Effects of Environmental Pollutants on Tryptophan Metabolism
Abstract
:1. Introduction
2. Material and Methods
3. Tryptophan
3.1. Source of Trp
3.2. The Pathway of Trp Metabolism
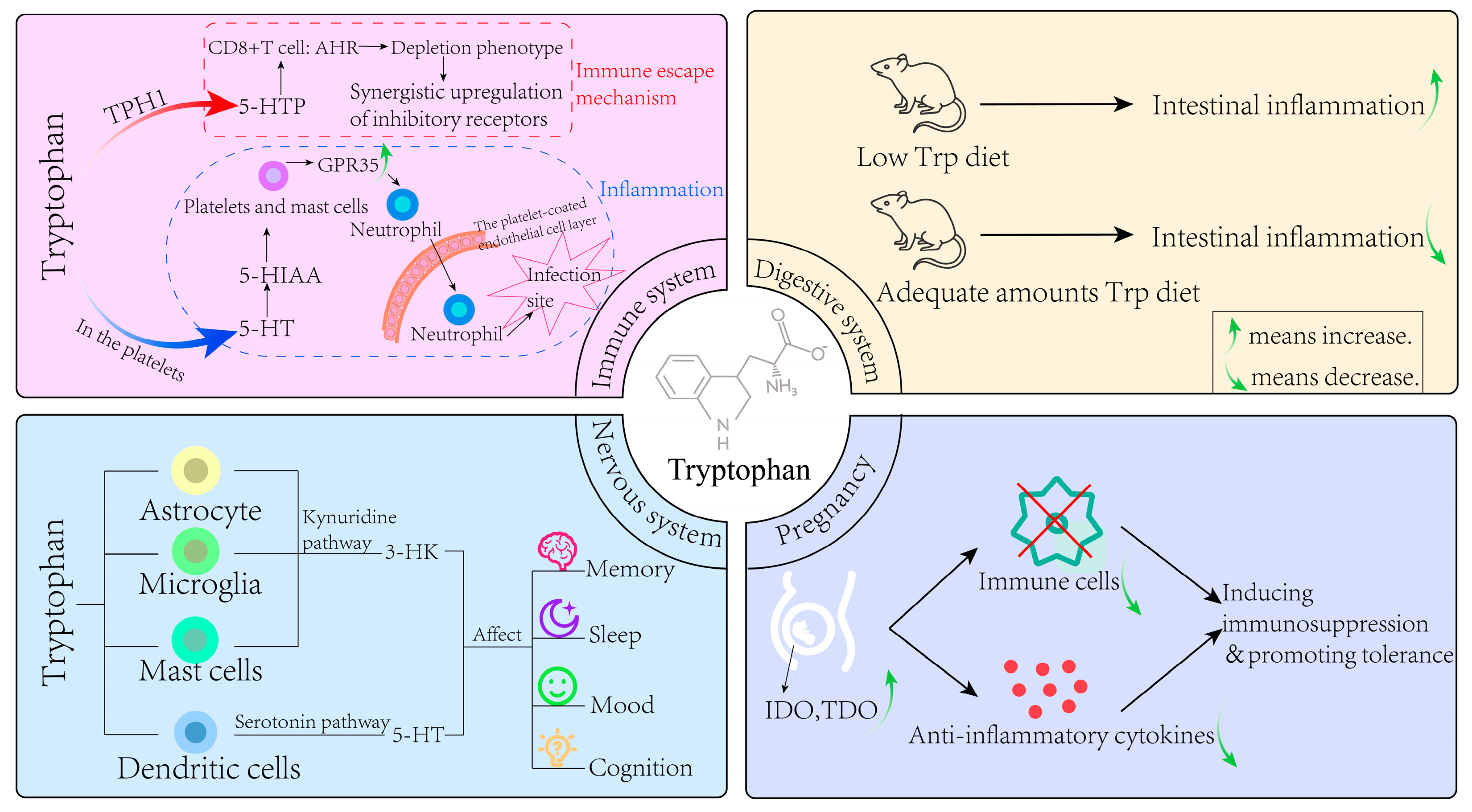
4. The Effect of Trp Metabolism in Various Physiological Systems
4.1. Nervous System
4.2. Immune System
4.3. Digestive System
4.4. Pregnancy
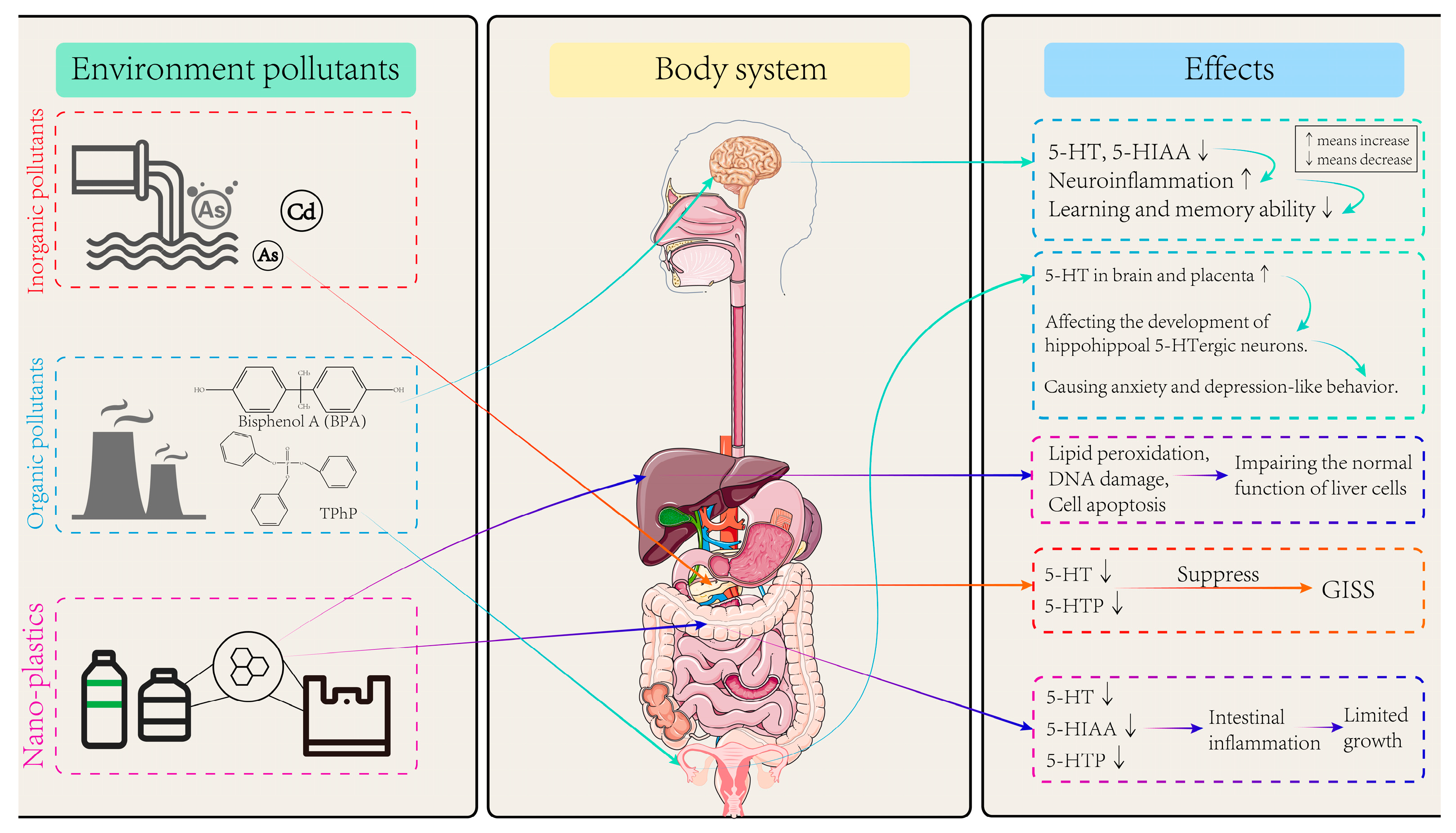
5. Effects of Environmental Pollutants on Trp Metabolism
5.1. Effects of Inorganic Pollutants on Trp Metabolism
5.2. Effects of Organic Pollutants on Trp Metabolism
5.3. Effects of Microplastics and Nanoplastics on Trp Metabolism
6. Conclusions and Perspectives
- (1)
- Currently, the evidence regarding the impact of environmental pollutants on human health through interference with the tryptophan metabolism pathway primarily stems from in vitro studies and whole-animal experiments. Research based on population-level data remains limited, especially regarding how tryptophan metabolism mediates diseases caused by environmental pollutants. This gap is more pronounced in diverse populations with varying physiological conditions. Further investigation is warranted in this area.
- (2)
- Despite growing recognition of these connections, critical knowledge gaps persist regarding how specific environmental pollutants alter tryptophan (Trp) metabolic pathways and subsequently affect human health. Further investigations—using in silico, in vitro, or in vivo approaches—are needed.
- (3)
- To better investigate the relationship between Trp metabolism and environmental pollutants, integrating multi-omics data and epigenetic changes is worth considering. These approaches enable the identification of novel regulatory nodes within the Trp metabolic network that are perturbed by environmental pollutants, and serve as biomarkers or therapeutic targets for pollutant-induced disease. Advancements in in vitro models, multi-omics approaches, and computational toxicology will facilitate high-throughput screening and mechanistic studies, improving the accuracy of Trp metabolism.
- (4)
- Strengthening environmental regulations, improving pollutant management, and promoting Trp-rich dietary interventions could mitigate health risks and support the development of targeted prevention strategies, bridging mechanistic research with practical public health policies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.X.; Zhang, X.M.; Xu, Q.S. Physiological and biochemical effects of tryptophan and its application. Amino Acids Biol. Resour. 2005, 3, 58–62. [Google Scholar]
- Food and Nutrition Board. Recommended Dietary Allowances, 8th ed.; National Academy of Sciences: Washington, DC, USA, 1974. [Google Scholar]
- Huang, Y.; Wu, D. Research progress of indole-acetic acid synthesis by microorganisms and its effects on plants. Zhejiang Agric. Sci. 2019, 65, 2659–2664. [Google Scholar]
- Schröcksnadel, K.; Wirleitner, B.; Winkler, C.; Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta 2006, 364, 82–90. [Google Scholar] [CrossRef]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, eaaf9794. [Google Scholar] [CrossRef]
- Gao, F. The Role of Serotonin in the Intestinal Injury and Digestive Mechanism of Daphnia Magna Induced by Cadmium. Ph.D. Thesis, Shanxi University, Taiyuan, China, 2020. [Google Scholar]
- Ye, D.; Xu, H.; Tang, Q.; Xia, H.; Zhang, C.; Bi, F. The role of 5-HT metabolism in cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188618. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Albreht, A. Kinetics, mechanism, and inhibition of monoamine oxidase. J. Neural Transm. 2018, 125, 1659–1683. [Google Scholar] [CrossRef]
- Wei, G.Z.; Martin, K.A.; Xing, P.Y. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2021091118. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Pihel, K.; Hsieh, S.; Jorgenson, J.W.; Wightman, R.M. Quantal corelease of histamine and 5-hydroxytryptamine from mast cells and the effects of prior incubation. Biochemistry 1998, 37, 1046–1052. [Google Scholar] [CrossRef]
- Purcell, W.M.; Atterwill, C.K. Mast cells in neuroimmune function: Neurotoxicological and neuropharmacological perspectives. Neurochem. Res. 1995, 20, 521–532. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; McDougle, C.J.; Schumacher, M.; Olcese, J.; Mason, J.W.; Heninger, G.R.; Price, L.H. Effects of acute tryptophan depletion on nocturnal melatonin secretion in humans. J. Clin. Endocrinol. Metab. 1993, 76, 1160–1164. [Google Scholar] [PubMed]
- Lucki, I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 1998, 44, 151–162. [Google Scholar] [CrossRef]
- Lesch, K.P. Gene-environment interaction and the genetics of depression. J. Psychiatry Neurosci. 2004, 29, 174–184. [Google Scholar]
- Schwarcz, R.; Pellicciari, R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002, 303, 1–10. [Google Scholar] [CrossRef]
- Stone, T.W. Kynurenines in the CNS: From endogenous obscurity to therapeutic importance. Prog. Neurobiol. 2001, 64, 185–218. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug. Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef]
- Perkins, M.N.; Stone, T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Stone, T.W.; Connick, J.H.; Winn, P.; Hastings, M.H.; English, M. Endogenous excitotoxic agents. Ciba Found Symp. 1987, 126, 204–220. [Google Scholar]
- Nakagami, Y.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine toxicity on the rat striatum in vivo. Jpn. J. Pharmacol. 1996, 71, 183–186. [Google Scholar] [CrossRef]
- Okuda, S.; Nishiyama, N.; Saito, H.; Katsuki, H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998, 70, 299–307. [Google Scholar] [CrossRef]
- Birch, P.J.; Grossman, C.J.; Hayes, A.G. Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur. J. Pharmacol. 1988, 154, 85–87. [Google Scholar] [CrossRef]
- Nichols, A.C.; Yielding, K.L. Anticonvulsant activity of antagonists for the NMDA-associated glycine binding site. Mol. Chem. Neuropathol. 1993, 19, 269–282. [Google Scholar] [CrossRef]
- Shedpure, M.; Pati, A.K. The pineal gland: Structural and functional diversity. Indian J. Exp. Biol. 1995, 33, 625–640. [Google Scholar]
- Waldhauser, F.; Ehrhart, B.; Förster, E. Clinical aspects of the melatonin action: Impact of development, aging, and puberty, involvement of melatonin in psychiatric disease and importance of neuroimmunoendocrine interactions. Experientia 1993, 49, 671–681. [Google Scholar] [CrossRef]
- Lemos, H.; Huang, L.; Prendergast, G.C.; Mellor, A.L. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat. Rev. Cancer 2019, 19, 162–175. [Google Scholar] [CrossRef]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat. Commun. 2020, 11, 4011. [Google Scholar] [CrossRef]
- Munn, D.H.; Sharma, M.D.; Baban, B.; Harding, H.P.; Zhang, Y.H.; Ron, D.; Mellor, A.L. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005, 22, 633–642. [Google Scholar] [CrossRef]
- Ravishankar, B.; Liu, H.; Shinde, R.; Chaudhary, K.; Xiao, W.; Bradley, J.; Koritzinsky, M.; Madaio, M.P.; McGaha, T.L. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc. Natl. Acad. Sci. USA 2015, 112, 10774–10779. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, C.E.; Park, H.Y.; Yoon, E.H.; Won, H.J.; Ahn, J.M.; Nguyen, N.Z.N.; Kim, M.; Jang, W.J.; Lee, W.S.; et al. Aryl hydrocarbon receptor-targeted therapy for CD4+ T cell-mediated idiopathic pneumonia syndrome in mice. Blood 2022, 139, 3325–3339. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, H.Y.; Suh, Y.S.; Yoon, E.H.; Kim, J.; Jang, W.H.; Lee, W.S.; Park, S.G.; Choi, I.W.; Choi, I.; et al. Inhibition of acute lethal pulmonary inflammation by the IDO-AhR pathway. Proc. Natl. Acad. Sci. USA 2017, 114, E5881–E5890. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, B.; Shinde, R.; Liu, H.; Chaudhary, K.; Bradley, J.; Lemos, H.P.; Chandler, P.; Tanaka, M.; Munn, D.H.; Mellor, A.L.; et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc. Natl. Acad. Sci. USA 2014, 111, 4215–4220. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, N.; Zhou, L.; Wang, J.; Zhou, Y.; Zhang, T.; Fang, Y.; Deng, J.W.; Gao, Y.F.; Liang, X.Y.; et al. IL-2 regulates tumor-reactive CD8+ T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 2021, 22, 358–369. [Google Scholar] [CrossRef]
- Karmakar, S.; Lal, G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics 2021, 11, 5296–5312. [Google Scholar] [CrossRef]
- Duerschmied, D.; Suidan, G.L.; Demers, M.; Herr, N.; Carbo, C.; Brill, A.; Cifuni, S.M.; Mauler, M.; Cicko, S.; Bader, M.; et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013, 121, 1008–1015. [Google Scholar] [CrossRef]
- De Giovanni, M.; Tam, H.; Valet, C.; Xu, Y.; Looney, M.R.; Cyster, J.G. GPR35 promotes neutrophil recruitment in response to serotonin metabolite 5-HIAA. Cell 2022, 185, 815–830. [Google Scholar] [CrossRef]
- Haq, S.; Grondin, J.A.; Khan, W.I. Tryptophan-derived serotonin-kynurenine balance in immune activation and intestinal inflammation. FASEB J. 2021, 35, e21888. [Google Scholar] [CrossRef]
- Shi, Z.C.; Devasagayaraj, A.; Gu, K.; Jin, H.; Marinelli, B.; Samala, L.; Scott, S.; Stouch, T.; Tunoori, A.; Wang, Y. Modulation of peripheral serotonin levels by novel tryptophan hydroxylase inhibitors for the potential treatment of functional gastrointestinal disorders. J. Med. Chem. 2008, 51, 3684–3687. [Google Scholar] [CrossRef]
- Margolis, K.G.; Stevanovic, K.; Li, Z.S.; Yang, Q.M.; Oravecz, T.; Zambrowicz, B.; Jhaver, K.G.; Diacou, A.; Gershon, M.D. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 2014, 63, 928–937. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Sun, X.C.; Shao, Y.Y.; Jin, Y.; Huai, J.P.; Zhou, Q.; Huang, Z.M.; Wu, J.S. Melatonin reduces bacterial translocation by preventing damage to the intestinal mucosa in an experimental severe acute pancreatitis rat model. Exp. Ther. Med. 2013, 6, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Sommansson, A.; Nylander, O.; Sjöblom, M. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J. Pineal Res. 2013, 54, 282–291. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D. Opinion: Understanding “global” systems biology: Metabonomics and the continuum of metabolism. Nat. Rev. Drug. Discov. 2003, 2, 668–676. [Google Scholar] [CrossRef]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Marsland, B.J. Regulating inflammation with microbial metabolites. Nat. Med. 2016, 22, 581–583. [Google Scholar] [CrossRef]
- Vickers, C.; Hales, P.; Kaushik, V.; Dick, L.; Gavin, J.; Tang, J.; Godbout, K.; Parsons, T.; Baronas, E.; Hsieh, F.; et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002, 277, 14838–14843. [Google Scholar] [CrossRef]
- Zelante, T.; Lannitti, R.G.; Cunha, C.; Luca, A.D.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; Angelo, C.D.; Benedetti, C.M.; Fallarino, F.; et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef]
- Dai, X.; Zhu, B.T. Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: Implications for its biological functions. J. Histochem. Cytochem. 2010, 58, 17–28. [Google Scholar] [CrossRef]
- Niño-Castro, A.; Abdullah, Z.; Popov, A.; Thabet, Y.; Beyer, M.; Knolle, P.; Domann, E.; Chakraborty, T.; Schmidt, S.V.; Schultze, J.L. The IDO1-induced kynurenines play a major role in the antimicrobial effect of human myeloid cells against Listeria monocytogenes. Innate Immun. 2014, 20, 401–411. [Google Scholar] [CrossRef]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Servillo, G.; Brunacci, C.; Bicciato, S.; Mazza, E.M.C.; Macchiarulo, A.; Vacca, C.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2014, 511, 184–190. [Google Scholar] [CrossRef]
- Ranzil, S.; Walker, D.W.; Borg, A.J.; Wallace, E.M.; Ebeling, P.R.; Murthi, P. The relationship between the placental serotonin pathway and fetal growth restriction. Biochimie 2019, 161, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Sundström, E.; Kölare, S.; Souverbic, F.; Samuelsson, E.B.; Pschera, H.; Lunell, N.O.; Seiger, Å. Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res. Dev. Brain Res. 1993, 75, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bonnin, A.; Goeden, N.; Chen, K.; Wilson, M.L.; King, J.; Shih, J.C.; Blakely, R.D.; Deneris, E.S.; Levitt, P. A transient placental source of serotonin for the fetal forebrain. Nature 2011, 472, 347–350. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D hormone regulates serotonin synthesis. Part 1: Relevance for autism. FASEB J. 2014, 28, 2398–2413. [Google Scholar] [CrossRef]
- Bonney, E.A.; Matzinger, P. Much IDO about pregnancy. Nat. Med. 1998, 4, 1128–1129. [Google Scholar] [CrossRef]
- Sedlmayr, P.; Blaschitz, A.; Stocker, R. The role of placental tryptophan catabolism. Front. Immunol. 2014, 5, 230. [Google Scholar] [CrossRef]
- Hsu, P.; Nanan, R. Foetal immune programming: Hormones, cytokines, microbes and regulatory T cells. J. Reprod. Immunol. 2014, 104–105, 2–7. [Google Scholar] [CrossRef]
- Tatsumi, K.; Higuchi, T.; Fujiwara, H.; Nakayama, T.; Egawa, H.; Itoh, K.; Fujii, S.; Fujita, J. Induction of tryptophan 2,3-dioxygenase in the mouse endometrium during implantation. Biochem. Biophys. Res. Commun. 2000, 274, 166–170. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef]
- Esser, C. The Aryl Hydrocarbon Receptor in Immunity: Tools and Potential. Methods Mol. Biol. 2016, 1371, 239–257. [Google Scholar]
- DiNatale, B.C.; Murray, L.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Däubener, W.; Schmidt, S.K.; Heseler, K.; Spekker, K.H.; MacKenzie, C.R. Antimicrobial and immunoregulatory effector mechanisms in human endothelial cells. Indoleamine 2,3-dioxygenase versus inducible nitric oxide synthase. Thromb. Haemost. 2009, 102, 1110–1116. [Google Scholar] [PubMed]
- Broekhuizen, M.; Klein, T.; Hitzerd, E.; Rijke, Y.B.; Schoenmakers, S.; Sedlmayr, P.; Danser, A.H.J.; Merkus, D.; Reiss, I.K.M. l-Tryptophan-Induced Vasodilation Is Enhanced in Preeclampsia: Studies on Its Uptake and Metabolism in the Human Placenta. Hypertension 2020, 76, 184–194. [Google Scholar] [CrossRef]
- Moschella, P.S.; Laane, R.P.; Bäck, S.; Behrendt, H.; Bendoricchio, G.; Georgiou, S.; Herman, P.M.; Lindeboom, H.; Skourtous, M.S.; Tett, P.; et al. Group report: Methodologies to support implementation of the water framework directive. In Managing European Coasts: Past, Present and Future; Springer: Berlin/Heidelberg, Germany, 2005; pp. 137–152. [Google Scholar]
- Chormare, R.; Kumar, M.A. Environmental health and risk assessment metrics with special mention to biotransfer, bioaccumulation and biomagnification of environmental pollutants. Chemosphere 2022, 302, 134836. [Google Scholar] [CrossRef]
- Mathew, B.B.; Singh, H.; Biju, V.G.; Krishnamurthy, N.B. Classification, Source, and Effect of Environmental Pollutants and Their Biodegradation. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 55–71. [Google Scholar] [CrossRef]
- Zeng, T.; Liang, Y.; Chen, J.; Cao, G.; Yang, Z.; Zhao, X.; Tian, J.; Xin, X.; Lei, B.; Cai, Z. Urinary metabolic characterization with nephrotoxicity for residents under cadmium exposure. Environ. Int. 2021, 154, 106646. [Google Scholar] [CrossRef]
- Wang, W.J.; Sun, B.F.; Luo, D.P.; Chen, X.; Yao, M.L.; Zhang, A.H. Biological markers of arsenic-induced cognitive impairment based on plasma neurotransmitter metabolomics. In Proceedings of the 11th National Toxicology Conference of the Chinese Society of Toxicology, Hangzhou, China, 20–23 September 2024; Key Laboratory of Environmental Pollution and Disease Control, Ministry of Education/School of Public Health and Health, Guizhou Medical University: Hangzhou, China, 2024. [Google Scholar]
- Du, X.; Zhang, J.; Zhang, X.; Schramm, K.W.; Nan, B.; Huang, Q.; Tian, M.; Shen, H. Persistence and reversibility of arsenic-induced gut microbiome and metabolome shifts in male rats after 30-days recovery duration. Sci. Total Environ. 2021, 776, 145972. [Google Scholar] [CrossRef]
- Chen, L.; Dang, Z.Z.; Liu, C.L.; Wang, T.; Zhen, Y.G.; Zhang, X.F.; Qin, G.X.; Sun, J. Research progress on production, determination and metabolic pathways of 5-hydroxytryptophan and its application. J. Econ. Anim. 2024, 28, 50–56. [Google Scholar]
- Yang, C.J.; Tan, H.P.; Du, Y.J. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience 2014, 267, 1–10. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.D.; Gao, X.S.; Zhu, J.S.; Wang, L.; Cao, S.Y.; Wu, Q.; Qiao, S.L.; Zhang, Z.; Li, L. Comparative effects of mercury chloride and methylmercury exposure on early neurodevelopment in zebrafish larvae. RSC Adv. 2019, 9, 10766–10775. [Google Scholar] [CrossRef]
- Rose, M.; Filiatreault, A.; Guénette, J.; Williams, A.; Thomson, E.M. Ozone increases plasma kynurenine-tryptophan ratio and impacts hippocampal serotonin receptor and neurotrophic factor expression: Role of stress hormones. Environ. Res. 2020, 185, 109483. [Google Scholar] [CrossRef] [PubMed]
- Pyatha, S.; Kim, H.; Lee, D. Co-exposure to lead, K.K.; mercury, and cadmium induces neurobehavioral impairments in mice by interfering with dopaminergic and serotonergic neurotransmission in the striatum. Front. Public Health 2023, 11, 1265864. [Google Scholar] [CrossRef]
- Carmean, C.M.; Yokoi, N.; Takahashi, H.; Oduori, O.S.; Kang, C.; Kanagawa, A.; Kirkley, A.G.; Han, G.; Landeche, M.; Hidaka, S.; et al. Arsenic modifies serotonin metabolism through glucuronidation in pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E464–E474. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Occurrence of environmental pollutants in foodstuffs: A review of organic vs. conventional food. Food Chem Toxicol. 2019, 125, 370–375. [Google Scholar] [CrossRef]
- Ducroq, S.; Duplus, E.; Penalva-Mousset, L.; Trivelloni, F.; L’honoré, A.; Chabat-Courrède, C.; Nemazanyy, I.; Grange-Messent, V.; Petropoulos, I.; Mhaouty-Kodja, S. Behavior, Neural Structure, and Metabolism in Adult Male Mice Exposed to Environmentally Relevant Doses of Di(2-ethylhexyl) Phthalate Alone or in a Phthalate Mixture. Environ. Health. Perspect. 2023, 131, 77008. [Google Scholar] [CrossRef]
- Hong, J.; Lu, X.; Wang, J.; Jiang, M.; Liu, Q.; Lin, J.; Sun, W.; Zhang, J.; Shi, Y.; Liu, X. Triphenyl phosphate disturbs placental tryptophan metabolism and induces neurobehavior abnormal in male offspring. Ecotoxicol. Environ. Saf. 2022, 243, 113978. [Google Scholar] [CrossRef]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef]
- Lizuka, T.; Yin, P.; Zuberi, A.; Kujawa, S.; Coon, J.S.; Björvang, R.D.; Damdimopoulou, P.; Pacyga, D.C.; Strakovsky, R.S.; Flaws, J.A.; et al. Mono-(2-ethyl-5-hydroxyhexyl) phthalate promotes uterine leiomyoma cell survival through tryptophan-kynurenine-AHR pathway activation. Proc. Natl. Acad. Sci. USA 2022, 119, e2208886119. [Google Scholar]
- Liang, F.; Wang, G.Z.; Wang, Y.; Yang, Y.N.; Wen, Z.S.; Chen, D.N.; Fang, W.F.; Zhang, B.; Yang, L.; Zhang, C.; et al. Tobacco carcinogen induces tryptophan metabolism and immune suppression via induction of indoleamine 2,3-dioxygenase 1. Signal Transduct. Target Ther. 2022, 7, 311. [Google Scholar] [CrossRef]
- Bobori, D.C.; Feidantsis, K.; Dimitriadi, A.; Datsi, N.; Ripis, P.; Kalogiannis, S.; Sampsonidis, I.; Kastrinaki, G.; Ainali, N.M.; Lambropoulou, D.A.; et al. Dose-Dependent Cytotoxicity of Polypropylene Microplastics (PP-MPs) in Two Freshwater Fishes. Int. J. Mol. Sci. 2022, 23, 13878. [Google Scholar] [CrossRef]
- Rajendran, D.; Chandrasekaran, N. Molecular Interaction of Functionalized Nanoplastics with Human Hemoglobin. J. Fluoresc. 2023, 33, 2257–2272. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Zhao, X.; Wang, C.; Wang, C.; White, J.C.; Zhao, W.; Zhou, L.; Duan, M.; Wu, F. Polystyrene Nanoplastics Toxicity to Zebrafish: Dysregulation of the Brain-Intestine-Microbiota Axis. ACS Nano 2022, 16, 8190–8204. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, D.; Chandrasekaran, N.; Waychal, Y.; Mukherjee, A. Nanoplastics alter the conformation and activity of human serum albumin. NanoImpact 2022, 27, 100412. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Félix, L.; Bellas, J.; Monteiro, S.M. Oxidative stress, apoptosis and serotonergic system changes in zebrafish (Danio rerio) gills after long-term exposure to microplastics and copper. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 258, 109363. [Google Scholar] [CrossRef]


| Total Number of Items Retrieved | Number of Items After Removal of Duplicates and Selected on the Basis of Title and Abstract | Number of Studies Selected After Full Text Revision |
|---|---|---|
| 200 | 100 | 88 |
| Types | References | Species and Cell Line | Dose of Exposure | Routes of Exposure | Length of Exposure |
|---|---|---|---|---|---|
| Inorganic pollutants | [69] | Human | 3.08 ± 1.40 μg/L of Cd | Oral administration | 15 years |
| [71] | Wistar rats | 0.05, 0.25, 1.25, 6.25 mg/L of As | Oral administration | 30 days | |
| [74] | Zebrafish | 0, 4, 40, 400 nM of HgCl2 | Water exposure | 96 h | |
| [75] | Male Fischer-344 rats | 0, 0.8 ppm of ozone | Respiratory infection | 4 h | |
| [76] | C57BL/6 male mice | 25 mg/L of Pb, 10 mg/L of Hg, and 15 of mg/L Cd | Oral administration | 28 days | |
| [77] | MIN6-K8 mouse islet β cells | 0.1–1 μM of As | Cytotoxicity | 30 days | |
| Organic pollutants | [79] | C57BL/6J male mice | 0, 5, 50 μg/kg/d of DEHP | Oral administration | 8 weeks |
| [80] | C57BL/6 prenatal mice | 1, 5 mg/kg of TPHP | Oral administration | 56 days | |
| [81] | C57BL/6J mice | 0.5, 5, 50 mg/kg of BPA | Oral administration | 22 weeks | |
| [82] | Uterine leiomyoma cell | 0.16, 1.6, 16 μM of MEHHP | Cytotoxicity | 48 and 72 h | |
| [83] | A/J female mice | 50 mg/kg of NNK | Oral administration | 5 weeks | |
| Microplastics and nanoplastics | [84] | adult specimens of D. rerio and P. fluviatilis | 1, 10 mg/g of 8–10 μm PP-MPs | Water exposure | 21 days |
| [86] | Zebrafish | 1, 10, 100 μg/L of 44 nm PP-NPs | Water exposure | 30 days | |
| [88] | Zebrafish | 2 mg/L of microplastics | Water exposure | 30 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Lu, X.; Wu, M.; Bai, Z.; Liu, X. Effects of Environmental Pollutants on Tryptophan Metabolism. Toxics 2025, 13, 311. https://doi.org/10.3390/toxics13040311
Hu H, Lu X, Wu M, Bai Z, Liu X. Effects of Environmental Pollutants on Tryptophan Metabolism. Toxics. 2025; 13(4):311. https://doi.org/10.3390/toxics13040311
Chicago/Turabian StyleHu, Hongyang, Xiaoxun Lu, Miaoliang Wu, Zhi Bai, and Xiaoshan Liu. 2025. "Effects of Environmental Pollutants on Tryptophan Metabolism" Toxics 13, no. 4: 311. https://doi.org/10.3390/toxics13040311
APA StyleHu, H., Lu, X., Wu, M., Bai, Z., & Liu, X. (2025). Effects of Environmental Pollutants on Tryptophan Metabolism. Toxics, 13(4), 311. https://doi.org/10.3390/toxics13040311







