Acrylamide and Its Metabolite Glycidamide Induce Reproductive Toxicity During In Vitro Maturation of Bovine Oocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Reagents
2.2. Oocyte Collection and In Vitro Maturation (IVM)
2.3. In Vitro Fertilization (IVF) and Culture
2.4. Visualization of Cytoskeleton
2.5. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL)
2.6. Immunofluorescence Analysis
2.7. RNA Extraction and RT-qPCR
2.8. Statistical Analysis
3. Results
3.1. Effect of Acrylamide and Glycidamide on Bovine Embryo Development
3.2. Effect of Acrylamide and Glycidamide on Actin Cytoskeleton Stabilization and Oocyte Quality
3.3. Impact of Acrylamide and Glycidamide on Key Epigenetic Regulators
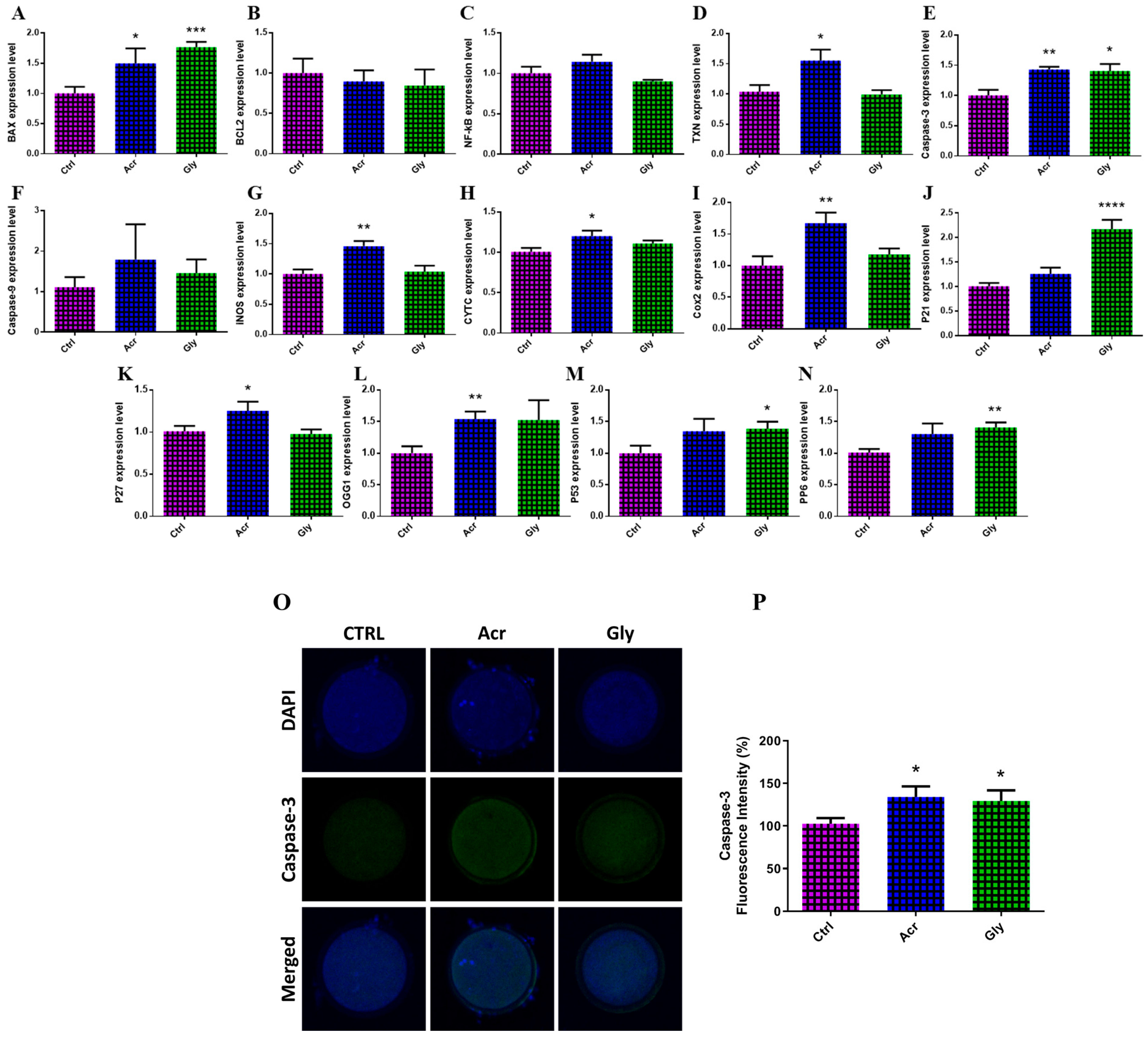
3.4. Effect of Acrylamide and Glycidamide on Expression of Genes and Protein Involved in Apoptosis and DNA Damage Responses
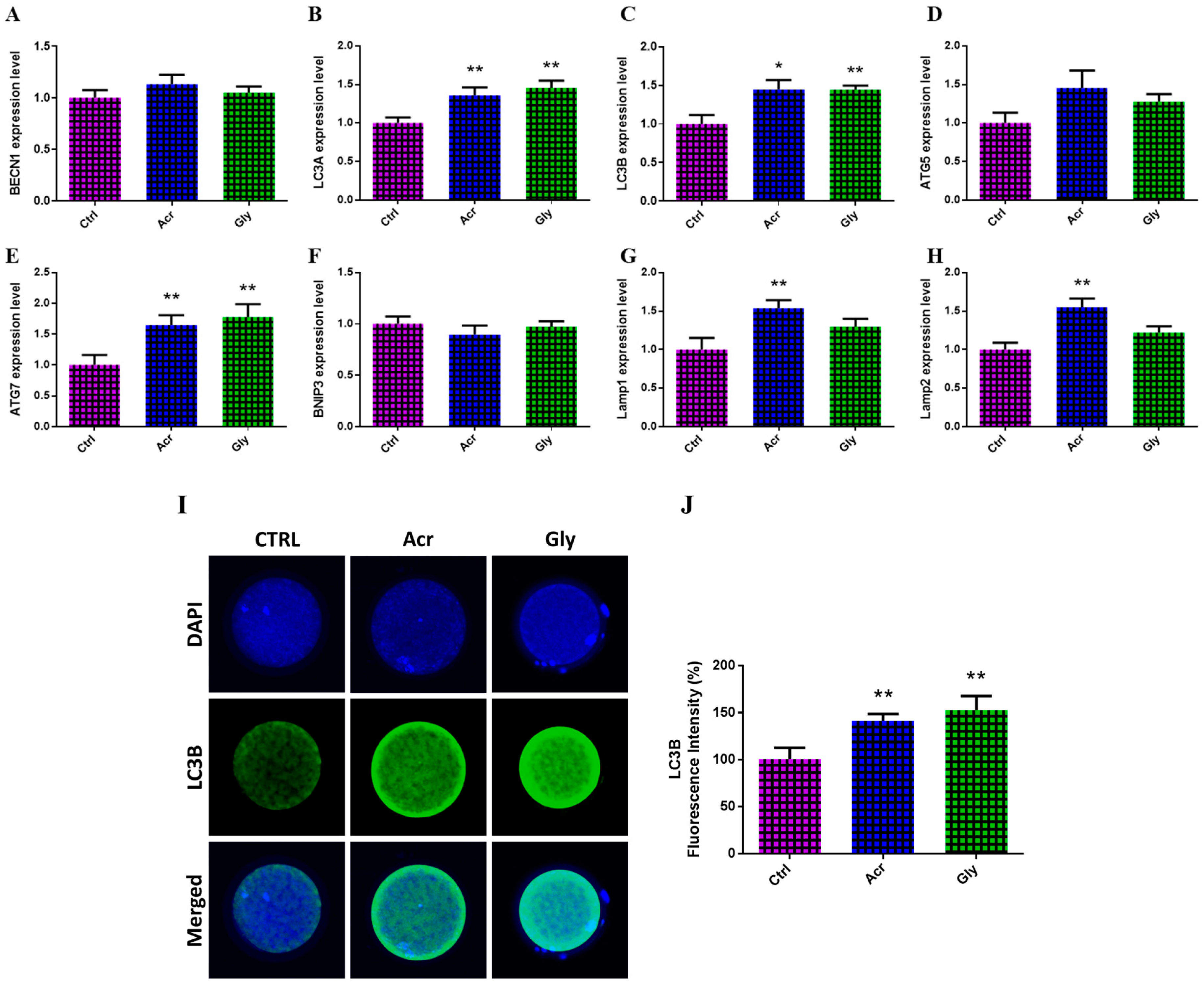
3.5. Effect of Acrylamide and Glycidamide on mRNA and Protein Levels of the Autophagy-Specific Markers
3.6. Effect of Acrylamide and Glycidamide on Expression of Genes Involved in MAPK Signaling Pathway
3.7. Effect of Acrylamide and Glycidamide on the Quality of Developed Blastocysts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, E.A.; Prues, S.L.; Oehme, F.W. Environmental degradation of polyacrylamides. 1. Effects of artificial environmental conditions: Temperature, light, and pH. Ecotoxicol. Environ. Saf. 1996, 35, 121–135. [Google Scholar] [CrossRef]
- Smith, E.A.; Prues, S.L.; Oehme, F.W. Environmental degradation of polyacrylamides. II. Effects of environmental (outdoor) exposure. Ecotoxicol. Environ. Saf. 1997, 37, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.N.; Hewavitharana, G.G.; Navaratne, S.B. Comprehensive Study on the Acrylamide Content of High Thermally Processed Foods. Biomed. Res. Int. 2021, 2021, 6258508. [Google Scholar] [CrossRef]
- Aras, D.; Cakar, Z.; Ozkavukcu, S.; Can, A.; Cinar, O. In Vivo acrylamide exposure may cause severe toxicity to mouse oocytes through its metabolite glycidamide. PLoS ONE 2017, 12, e0172026. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and safety of acrylamide. A review. J. Agric. Food Chem. 2003, 51, 4504–4526. [Google Scholar] [CrossRef] [PubMed]
- Mojska, H.; Gielecinska, I.; Cendrowski, A. Acrylamide content in cigarette mainstream smoke and estimation of exposure to acrylamide from tobacco smoke in Poland. Ann. Agric. Environ. Med. 2016, 23, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Kontas Yedier, S.; Atli Sekeroglu, Z.; Sekeroglu, V.; Aydin, B. Cytotoxic, genotoxic, and carcinogenic effects of acrylamide on human lung cells. Food Chem. Toxicol. 2022, 161, 112852. [Google Scholar] [CrossRef]
- Gao, J.G.; Yang, J.K.; Zhu, L.; Xu, C.; Nie, L.W. Acrylamide impairs the developmental potential of germinal vesicle oocytes by inducing mitochondrial dysfunction and autophagy/apoptosis in mice. Hum. Exp. Toxicol. 2021, 40, S370–S380. [Google Scholar] [CrossRef]
- Yan, F.; Wang, L.; Zhao, L.; Wang, C.; Lu, Q.; Liu, R. Acrylamide in food: Occurrence, metabolism, molecular toxicity mechanism and detoxification by phytochemicals. Food Chem. Toxicol. 2023, 175, 113696. [Google Scholar] [CrossRef]
- Holzl-Armstrong, L.; Kucab, J.E.; Moody, S.; Zwart, E.P.; Loutkotova, L.; Duffy, V.; Luijten, M.; Gamboa da Costa, G.; Stratton, M.R.; Phillips, D.H.; et al. Mutagenicity of acrylamide and glycidamide in human TP53 knock-in (Hupki) mouse embryo fibroblasts. Arch. Toxicol. 2020, 94, 4173–4196. [Google Scholar] [CrossRef]
- Besaratinia, A.; Pfeifer, G.P. Genotoxicity of acrylamide and glycidamide. J. Natl. Cancer Inst. 2004, 96, 1023–1029. [Google Scholar] [CrossRef]
- Martins, C.; Oliveira, N.G.; Pingarilho, M.; Gamboa da Costa, G.; Martins, V.; Marques, M.M.; Beland, F.A.; Churchwell, M.I.; Doerge, D.R.; Rueff, J.; et al. Cytogenetic damage induced by acrylamide and glycidamide in mammalian cells: Correlation with specific glycidamide-DNA adducts. Toxicol. Sci. 2007, 95, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Orta Yilmaz, B.; Aydin, Y. Dynamic assessment of the relationship between oxidative stress and apoptotic pathway in embryonic fibroblast cells exposed to glycidamide: Possible protective role of hesperidin. Environ. Sci. Pollut. Res. Int. 2023, 30, 53295–53308. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, L.; Zhong, T.; Kong, S.; Zheng, R.; Kong, F.; Zhang, C.; Zhang, L.; An, L. Effect of Acrylamide on Oocyte Nuclear Maturation and Cumulus Cells Apoptosis in Mouse In Vitro. PLoS ONE 2015, 10, e0135818. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Q.C.; Chen, K.L.; Zhu, C.C.; Liu, J.; Sun, S.C. Acrylamide toxic effects on mouse oocyte quality and fertility in vivo. Sci. Rep. 2015, 5, 11562. [Google Scholar] [CrossRef] [PubMed]
- Favor, J.; Shelby, M.D. Transmitted mutational events induced in mouse germ cells following acrylamide or glycidamide exposure. Mutat. Res. 2005, 580, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Deng, H.; Gregotti, C.; Manzo, L.; Faustman, E.M.; Bergmark, E.; Calleman, C.J. Comparative studies on the neuro-and reproductive toxicity of acrylamide and its epoxide metabolite glycidamide in the rat. Neurotoxicology 1992, 13, 219–224. [Google Scholar]
- Sakamoto, J.; Hashimoto, K. Reproductive toxicity of acrylamide and related compounds in mice—Effects on fertility and sperm morphology. Arch. Toxicol. 1986, 59, 201–205. [Google Scholar] [CrossRef]
- Wang, H.; Huang, P.; Lie, T.; Li, J.; Hutz, R.J.; Li, K.; Shi, F. Reproductive toxicity of acrylamide-treated male rats. Reprod. Toxicol. 2010, 29, 225–230. [Google Scholar] [CrossRef]
- Zenick, H.; Hope, E.; Smith, M.K. Reproductive toxicity associated with acrylamide treatment in male and female rats. J. Toxicol. Environ. Health 1986, 17, 457–472. [Google Scholar] [CrossRef]
- Hashimoto, K.; Sakamoto, J.; Tanii, H. Neurotoxicity of acrylamide and related compounds and their effects on male gonads in mice. Arch. Toxicol. 1981, 47, 179–189. [Google Scholar] [CrossRef]
- Duranthon, V.; Renard, J.P. The developmental competence of mammalian oocytes: A convenient but biologically fuzzy concept. Theriogenology 2001, 55, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Verlhac, M.H.; Lefebvre, C.; Guillaud, P.; Rassinier, P.; Maro, B. Asymmetric division in mouse oocytes: With or without Mos. Curr. Biol. 2000, 10, 1303–1306. [Google Scholar] [CrossRef] [PubMed]
- Strege, P.R.; Holm, A.N.; Rich, A.; Miller, S.M.; Ou, Y.; Sarr, M.G.; Farrugia, G. Cytoskeletal modulation of sodium current in human jejunal circular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2003, 284, C60–C66. [Google Scholar] [CrossRef]
- Wei, Q.; Li, J.; Li, X.; Zhang, L.; Shi, F. Reproductive toxicity in acrylamide-treated female mice. Reprod. Toxicol. 2014, 46, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Beker van Woudenberg, A.; Grollers-Mulderij, M.; Snel, C.; Jeurissen, N.; Stierum, R.; Wolterbeek, A. The bovine oocyte in vitro maturation model: A potential tool for reproductive toxicology screening. Reprod. Toxicol. 2012, 34, 251–260. [Google Scholar] [CrossRef]
- Campbell, B.K.; Souza, C.; Gong, J.; Webb, R.; Kendall, N.; Marsters, P.; Robinson, G.; Mitchell, A.; Telfer, E.E.; Baird, D.T. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod. Suppl. 2003, 61, 429–443. [Google Scholar] [CrossRef]
- Banliat, C.; Mahe, C.; Lavigne, R.; Com, E.; Pineau, C.; Labas, V.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. The proteomic analysis of bovine embryos developed in vivo or in vitro reveals the contribution of the maternal environment to early embryo. BMC Genom. 2022, 23, 839. [Google Scholar] [CrossRef]
- Roth, Z.; Komsky-Elbaz, A.; Kalo, D. Effect of environmental contamination on female and male gametes—A lesson from bovines. Anim. Reprod. 2020, 17, e20200041. [Google Scholar] [CrossRef]
- Komsky-Elbaz, A.; Kalo, D.; Roth, Z. New evidence for deleterious effects of environmental contaminants on the male gamete. Anim. Reprod. Sci. 2022, 246, 106886. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, Q.; Sun, Q.Y. Histone modifications during mammalian oocyte maturation: Dynamics, regulation and functions. Cell Cycle 2010, 9, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhang, K.; Xie, W. Epigenetic Reprogramming in Early Animal Development. Cold Spring Harb. Perspect. Biol. 2022, 14, a039677. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef]
- Tomizawa, S.; Nowacka-Woszuk, J.; Kelsey, G. DNA methylation establishment during oocyte growth: Mechanisms and significance. Int. J. Dev. Biol. 2012, 56, 867–875. [Google Scholar] [CrossRef]
- Mesalam, A.; Lee, K.L.; Khan, I.; Chowdhury, M.M.R.; Zhang, S.; Song, S.H.; Joo, M.D.; Lee, J.H.; Jin, J.I.; Kong, I.K. A combination of bovine serum albumin with insulin-transferrin-sodium selenite and/or epidermal growth factor as alternatives to fetal bovine serum in culture medium improves bovine embryo quality and trophoblast invasion by induction of matrix metalloproteinases. Reprod. Fertil. Dev. 2019, 31, 333–346. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Zou, F.; Bai, S.; Jiang, X.; Tian, L.; Ou, S.; Jiao, R.; Bai, W. Protection of cyanidin-3-O-glucoside against acrylamide-and glycidamide-induced reproductive toxicity in leydig cells. Food Chem. Toxicol. 2018, 119, 268–274. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Schatten, H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction 2006, 131, 193–205. [Google Scholar] [CrossRef]
- Su, Y.Q.; Sun, F.; Handel, M.A.; Schimenti, J.C.; Eppig, J.J. Meiosis arrest female 1 (MARF1) has nuage-like function in mammalian oocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 18653–18660. [Google Scholar] [CrossRef]
- De Castro, F.C.; Cruz, M.H.; Leal, C.L. Role of Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15 in Ovarian Function and Their Importance in Mammalian Female Fertility—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1065–1074. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Ritter, L.J.; Cranfield, M.; Jeffery, L.A.; Amato, F.; Scott, S.J.; Myllymaa, S.; Kaivo-Oja, N.; Lankinen, H.; Mottershead, D.G.; et al. Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity. Biol. Reprod. 2004, 71, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Tu, Z.; Chen, D.; Zhang, S. Histone modifications in embryo implantation and placentation: Insights from mouse models. Front. Endocrinol. 2023, 14, 1229862. [Google Scholar] [CrossRef] [PubMed]
- Shacfe, G.; Turko, R.; Syed, H.H.; Masoud, I.; Tahmaz, Y.; Samhan, L.M.; Alkattan, K.; Shafqat, A.; Yaqinuddin, A. A DNA Methylation Perspective on Infertility. Genes 2023, 14, 2132. [Google Scholar] [CrossRef]
- Zhao, Y.; Bai, D.; Wu, Y.; Zhang, D.; Liu, M.; Tian, Y.; Lu, J.; Wang, H.; Gao, S.; Lu, Z. Maternal Ezh1/2 deficiency in oocyte delays H3K27me2/3 restoration and impairs epiblast development responsible for embryonic sub-lethality in mouse. Development 2022, 149, dev200316. [Google Scholar] [CrossRef]
- Cai, Q.; Niu, H.; Zhang, B.; Shi, X.; Liao, M.; Chen, Z.; Mo, D.; He, Z.; Chen, Y.; Cong, P. Effect of EZH2 knockdown on preimplantation development of porcine parthenogenetic embryos. Theriogenology 2019, 132, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, C.; Xu, J.; Liu, S.; Yu, L.; Chen, S.; Wen, H.; Li, Z.; Liu, N. Dppa3 facilitates self-renewal of embryonic stem cells by stabilization of pluripotent factors. Stem. Cell Res. Ther. 2022, 13, 169. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, M.; Cui, Y.; Xia, Q.; Jiang, L.; Yin, H.; Zhao, L. Acrylamide induces intrinsic apoptosis and inhibits protective autophagy via the ROS mediated mitochondrial dysfunction pathway in U87-MG cells. Drug Chem. Toxicol. 2022, 45, 2601–2612. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, B.; Deng, L. The Mechanism of Acrylamide-Induced Neurotoxicity: Current Status and Future Perspectives. Front. Nutr. 2022, 9, 859189. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Gimeno, I.; Canon-Beltran, K.; Gutierrez-Adan, A.; Rizos, D.; Gomez, E. Senescence and Apoptosis During in vitro Embryo Development in a Bovine Model. Front. Cell Dev. Biol. 2020, 8, 619902. [Google Scholar] [CrossRef]
- Pang, P.; Zhang, X.; Yuan, J.; Yan, H.; Yan, D. Acrylamide interferes with autophagy and induces apoptosis in Neuro-2a cells by interfering with TFEB-regulated lysosomal function. Food Chem. Toxicol. 2023, 177, 113818. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, L.; Zhang, X.; Jiao, Y.; Liu, Y.; Dai, L.; Yan, H. Effect of long-term exposure to acrylamide on endoplasmic reticulum stress and autophagy in rat cerebellum. Ecotoxicol. Environ. Saf. 2021, 224, 112691. [Google Scholar] [CrossRef]
- Matoso, V.; Bargi-Souza, P.; Ivanski, F.; Romano, M.A.; Romano, R.M. Acrylamide: A review about its toxic effects in the light of Developmental Origin of Health and Disease (DOHaD) concept. Food Chem. 2019, 283, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.; Yang, L.; Luo, Y.; Chen, F. MiR-27a-5p regulates acrylamide-induced mitochondrial dysfunction and intrinsic apoptosis via targeting Btf3 in rats. Food Chem. 2022, 368, 130816. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Polo, R.A.; Boya, P.; Pauleau, A.L.; Jalil, A.; Larochette, N.; Souquere, S.; Eskelinen, E.L.; Pierron, G.; Saftig, P.; Kroemer, G. The apoptosis/autophagy paradox: Autophagic vacuolization before apoptotic death. J. Cell Sci. 2005, 118, 3091–3102. [Google Scholar] [CrossRef]
- Nakahira, K.; Cloonan, S.M.; Mizumura, K.; Choi, A.M.; Ryter, S.W. Autophagy: A crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid. Redox Signal. 2014, 20, 474–494. [Google Scholar] [CrossRef]
- Song, G.; Liu, Z.; Wang, L.; Shi, R.; Chu, C.; Xiang, M.; Tian, Q.; Liu, X. Protective effects of lipoic acid against acrylamide-induced neurotoxicity: Involvement of mitochondrial energy metabolism and autophagy. Food Funct. 2017, 8, 4657–4667. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.; Liu, S.; Xie, K.; Zhang, H. Acrylamide induces human chondrocyte cell death by initiating autophagy-dependent ferroptosis. Exp. Ther. Med. 2023, 25, 246. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Lopez, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Yin, J.; Luo, Y.; Huang, S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol. 2009, 41, 1284–1295. [Google Scholar] [CrossRef]
- Hattori, K.; Naguro, I.; Runchel, C.; Ichijo, H. The roles of ASK family proteins in stress responses and diseases. Cell Commun. Signal. 2009, 7, 9. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheikh, M.; Mesalam, A.A.; Mesalam, A.; Kong, I.-K. Acrylamide and Its Metabolite Glycidamide Induce Reproductive Toxicity During In Vitro Maturation of Bovine Oocytes. Toxics 2025, 13, 223. https://doi.org/10.3390/toxics13030223
El-Sheikh M, Mesalam AA, Mesalam A, Kong I-K. Acrylamide and Its Metabolite Glycidamide Induce Reproductive Toxicity During In Vitro Maturation of Bovine Oocytes. Toxics. 2025; 13(3):223. https://doi.org/10.3390/toxics13030223
Chicago/Turabian StyleEl-Sheikh, Marwa, Ahmed Atef Mesalam, Ayman Mesalam, and Il-Keun Kong. 2025. "Acrylamide and Its Metabolite Glycidamide Induce Reproductive Toxicity During In Vitro Maturation of Bovine Oocytes" Toxics 13, no. 3: 223. https://doi.org/10.3390/toxics13030223
APA StyleEl-Sheikh, M., Mesalam, A. A., Mesalam, A., & Kong, I.-K. (2025). Acrylamide and Its Metabolite Glycidamide Induce Reproductive Toxicity During In Vitro Maturation of Bovine Oocytes. Toxics, 13(3), 223. https://doi.org/10.3390/toxics13030223








