Gyasi, B.; Hall, J.E.; Shekhar, S. The Thyroid Hormone Axis and Female Reproduction. Int. J. Mol. Sci. 2023, 24, 9815. [Google Scholar] [CrossRef]
- Cicatiello, A.G.; Di Girolamo, D.; Dentice, M. Metabolic Effects of the Intracellular Regulation of Thyroid Hormone: Old Players, New Concepts. Front. Endocrinol. 2018, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef]
- Groeneweg, S.; van Geest, F.S.; Peeters, R.P.; Heuer, H.; Visser, W.E. Thyroid Hormone Transporters. Endocr. Rev. 2020, 41, bnz008. [Google Scholar] [CrossRef]
- van der Spek, A.H.; Fliers, E.; Boelen, A. The classic pathways of thyroid hormone metabolism. Mol. Cell. Endocrinol. 2017, 458, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, A.; Lee, J.W.; Kim, N.H.; Liu, Y.Y.; Yang, A.; Sedrakyan, S.; Kahng, A.; Cervantes, V.; Tripuraneni, N.; Cheng, S.Y.; et al. Thyroid Hormone Receptor α Plays an Essential Role in Male Skeletal Muscle Myoblast Proliferation, Differentiation, and Response to Injury. Endocrinology 2016, 157, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Minakhina, S.; Bansal, S.; Zhang, A.; Brotherton, M.; Janodia, R.; De Oliveira, V.; Tadepalli, S.; Wondisford, F.E. A Direct Comparison of Thyroid Hormone Receptor Protein Levels in Mice Provides Unexpected Insights into Thyroid Hormone Action. Thyroid 2020, 30, 1193–1204. [Google Scholar] [CrossRef]
- Nappi, A.; Murolo, M.; Cicatiello, A.G.; Sagliocchi, S.; Di Cicco, E.; Raia, M.; Stornaiuolo, M.; Dentice, M.; Miro, C. Thyroid Hormone Receptor Isoforms Alpha and Beta Play Convergent Roles in Muscle Physiology and Metabolic Regulation. Metabolites 2022, 12, 405. [Google Scholar] [CrossRef]
- Giammanco, M.; Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals. Int. J. Mol. Sci. 2020, 21, 4140. [Google Scholar] [CrossRef]
- Gonzalez, M.L.; Chernock, R.D.; Mansour, M. Environmental factors and anatomic pathology of the thyroid gland: Review of literature. Diagn. Histopathol. 2020, 26, 207–215. [Google Scholar] [CrossRef]
- Murthy, M.B.; Murthy, B.K. Thyroid disruptors and their possible clinical implications. Indian J. Pharmacol. 2012, 44, 542–543. [Google Scholar] [CrossRef]
- Oliveira, K.J.; Chiamolera, M.I.; Giannocco, G.; Pazos-Moura, C.C.; Ortiga-Carvalho, T.M. Thyroid Function Disruptors: From nature to chemicals. J. Mol. Endocrinol. 2018, JME-18-0081. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Iervasi, G.; Coi, A.; Pitto, L.; Bianchi, F. The Role of Polybrominated Diphenyl Ethers in Thyroid Carcinogenesis: Is It a Weak Hypothesis or a Hidden Reality? From Facts to New Perspectives. Int. J. Environ. Res. Public Health 2018, 15, 1834. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Bustaffa, E.; Coi, A.; Iervasi, G.; Bianchi, F. Bisphenols as Environmental Triggers of Thyroid Dysfunction: Clues and Evidence. Int. J. Environ. Res. Public Health 2020, 17, 2654. [Google Scholar] [CrossRef]
- Street, M.E.; Shulhai, A.M.; Petraroli, M.; Patianna, V.; Donini, V.; Giudice, A.; Gnocchi, M.; Masetti, M.; Montani, A.G.; Rotondo, R.; et al. The impact of environmental factors and contaminants on thyroid function and disease from fetal to adult life: Current evidence and future directions. Front. Endocrinol. 2024, 15, 1429884. [Google Scholar] [CrossRef]
- Statista Research Department. Global Plasticizers Market Volume 2015–2030. 2023. Available online: https://www.statista.com/statistics/1245193/plasticizer-market-volume-worldwide/#:~:text=In%202022%2C%20market%20volume%20of,it%20softer%20and%20more%20flexible (accessed on 7 November 2024).
- Naveen, K.V.; Saravanakumar, K.; Zhang, X.; Sathiyaseelan, A.; Wang, M.H. Impact of environmental phthalate on human health and their bioremediation strategies using fungal cell factory—A review. Environ. Res. 2022, 214, 113781. [Google Scholar] [CrossRef]
- Baloyi, N.D.; Tekere, M.; Maphangwa, K.W.; Masindi, V. Insights into the Prevalence and Impacts of Phthalate Esters in Aquatic Ecosystems. Front. Environ. Sci. 2021, 9, 684190. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Li, Z.; Tao, Y.; Yang, Y. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci. Total Environ. 2021, 771, 145418. [Google Scholar] [CrossRef]
- Arrigo, F.; Impellitteri, F.; Piccione, G.; Faggio, C. Phthalates and their effects on human health: Focus on erythrocytes and the reproductive system. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 270, 109645. [Google Scholar] [CrossRef]
- Gao, D.W.; Wen, Z.D. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci. Total Environ. 2016, 541, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate Exposure and Long-Term Epigenomic Consequences: A Review. Front. Genet. 2020, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.S.; Clark, J.M.; Pearce, J.L.; Ferguson, P.L.; Newman, R.B.; Roberts, J.R.; Grobman, W.A.; Sciscione, A.C.; Skupski, D.W.; Garcia, K.; et al. Impact of Skin Care Products on Phthalates and Phthalate Replacements in Children: The ECHO-FGS. Environ. Health Perspect. 2024, 132, 97001. [Google Scholar] [CrossRef]
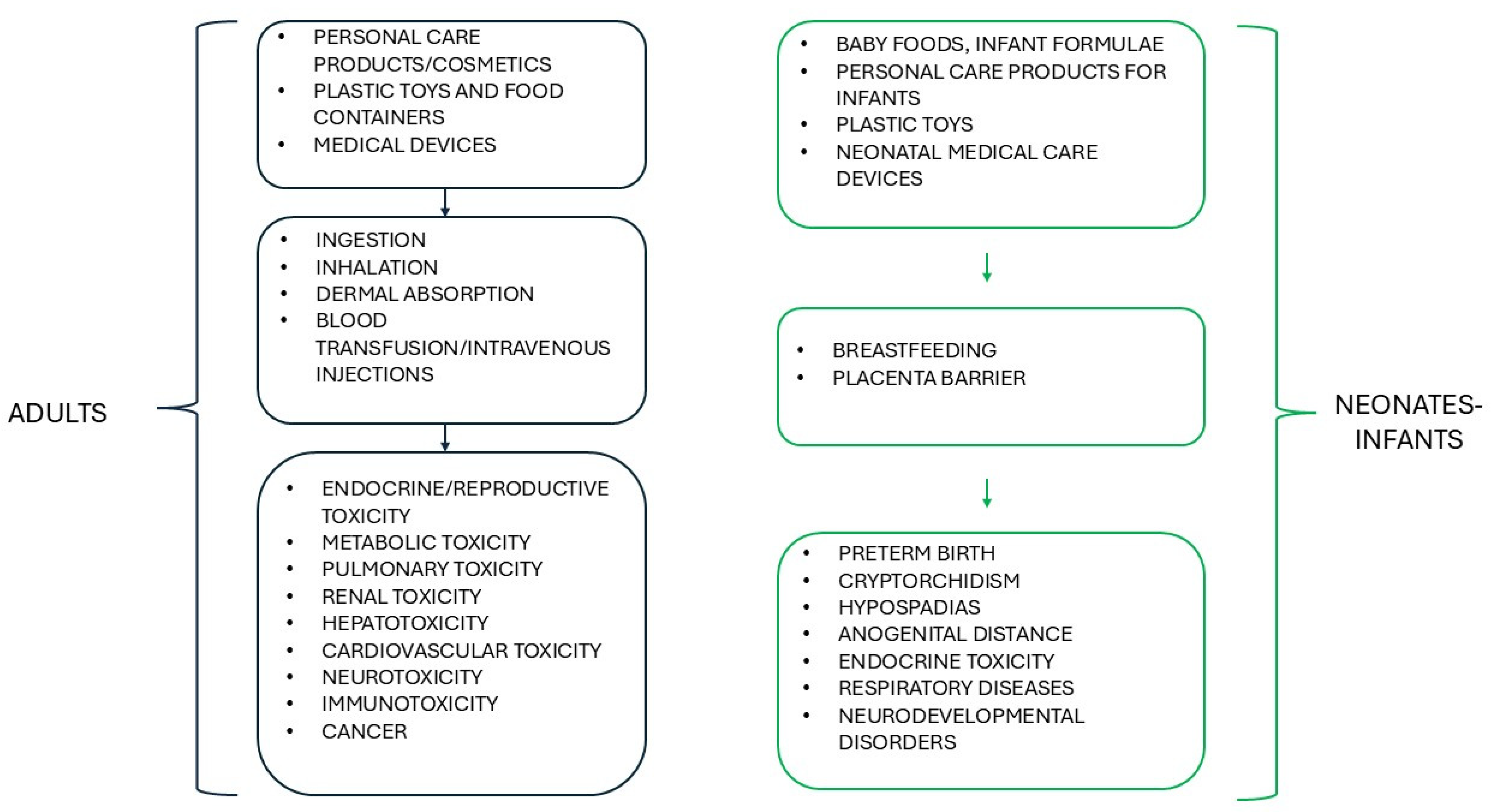
- Qian, Y.; Shao, H.; Ying, X.; Huang, W.; Hua, Y. The Endocrine Disruption of Prenatal Phthalate Exposure in Mother and Offspring. Front. Public Health 2020, 8, 366. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Šimunović, A.; Tomić, S.; Kranjčec, K. Medical devices as a source of phthalate exposure: A review of current knowledge and alternative solutions. Arch. Ind. Hyg. Toxicol. 2022, 73, 179–190. [Google Scholar] [CrossRef]
- Genuis, S.J.; Beesoon, S.; Lobo, R.A.; Birkholz, D. Human elimination of phthalate compounds: Blood, urine, and sweat (BUS) study. Sci. World J. 2012, 2012, 615068. [Google Scholar] [CrossRef]
- Samandar, E.; Silva, M.J.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Temporal stability of eight phthalate metabolites and their glucuronide conjugates in human urine. Environ Res. 2009, 109, 641–646. [Google Scholar] [CrossRef]
- Chen, Y.; Zhen, Z.; Li, G.; Li, H.; Wei, T.; Huang, F.; Li, T.; Yang, C.; Ren, L.; Liang, Y.; et al. Di-2-ethylhexyl phthalate (DEHP) degradation and microbial community change in mangrove rhizosphere gradients. Sci. Total Environ. 2023, 871, 162022. [Google Scholar] [CrossRef]
- ATSDR (Agency for Toxic Substances and Disease Registry). ToxFAQs™ for Di(2-ethylhexyl)phthalate (DEHP). 2022. Available online: https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=377&toxid=65 (accessed on 8 November 2024).
- ECHA (European Chemical Agency). Phthalates. Available online: https://echa.europa.eu/hot-topics/phthalates (accessed on 11 November 2024).
- INERIS. Phthalates Substitution. Available online: https://substitution-phtalates.ineris.fr/en/regulatory-information#:~:text=Fourteen%20phthalates%20are%20included%20in,1%2C2%2DBenzenedicarboxylic%20acid%2C (accessed on 13 November 2024).
- EFSA (European Food Safety Agency). EFSA: Updated Risk Assessment of Five Phthalates. 2019. Available online: https://foodpackagingforum.org/news/efsa-updated-risk-assessment-of-five-phthalates (accessed on 11 November 2024).
- U.S. Food & Drug Administration. Phthalates in Food Packaging and Food Contact Applications. 2024. Available online: https://www.fda.gov/food/food-additives-and-gras-ingredients-information-consumers/phthalates-food-packaging-and-food-contact-applications (accessed on 11 November 2024).
- Ding, S.; Zhang, Z.; Chen, Y.; Qi, W.; Zhang, Y.; Xu, Q.; Liu, H.; Zhang, T.; Zhao, Y.; Han, X.; et al. Urinary levels of phthalate metabolites and their association with lifestyle behaviors in Chinese adolescents and young adults. Ecotoxicol. Environ. Saf. 2019, 183, 109541. [Google Scholar] [CrossRef]
- Frederiksen, H.; Nielsen, O.; Koch, H.M.; Skakkebaek, N.E.; Juul, A.; Jørgensen, N.; Andersson, A.M. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int. J. Hyg. Environ. Health 2020, 223, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Fruh, V.; Preston, E.V.; Quinn, M.R.; Hacker, M.R.; Wylie, B.J.; O’Brien, K.; Hauser, R.; James-Todd, T.; Mahalingaiah, S. Urinary phthalate metabolite concentrations and personal care product use during pregnancy—Results of a pilot study. Sci. Total Environ. 2022, 835, 155439. [Google Scholar] [CrossRef]
- Domínguez-Romero, E.; Komprdová, K.; Kalina, J.; Bessems, J.; Karakitsios, S.; Sarigiannis, D.A.; Scheringer, M. Time-trends in human urinary concentrations of phthalates and substitutes DEHT and DINCH in Asian and North American countries (2009–2019). J. Expo. Sci. Environ. Epidemiol. 2023, 33, 244–254. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Sheikh, I.A.; Beg, M.A. Insights into the Endocrine Disrupting Activity of Emerging Non-Phthalate Alternate Plasticizers against Thyroid Hormone Receptor: A Structural Perspective. Toxics 2022, 10, 263. [Google Scholar] [CrossRef]
- Gran View Research. Non-Phthalate Plasticizers Market Size, Share & Trends Analysis Report by Type (Adipates, Trimellitates, Benzoates, Epoxies), by Application (Flooring & Wall Coverings, Wires & Cables), by Region, and Segment Forecasts, 2024—2030. Available online: https://www.grandviewresearch.com/industry-analysis/non-phthalate-plasticizers-market-report#:~:text=Non%2Dphthalate%20Plasticizers%20Market%20Trends,seek%20out%20non%2Dphthalate%20options (accessed on 11 November 2024).
- Harmon, P.; Otter, R. A review of common non-ortho-phthalate plasticizers for use in food contact materials. Food Chem. Toxicol. 2022, 164, 112984. [Google Scholar] [CrossRef] [PubMed]
- ECHA (European Chemical Agency). 1,2-Cyclohexanedicarboxylic Acid, Diisononyl Ester, Reaction Products of Hydrogenation of Di-Isononylphthalates (n-butenes Based). 2024. Available online: https://echa.europa.eu/it/substance-information/-/substanceinfo/100.103.017 (accessed on 11 November 2024).
- ECHA (European Chemical Agency). Bis(2-Ethylhexyl) Adipate. 2024. Available online: https://echa.europa.eu/it/substance-information/-/substanceinfo/100.002.810 (accessed on 12 November 2024).
- Qadeer, A.; Kirsten, K.L.; Ajmal, Z.; Jiang, X.; Zhao, X. Alternative Plasticizers As Emerging Global Environmental and Health Threat: Another Regrettable Substitution? Environ. Sci. Technol. 2022, 56, 1482–1488. [Google Scholar] [CrossRef]
- Nehring, A.; Bury, D.; Ringbeck, B.; Kling, H.W.; Otter, R.; Weiss, T.; Brüning, T.; Koch, H.M. Metabolism and urinary excretion kinetics of di(2-ethylhexyl) adipate (DEHA) in four human volunteers after a single oral dose. Toxicol. Lett. 2020, 321, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.; McCray, N.L.; VanNoy, B.N.; Yau, A.; Geller, R.J.; Adamkiewicz, G.; Zota, A.R. Phthalate and novel plasticizer concentrations in food items from U.S. fast food chains: A preliminary analysis. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 366–373. [Google Scholar] [CrossRef]
- Coltro, L.; Borghetti Pitta, J.; da Costa, P.A.; Fávaro Perez, M.A.; Aparecida de Araújo, V.; Rodrigues, R. Migration of conventional and new plasticizers from PVC films into food simulants: A comparative study. Food Control. 2014, 44, 118–129. [Google Scholar] [CrossRef]
- Behairy, A.; Abd El-Rahman, G.I.; Aly, S.S.H.; Fahmy, E.M.; Abd-Elhakim, Y.M. Di(2-ethylhexyl) adipate plasticizer triggers hepatic, brain, and cardiac injury in rats: Mitigating effect of Peganum harmala oil. Ecotoxicol. Environ. Saf. 2021, 208, 111620. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Agency). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission on the application of A Total Reduction Factor of 5 for di(2-ethylhexyl)adipate used as plasticizer in flexible PVC food packaging films. EFSA J. 2005, 217, 1–5. [Google Scholar]
- EFSA (European Food Safety Agency). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request related to a 12th list of substances for food contact materials. EFSA J. 2006, 395–401, 1–21. [Google Scholar]
- sEFSA (European Food Safety Agency). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request related to a 18th list of substances for food contact materials. EFSA J. 2008, 628–633, 1–19. [Google Scholar]
- Ketema, R.M.; Kasper-Sonnenberg, M.; Ait Bamai, Y.; Miyashita, C.; Koch, H.M.; Pälmke, C.; Kishi, R.; Ikeda, A. Exposure Trends to the Non-phthalate Plasticizers DEHTP, DINCH, and DEHA in Children from 2012 to 2017: The Hokkaido Study. Environ. Sci. Technol. 2023, 57, 11926–11936. [Google Scholar] [CrossRef] [PubMed]
- Campioli, E.; Lee, S.; Lau, M.; Marques, L.; Papadopoulos, V. Effect of prenatal DINCH plasticizer exposure on rat offspring testicular function and metabolism. Sci. Rep. 2017, 7, 11072. [Google Scholar] [CrossRef]
- Schaffert, A.; Arnold, J.; Karkossa, I.; Blüher, M.; von Bergen, M.; Schubert, K. The Emerging Plasticizer Alternative DINCH and Its Metabolite MINCH Induce Oxidative Stress and Enhance Inflammatory Responses in Human THP-1 Macrophages. Cells 2021, 10, 2367. [Google Scholar] [CrossRef]
- Saad, N.; Bereketoglu, C.; Pradhan, A. Di(isononyl) cyclohexane-1,2-dicarboxylate (DINCH) alters transcriptional profiles, lipid metabolism and behavior in zebrafish larvae. Heliyon 2021, 7, e07951. [Google Scholar] [CrossRef]
- Kasper-Sonnenberg, M.; Koch, H.M.; Apel, P.; Rüther, M.; Pälmke, C.; Brüning, T.; Kolossa-Gehring, M. Time trend of exposure to the phthalate plasticizer substitute DINCH in Germany from 1999 to 2017: Biomonitoring data on young adults from the Environmental Specimen Bank (ESB). Int. J. Hyg. Environ. Health 2019, 222, 1084–1092. [Google Scholar] [CrossRef]
- Silva, M.J.; Wong, L.Y.; Samandar, E.; Preau, J.L., Jr.; Jia, L.T.; Calafat, A.M. Exposure to di-2-ethylhexyl terephthalate in the U.S. general population from the 2015–2016 National Health and Nutrition Examination Survey. Environ. Int. 2019, 123, 141–147. [Google Scholar] [CrossRef]
- Vogel, N.; Frederiksen, H.; Lange, R.; Jørgensen, N.; Koch, H.M.; Weber, T.; Andersson, A.M.; Kolossa-Gehring, M. Urinary excretion of phthalates and the substitutes DINCH and DEHTP in Danish young men and German young adults between 2000 and 2017—A time trend analysis. Int. J. Hyg. Environ. Health 2023, 248, 114080. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, S.; Oh, B.C.; Jung, D.; Choi, K.; Park, Y.J. Association Between Diethylhexyl Phthalate Exposure and Thyroid Function: A Meta-Analysis. Thyroid 2019, 29, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.E.; Eliot, M.N.; Zoeller, R.T.; Hoofnagle, A.N.; Calafat, A.M.; Karagas, M.R.; Yolton, K.; Chen, A.; Lanphear, B.P.; Braun, J.M. Maternal urinary phthalate metabolites during pregnancy and thyroid hormone concentrations in maternal and cord sera: The HOME Study. Int. J. Hyg. Environ. Health 2018, 221, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Cathey, A.L.; Watkins, D.; Rosario, Z.Y.; Vélez, C.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Associations of Phthalates and Phthalate Replacements with CRH and Other Hormones Among Pregnant Women in Puerto Rico. J. Endocr. Soc. 2019, 3, 1127–1149. [Google Scholar] [CrossRef]
- Villanger, G.D.; Drover, S.S.M.; Nethery, R.C.; Thomsen, C.; Sakhi, A.K.; Øvergaard, K.R.; Zeiner, P.; Hoppin, J.A.; Reichborn-Kjennerud, T.; Aase, H.; et al. Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ. Int. 2020, 137, 105509. [Google Scholar] [CrossRef] [PubMed]
- Abiri, B.; Ahmadi, A.R.; Mahdavi, M.; Amouzegar, A.; Valizadeh, M. Association between thyroid function and obesity phenotypes in healthy euthyroid individuals: An investigation based on Tehran Thyroid Study. Eur. J. Med. Res. 2023, 28, 179. [Google Scholar] [CrossRef]
- Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. Int. J. Mol. Sci. 2024, 25, 675. [Google Scholar] [CrossRef]
- Choi, S.; Kim, M.J.; Park, Y.J.; Kim, S.; Choi, K.; Cheon, G.J.; Cho, Y.H.; Jeon, H.L.; Yoo, J.; Park, J. Thyroxine-binding globulin, peripheral deiodinase activity, and thyroid autoantibody status in association of phthalates and phenolic compounds with thyroid hormones in adult population. Environ. Int. 2020, 140, 105783. [Google Scholar] [CrossRef]
- Derakhshan, A.; Shu, H.; Broeren, M.A.C.; Lindh, C.H.; Peeters, R.P.; Kortenkamp, A.; Demeneix, B.; Bornehag, C.G.; Korevaar, T.I.M. Association of phthalate exposure with thyroid function during pregnancy. Environ. Int. 2021, 157, 106795. [Google Scholar] [CrossRef]
- Yang, Z.; Shan, D.; Zhang, T.; Li, L.; Wang, S.; Du, R.; Li, Y.; Wu, S.; Jin, L.; Zhao, Y.; et al. Associations between exposure to phthalates and subclinical hypothyroidism in pregnant women during early pregnancy: A pilot case-control study in China. Environ. Pollut. 2023, 320, 121051. [Google Scholar] [CrossRef]
- Huang, P.C.; Chang, W.H.; Wu, M.T.; Chen, M.L.; Wang, I.J.; Shih, S.F.; Hsiung, C.A.; Liao, K.W. Characterization of phthalate exposure in relation to serum thyroid and growth hormones, and estimated daily intake levels in children exposed to phthalate-tainted products: A longitudinal cohort study. Environ. Pollut. 2020, 264, 114648. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Elkhatib, R.; Alghamdi, R.; Alrushud, N.; Alnuwaysir, H.; Alnemer, M.; Aldhalaan, H.; Shoukri, M. Phthalate exposure during pregnancy and its association with thyroid hormones: A prospective cohort study. Int. J. Hyg. Environ. Health 2024, 261, 114421. [Google Scholar] [CrossRef] [PubMed]
- Sur, U.; Erkekoglu, P.; Bulus, A.D.; Andiran, N.; Kocer-Gumusel, B. Oxidative stress markers, trace elements, and endocrine disrupting chemicals in children with Hashimoto’s thyroiditis. Toxicol. Mech. Methods 2019, 29, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yuan, J.; Yao, Q.; Yan, N.; Song, R.; Jiang, W.; Li, D.; Shi, L.; Zhang, J.A. A case-control study of selenoprotein genes polymorphisms and autoimmune thyroid diseases in a Chinese population. BMC Med. Genet. 2017, 18, 54. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Ahmadpour, F.; Chahardoli, B.; Malekpour-Dehkordi, Z.; Nourbakhsh, M.; Hosseini-Fard, S.R.; Doustimotlagh, A.; Golestani, A.; Razzaghy-Azar, M. Selenium and its relationship with selenoprotein P and glutathione peroxidase in children and adolescents with Hashimoto’s thyroiditis and hypothyroidism. J. Trace Elem. Med. Biol. 2016, 34, 10–14. [Google Scholar] [CrossRef]
- Erkekoglu, P.; Giray, B.K.; Kızilgün, M.; Rachidi, W.; Hininger-Favier, I.; Roussel, A.M.; Favier, A.; Hincal, F. Di(2-ethylhexyl)phthalate-induced renal oxidative stress in rats and protective effect of selenium. Toxicol. Mech. Methods 2012, 22, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Erkekoglu, P.; Zeybek, N.D.; Giray, B.K.; Rachidi, W.; Kızılgün, M.; Hininger-Favier, I.; Favier, A.; Asan, E.; Hincal, F. The effects of di(2-ethylhexyl)phthalate on rat liver in relation to selenium status. Int. J. Exp. Pathol. 2014, 95, 64–77. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R.; Cooper, D.S. Subclinical Hypothyroidism: A Review. JAMA 2019, 322, 153–160. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- Yalçin, S.S.; Erdal, İ.; Çetinkaya, S.; Oğuz, B. Urinary levels of phthalate esters and heavy metals in adolescents with thyroid colloid cysts. Int. J. Environ. Health Res. 2022, 32, 1359–1372. [Google Scholar] [CrossRef]
- Rho, M.H.; Kim, D.W. Long-Term Ultrasonography Follow-Up of Thyroid Colloid Cysts at the Health Center: A Single-Center Study. Int. J. Endocrinol. 2015, 2015, 324581. [Google Scholar] [CrossRef]
- Marotta, V.; Russo, G.; Gambardella, C.; Grasso, M.; La Sala, D.; Chiofalo, M.G.; D’Anna, R.; Puzziello, A.; Docimo, G.; Masone, S.; et al. Human exposure to bisphenol AF and diethylhexylphthalate increases susceptibility to develop differentiated TC in patients with thyroid noduless. Chemosphere 2019, 218, 885–894. [Google Scholar] [CrossRef]
- Houten, P.V.; Netea-Maier, R.T.; Smit, J.W. Differentiated thyroid carcinoma: An update. Best Pr. Res. Clin. Endocrinol. Metab. 2023, 37, 101687. [Google Scholar] [CrossRef] [PubMed]
- Alaraifi, A.K.; Alessa, M.; Hijazi, L.O.; Alayed, A.M.; Alsalem, A.A. TSH level as a risk factor of thyroid malignancy for noduless in euthyroid patients. Acta Otorhinolaryngol. Ital. 2023, 43, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Deng, Y.L.; Zheng, T.Z.; Yang, P.; Jiang, X.Q.; Liu, E.N.; Miao, X.P.; Wang, L.Q.; Jiang, M.; Zeng, Q. Urinary biomarkers of phthalates exposure and risks of TC and benign nodules. J. Hazard. Mater. 2020, 383, 121189. [Google Scholar] [CrossRef]
- Lam, A.K. Papillary Thyroid Carcinoma: Current Position in Epidemiology, Genomics, and Classification. Methods Mol. Biol. 2022, 2534, 1–15. [Google Scholar] [PubMed]
- Li, Y.; Che, W.; Yu, Z.; Zheng, S.; Xie, S.; Chen, C.; Qiao, M.; Lyu, J. The Incidence Trend of Papillary Thyroid Carcinoma in the United States During 2003–2017. Cancer Control. 2022, 29, 10732748221135447. [Google Scholar] [CrossRef]
- Miao, H.; Liu, X.; Li, J.; Zhang, L.; Zhao, Y.; Liu, S.; Ni, S.; Wu, Y. Associations of urinary phthalate metabolites with risk of papillary TC. Chemosphere 2020, 241, 125093. [Google Scholar] [CrossRef]
- Iglesias, M.L.; Schmidt, A.; Ghuzlan, A.A.; Lacroix, L.; Vathaire, F.; Chevillard, S.; Schlumberger, M. Radiation exposure and TC: A review. Arch. Endocrinol. Metab. 2017, 61, 180–187. [Google Scholar] [CrossRef]
- Saenko, V.; Mitsutake, N. Radiation-Related TC. Endocr. Rev. 2024, 45, 1–29. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, W.; Xu, Q.; Li, X.; Zhou, L.; Ye, L. Di(2-ethylhexyl) phthalate (DEHP) and thyroid: Biological mechanisms of interference and possible clinical implications. Environ. Sci. Pollut. Res. Int. 2022, 29, 1634–1644. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Wei, L.; Li, L. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environ. Sci. Pollut. Res. Int. 2015, 22, 12711–12719. [Google Scholar] [CrossRef]
- Zhai, W.; Huang, Z.; Chen, L.; Feng, C.; Li, B.; Li, T. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS ONE 2014, 9, e92465. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhou, L.; Wang, S.; Liu, T.; Zhu, J.; Jia, Y.; Xu, J.; Chen, H.; Wang, Q.; Xu, F.; et al. Effect of Di-(2-ethylhexyl) phthalate on the hypothalamus-pituitary-thyroid axis in adolescent rat. Endocr. J. 2022, 69, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, X.; Hu, G.; Hao, Y.; Zhang, X.; Liu, H.; Wei, S.; Wang, X.; Yu, H. Bioanalytical and instrumental analysis of thyroid hormone disrupting compounds in water sources along the Yangtze River. Environ. Pollut. 2011, 159, 441–448. [Google Scholar] [CrossRef]
- Hu, X.; Shi, W.; Zhang, F.; Cao, F.; Hu, G.; Hao, Y.; Wei, S.; Wang, X.; Yu, H. In vitro assessment of thyroid hormone disrupting activities in drinking water sources along the Yangtze River. Environ. Pollut. 2013, 173, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Miccadei, S.; De Leo, R.; Zammarchi, E.; Natali, P.G.; Civitareale, D. The synergistic activity of thyroid transcription factor 1 and Pax 8 relies on the promoter/enhancer interplay. Mol. Endocrinol. 2002, 16, 837–846. [Google Scholar] [CrossRef]
- Li, L.; Xia, Y.; Chen, J.; Han, X.; Hao, L.; Li, D.; Liu, Y. DBP exposure induces thyroid inflammatory impairment through activating AKT/NF-κB/NLRP3 signaling. Ecotoxicol. Environ. Saf. 2023, 264, 115385. [Google Scholar] [CrossRef]
- Faria, M.; Domingues, R.; Paixão, F.; Bugalho, M.J.; Matos, P.; Silva, A.L. TNFα-mediated activation of NF-κB downregulates sodium-iodide symporter expression in thyroid cells. PLoS ONE 2020, 15, e0228794. [Google Scholar] [CrossRef]
- Crescenzi, E.; Leonardi, A.; Pacifico, F. NF-κB in Thyroid Cancer: An Update. Int. J. Mol. Sci. 2024, 25, 11464. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Wu, H.; Ma, K.; Na, X. Rosmarinic acid alleviates di-2-ethylhexyl phthalate (DEHP) -induced thyroid dysfunction via multiple inflammasomes activation. J. Toxicol. Sci. 2020, 45, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Matico, R.E.; Yu, X.; Miller, R.; Somani, S.; Ricketts, M.D.; Kumar, N.; Steele, R.A.; Medley, Q.; Berger, S.; Faustin, B.; et al. Structural basis of the human NAIP/NLRC4 inflammasome assembly and pathogen sensing. Nat. Struct. Mol. Biol. 2024, 31, 82–91. [Google Scholar] [CrossRef]
- Kumari, P.; Russo, A.J.; Shivcharan, S.; Rathinam, V.A. AIM2 in health and disease: Inflammasome and beyond. Immunol. Rev. 2020, 297, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Franklin, B.S.; Bossaller, L.; De Nardo, D.; Ratter, J.M.; Stutz, A.; Engels, G.; Brenker, C.; Nordhoff, M.; Mirandola, S.R.; Al-Amoudi, A.; et al. The adaptor ASC has extracellular and ’prionoid’ activities that propagate inflammation. Nat. Immunol. 2014, 15, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- He, W.T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.H.; Zhong, C.Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Liu, M.; Zhang, M.; Jia, X. NEK7: A new target for the treatment of multiple tumors and chronic inflammatory diseases. Inflammopharmacology 2022, 30, 1179–1187. [Google Scholar] [CrossRef]
- Rosa, C.P.; Belo, T.C.A.; Santos, N.C.M.; Silva, E.N.; Gasparotto, J.; Corsetti, P.P.; de Almeida, L.A. Reactive oxygen species trigger inflammasome activation after intracellular microbial interaction. Life Sci. 2023, 331, 122076. [Google Scholar] [CrossRef]
- Hua, K.F.; Chou, J.C.; Ka, S.M.; Tasi, Y.L.; Chen, A.; Wu, S.H.; Chiu, H.W.; Wong, W.T.; Wang, Y.F.; Tsai, C.L.; et al. Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1β production. J. Cell Physiol. 2015, 30, 863–874. [Google Scholar] [CrossRef]
- Deng, J.; Pan, T.; Liu, Z.; McCarthy, C.; Vicencio, J.M.; Cao, L.; Alfano, G.; Suwaidan, A.A.; Yin, M.; Beatson, R.; et al. The role of TXNIP in cancer: A fine balance between redox, metabolic, and immunological tumor control. Br. J. Cancer 2023, 129, 1877–1892. [Google Scholar] [CrossRef]
- De Felice, M.; Postiglione, M.P.; Di Lauro, R. Minireview: Thyrotropin receptor signaling in development and differentiation of the thyroid gland: Insights from mouse models and human diseases. Endocrinology 2004, 145, 4062–4067. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, J.; Zhao, Y.; Guo, J.; Wang, Z.; Liu, M.; Zhang, Y.; Na, X. Effects of Long-Term In Vivo Exposure to Di-2-Ethylhexylphthalate on Thyroid Hormones and the TSH/TSHR Signaling Pathways in Wistar Rats. Int. J. Environ. Res. Public Health 2017, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Zhao, X.; Tang, L.; Chen, J.; Zhao, J.; Guo, M.; Chen, C.; Zhou, Y.; Xu, L. Thyroid Transcription Factor-1: Structure, Expression, Function and Its Relationship with Disease. Biomed. Res. Int. 2021, 2021, 9957209. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.Y.; Yoo, Y.J.; Jeong, J.S.; Nam, K.T.; Jee, S.H.; Lim, K.M.; Lee, Y.S. Di-2-ethylhexylphthalate promotes thyroid cell proliferation and DNA damage through activating thyrotropin-receptor-mediated pathways in vitro and in vivo. Food Chem. Toxicol. 2019, 124, 265–272. [Google Scholar] [CrossRef]
- Nozhat, Z.; Hedayati, M. PI3K/AKT Pathway and Its Mediators in Thyroid Carcinomas. Mol. Diagn. Ther. 2016, 20, 13–26. [Google Scholar] [CrossRef]
- Gorini, F.; Tonacci, A. Vitamin C in the Management of Thyroid Cancer: A Highway to New Treatment? Antioxidants 2024, 13, 1242. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.H.; Waxman, D.J. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003, 74, 297–308. [Google Scholar] [CrossRef]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef]
- Wood, W.M.; Sharma, V.; Bauerle, K.T.; Pike, L.A.; Zhou, Q.; Fretwell, D.L.; Schweppe, R.E.; Haugen, B.R. PPARγ Promotes Growth and Invasion of Thyroid Cancer Cells. PPAR Res. 2011, 2011, 171765. [Google Scholar] [CrossRef]
- Romitti, M.; Ceolin, L.; Siqueira, D.R.; Ferreira, C.V.; Wajner, S.M.; Maia, A.L. Signaling pathways in follicular cell-derived thyroid carcinomas (review). Int. J. Oncol. 2013, 42, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, X.; Zhang, X.; Ye, J.; Zhao, W.; Zhang, M.; Xing, J.; Qi, W.; Ye, L. Role of Notch pathway in effect of mono-2-ethylhexyl phthalate on the proliferation and cell cycle of SH-SY5Y cell. Environ. Toxicol. 2021, 36, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Guenter, R.; Patel, Z.; Chen, H. Notch Signaling in Thyroid Cancer. Adv. Exp. Med. Biol. 2021, 1287, 155–168. [Google Scholar] [PubMed]
- Shi, Q.; Xue, C.; Zeng, Y.; Yuan, X.; Chu, Q.; Jiang, S.; Wang, J.; Zhang, Y.; Zhu, D.; Li, L. Notch signaling pathway in cancer: From mechanistic insights to targeted therapies. Signal Transduct. Target. Ther. 2024, 9, 128. [Google Scholar] [CrossRef]
- Mosteiro, L.; Nguyen, T.T.T.; Hankeova, S.; Alvarez-Sierra, D.; Reichelt, M.; Vandriel, S.M.; Lai, Z.; Choudhury, F.K.; Sangaraju, D.; Kamath, B.M.; et al. Notch signaling in thyrocytes is essential for adult thyroid function and mammalian homeostasis. Nat. Metab. 2023, 5, 2094–2110. [Google Scholar] [CrossRef]
- Tang, X.; Wu, S.; Shen, L.; Wei, Y.; Cao, X.; Wang, Y.; Long, C.; Zhou, Y.; Li, D.; Huang, F.; et al. Di-(2-ethylhexyl) phthalate (DEHP)-induced testicular toxicity through Nrf2-mediated Notch1 signaling pathway in Sprague-Dawley rats. Environ. Toxicol. 2018, 33, 720–728. [Google Scholar] [CrossRef]
- Chen, X.; Xu, S.; Tan, T.; Lee, S.T.; Cheng, S.H.; Lee, F.W.; Xu, S.J.; Ho, K.C. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. Int. J. Environ. Res. Public Health 2014, 11, 3156–3168. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Bastari, G.; Cipolletti, M.; Leone, S.; Acconcia, F.; Marino, M. The Peculiar Estrogenicity of Diethyl Phthalate: Modulation of Estrogen Receptor α Activities in the Proliferation of Breast Cancer Cells. Toxics 2021, 9, 237. [Google Scholar] [CrossRef]
- Pérez, P.A.; Toledo, J.; Sosa, L.D.V.; Peinetti, N.; Torres, A.I.; De Paul, A.L.; Gutiérrez, S. The phthalate DEHP modulates the estrogen receptors α and β increasing lactotroph cell population in female pituitary glands. Chemosphere 2020, 258, 127304. [Google Scholar] [CrossRef]
- Pérez, P.A.; Toledo, J.; Picech, F.; Petiti, J.P.; Mukdsi, J.H.; Diaz-Torga, G.; Torres, A.I.; De Paul, A.L.; Gutiérrez, S. Perinatal DEHP exposure modulates pituitary estrogen receptor α and β expression altering lactotroph and somatotroph cell growth in prepuberal and adult male rats. Food Chem. Toxicol. 2021, 158, 112649. [Google Scholar] [CrossRef]
- Prokai-Tatrai, K.; Prokai, L. The impact of 17β-estradiol on the estrogen-deficient female brain: From mechanisms to therapy with hot flushes as target symptoms. Front. Endocrinol. 2024, 14, 1310432. [Google Scholar] [CrossRef] [PubMed]
- Al-Shami, K.; Awadi, S.; Khamees, A.; Alsheikh, A.M.; Al-Sharif, S.; Ala’ Bereshy, R.; Al-Eitan, S.F.; Banikhaled, S.H.; Al-Qudimat, A.R.; Al-Zoubi, R.M.; et al. Estrogens and the risk of breast cancer: A narrative review of literature. Heliyon 2023, 9, e20224. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Discov. Oncol. 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int. Mol. Sci. 2023, 24, 14673. [Google Scholar] [CrossRef] [PubMed]
- Derwahl, M.; Nicula, D. Estrogen and its role in thyroid cancer. Endocr. Relat. Cancer 2014, 21, T273–T283. [Google Scholar] [CrossRef]
- Rubio, G.A.; Catanuto, P.; Glassberg, M.K.; Lew, J.I.; Elliot, S.J. Estrogen receptor subtype expression and regulation is altered in papillary thyroid cancer after menopause. Surgery 2018, 163, 143–149. [Google Scholar] [CrossRef]
- Zaballos, M.A.; Santisteban, P. Key signaling pathways in thyroid cancer. J. Endocrinol. 2017, 235, R43–R61. [Google Scholar] [CrossRef]
- Horie, Y.; Yap, K.C.; Okamura, H. Developmental toxicity and thyroid hormone-disrupting effects of acetyl tributyl citrate in zebrafish and Japanese medaka. J. Hazard. Mater. Adv. 2022, 8, 100199. [Google Scholar] [CrossRef]
- Horie, Y.; Ramaswamy, B.R.; Ríos, J.M.; Yap, C.K.; Okamura, H. Effects of plasticizer diisobutyl adipate on the Japanese medaka (Oryzias latipes) endocrine system. J. Appl. Toxicol. 2023, 43, 982–992. [Google Scholar] [CrossRef]
- Horie, Y.; Nomura, M.; Ramaswamy, B.R.; Harino, H.; Yap, C.K.; Okamura, H. Effects of non-phthalate plasticizer bis(2-ethylhexyl) sebacate (DEHS) on the endocrine system in Japanese medaka (Oryzias latipes). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 264, 109531. [Google Scholar] [CrossRef]
- Horie, Y.; Nomura, M.; Ramaswamy, B.R.; Harino, H.; Yap, C.K.; Okamura, H. Thyroid hormone disruption by bis-(2-ethylhexyl) phthalate (DEHP) and bis-(2-ethylhexyl) adipate (DEHA) in Japanese medaka Oryzias latipes. Aquat. Toxicol. 2022, 252, 106312. [Google Scholar] [CrossRef] [PubMed]
- Houbrechts, A.M.; Delarue, J.; Gabriëls, I.J.; Sourbron, J.; Darras, V.M. Permanent Deiodinase Type 2 Deficiency Strongly Perturbs Zebrafish Development, Growth, and Fertility. Endocrinology 2016, 157, 3668–3681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, X.; Lu, S.; He, J.; Wu, Q.; Liu, X.; Han, Z.; Xie, P. Perfluorohexanoic acid caused disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Ecotoxicol. Environ. Saf. 2022, 232, 113283. [Google Scholar] [CrossRef]
- Baumann, L.; Ros, A.; Rehberger, K.; Neuhauss, S.C.; Segner, H. Thyroid disruption in zebrafish (Danio rerio) larvae: Different molecular response patterns lead to impaired eye development and visual functions. Aquat. Toxicol. 2016, 172, 44–55. [Google Scholar] [CrossRef]
- Ghisari, M.; Bonefeld-Jorgensen, E.C. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol. Lett. 2009, 189, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Tama, R.T.; Dona, H.A.; Hoque, N.S.; Rahaman, M.A.; Alam, M.A. Comprehensive review of phthalate exposure: Health implications, biomarker detection and regulatory standards. J. Steroid Biochem. Mol. Biol. 2025, 247, 106671. [Google Scholar] [CrossRef]
- Jarošová, A.; Bogdanovičová, S. Phthalates in meat products in dependence on the fat content. Potravin. Slovak J. Food Sci. 2016, 10, 378–383. [Google Scholar] [CrossRef]
- Tsai, M.Y.; Ho, C.H.; Chang, H.Y.; Yang, W.; Lin, C.F.; Lin, C.T.; Xue, Y.J.; Lai, J.; Wang, J.; Chang, G.R. Analysis of Pollution of Phthalates in Pork and Chicken in Taiwan Using Liquid Chromatography–Tandem Mass Spectrometry and Assessment of Health Risk. Molecules 2019, 24, 3817. [Google Scholar] [CrossRef]
- Ceballos-Luna, V.; Chávez-Flores, D.; Martínez-Serrano, I.; Rocha-Gutierrez, B.A.; Nevárez-Rodríguez, M.C.; Beltrán, B.G. Bisphenol and Phthalate Migration Test from Mexican Meat Packaging Using HPLC-DAD Technique. J. Chem. 2022, 2022, 2688236. [Google Scholar] [CrossRef]
- Jarošová, A.; Komprda, T.; Bogdanovičová, S.; Krejčíková, M.; Cwiková, O.; Gregor, T. Effect of milking method and season on phthalate content in cow milk from organic production. J. Food Compos. Anal. 2024, 129, 106096. [Google Scholar] [CrossRef]
- Sharman, M.; Read, W.A.; Castle, L.; Gilbert, J. Levels of di-(2-ethylhexyl) phthalate and total phthalate esters in milk, cream, butter and cheese. Food Addit. Contam. 1994, 11, 375–385. [Google Scholar] [CrossRef]
- MeeKyung, K.; Seon, J.Y.; Gab-Soo, C. Determination of phthalates in raw bovine milk by gas chromatography/time-of-flight mass spectrometry (GC/TOF-MS) and dietary intakes. Food Addit. Contam. 2009, 26, 134–138. [Google Scholar]
- Fierens, T.; Van Holderbeke, M.; Willems, H.; De Henauw, S.; Sioen, I. Phthalates in Belgian cow’s milk and the role of feed and other contamination pathways at farm level. Food Chem. Toxicol. 2012, 50, 2945–2953. [Google Scholar] [CrossRef] [PubMed]
- Lü, H.; Mo, C.H.; Zhao, H.M.; Xiang, L.; Katsoyiannis, A.; Li, Y.W.; Cai, Q.Y.; Wong, M. Soil contamination and sources of phthalates and its health risk in China: A review. Environ. Res. 2018, 164, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Nagorka, R.; Birmili, W.; Schulze, J.; Koschorreck, J. Diverging trends of plasticizers (phthalates and non-phthalates) in indoor and freshwater environments—Why? Environ. Sci. Eur. 2022, 34, 46. [Google Scholar] [CrossRef]
- Sun, J.; Wu, X.; Gan, J. Uptake and Metabolism of Phthalate Esters by Edible Plants. Environ. Sci. Technol. 2015, 49, 8471–8478. [Google Scholar] [CrossRef]
- Chen, N.; Shuai, W.; Hao, X.; Zhang, H.; Zhou, D.; Gao, J. Contamination of Phthalate Esters in Vegetable Agriculture and Human Cumulative Risk Assessment. Pedosphere 2017, 27, 439–451. [Google Scholar] [CrossRef]
- Zhang, L.E.; Ruan, Z.; Jing, J.; Yang, Y.; Li, Z.; Zhang, S.; Yang, J.; Ai, S.; Luo, N.; Peng, Y.; et al. High-temperature soup foods in plastic packaging are associated with phthalate body burden and expression of inflammatory mRNAs: A dietary intervention study. Environ. Sci. Technol. 2022, 56, 8416–8427. [Google Scholar] [CrossRef]
- Datta, S.; Chauhan, A.; Ranjan, A.; Sardar, A.H.; Tuli, H.S.; Jairoun, A.A.; Shahwan, M.; Sharma, U.; Jindal, T. Bisphenol A in Indian Take-Out Soups: Compliance, Implications and Sustainable Solutions. Nat. Environ. Pollut. Technol. 2024, 23, 2403–2409. [Google Scholar] [CrossRef]
- Marega, M.; Grob, K.; Moret, S.; Purcaro, G.; Conte, L.S. Phtalate contamination in olive oil production chain. In Proceedings of the Workshop Contaminants of Edible Fats and Oils: Analytical, Normative Issues and Prevention, Udine, Italy, 12–13 November 2009. [Google Scholar]
- Nanni, N.; Fiselier, K.; Grob, K.; Di Pasquale, M.; Fabrizi, L.; Aureli, P.; Coni, E. Contamination of vegetable oils marketed in Italy by phthalic acid esters. Food Control. 2011, 22, 209–214. [Google Scholar] [CrossRef]
- Lacoste, F. Undesirable substances in vegetable oils: Anything to declare? OCL 2014, 21, 1–9. [Google Scholar] [CrossRef]
- Oh, M.S.; Lee, S.W.; Moon, M.; Lee, D.; Park, H.M. Simultaneous analysis of phthalates, adipate and polycyclic aromatic hydrocarbons in edible oils using isotope dilution-gas chromatography-mass spectrometry. Food Addit. Contam. Part B Surveill. 2014, 7, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Phillips, C.A.; Mitro, S.D. Recent Fast Food Consumption and Bisphenol A and Phthalates Exposures among the U.S. Population in NHANES, 2003–2010. Environ. Health Perspect. 2016, 124, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, J.R.; Morello-Frosch, R.; Woodruff, T.J.; Zota, A.R. Dietary sources of cumulative phthalates exposure among the U.S. general population in NHANES 2005–2014. Environ. Int. 2018, 115, 417–429. [Google Scholar] [CrossRef]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Khandpur, N.; da Costa Louzada, M.L.; Monteiro, C.A. Association between dietary contribution of ultra-processed foods and urinary concentrations of phthalates and bisphenol in a nationally representative sample of the US population aged 6 years and older. PLoS ONE 2020, 15, e0236738. [Google Scholar] [CrossRef]
- Sugita, T.; Hirayama, K.; Nino, R.; Ishibashi, T.; Yamada, T. Contents of phthalate in polyvinyl chloride toys. Shokuhin Eiseigaku Zasshi. J. Food Hyg. Soc. Jpn. 2001, 42, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Biscardi, D.; Monarca, S.; De Fusco, R.; Senatore, F.; Poli, P.; Buschini, A.; Rossi, C.; Zani, C. Evaluation of the migration of mutagens/carcinogens from PET bottles into mineral water by Tradescantia/micronuclei test, Comet assay on leukocytes and GC/MS. Sci. Total Environ. 2003, 302, 101–108. [Google Scholar] [CrossRef]
- Amiridou, D.; Voutsa, D. Alkylphenols and phthalates in bottled waters. J. Hazard. Mater. 2011, 185, 281–286. [Google Scholar] [CrossRef]
- Plotan, M.; Frizzell, C.; Robinson, V.; Elliott, C.T.; Connolly, L. Endocrine disruptor activity in bottled mineral and flavoured water. Food Chem. 2013, 136, 1590–1596. [Google Scholar] [CrossRef]
- Casajuana, N.; Lacorte, S. Presence and release of phthalic esters and other endocrine disrupting compounds in drinking water. Chromatographia 2003, 57, 649–655. [Google Scholar] [CrossRef]
- Schmid, P.; Kohler, M.; Meierhofer, R.; Luzi, S.; Wegelin, M. Does the reuse of PET bottles during solar water disinfection pose a health risk due to the migration of plasticisers and other chemicals into the water? Water Res. 2008, 42, 5054–5060. [Google Scholar] [CrossRef] [PubMed]
- Bošnir, J.; Puntarić, D.; Galić, A.; Škes, I.; Dijanić, T.; Klarić, M.; Grgić, M.; Čurković, M.; Šmit, Z. Migration of phthalates from plastic containers into soft drinks and mineral water. Food Technol. Biotechnol. 2007, 45, 91–95. [Google Scholar]
- Wu, C.F.; Chang-Chien, G.P.; Su, S.W.; Chen, B.H.; Wu, M.T. Findings of 2731 suspected phthalate-tainted foodstuffs during the 2011 phthalates incident in Taiwan. J. Formos. Med. Assoc. 2014, 113, 600–605. [Google Scholar] [CrossRef]
- De Toni, L.; Tisato, F.; Seraglia, R.; Roverso, M.; Gandin, V.; Marzano, C.; Padrini, R.; Foresta, C. Phthalates and heavy metals as endocrine disruptors in food: A study on pre-packed coffee products. Toxicol. Rep. 2017, 4, 234–239. [Google Scholar] [CrossRef]
- Troisi, J.; Richards, S.; Symes, S.; Ferretti, V.; Di Maio, A.; Amoresano, A.; Daniele, B.; Aliberti, F.; Guida, M.; Trifuoggi, M. A comparative assessment of metals and phthalates in commercial tea infusions: A starting point to evaluate their tolerance limits. Food Chem. 2019, 288, 193–200. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, B.; Wang, L.; Li, T.; Ye, H.; Li, S.; Huang, M.; Zhang, X. Adsorption of Phthalate Acid Esters by Activated Carbon: The Overlooked Role of the Ethanol Content. Foods 2022, 11, 2114. [Google Scholar] [CrossRef]
- Plank, C.M.; Trela, B.C. A Review of Plastics Use in Winemaking: HACCP Considerations. Am. J. Enol. Vitic. 2018, 69, 307–320. [Google Scholar] [CrossRef]
- Chatonnet, P.; Boutou, S.; Plana, A. Contamination of wines and spirits by phthalates: Types of contaminants present, contamination sources and means of prevention. Food Addit. Contam. Part A 2014, 31, 1605–1615. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, S.; Xie, Q. Rapid determination of phthalate esters in alcoholic beverages by conventional ionic liquid dispersive liquid–liquid microextraction coupled with high performance liquid chromatography. Talanta 2014, 119, 291–298. [Google Scholar] [CrossRef]
- March, J.G.; Cerdà, V. An innovative arrangement for in-vial membrane-assisted liquid-liquid microextraction: Application to the determination of esters of phthalic acid in alcoholic beverages by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4213–4217. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, S.; Chaudhary, B.U.; Tewari, S.; Meshram, R.; Kale, R.D. Utilization of agricultural waste as an alternative for packaging films. Ind. Crops Prod. 2022, 188, 115685. [Google Scholar] [CrossRef]
- Birania, S.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Rohilla, P.; Kumar, R. Advances in development of biodegradable food packaging material from agricultural and agro-industry waste. J. Food Process Eng. 2022, 45, e13930. [Google Scholar] [CrossRef]
- Duguma, H.T.; Khule, P.; McArdle, A.; Fennell, K.; Almenar, E. Turning agricultural waste into packages for food: A literature review from origin to end-of-life. Food Packag. Shelf Life 2023, 40, 101166. [Google Scholar] [CrossRef]
- Modesti, M.; Taglieri, I.; Bianchi, A.; Tonacci, A.; Sansone, F.; Bellincontro, A.; Venturi, F.; Sanmartin, C. E-Nose and Olfactory Assessment: Teamwork or a Challenge to the Last Data? The Case of Virgin Olive Oil Stability and Shelf Life. Appl. Sci. 2021, 11, 8453. [Google Scholar] [CrossRef]
- Rodríguez-Ramos, R.; Santana-Mayor, A.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.A. Recent advances in the analysis of plastic migrants in food. TrAC Trends Anal. Chem. 2024, 178, 117847. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pipliya, S.; Karunanithi, S.; Eswaran, U.G.M.; Kumar, S.; Mandliya, S.; Srivastav, P.P.; Suthar, T.; Shaikh, A.M.; Harsányi, E.; et al. Migration of Chemical Compounds from Packaging Materials into Packaged Foods: Interaction, Mechanism, Assessment, and Regulations. Foods 2024, 13, 3125. [Google Scholar] [CrossRef]
- Piergiovanni, L.; Limbo, S. Food Packaging Materials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–75. [Google Scholar]
- Alamri, M.S.; Qasem, A.A.A.; Mohamed, A.A.; Hussain, S.; Ibraheem, M.A.; Shamlan, G.; Alqah, H.A.; Qasha, A.S. Food packaging’s materials: A food safety perspective. Saudi J. Biol. Sci. 2021, 28, 4490–4499. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry. Use of Recycled Plastics in Food Packaging: Chemistry Considerations. CFSAN. 2021. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-use-recycled-plastics-food-packaging-chemistry-considerations (accessed on 16 March 2025).
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of known plastic packaging-associated chemicals and their hazards. Sci. Total. Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Undas, A.K.; Groenen, M.; Peters, R.J.; van Leeuwen, S.P. Safety of recycled plastics and textiles: Review on the detection, identification and safety assessment of contaminants. Chemosphere 2023, 312, 137175. [Google Scholar] [CrossRef]
- Van Willige, R.; Schoolmeester, D.; Van Ooij, A.; Linssen, J.; Voragen, A. Influence of storage time and temperature on absorption of flavor compounds from solutions by plastic packaging materials. J. Food Sci. 2002, 67, 2023–2031. [Google Scholar] [CrossRef]
- Li, M.; Zhao, X.; Shi, H.; Zhang, Q.; Wang, Z.; Lv, Q. Non-targeted identification and risk assessment of unknown substances in disposable plastic tableware by GC-Orbitrap HRMS. Food Chem. 2024, 454, 139837. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A review of the endocrine disrupting effects of micro and nano plastic and their associated chemicals in mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef] [PubMed]


| Clues | Reference | Pitfalls | Reference |
|---|---|---|---|
| Urinary MEHP and MEHPP levels inversely correlated with serum levels of TT4 | [91] | Differences across adults, pregnant women, and children in the associations between phthalate metabolites and levels and thyroid parameters | [91] |
| Urinary MEOHP concentration positively associated with serum levels of TSH | [91] | Single measurement of urinary phthalate metabolites | [3,91,94,98,99,100] |
| Each 10-fold increase in maternal urinary MEP negatively associated with serum TT4 | [92] | Phthalate metabolites and THs measured by different methods in the studies included | [91] |
| Each 10-fold increase in average maternal urinary MBzP inversely correlated with cord serum TSH | [92] | Lack of correction for urinary creatinine and/or specific gravity | [91,93,94,97,99] |
| Phthalate index (MEP and MCPP the most contributors) associated with decreased maternal TT4 | [92] | Possibility that the analytical method cannot detect the non-linear relationship between phthalate exposure and thyroid function | [91,94] |
| Phthalate index (MBzP and MiBP the most contributors) associated with decreased cord serum TT4 and TSH | [92] | Conflicting results on the modifying effect of iodine in the relationship between phthalate exposure and thyroid parameters | [92,93,94] |
| IQR increase in several phthalate metabolites significantly associated with increased levels of TT3 in each study visit | [93] | The use of WQS does not consider the direction of association of single metabolites and assumes both a linear relationship between exposure and outcomes and the absence of interactions between metabolites within cumulative index | [92] |
| IQR increase in MCOP and MCPP significantly associated with increased levels of fT4 and TT4 | [93] | Lack of evaluation of BMI, iodine intake, thyroid autoantibodies, or pre-existing thyroid dysfunction | [3,92,93,94,97,98,99,101] |
| Measurements of phthalates and THs at two times/multiple points during pregnancy | [92,100] | Differences in the timing of sample collection during pregnancy and consequent inaccuracy in TH measurements due to variations in TH levels at various gestational age | [92,93,94,98,99] |
| Factor 1 (MiBP, MnBP, and MBzP as the most contributors) significantly associated with increased levels of TT3 and fT3 in pregnant women | [94] | Possibility of residual bias by the oversampling of mothers with ADHD children | [94] |
| Significantly inverse association between factor 1 (MiBP, MnBP, and MBzP the most contributors) and ft3 and TT3 in pregnant women with sufficient iodine intake | [94] | Cross-sectional design | [3,91,92,94,97,99] |
| Factor 2 (molar sum of DEHP and DiNP metabolites the most contributors) significantly associated with decreased levels of fT3 and TT3 independently from iodine intake of pregnant women | [94] | Lack of significant associations between phthalate metabolites and TSH or thyroid autoantibodies | [93,97,98] |
| Use of factor analysis and of information on the habitual iodine intake to investigate phthalate-thyroid function relationship | [94] | Small number of subjects with positive thyroid autoantibodies | [97] |
| All phthalate metabolites significantly associated with higher levels of TT3 and, all except for MnBP and MCPP, negatively associated with fT3 | [97] | Lack of measurements of albumin, transthyretin, or thyroxine-binding globulin | [98] |
| All metabolites significantly associated with increased DIO activity | [97] | Small sample size | [99,100,101] |
| DEHP metabolites significantly and positively associated with TBG levels | [97] | Possibility of recall bias | [100] |
| Thyroid autoimmunity status able to modify the association between phthalate metabolites and fT3, fT4, TSG, and TBG | [97] | Loss of participants during the follow-up | [100] |
| Factor (molar sum of MEHHP, MEHOP, MECPP, MnBP, and MBzP) and factor 2 (molar sum of MCOP, MCNP, and MCPP) significantly negatively associated with fT3 and significantly positively associated with TT3, TBG, and DIO activity | [97] | ||
| Higher levels of the molar of DEHP metabolites significantly associated with lower fT4 levels and a higher TSH7fT4 ratio in pregnant women | [98] | ||
| Higher levels of the molar of DiNP metabolites significantly associated with lower TT4 levels and lower TT4/fT4 and TT4/TT3 ratios in pregnant women | [98] | ||
| BBzP and DBP significantly and positively associated with lower TT4/TT3 and fT4/fT3 ratios and with higher fT4/TT4 and fT3/TT3 ratios in pregnant women | [98] | ||
| MEP, MECPP, MEOHP, and molar sum of DEHP significantly associated with a higher risk of SCH during pregnancy | [99] | ||
| MCOP significantly positively correlated with TT3 and TSH in adolescents | [3] | ||
| MCNP significantly inversely correlated with TSH in adolescents | [3] | ||
| Significant negative correlation between levels of MMP and TT3, TT4 and fT4 in children | [100] | ||
| The molar sum of DEHP metabolites, MEHHP and MEHOP, significantly positively correlated with fT4 | [100] | ||
| The highest quartile of MEP, MECPP, MEHHP, molar sum of all metabolites, DEHP, two DEP metabolites low- and high-molecular-weight metabolites significantly associated with increased levels of fT4 compared to the lowest quartile | [101] | ||
| The highest quartile of all metabolites (except for MEHHP) and their sum positively associated with increased levels of TSH compared to the lowest quartiles | [101] | ||
| The highest quartile of MEP and molar sum of DBP metabolites positively and inversely correlated with TT3, respectively | [101] | ||
| The highest quartiles (third or fourth) of MEP, MiBP, MnBP, the molar sum of all metabolites, DBP, and low-molecular-weight metabolites significantly associated with a higher TSH/fT4 ratio | [101] |
| Regulatory Bodies Policy | Household Best Practices |
|---|---|
| Enforce strict limits on plasticizer usage and ensure compliance by manufacturers, with continuous monitoring and periodic updates of regulatory guidelines based on new scientific data. | Avoid extensive reuse of packaging as specific storage conditions that can affect migration, e.g., the aging process of the material as well as mechanical damage due to the repeated use and frequent washing cycles. |
| Targeted efforts to reduce plasticizer use in vulnerable sectors such as medical devices, children’s playthings, food packaging, and kitchenware, promoting safer alternatives and educating consumers on their correct usage. | Adhere strictly to the usage recommendations provided by the manufacturer on the package label, including guidelines on temperature ranges, cooking and washing utensils, and types of foods to store. |
| Large-scale investment in biodegradable microorganisms capable of degrading phthalates should be prioritized, alongside research to optimize microbial efficiency. | Reduce exposure to non-dietary sources of plasticizers: thermal paper (dermal route), pharmaceuticals (dermal route/ingestion), cosmetics (dermal route), toys (oral route/ingestion), pacifiers (oral route), surface water (while swimming) (oral route/dermal route), dental fillings (oral route), and dust (inhalation). |
| Advancements in detection techniques, such as LC and HPLC-MS/MS, are critical to accurately monitor phthalate levels in various environments, enabling better regulatory enforcement and public health protection. | Minimize or eliminate the use of plastic materials in activities for children and toddlers. |
| Comprehensive strategies involving stricter regulations, citizens’ involvement, public awareness campaigns, and collaboration between actors (governments, NGOs, private sector) are essential to reduce the harmful impacts of phthalates on human health, animals, and ecosystems. | Strictly follow the recycling recommendations provided by the manufacturer on the packaging label and/or by regulatory bodies. |
| Improve policies to regulate the collection and recycling of food waste, aiming to produce plastic recycled materials for use in FCMs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorini, F.; Tonacci, A.; Sanmartin, C.; Venturi, F. Phthalates and Non-Phthalate Plasticizers and Thyroid Dysfunction: Current Evidence and Novel Strategies to Reduce Their Spread in Food Industry and Environment. Toxics 2025, 13, 222. https://doi.org/10.3390/toxics13030222
Gorini F, Tonacci A, Sanmartin C, Venturi F. Phthalates and Non-Phthalate Plasticizers and Thyroid Dysfunction: Current Evidence and Novel Strategies to Reduce Their Spread in Food Industry and Environment. Toxics. 2025; 13(3):222. https://doi.org/10.3390/toxics13030222
Chicago/Turabian StyleGorini, Francesca, Alessandro Tonacci, Chiara Sanmartin, and Francesca Venturi. 2025. "Phthalates and Non-Phthalate Plasticizers and Thyroid Dysfunction: Current Evidence and Novel Strategies to Reduce Their Spread in Food Industry and Environment" Toxics 13, no. 3: 222. https://doi.org/10.3390/toxics13030222
APA StyleGorini, F., Tonacci, A., Sanmartin, C., & Venturi, F. (2025). Phthalates and Non-Phthalate Plasticizers and Thyroid Dysfunction: Current Evidence and Novel Strategies to Reduce Their Spread in Food Industry and Environment. Toxics, 13(3), 222. https://doi.org/10.3390/toxics13030222










