Developmental Toxicity of Micro(Nano)Plastics (MNPs) Exposure in Mammals: A Mini-Review
Abstract
1. Introduction
2. Methods
3. Physicochemical Properties and Exposure Characteristics of MNPs
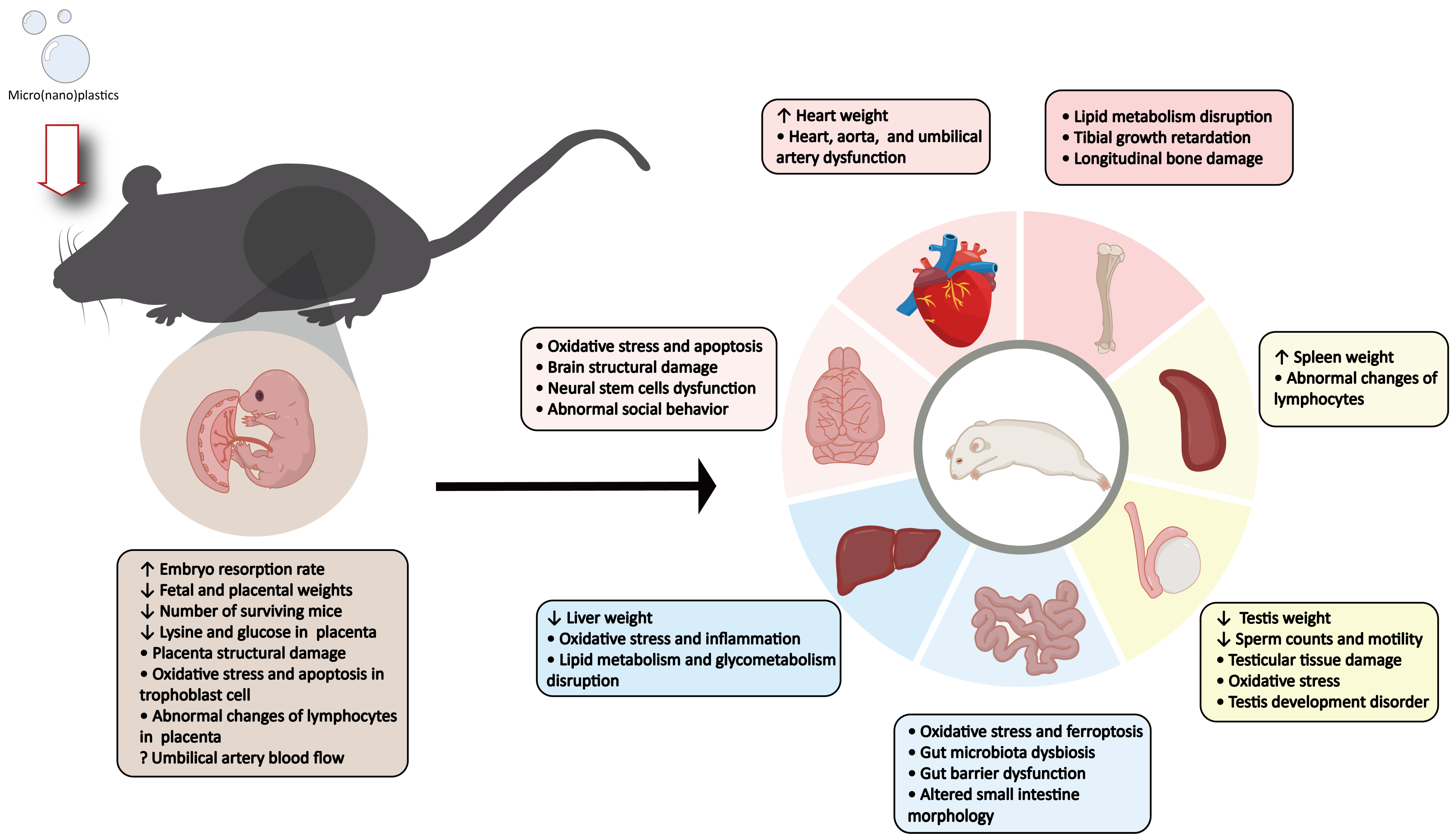
4. Impact of MNPs Exposure on Mammalian Embryo, Fetal, and Placental Development
4.1. Effects on Embryo and Fetal Development
4.2. Effects on Placental Development
| MNPs | Animals | Exposure | Effects | References | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Diameter | Abundance (MNPs/g) | Route | Concentration | Time | |||
| PS | 20 nm | 2.27 × 1017 | SD rats | Intratracheal instillation | 2.64 × 1014 particles | GD 19 to GD 20 | Reduced fetal and placental weights; NPs were observed in the placenta, fetal liver, lungs, heart, kidney, and brain | [26] |
| PS | 1 μm | 1.82 × 1012 | C57BL/6J mice | Drink | 0.1, 1, 10 mg/L | GD 0.5 to GD 18.5 | Increased embryo resorption rate; decreased fetal body and tail length; placental malfunction | [28] |
| PE | 40–48 μm | 1.82 × 107–3.14 × 107 | ICR mice | Gavage | 0.125, 0.5, 2 mg/day | 90 days parental exposure and to dams until lactation | Altered numbers of live births per dam, the sex ratio of pups, and body weight of pups | [29] |
| PS | 100 nm | 1.82 × 1015 | Kunming mice | Drink | 0.1, 1, 10 mg/L | GD 0 to PND 21 | Decreased birth and postnatal body weight in offspring mice | [30] |
| PS | 5 μm, 50 nm | 1.46 × 1010, 1.46 × 1016 | CD-1 mice | Drink | 102, 104, 106 ng/L | E 0.5 to E 17.5 | Fetal growth restriction; reduced umbilical cord length and fetal weights | [31] |
| PS | 80 nm | 3.55 × 1015 | C57BL/6J mice | Oropharyngeal aspiration | 1, 5, 25 μg/μL | GD 0 to GD 21 | Reduced birth weight of female mice and elevated body weights of adult offspring | [32] |
| PE | 10–45 µm | 2.21 × 107–2.01 × 109 | ICR mice | Intragastric administration | 0.01, 0.1 mg/mouse/day | GD 9 to PND 7 | Declining trend in the weight gain and organ weight of neonates; increased serum acetylcholinesterase and glutathione peroxidase levels | [33] |
| PE | 10–150 μm | 5.96 × 105–2.01 × 109 | Kunming mice | Oral administration | 0.4, 4, 40 mg/kg/day | GD 0 to PND 21 | Reduced birth and postnatal body weight; reduced number of surviving mice | [34] |
| PE | 740–4990 nm | 1.62 × 1010–4.96 × 1012 | CD-1 mice | Drink | 106 ng/L | E 0.5 to E 17.5 | Increased umbilical artery blood flow | [35] |
| PS | 10 μm | 1.82 × 109 | C57BL/6-mated BALB/c mice | Intraperitoneal injection | 1.25 μg/μL | Injected on GD 5.5 and GD 7.5 | Elevated embryo resorption rate; reduced number and diameter of uterine arterioles; reduced percentage of decidual NK cells and increased helper T cells in the placenta; reversed M1/M2 ratio in placental macrophages | [37] |
| PS | 5 μm, 50 nm | 1.46 × 1010, 1.46 × 1016 | CD-1 mice | Drink | 106 ng/L | GD 0.5 to GD 17.5 | Increased umbilical artery blood flow in the MPs-exposed mice and decreased umbilical artery blood flow in the NPs-exposed mice | [38] |
| PS | 5 μm | 1.46 × 1010 | CD-1 mice | Drink | 102, 104, 106 ng/L | GD 0.5 to GD 17.5 | Reduced fetal weight; decreased lysine and glucose in the placenta; perturbated biotin metabolism, lysine degradation, and glycolysis/gluconeogenesis pathways in placenta | [39] |
| PS | 100 nm | 1.82 × 1015 | C57BL/6 mice | Drink | 1, 10 mg/L | GD 0 to GD 17 | Reduced fetal weight; abnormal morphologies of cells in the placenta; disturbed cholesterol metabolism and dysregulated complement and coagulation cascades pathways in the placenta | [40] |
| PS | 50 nm | 1.46 × 1016 | C57BL/6 mice | Gavage | 25, 50, 100 mg/kg/day | GD 1 to GD 14 | Increased miscarriage rates; oxidative stress; decreased mitochondrial membrane potential; and increased apoptosis in trophoblast cells | [41] |
| PS | 30 nm | 6.74 × 1016 | ICR mice | Gavage | 0.1, 1, 10 mg/kg/day | GD 0.5 to GD 18.5 | Fetal death; reduced weight and the thickness of the trophoblastic layer in the placenta; increased immature red blood cells in the placental vasculature; diminished invasion capabilities of nourishing cells | [42] |
5. Neurodevelopmental Toxicity of MNPs
| MNPs | Animals | Exposure | Effects | References | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Diameter | Abundance (MNPs/g) | Route | Concentration | Time | |||
| PS | 25 nm, 50 nm | 1.16 × 1017, 1.46 × 1016 | SD rats | Gavage | 0.5, 2.5, 10, 50 mg/ kg | GD 0.5 to GD 17.5, GD 0.5 to GD 21.5 | PS-NPs accumulated in brain regions of fetal rats, especially the cerebellum; reduced MBP and MOG expression; decreased myelin thickness; oligodendrocyte apoptosis; impaired motor coordination | [25] |
| PS | 100 nm, 1000 nm | 1.82 × 1015, 1.82 × 1012 | C57BL mice | Gavage | 1 mg/day | GD 1 to GD 17 | Induced anxiety-like behavior; reduced GABA in the prefrontal cortex and amygdala | [43] |
| PS | 5 μm, 50 nm | 1.46 × 1010, 1.46 × 1016 | CD-1 mice | Drink | 106 ng/L | GD 0.5 to GD 17.5 | Decreased middle cerebral artery pulsatility index | [38] |
| PS | 50 nm | 1.46 × 1016 | CD-1 mice | Drink | 106 ng/L | GD 0.5 to GD 17.5 | Reduced GABA, creatine and glucose in the fetal brain; altered asparagine concentration, with variations influenced by fetal sex | [44] |
| PS | 25, 50 nm | 1.16 × 1017, 1.46 × 1016 | SD rats | Gavage | 0.5, 2.5, 10, 50 mg/kg | GD 1 to GD 18 | Increased levels of IL-1β, IL-6; decreased CAT, SOD and GSH-PX activity; increased MDA content in the prefrontal cortex, hippocampus and striatum | [45] |
| PS | 50, 500 nm | 1.46 × 1016, 1.46 × 1013 | C57BL/6J mice | Oral administration | 0.5, 10, 100, 500, 1000 μg/day | GD 8 to PND 14 | Altered the functioning of NSCs, neural cell compositions, and brain histology; induced neurophysiological and cognitive deficits in a gender-specific manner | [46] |
| PS | 50 nm | 1.46 × 1016 | SD rats | Gavage | 2.5 mg/kg/day | GD 0.5 to PND 22 | Downregulation of neural developmental proteins and upregulation of inhibitory proteins in the hippocampus; KEGG pathway analysis highlighted ferroptosis enrichment | [47] |
| PS | 50 nm | 1.46 × 1016 | SD rats | Gavage | 2.5 mg/kg/day | Gestational, lactational exposure | Diminished cortical thickness; heightened cortical cell proliferation; disrupted neocortical migration; altered monoamine neurotransmitters within the cortex and amino acid neurotransmitters within the hippocampus; widened synaptic clefts; diminished postsynaptic density; deficits in anxiolytic-like behaviors and spatial memory | [48] |
| PS | 100 nm | 1.82 × 1015 | C57BL/6 mice | Drink | 10 mg/L | GD 0.5 to PND 21 | Defective neural retinal development; delayed retinal vessel development; abnormal ERG responses; oxidative stress in retina | [49] |
| PS | 40, 193 nm | 2.84 × 1016, 2.53 × 1014 | C57BL/6J mice | Drink | 5, 10 mg/L | Dams: GD 9.5 to PND 28.5, Offspring: PND 28.5 to PND113 | Downregulated Gabra2 expression in the brain; abnormal social behavior, anxiety- and depression-like behavior | [50] |
| PS | 2 µm | 2.27 × 1011 | C57BL/6J mice | Drink | 1 mg/L | Dams: GD 0.5 to GD 28.5, Offspring: GD 28.5 to PND 168 | Reduced dendritic length; impaired social novelty preferences | [51] |
| PS | 200 nm, 1 μm, 5 μm | 2.27 × 1014, 1.82 × 1012, 1.46 × 1010 | C57BL/6J mice | Oral administration, intragastric injection | 5 mg/kg/day | Dams: GD 12.5 to GD 16.5, Offspring: PND 1 to PND 5, PND 14 to PND 18, PND 28 to PND 32 | Impair microglia-mediated synaptic pruning; social behavioral defects in adulthood | [52] |
5.1. Impact of Gestational Exposure to MNPs on Neurodevelopment
5.2. Impact of Exposure to MNPs During Both Pregnancy and Lactation Period on Neurodevelopment
5.3. Long-Term Impact of MNPs Exposure on Neurodevelopment
6. The Impact of Parental Exposure to MNPs on Other Tissues in Offspring
6.1. Impact on the Liver in Offspring
6.2. Impact on Intestinal Health in Offspring
| MNPs | Animals | Exposure | Effects | References | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Diameter | Abundance (MNPs/g) | Route | Concentration | Time | |||
| Liver and metabolic functions | ||||||||
| PS | 100 nm | 1.82 × 1015 | Kunming mice | Drink | 0.1, 1 and 10 mg/L | GD 0 to PND 21 | Reduced liver weight in male offspring; oxidative stress, inflammatory cell infiltration, and disturbed glycometabolism in the liver of male offspring | [30] |
| PS | 71.09 ± 6.63 nm | 5.30 × 1015 | C57BL/6J mice | Oropharyngeal aspiration | 1, 5, 25 μg/μL | GD 0 to PND 0 | Hepatic steatosis in adult female offspring | [53] |
| PS | 5 μm | 1.46 × 1010 | ICR mice | Drink | 100, 1000 μg/L | Gestational, lactational exposure for 6 weeks | Hepatic transcriptome and serum metabolite changes in F1 generation; potential of hepatic lipid accumulation in adult F1 generation | [54] |
| PS | 0.5, 5 μm | 1.46 × 1013– 1.46 × 1010 | ICR mice | Drink | 100, 1000 μg/L | GD 0 to PND 0 | Altered serum TG, TC, HDL-C and LDL-C levels and hepatic TC, TG levels in F1 generation; FA metabolism disorder in the F1 generation | [55] |
| PS | 40–100 μm | 1.82 × 106, 2.84 × 107 | C57BL/6J mice | Feed | 50, 500 mg/kg MP-contained food | Paternal exposure for 21 weeks before pregnancy | Dysregulated lipid metabolism in the liver and plasma of F1 generationin in a dose-, gender-, and tissue-specific pattern | [56] |
| Intestinal health | ||||||||
| PE | 200 nm | 2.52 × 1014 | C57BL/6J mice | Gavage | 2 mg/L | Paternal exposure for 35 days before pregnancy | Gut microbial dysbiosis in F0 and F1 generation | [57] |
| PS | 5 μm | 1.46 × 1010 | ICR mice | Drink | 100, 1000 μg/L | Gestational, lactational exposure for 6 weeks | Gut microbiota dysbiosis and gut barrier dysfunction in dams | [54] |
| PS, PP | 50 nm (PS), 500 nm (PP) | 1.46 × 1016, 1.68 × 1013 | C57BL/6J mice | Feed | 0.5, 10, 100, 500 μg/day | GD 8 to PND 14 | Induced shifts in the distribution of intestinal microbes. | [58] |
| PS | 80 nm | 3.55 × 1015 | C57/BL6J mice | Oropharyngeal aspiration | 1, 5, 25 μg/μL | GD 0 to GD 21 | Altered small intestine morphology; induced oxidative stress and initiated ferroptosis in the small intestines; female offspring showed higher small intestinal damage than males | [32] |
| Reproductive health | ||||||||
| PS | 100 nm | 1.82 × 1015 | Kunming mice | Drink | 0.1, 1, 10 mg/L | GD 0 to PND 21 | Diminished testis weight; decreased sperm counts; disrupted seminiferous epithelium; oxidative stress in the testis | [30] |
| PE | 200 nm | 2.52 × 1014 | C57BL/6J mice | Gavage | 2 mg/L | Paternal exposure for 35 days before pregnancy | Abnormal growth phenotypes and sex hormone levels; histological damage in the testicular tissue; reduced total sperm counts and motility; sperm abnormality; altered microRNA profiles in the sperm | [57] |
| PS | 0.5 μm | 1.46 × 1013 | ICR mice | Drink | 0.5, 5, 50 mg/L | GD 1 to PND 35 or PND 70 | Testis development disorder; spermatogenesis dysfunction | [59] |
| PS | 1 μm | 1.82 × 1012 | ICR mice | Gavage | 1.357 ng/g/day, 1.357 μg/g/day | F0 PND 0 to PND 21 | Decreased sperm count and viability in F1 male offspring; decreased sperm count in the F2 male offspring | [60] |
| PE | 10–150 μm | 5.96 × 105– 2.01 × 109 | Kunming mice | Oral administration | 0.4, 4, 40 mg/kg/day | GD 0 to PND 21 | Reduced oocyte maturation, fertilization rate, and embryonic development in female offspring; oxidative stress in the ovaries | [34] |
| Spleen immunity | ||||||||
| PS | 1 μm | 1.82 × 1012 | ICR mice | Intragastric administration | 1.36 × 10−6, 1.36 × 10−3 mg/g/day | GD 0 to PND 21 | Increased spleen weight in offspring; elevated number of B cells, Th cells and Tregs in spleen of male offspring; elevated ratio of Th17/Tregs and Th1/Th2 cells in F1 male offspring | [61] |
| PE | 16.9 ± 1.9 μm | 4.17 × 108 | ICR mice | Gavage | 0.125, 0.5, 2 mg/day | 90 days parental exposure and to dams until lactation | Decreased proportion of Tregs within the spleen in the female pups; increased proportion of Th cells within the spleen in both sexes of pups; inhibited maturation of dendritic cells in splenocytes of male pups, while it was enhanced in female pups | [29] |
| Bone and skeletal muscle health | ||||||||
| PS | 100 nm | 1.82 × 1015 | C57BL/6 mice | Drink | 1, 10 mg/L | GD 0 to GD 17 | Disturbed cholesterol metabolism, complement and coagulation cascade and muscle tissue formation in fetal skeletal muscle | [40] |
| PS | 1 μm | 1.82 × 1012 | SD rats | Gavage | 2 mg/kg/day | 28 days | Shortened tibial length and altered blood calcium and phosphorus metabolism; altered expression of the transcription factors involved in chondrocyte proliferation, differentiation, apoptosis, and matrix secretion in tibial proximal growth plate tissue | [62] |
| [63] | ||||||||
| PS | 20 nm | 2.27 × 1017 | SD rats | Intratracheal instillation | 2.64 × 1014 particles | GD 19 to GD 20 | Increased heart weight and vascular dysfunction in the aorta of dams; vascular dysfunction in the radial artery of the uterus in offspring; dysfunction of the fetal heart, fetal aorta, and umbilical artery | |
6.3. Impact on Reproductive Health in Offspring
6.4. Disruption of Spleen Immunity in Offspring
6.5. Impact of Parental MNPs Exposure on Bone and Skeletal Muscle Health in Offspring
6.6. Impact on Cardiovascular Function in Offspring
7. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, F.; Hubbart, J.A. The Occurrence and Transport of Microplastics: The State of the Science. Sci. Total Environ. 2021, 758, 143936. [Google Scholar] [CrossRef] [PubMed]
- Lim, X. Microplastics Are Everywhere—But Are They Harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as Contaminants in the Marine Environment: A Review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current Opinion: What Is a Nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Zhu, L.; Kang, Y.; Ma, M.; Wu, Z.; Zhang, L.; Hu, R.; Xu, Q.; Zhu, J.; Gu, X.; An, L. Tissue Accumulation of Microplastics and Potential Health Risks in Human. Sci. Total Environ. 2024, 915, 170004. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health Impacts of Environmental Contamination of Micro- and Nanoplastics: A Review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.; Zhang, Y.; Luo, X.; Kang, Q.; Chen, P.; Guo, J.; Hu, Z.; Yang, Z.; Zheng, H.; et al. Microplastics in Glaciers of Tibetan Plateau: Characteristics and Potential Sources. Sci. Total Environ. 2024, 954, 176370. [Google Scholar] [CrossRef]
- Vdovchenko, A.; Resmini, M. Mapping Microplastics in Humans: Analysis of Polymer Types, and Shapes in Food and Drinking Water-A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7074. [Google Scholar] [CrossRef]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine Mammals and Microplastics: A Systematic Review and Call for Standardisation. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, J.; Xiong, F.; Xu, K.; Pu, Y.; Huang, J.; Zhang, J.; Pu, Y.; Sun, R.; Cheng, K. Research Advances of Microplastics and Potential Health Risks of Microplastics on Terrestrial Higher Mammals: A Bibliometric Analysis and Literature Review. Environ. Geochem. Health 2023, 45, 2803–2838. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.; Zeta-Flores, M.; Ramos-Baldárrago, S.; Tume-Ruiz, J.; Rangel-Vega, A.; Reyes, E.; Quinde, E.; De-la-Torre, G.E.; Lajo-Salazar, L.; Cárdenas-Alayza, S. Terrestrial Mammals of the Americas and Their Interactions with Plastic Waste. Environ. Sci. Pollut. Res. Int. 2023, 30, 57759–57770. [Google Scholar] [CrossRef] [PubMed]
- Gallitelli, L.; Battisti, C.; Pietrelli, L.; Scalici, M. Anthropogenic Particles in Coypu (Myocastor Coypus; Mammalia, Rodentia)’ Faeces: First Evidence and Considerations about Their Use as Track for Detecting Microplastic Pollution. Environ. Sci. Pollut. Res. Int. 2022, 29, 55293–55301. [Google Scholar] [CrossRef]
- Tyl, R.W. Toxicity Testing, Developmental. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 656–668. ISBN 978-0-12-386455-0. [Google Scholar]
- Huang, W.; Mo, J.; Li, J.; Wu, K. Exploring Developmental Toxicity of Microplastics and Nanoplastics (MNPS): Insights from Investigations Using Zebrafish Embryos. Sci. Total Environ. 2024, 933, 173012. [Google Scholar] [CrossRef]
- Sulaiman, R.N.R.; Bakar, A.A.; Ngadi, N.; Kahar, I.N.S.; Nordin, A.H.; Ikram, M.; Nabgan, W. Microplastics in Malaysia’s Aquatic Environment: Current Overview and Future Perspectives. Glob. Chall. 2023, 7, 2300047. [Google Scholar] [CrossRef]
- Kiran, B.R.; Kopperi, H.; Venkata Mohan, S. Micro/Nano-Plastics Occurrence, Identification, Risk Analysis and Mitigation: Challenges and Perspectives. Rev. Environ. Sci. Biotechnol. 2022, 21, 169–203. [Google Scholar] [CrossRef]
- Fan, P.; Tan, W.; Yu, H. Effects of Different Concentrations and Types of Microplastics on Bacteria and Fungi in Alkaline Soil. Ecotoxicol. Environ. Saf. 2022, 229, 113045. [Google Scholar] [CrossRef]
- Almeida, J.M.; Singdahl-Larsen, C.; Buenaventura, N.; Gomes, L.; Morgado, V.; Olsen, M.; Bettencourt da Silva, R.; Palma, C. Assessment and Comparison of Microplastic Contamination in Atlantic Navigation Routes with Known Uncertainty. Environ. Sci. Technol. 2023, 57, 10062–10069. [Google Scholar] [CrossRef]
- Dybka-Stępień, K.; Antolak, H.; Kmiotek, M.; Piechota, D.; Koziróg, A. Disposable Food Packaging and Serving Materials—Trends and Biodegradability. Polymers 2021, 13, 3606. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Liu, Z.; Xu, C.; Zhang, Y.; Liu, P. Microplastic Injection? Identification and Quantification of Plastic Particles in Medical Injections. Sci. Total Environ. 2024, 954, 176468. [Google Scholar] [CrossRef]
- Ge, Y.; Yang, S.; Zhang, T.; Wan, X.; Zhu, Y.; Yang, F.; Yin, L.; Pu, Y.; Liang, G. The Hepatotoxicity Assessment of Micro/Nanoplastics: A Preliminary Study to Apply the Adverse Outcome Pathways. Sci. Total Environ. 2023, 902, 165659. [Google Scholar] [CrossRef]
- Yang, S.; Li, M.; Kong, R.Y.C.; Li, L.; Li, R.; Chen, J.; Lai, K.P. Reproductive Toxicity of Micro- and Nanoplastics. Environ. Int. 2023, 177, 108002. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, L.; Chen, J.; Liu, X.; Li, K.; Liu, H.; Lai, W.; Shi, Y.; Lin, B.; Xi, Z. Selective Bioaccumulation of Polystyrene Nanoplastics in Fetal Rat Brain and Damage to Myelin Development. Ecotoxicol. Environ. Saf. 2024, 278, 116393. [Google Scholar] [CrossRef]
- Fournier, S.B.; D’Errico, J.N.; Adler, D.S.; Kollontzi, S.; Goedken, M.J.; Fabris, L.; Yurkow, E.J.; Stapleton, P.A. Nanopolystyrene Translocation and Fetal Deposition after Acute Lung Exposure during Late-Stage Pregnancy. Part. Fibre Toxicol. 2020, 17, 55. [Google Scholar] [CrossRef]
- You, H.-J.; Jo, Y.-J.; Kim, G.; Kwon, J.; Yoon, S.-B.; Youn, C.; Kim, Y.; Kang, M.-J.; Cho, W.-S.; Kim, J.-S. Disruption of Early Embryonic Development in Mice by Polymethylmethacrylate Nanoplastics in an Oxidative Stress Mechanism. Chemosphere 2024, 361, 142407. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, Y.; Nie, P.; Wang, W.; Wu, H.; Wan, X.; Xu, H.; Fu, F. Polystyrene Microplastics Disturb Maternal Glucose Homeostasis and Induce Adverse Pregnancy Outcomes. Ecotoxicol. Environ. Saf. 2024, 279, 116492. [Google Scholar] [CrossRef]
- Park, E.-J.; Han, J.-S.; Park, E.-J.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-Oral Dose Toxicity of Polyethylene Microplastics and the Possible Implications on Reproduction and Development of the next Generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, W.; Lin, T.; Liu, S.; Sun, Z.; Liu, F.; Yuan, Y.; Xiang, X.; Kuang, H.; Yang, B.; et al. Maternal Exposure to Polystyrene Nanoplastics during Gestation and Lactation Induces Hepatic and Testicular Toxicity in Male Mouse Offspring. Food Chem. Toxicol. 2022, 160, 112803. [Google Scholar] [CrossRef]
- Aghaei, Z.; Sled, J.G.; Kingdom, J.C.; Baschat, A.A.; Helm, P.A.; Jobst, K.J.; Cahill, L.S. Maternal Exposure to Polystyrene Micro- and Nanoplastics Causes Fetal Growth Restriction in Mice. Environ. Sci. Technol. Lett. 2022, 9, 426–430. [Google Scholar] [CrossRef]
- Tang, J.; Bu, W.; Hu, W.; Zhao, Z.; Liu, L.; Luo, C.; Wang, R.; Fan, S.; Yu, S.; Wu, Q.; et al. Ferroptosis Is Involved in Sex-Specific Small Intestinal Toxicity in the Offspring of Adult Mice Exposed to Polystyrene Nanoplastics during Pregnancy. ACS Nano 2023, 17, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Kim, C. Toxicities Demonstrated in Dams and Neonates Following Intragastric Intubation of Polyethylene Microplastics to Pregnant Mice. J. Environ. Health Sci. 2021, 47, 446–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhao, Y.; Zhao, J.; Yu, T.; Yao, Y.; Zhao, R.; Yu, R.; Liu, J.; Su, J. Reproductive Toxicity of Microplastics in Female Mice and Their Offspring from Induction of Oxidative Stress. Environ. Pollut. 2023, 327, 121482. [Google Scholar] [CrossRef]
- Hanrahan, J.; Steeves, K.L.; Locke, D.P.; O’Brien, T.M.; Maekawa, A.S.; Amiri, R.; Macgowan, C.K.; Baschat, A.A.; Kingdom, J.C.; Simpson, A.J.; et al. Maternal Exposure to Polyethylene Micro- and Nanoplastics Impairs Umbilical Blood Flow but Not Fetal Growth in Pregnant Mice. Sci. Rep. 2024, 14, 399. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Sferruzzi-Perri, A.N. Human Placental Development and Function. Semin. Cell Dev. Biol. 2022, 131, 66–77. [Google Scholar] [CrossRef]
- Hu, J.; Qin, X.; Zhang, J.; Zhu, Y.; Zeng, W.; Lin, Y.; Liu, X. Polystyrene Microplastics Disturb Maternal-Fetal Immune Balance and Cause Reproductive Toxicity in Pregnant Mice. Reprod. Toxicol. 2021, 106, 42–50. [Google Scholar] [CrossRef]
- Dibbon, K.C.; Mercer, G.V.; Maekawa, A.S.; Hanrahan, J.; Steeves, K.L.; Ringer, L.C.M.; Simpson, A.J.; Simpson, M.J.; Baschat, A.A.; Kingdom, J.C.; et al. Polystyrene Micro- and Nanoplastics Cause Placental Dysfunction in Mice†. Biol. Reprod. 2024, 110, 211–218. [Google Scholar] [CrossRef]
- Aghaei, Z.; Mercer, G.V.; Schneider, C.M.; Sled, J.G.; Macgowan, C.K.; Baschat, A.A.; Kingdom, J.C.; Helm, P.A.; Simpson, A.J.; Simpson, M.J.; et al. Maternal Exposure to Polystyrene Microplastics Alters Placental Metabolism in Mice. Metabolomics 2022, 19, 1. [Google Scholar] [CrossRef]
- Chen, G.; Xiong, S.; Jing, Q.; van Gestel, C.A.M.; van Straalen, N.M.; Roelofs, D.; Sun, L.; Qiu, H. Maternal Exposure to Polystyrene Nanoparticles Retarded Fetal Growth and Triggered Metabolic Disorders of Placenta and Fetus in Mice. Sci. Total Environ. 2023, 854, 158666. [Google Scholar] [CrossRef]
- Wan, S.; Wang, X.; Chen, W.; Wang, M.; Zhao, J.; Xu, Z.; Wang, R.; Mi, C.; Zheng, Z.; Zhang, H. Exposure to High Dose of Polystyrene Nanoplastics Causes Trophoblast Cell Apoptosis and Induces Miscarriage. Part. Fibre Toxicol. 2024, 21, 13. [Google Scholar] [CrossRef]
- Lv, J.; He, Q.; Yan, Z.; Xie, Y.; Wu, Y.; Li, A.; Zhang, Y.; Li, J.; Huang, Z. Inhibitory Impact of Prenatal Exposure to Nano-Polystyrene Particles on the MAP2K6/P38 MAPK Axis Inducing Embryonic Developmental Abnormalities in Mice. Toxics 2024, 12, 370. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, J.; Zhou, X.; Pan, D.; Nan, S.; Yin, R.; Lei, Q.; Ma, N.; Zhu, H.; Chen, J.; et al. Polystyrene Micro- and Nano-Particle Coexposure Injures Fetal Thalamus by Inducing ROS-Mediated Cell Apoptosis. Environ. Int. 2022, 166, 107362. [Google Scholar] [CrossRef]
- Mercer, G.V.; Harvey, N.E.; Steeves, K.L.; Schneider, C.M.; Sled, J.G.; Macgowan, C.K.; Baschat, A.A.; Kingdom, J.C.; Simpson, A.J.; Simpson, M.J.; et al. Maternal Exposure to Polystyrene Nanoplastics Alters Fetal Brain Metabolism in Mice. Metabolomics 2023, 19, 96. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Tian, L.; Xie, X.-Q.; Wang, Y.-T.; Lyu, P.; Xi, Z.-G. [Effects of nanopolystyrene nanoplastic exposure on the development and neurotoxicity of fetal rats during gestation]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2022, 38, 760–765. [Google Scholar] [CrossRef]
- Jeong, B.; Baek, J.Y.; Koo, J.; Park, S.; Ryu, Y.-K.; Kim, K.-S.; Zhang, S.; Chung, C.; Dogan, R.; Choi, H.-S.; et al. Maternal Exposure to Polystyrene Nanoplastics Causes Brain Abnormalities in Progeny. J. Hazard. Mater. 2022, 426, 127815. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Liu, X.; Li, K.; Liu, H.; Lai, W.; Shi, Y.; Xi, Z.; Yan, L.; Tian, L.; et al. Effects of Exposure to Nano-Plastic Drinking during Pregnancy on Cognitive Related Proteins in Offspring of SD Rats. Environ. Pollut. Bioavailab. 2024, 36, 2292104. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Chen, J.; Liu, X.; Nie, H.; Li, K.; Liu, H.; Lai, W.; Shi, Y.; Xi, Z.; et al. Effects of Nanoplastic Exposure during Pregnancy and Lactation on Neurodevelopment of Rat Offspring. J. Hazard. Mater. 2024, 474, 134800. [Google Scholar] [CrossRef]
- Xiong, S.; He, J.; Qiu, H.; van Gestel, C.A.M.; He, E.; Qiao, Z.; Cao, L.; Li, J.; Chen, G. Maternal Exposure to Polystyrene Nanoplastics Causes Defective Retinal Development and Function in Progeny Mice by Disturbing Metabolic Profiles. Chemosphere 2024, 352, 141513. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, S.H.; Moon, H.J.; So, Y.H.; Lee, H.R.; Lee, E.-H.; Jung, E.-M. Exposure to Polystyrene Particles Causes Anxiety-, Depression-like Behavior and Abnormal Social Behavior in Mice. J. Hazard. Mater. 2023, 454, 131465. [Google Scholar] [CrossRef]
- So, Y.H.; Shin, H.S.; Lee, S.H.; Moon, H.J.; Jang, H.J.; Lee, E.-H.; Jung, E.-M. Maternal Exposure to Polystyrene Microplastics Impairs Social Behavior in Mouse Offspring with a Potential Neurotoxicity. Neurotoxicology 2023, 99, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Xu, X.; Wang, Y.; Lin, F.; Zhang, C.; Liu, R.; Hou, X.; Wang, J.; Jiang, X.; Zhang, Q.; et al. Neonatal Exposure to Polystyrene Nanoplastics Impairs Microglia-Mediated Synaptic Pruning and Causes Social Behavioral Defects in Adulthood. Environ. Sci. Technol. 2024, 58, 11945–11957. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Z.; Wang, X.; Hu, W.; Luo, C.; Chu, X.; Qian, M.; Wang, R.; Yu, S.; Wu, Q.; et al. Effects of Polystyrene Nanoplastic Gestational Exposure on Mice. Chemosphere 2023, 324, 138255. [Google Scholar] [CrossRef]
- Luo, T.; Wang, C.; Pan, Z.; Jin, C.; Fu, Z.; Jin, Y. Maternal Polystyrene Microplastic Exposure during Gestation and Lactation Altered Metabolic Homeostasis in the Dams and Their F1 and F2 Offspring. Environ. Sci. Technol. 2019, 53, 10978–10992. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Wang, C.; Wang, X.; Zhou, J.; Shen, M.; Zhao, Y.; Fu, Z.; Jin, Y. Maternal Exposure to Different Sizes of Polystyrene Microplastics during Gestation Causes Metabolic Disorders in Their Offspring. Environ. Pollut. 2019, 255, 113122. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, H.; Huang, Y.; Wang, Q.; Chen, W.; Chen, D. Polystyrene Microplastics Affect the Reproductive Performance of Male Mice and Lipid Homeostasis in Their Offspring. Environ. Sci. Technol. Lett. 2022, 9, 752–757. [Google Scholar] [CrossRef]
- Sun, J.; Teng, M.; Zhu, W.; Zhao, X.; Zhao, L.; Li, Y.; Zhang, Z.; Liu, Y.; Bi, S.; Wu, F. MicroRNA and Gut Microbiota Alter Intergenerational Effects of Paternal Exposure to Polyethylene Nanoplastics. ACS Nano 2024, 18, 18085–18100. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, J.-S.; Kwon, A.R.; Lee, J.; Park, S.; Koo, J.; Lee, W.S.; Baek, J.Y.; Shin, W.-H.; Lee, J.-S.; et al. Maternal Nanoplastic Ingestion Induces an Increase in Offspring Body Weight through Altered Lipid Species and Microbiota. Environ. Int. 2024, 185, 108522. [Google Scholar] [CrossRef]
- Zhao, T.; Shen, L.; Ye, X.; Bai, G.; Liao, C.; Chen, Z.; Peng, T.; Li, X.; Kang, X.; An, G. Prenatal and Postnatal Exposure to Polystyrene Microplastics Induces Testis Developmental Disorder and Affects Male Fertility in Mice. J. Hazard. Mater. 2023, 445, 130544. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, M.; Zhang, H.; Zhang, C.; Feng, L.; Hu, J.; Gao, Y.; Yuan, X.-Z.; Zhao, Y.; Zhao, H.; et al. Lactating Exposure to Microplastics at the Dose of Infants Ingested during Artificial Feeding Induced Reproductive Toxicity in Female Mice and Their Offspring. Sci. Total Environ. 2024, 949, 174972. [Google Scholar] [CrossRef]
- Shang, Q.; Wu, H.; Wang, K.; Zhang, M.; Dou, Y.; Jiang, X.; Zhao, Y.; Zhao, H.; Chen, Z.-J.; Wang, J.; et al. Exposure to Polystyrene Microplastics during Lactational Period Alters Immune Status in Both Male Mice and Their Offspring. Sci. Total Environ. 2024, 951, 175371. [Google Scholar] [CrossRef]
- Zhang, Q.; Lang, Y.; Tang, X.; Cheng, W.; Cheng, Z.; Rizwan, M.; Xie, L.; Liu, Y.; Xu, H.; Liu, Y. Polystyrene Microplastic-Induced Endoplasmic Reticulum Stress Contributes to Growth Plate Endochondral Ossification Disorder in Young Rat. Environ. Toxicol. 2024, 39, 3314–3329. [Google Scholar] [CrossRef] [PubMed]
- Cary, C.M.; Fournier, S.B.; Adams, S.; Wang, X.; Yurkow, E.J.; Stapleton, P.A. Single Pulmonary Nanopolystyrene Exposure in Late-Stage Pregnancy Dysregulates Maternal and Fetal Cardiovascular Function. Toxicol. Sci. 2024, 199, 149–159. [Google Scholar] [CrossRef]
- Amereh, F.; Amjadi, N.; Mohseni-Bandpei, A.; Isazadeh, S.; Mehrabi, Y.; Eslami, A.; Naeiji, Z.; Rafiee, M. Placental Plastics in Young Women from General Population Correlate with Reduced Foetal Growth in IUGR Pregnancies. Environ. Pollut. 2022, 314, 120174. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xu, Z.; Hu, X.; Lu, Y.; Zhao, Y.; Zhang, H. Microplastics in Maternal Amniotic Fluid and Their Associations with Gestational Age. Sci. Total Environ. 2024, 920, 171044. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, G.; Wan, T.; Chen, Z.; Liu, C.; Li, R. Developmental Toxicity of Micro(Nano)Plastics (MNPs) Exposure in Mammals: A Mini-Review. Toxics 2025, 13, 224. https://doi.org/10.3390/toxics13030224
Xia G, Wan T, Chen Z, Liu C, Li R. Developmental Toxicity of Micro(Nano)Plastics (MNPs) Exposure in Mammals: A Mini-Review. Toxics. 2025; 13(3):224. https://doi.org/10.3390/toxics13030224
Chicago/Turabian StyleXia, Gongxiang, Teng Wan, Zhuan Chen, Cuiqing Liu, and Ran Li. 2025. "Developmental Toxicity of Micro(Nano)Plastics (MNPs) Exposure in Mammals: A Mini-Review" Toxics 13, no. 3: 224. https://doi.org/10.3390/toxics13030224
APA StyleXia, G., Wan, T., Chen, Z., Liu, C., & Li, R. (2025). Developmental Toxicity of Micro(Nano)Plastics (MNPs) Exposure in Mammals: A Mini-Review. Toxics, 13(3), 224. https://doi.org/10.3390/toxics13030224






