Molecular Mechanisms Contributing to the Impairment of Steroid Hormones, Sperm Characteristics, and Testicular Architecture in Male Rabbits After Chronic Exposure to Cadmium: Role of Gallic Acid and Selenium as Antioxidants
Abstract
1. Introduction
2. Materials
2.1. Animals
2.2. Research Design
3. Methods
3.1. Preparation of the Microsomal Fraction
3.2. Biochemical Assays
3.3. Semen Analysis
3.4. Sperm Motility
3.5. Sperm Count
3.6. Sperm Morphology
3.7. Blood Samples
3.8. Hormonal Assays
3.9. Western Immunoblotting for the Identification of the Immobilized Proteins
3.10. Histopathology
3.11. Statistical Analyses
4. Results
4.1. Cadmium and Steroid Hormones
4.2. Effect of Cadmium on 17β-Hydroxysteroid Dehydrogenase Activity and the Protein Expression of Cytochrome P450 Isozymes in Testes Tissues
4.3. Cadmium and Antioxidant Enzyme Activities in Testes Tissues
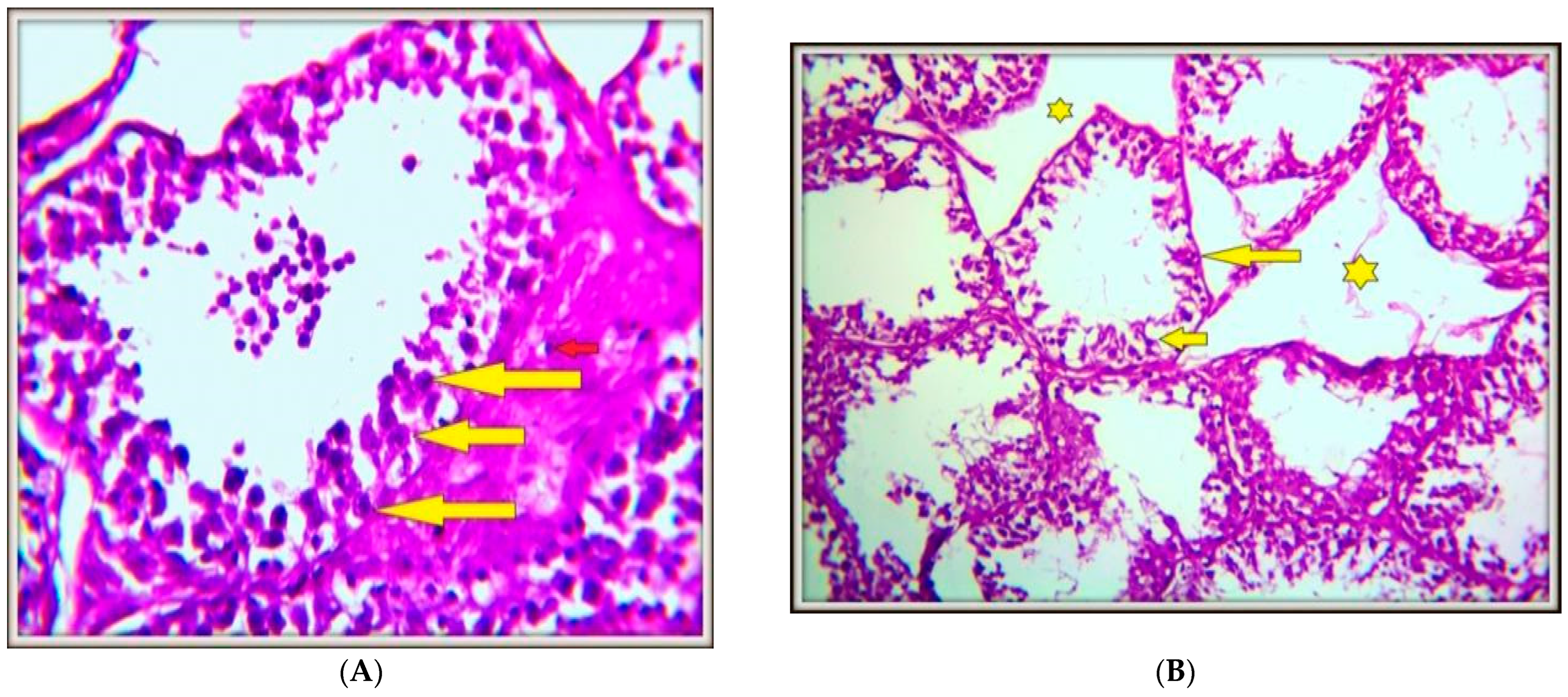
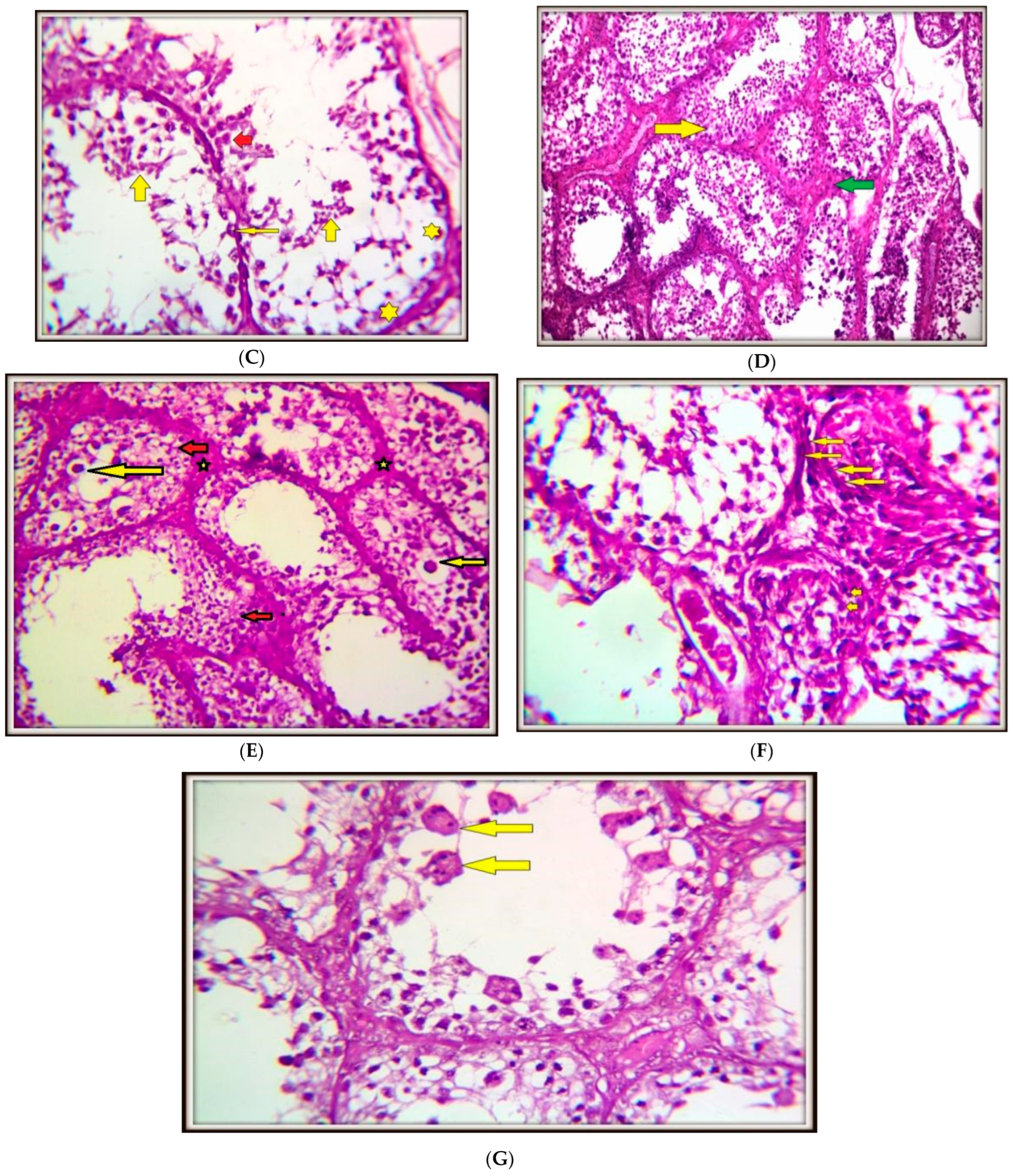
4.4. Histopathology of Testicular Tissues
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Dubin, L.; Amelar, R.D. Varicocele. Urol. Clin. N. Am. 1978, 5, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. 2019, 77, 210–217. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Sharma, R.K.; Said, T.M.; Sikka, S.C.; Thomas, A.J.J. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil. Steril. 2003, 80, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Vernet, P.; Aitken, R.J.; Drevet, J.R. Antioxidant strategies in the epididymis. Mol. Cell Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.Z.; Ranasinghe, S.; Sjaarda, L.A.; Mumford, S.L. Cadmium and reproductive health in women: A systematic review of the epidemiologic evidence. Curr. Environ. Health Rep. 2014, 1, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-L.; Ru, Y.-F.; Liu, M.; Tang, J.-N.; Zheng, J.-F.; Wu, B.; Gu, Y.-H.; Shi, H.-J. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef]
- De Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The environment and male reproduction: The effect of cadmium exposure on reproductive function and its implication in fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Akinloye, O.; Arowojolu, A.O.; Shittu, O.B.; Anetor, J.I. Cadmium toxicity: A possible cause of male infertility in Nigeria. Reprod. Biol. 2006, 6, 17–30. [Google Scholar]
- Zhu, Q.; Li, X.; Ge, R.S. Toxicological Effects of Cadmium on Mammalian Testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benoff, S.H.; Millan, C.; Hurley, I.R.; Napolitano, B.; Marmar, J.L. Bilateral increased apoptosis and bilateral accumulation of cadmium in infertile men with left varicocele. Hum. Reprod. 2004, 19, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, J.; He, W.; Zhou, L.; Huang, Y. Parental exposure to cadmium chloride causes developmental toxicity and thyroid endocrine disruption in zebrafish offspring. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 234, 108782. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, Y.; Mo, A.; Yuan, Y. Selenium mitigated cadmium-induced ovarian retardation in female Procambarus clarkii by regulating vitellogenin synthesis and transfer in the hepatopancreas. Ecotoxicol. Environ. Saf. 2024, 288, 117339. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, S.; Wang, Y.; Wang, M.; Cui, Z.; Huang, H. Antagonistic effect of selenium on programmed necrosis of testicular Leydig cells caused by cadmium through endoplasmic reticulum stress in chicken. Environ. Sci. Pollut. Res. Int. 2023, 30, 112517–112535. [Google Scholar] [CrossRef] [PubMed]
- Maniradhan, M.; Sivagurunathan, N.; Unnikrishnan, A.K.; Anbiah, V.S.; Calivarathan, L. Selenium ameliorates oxidized phospholipid-mediated testicular dysfunction and epididymal sperm abnormalities following Bisphenol A exposure in adult Wistar rats. Reprod. Toxicol. 2024, 130, 108751. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S.; Sengul, E.; Aksu, E.H.; Cinar, İ.; Gelen, V.; Tekin, S.; Dag, Y. Selenium reduces acrylamide-induced testicular toxicity in rats by regulating HSD17B1, StAR, and CYP17A1 expression, oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Toxicol. 2024, 39, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, M.; Ebrahimzadeh-Bideskan, A.; Rajabian, A.; Alipour, F.; Raoofi, A.; Ebrahimi, V. Ameliorating effects of selenium nanoparticle coated by gallic acid on histological and biochemical parameters of testis in azoospermic rat model. Tissue Cell 2024, 91, 102550. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.L.D.S.; Lins, T.L.B.G.; Monte, A.P.O.D.; Andrade, K.O.d.; Barberino, R.d.S.; Silva, G.A.L.d.; Campinho, D.D.S.P.; Junior, R.C.P.; Matos, M.H.T. Protective effect of gallic acid on doxorubicin-induced ovarian toxicity in mouse. Reprod. Toxicol. 2023, 115, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of the literature. J. Hum. Reprod. Sci. 2015, 8, 191. [Google Scholar] [CrossRef]
- Owumi, S.E.; Adedara, I.A.; Akomolafe, A.P.; Farombi, E.O.; Oyelere, A.K. Gallic acid enhances reproductive function by modulating oxido-inflammatory and apoptosis mediators in rats exposed to aflatoxin-B1. Exp. Biol. Med. 2020, 245, 1016–1028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef]
- Aitken, R.J.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; El-Masry, M.S.R. Behenic acid protects the testosterone cycle and prevents the sperm apoptosis and protein loss in phthalate exposure by inhibiting oxidative stress and stimulating ATPase activity. Toxicol. Rep. 2024, 13, 101845. [Google Scholar] [CrossRef]
- Evgeni, E.; Charalabopoulos, K.; Asimakopoulos, B. Human sperm DNA fragmentation and its correlation with conventional semen parameters. J. Reprod. Infertil. 2014, 15, 2–14. [Google Scholar] [PubMed]
- Kim, J.; Sharma, R.P. Cadmium-induced apoptosis in murine macrophages is antagonized by antioxidants and caspase inhibitors. J. Toxicol. Environ. Health Part A 2006, 69, 1181–1201. [Google Scholar] [CrossRef]
- Wan, H.T.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. The apical ES-BTB-BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol. Med. 2013, 19, 396–405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, S.; Wang, L.; Zhang, W.; Xu, M.; Song, R.; Zou, H.; Gu, J.; Bian, J.; Yuan, Y.; Liu, Z. Cadmium-activated Fas induces mitochondrial apoptosis pathway mediated through Caspase-8 and JNK in rat cortical neurons. Metallomics 2021, 13, mfab042. [Google Scholar] [CrossRef]
- Dudek, P.; Kozakowski, J.; Zgliczynski, W. Late-onset hypogonadism. Prz. Menopauzalny 2017, 16, 66–69. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, S.; Gavrilović, A.; Li, D.; Tang, R. Selenium alleviates cadmium-induced oxidative stress, endoplasmic reticulum stress, and apoptosis in L8824 cells. Ecotoxicol. Environ. Saf. 2023, 262, 115337. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ge, R.S.; Zirkin, B.R. Leydig cells: From stem cells to aging. Mol. Cell. Endocrinol. 2009, 306, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I. Steroidogenic enzymes: Structure, function, and role in regulation of steroid hormone biosynthesis. J. Steroid Biochem. Mol. Biol. 1992, 43, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.H.; Youngblood, G.L. Regulation of expression of steroidogenic enzymes in Leydig cells. Biol. Reprod. 1995, 52, 217–225. [Google Scholar] [CrossRef]
- Honour, J.W. Steroid biosynthesis. In Steroids in the Laboratory and Clinical Practice; Honour, J.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 1.3; pp. 63–92. ISBN 9780128181249. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stocco, D.M.; Chen, W. Presence of identical mitochondrial proteins in unstimulated constitutive steroid-producing R2C rat Leydig tumor and stimulated nonconstitutive steroid-producing MA-10 mouse Leydig tumor cells. Endocrinology 1991, 128, 1918–1926. [Google Scholar] [CrossRef]
- Korytowski, W.; Pilat, A.; Schmitt, J.; Girotti, A. Deleterious Cholesterol Hydroperoxide Trafficking in Steroidogenic Acute Regulatory (StAR) Protein-expressing MA-10 Leydig Cells. J. Biol. Chem. 2013, 288, 11509–11519. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Utsunomiya, H.; Suzuki, T.; Saitou, S.; Akahira, J.; Okamura, K.; Yaegashi, N.; Sasano, H. 17Beta-hydroxysteroid dehydrogenases in human endometrium and its disorders. Mol. Cell Endocrinol. 2006, 248, 136–140. [Google Scholar] [CrossRef]
- Geissler, W.M.; Davis, D.L.; Wu, L.; Bradshaw, K.D.; Patel, S.; Mendonca, B.B.; Elliston, K.O.; Wilson, J.D.; Russell, D.W.; Andersson, S. Male pseudohermaphroditism caused by mutations of testicular 17 beta-hydroxysteroid dehydrogenase 3. Nat. Genet. 1994, 7, 34–39. [Google Scholar] [CrossRef]
- Hasegawa, E.; Nakagawa, S.; Sato, M.; Tachikawa, E.; Yamato, S. Effect of polyphenols on production of steroid hormones from human adrenocortical NCI-H295R cells. Biol. Pharm. Bull. 2013, 36, 228–237. [Google Scholar] [CrossRef]
- Newairy, A.A.; El-Sharaky, A.S.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Habtemariam, S.; Nabavi, S.F.; Sureda, A.; Daglia, M.; Moghaddam, A.H.; Amani, M.A. Protective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat’s kidney. Mol. Cell Biochem. 2013, 372, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Sarac, F.; Yeniocak, S.; Erbin, A.; Yucetas, E.; Altundal, K.; Ucpinar, B.; Saygili, A.; Koldas, M. Ischemia Modified Albumin and D-dimer in the Diagnosis of Testicular Torsion: An Experimental Model. Urol. J. 2019, 16, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; El-Dafrawi, Y.A.; El-Ghalid, O.A.; Ghoneim, A.A.; Wahid, A. Antioxidants (selenium and garlic) alleviated the adverse effects of tramadol on the reproductive system and oxidative stress markers in male rabbits. Sci. Rep. 2022, 12, 13958, Erratum in Sci. Rep. 2022, 12, 16871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bogovich, K.; Payne, A.H. Purification of rat testicular microsomal 17-ketosteroid reductase. Evidence that 17-ketosteroid reductase and 17 beta-hydroxysteroid dehydrogenase are distinct enzymes. J. Biol. Chem. 1980, 255, 5552–5559. [Google Scholar] [CrossRef]
- Suojanen, J.N.; Gay, R.J.; Hilf, R. Influence of estrogen on glutathione levels and glutathione-metabolizing enzymes in uteri and R3230AC mammary tumors of rats. Biochim. Biophys. Acta 1980, 630, 485–496. [Google Scholar] [CrossRef]
- Lee, C.Y.; Johnson, L.; Cox, R.H.; McKinney, J.D.; Lee, S.M. Mouse liver glutathione S-transferases. Biochemical and immunological characterization. J. Biol. Chem. 1981, 256, 8110–8116. [Google Scholar] [CrossRef]
- Chiu, D.T.; Stults, F.H.; Tappel, A.L. Purification and properties of rat lung soluble glutathione peroxidase. Biochim. Biophys. Acta 1976, 445, 558–566. [Google Scholar] [CrossRef]
- Luck, H. Estimation of catalase. In Methods of Enzymatic Analysis; Bergmayer, M.V., Ed.; Academic Press: New York, NY, USA, 1974; p. 885. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Tappel, A.; Zalkin, H. Inhibition of lipide peroxidation in mitochondria by vitamin E. Arch. Biochem. Biophys. 1959, 80, 333–336. [Google Scholar] [CrossRef]
- Atashfaraz, E.; Farokhi, F.; Najafi, G. Protective effect of ethyl pyruvate on epididymal sperm characteristics, oxidative stress and testosterone level in methotrexate treated mice. J. Reprod. Infertil. 2013, 14, 190–196. [Google Scholar] [PubMed]
- Freund, M.; Carol, B. Factors affecting hemocytometer counts of sperm concentration in human semen. J. Reprod. Fertil. 1964, 8, 149–155. [Google Scholar] [CrossRef]
- Narayana, K.; Prashanthi, N.; Nayanatara, A.; Kumar, H.H.; Abhilash, K.; Bairy, K.L. Effects of methyl parathion (o,o-dimethyl o-4-nitrophenyl phosphorothioate) on rat sperm morphology and sperm count, but not fertility, are associated with decreased ascorbic acid level in the testis. Mutat. Res. 2005, 588, 28–34. [Google Scholar] [CrossRef]
- Patton, C.J.; Crouch, S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Matsudaira, P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 1987, 262, 10035–10038. [Google Scholar] [CrossRef] [PubMed]
- Drury, A.; Wallington, E. Carleton’s Histological Technique, 5th ed.; Oxford University Press: New York, NY, USA; Toronto, ON, Canada, 1980. [Google Scholar]
- Wang, T.T.; Zhu, H.L.; Ouyang, K.W.; Wang, H.; Luo, Y.X.; Zheng, X.M.; Ling, Q.; Wang, K.W.; Zhang, J.; Chang, W.; et al. Environmental cadmium inhibits testicular testosterone synthesis via Parkin-dependent MFN1 degradation. J. Hazard. Mater. 2024, 470, 134142. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.K.; Sengupta, P.; Goswami, H.; Sarkar, M. Effects of dietary magnesium on testicular histology, steroidogenesis, spermatogenesis and oxidative stress markers in adult rats. Indian. J. Exp. Biol. 2013, 51, 37–47. [Google Scholar]
- Sheweita, S.A.; El Banna, Y.Y.; Balbaa, M.; Abdullah, I.A.; Hassan, H.E. N-nitrosamines induced infertility and hepatotoxicity in male rabbits. Environ. Toxicol. 2017, 32, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Udefa, A.L.; Beshel, F.N.; Nwangwa, J.N.; Mkpe, I.D.; Ofuru, O.S.; Sam-Ekpe, V.G.; Stephen GIVitamin, E. administration does not ameliorate tramadol-associated impairment of testicular function in Wistar rats. Andrologia 2020, 52, e13454. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Fu, J.; Zhen, L.; Zhao, N.; Yang, Q.; Li, S.; Li, X. Cadmium inhibits mouse sperm motility through inducing tyrosine phosphorylation in a specific subset of proteins. Reprod. Toxicol. 2016, 63, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Abilash, D.; Sridharan, T.B. Impact of air pollution and heavy metal exposure on sperm quality: A clinical prospective research study. Toxicol. Rep. 2024, 13, 101708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cong, Y.; Chi, Q.; Teng, X.; Li, S. The Protection of Selenium Against Cadmium-Induced Mitochondrial Damage via the Cytochrome P450 in the Livers of Chicken. Biol. Trace Elem. Res. 2019, 190, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gu, L.; Liang, X.; Cao, B.; Zhang, J.; Guo, X. The Effect of Selenium on CYP450 Isoform Activity and Expression in Pigs. Biol. Trace Elem. Res. 2020, 196, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Nikhil Kumar Tej, J.; Johnson, P.; Krishna, K.; Kaushik, K.; Gupta, P.S.P.; Nandi, S.; Mondal, S. Copper and Selenium stimulates CYP19A1 expression in caprine ovarian granulosa cells: Possible involvement of AKT and WNT signalling pathways. Mol. Biol. Rep. 2021, 48, 3515–3527. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.X.; Chu, X.L.; Zhang, T.; Cao, L.K. Effect of genistein on the gene expressions of androgen generating key enzymes StAR, P450scc and CYP19 in rat ovary. Pol. J. Vet. Sci. 2019, 22, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Maged, M.; Hairul-Islam, M.I.; Osama, I.A.; Maha, H.; Manal, A.; Hamza, H. Activation of aryl hydrocarbon receptor signaling by a novel agonist ameliorates autoimmune encephalomyelitis. PLoS ONE 2019, 14, e0215981, Erratum in PLoS ONE 2019, 14, e0223429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheweita, S.A.; Rafea, A.A.E.; Elbana, S.G. The deleterious effects of cadmium on oxidative stress markers, drug-metabolizing, and antioxidant enzyme activities: Role of Silymarin and Garlic as Antioxidants. Environ. Sci. Pollut. Res. Int. 2023, 30, 112490–112502. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Tilmisany, A.M.; Al-Sawaf, H. Mechanisms of male infertility: Role of antioxidants. Curr. Drug Metab. 2005, 6, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Zaher, A.O.; Abdel-Rahman, M.S.; Elwasei, F.M. Protective effect of Nigella sativa oil against tramadol-induced tolerance and dependence in mice: Role of nitric oxide and oxidative stress. Neurotoxicology 2011, 32, 725–733. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Cho, C.-L.; Agarwal, A. Role of sperm DNA fragmentation in male factor infertility: A systematic review. Arab. J. Urol. 2018, 16, 21–34. [Google Scholar] [CrossRef]
- Kopalli, S.R.; Cha, K.M.; Cho, J.Y.; Kim, S.K.; Koppula, S. Cordycepin mitigates spermatogenic and redox related expression in H2O2-exposed Leydig cells and regulates testicular oxidative apoptotic signalling in aged rats. Pharm. Biol. 2022, 60, 404–416. [Google Scholar] [CrossRef]
- Shal, E.; Selim, M.H. The effect of tramadol treatment on rat testes and the possible protective role of selenium (light and electron microscopic study). Al-Azhar Assiut Med. J. 2015, 13, 126–137. [Google Scholar]
- Akinrinde, A.S.; Adekanmbi, A.O.; Olojo, F.O. Nigella sativa oil protects against cadmium-induced intestinal toxicity via promotion of anti-inflammatory mechanisms, mucin expression and microbiota integrity. Avicenna J. Phytomed. 2022, 12, 241–256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Yang, L.; Su, P.; Chen, N. Curcumin protects against cadmium-induced germ cell death in the testis of rats. Toxicol. Res. 2024, 13, tfae082. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassan, E.; Kahilo, K.; Kamal, T.; El-Neweshy, M.; Hassan, M. Protective effect of diallyl sulfide against lead-mediated oxidative damage, apoptosis and down-regulation of CYP19 gene expression in rat testes. Life Sci. 2019, 226, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Guesmi, F.; Kharbech, O.; Hfaiedh, N.; Djebali, W. Gallic acid improves the antioxidant ability against cadmium toxicity: Impact on leaf lipid composition of sunflower (Helianthus annuus) seedlings. Ecotoxicol. Environ. Saf. 2021, 210, 111906. [Google Scholar] [CrossRef] [PubMed]
- Ozoani, H.; Ezejiofor, A.N.; Okolo, K.O.; Orish, C.N.; Cirovic, A.; Cirovic, A.; Orisakwe, O.E. Selenium and zinc alleviate hepatotoxicity induced by heavy metal mixture (cadmium, mercury, lead and arsenic) via attenuation of inflammo-oxidant pathways. Environ. Toxicol. 2023; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bassiony, M.M.; Youssef, U.M.; El-Gohari, H. Free Testosterone and Prolactin Levels and Sperm Morphology and Function Among Male Patients With Tramadol Abuse: A Case-Control Study. J. Clin. Psychopharmacol. 2020, 40, 405–408. [Google Scholar] [CrossRef]
- Ray, J.A.; Kushnir, M.M.; Meikle, A.W.; Sindt, J.E.; Strathmann, F.G. An exploratory study Evaluating the impact of opioid and non-opioid pain medications on serum/plasma free testosterone and free estradiol concentrations. Drug Test Anal. 2017, 9, 1555–1560. [Google Scholar] [CrossRef]
- Koohsari, M.; Ahangar, N.; Mohammadi, E.; Shaki, F. Ameliorative Effect of Melatonin Against Reproductive Toxicity of Tramadol in Rats via the Regulation of Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis-related Gene Expression Signaling Pathway. Addict. Health 2020, 12, 118–129. [Google Scholar]
- Ibrahim, M.A.-L.; Salah-Eldin, A.-E. Chronic Addiction to Tramadol and Withdrawal Effect on the Spermatogenesis and Testicular Tissues in Adult Male Albino Rats. Pharmacology 2019, 103, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reprod. Med. Biol. 2021, 20, 41–52. [Google Scholar] [CrossRef]
- Savran, M.; Asci, S.; Gulle, K.; Aslankoc, R.; Asci, H.; Karakuyu, N.F.; Erzurumlu, Y.; Kaynak, M. Agomelatine ameliorates doxorubicin-induced cortical and hippocampal brain injury via inhibition of TNF-alpha/NF-kB pathway. Toxicol. Mech. Methods 2024, 34, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Firdoos, S.; Dai, R.; Younas, Z.; Shah, F.A.; Gul, M.; Rasheed, M. Agomelatine-loaded nanostructured lipid carriers alleviate neuropathic pain in rats by Nrf2/HO-1 signalling pathway. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13922. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Katopodis, P.; Domali, E.; Potiris, A.; Stavros, S.; Zachariou, A. Impact of Reductive Stress on Human Infertility: Underlying Mechanisms and Perspectives. Int. J. Mol. Sci. 2024, 25, 11802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alqahtani, Y.S.; Chidrawar, V.R.; Shiromwar, S.; Singh, S.; Maheshwari, R.; Chitme, H.; Chilamakuru, N.B.; Mohite, P.; Aljameeli, A.M.; Khateeb, M.M. A multi-modal approach to investigate Desmodium gangeticum’s influence on stress-induced male infertility: In vivo, in vitro, and in silico assessments. Biomed. Pharmacother. 2024, 173, 116358. [Google Scholar] [CrossRef] [PubMed]
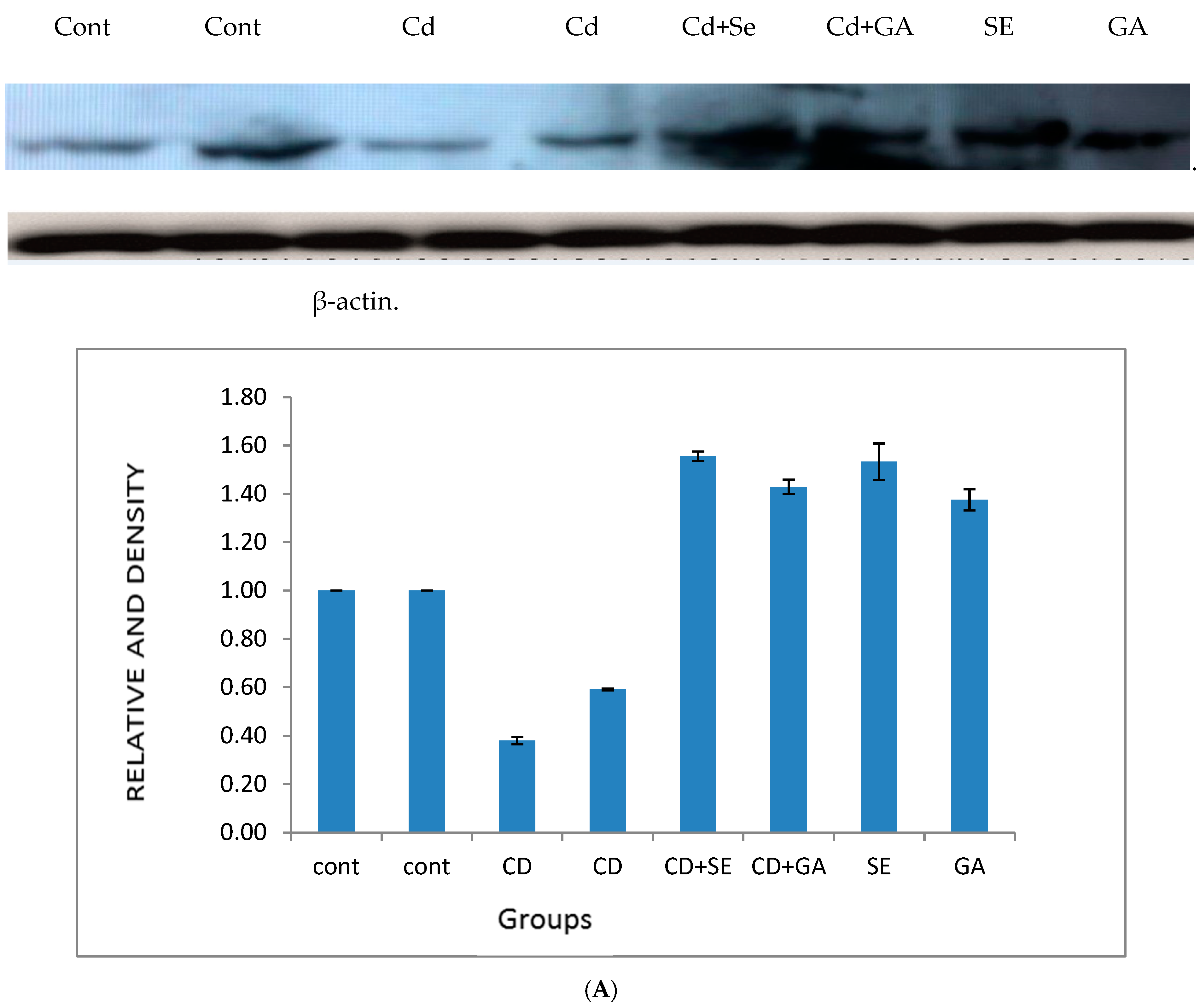

| Treatments | ||||||
|---|---|---|---|---|---|---|
| Weeks | Control | Cadmium | Cadmium + Gallic Acid | Cadmium + Selenium | Gallic Acid | Selenium |
| Testosterone level (ng/dL) | ||||||
| 4 | 5.72 ± 0.11 a | 5.0 ± 0.10 b | 5.8 ± 0.06 a | 6.04 ± 0.09 a | 6.12 ± 0.06 a | 6.08 ± 0.23 a |
| 8 | 6.26 ± 0.01 a | 4.20 ± 0.08 d | 5.04 ± 0.08 c | 5.34 ± 0.07 b | 6.28 ± 0.06 a | 6.38 ± 0.06 a |
| 12 | 6.46 ± 0.09 b | 3.38 ± 0.05 d | 4.92 ± 0.12 c | 4.76 ± 0.05 c | 6.54 ± 0.06 b | 6.88 ± 0.09 a |
| Estrogen (ng/dL) | ||||||
| 4 | 10.26 ± 0.12 b | 8.30 ± 0.14 d | 9.76 ± 0.16 c | 10.120 ± 0.09 bc | 10.76 ± 0.12 a | 10.96 ± 0.16 a |
| 8 | 10.48 ± 0.10 b | 5.40 ± 0.14 e | 8.84 ± 0.24 d | 9.34 ± 0.12 c | 11.40 ± 0.07 a | 11.32 ± 0.11 a |
| 12 | 11.26 ± 0.10 b | 4.12 ± 0.07 d | 8.88 ± 0.28 c | 8.54 ± 0.09 c | 11.70 ± 0.05 a | 11.80 ± 0.12 a |
| The volume of Siemens (Ejaculate) (mL) | ||||||
| 4 | 0.640 ± 0.024 a | 0.460 ± 024 c | 0.600 ± 0.031 ab | 0.520 ± 0.020 bc | 0.620 ± 0.037 a | 0.600 ± 0.031 ab |
| 8 | 0.670 ± 0.023 a | 0.54 ± 0.024 c | 0.62 ± 0.21 ab | 0.54 ± 0.23 c | 0.66 ± 0.024 a | 0.58 ± 0.020 bc |
| 12 | 0.70 ± 0.03 ab | 0.64 ± 0.024 ab | 0.66 ± 0.024 ab | 0.62 ± 0.02 b | 0.70 ± 0.031 ab | 0.72 ± 0.02 a |
| pH of Siemens | ||||||
| 4 | 7.5 ± 0.031 b | 7.7 ± 0.037 a | 7.7 ± 0.031 a | 7.5 ± 0.02 a | 7.6 ± 0.020 b | 7.5 ± 0.02 b |
| 8 | 7.5 ± 0.20 b | 7.7 ± 024 a | 7.7 ± 0.02 a | 7.7 ± 0.02 a | 7.6 ± 0.031 b | 7.5 ± 0.024 b |
| 12 | 7.5 ± 0.02 b | 7.7 ± 0.037 a | 7.6 ± 0.037 a | 7.6 ± 0.024 a | 7.5 ± 0.024 b | 7.5 ± 0.024 b |
| Sperm concentration (106/mL) | ||||||
| 4 | 168 ± 1.77 b | 123 ± 1.7 d | 135 ± 1.5 c | 133 ± 1.6 c | 168 ± 1.6 b | 189 ± 1.7 a |
| 8 | 186 ± 2.3 a | 111 ± 3.1 d | 134 ± 1.8 c | 148 ± 2.4 b | 185 ± 3.4 a | 192 ± 2.6 a |
| 12 | 190 ± 2.5 a | 100 ± 3.6 c | 141 ± 2.5 b | 146 ± 2.9 b | 193 ± 2.9 a | 198 ± 3.8 a |
| Motility | ||||||
| 4 | 70 ± 1.5 b | 38 ± 1.7 d | 62 ± 1.3 c | 59 ± 1.1 c | 75 ± 1.3 a | 72 ± 1.5 ab |
| 8 | 74 ± 1.7 a | 24 ± 2.04 c | 56 ± 1.3 b | 55 ± 1.7 b | 76 ± 1.5 a | 76 ± 1.6 a |
| 12 | 77 ± 1.7 a | 20 ± 1.5 c | 52 ± 1.4 b | 50 ± 1.9 b | 79 ± 1.6 a | 82 ± 1.3 a |
| Vitality% | ||||||
| 4 | 85.58 ± 1.47 a | 72.44 ± 1.11 b | 84.44 ± 1.14 a | 72.74 ± 1.26 b | 83.92 ± 1.57 a | 87.20 ± 1.77 a |
| 8 | 80.76 ± 1.44 a | 44.74 ± 2.09 d | 74.06 ± 1.7 b | 66.42 ± 1.65 c | 78.10 ± 1.83 ab | 81.62 ± 1.99 a |
| 12 | 78.32 ± 1.94 ab | 26.44 ± 2.95 d | 75.20 ± 1.16 b | 61.68 ± 1.90 c | 76.10 ± 1.56 ab | 81.82 ± 1.81 a |
| Enzymes | Treatments | |||||
|---|---|---|---|---|---|---|
| Control | Cadmium | Cadmium + Gallic Acid | Cadmium + Selenium | Gallic Acid | Selenium | |
| In Testes Tissues | ||||||
| 17 β-hydroxysteroid dehydrogenase (Unit/mg protein/min) | 2.59 ± 0.109 a | 0.76 ± 0.04 b | 1.8 ± 0.048 d | 2.1 ± 0.0244 e | 2.6 ± 0.048 a | 3.8 ± 0.037 c |
| Superoxide dismutase activity (U/mg protein) | 143.3 ± 6.09 b | 68.5 ± 6.28 d | 112.2 ± 1.97 c | 129.7 ± 4.60 b | 167.2 ± 3.65 a | 176.5 ± 1.97 a |
| Glutathione S-transferase (GST) (U/mg protein) | 2.88 ± 0.073 c | 1.66 ± 0.024 e | 2.56 ± 0.107 d | 2.88 ± 0.058 c | 4.60 ± 0.100 a | 3.98 ± 0.058 b |
| Glutathione reductase (µmol/g tissue) | 20.96 ± 1.88 c | 12.42 ± 0.73 e | 15.22 ± 1.18 de | 18.46 ± 1.50 cd | 26.78 ± 1.17 b | 35.54 ± 1.03 a |
| Thiobarbituric acid reactive substances (µmol/g tissue) | 2.60 ± 0.09 c | 3.97 ± 0.03 a | 2.74 ± 0.01 c | 2.57 ± 0.01 c | 1.48 ± 0.10 d | 0.75 ± 0.03 e |
| Glutathione peroxidase (GPx) (U/mg protein) | 70.70 ± 1.58 c | 41.12 ± 1.14 f | 65.44 ± 1.43 d | 71.56 ± 1.65 e | 93.22 ± 1.68 a | 84.80 ± 1.22 b |
| Glutathione level (µmol GSH/g tissue) | 5.98 ± 0.058 c | 3.82 ± 0.020 f | 5.91 ± 0.03 c | 5.90 ± 0.1 c | 7.90 ± 0.01 a | 6.94 ± 0.024 b |
| Catalase activity (H2O2/mg protein/min) | 64.6 ± 1.52 c | 29.7 ± 1.31 e | 41.8 ± 1.88 d | 47.6 ± 2.08 d | 67.2 ± 1.06 c | 94.4 ± 2.05 a |
| In Serum | ||||||
| Urea (mg/dL) | 27.60 ± 1.72 d | 84 ± 3.19 a | 66.20 ± 2.55 b | 51 ± 1.14 c | 30.6 ± 2.31 d | 33.80 ± 1.85 d |
| Creatinine (mg/dL) | 0.86 ± 0.05 d | 1.8 ± 0.10 a | 1.16 ± 0.04 b | 0.94 ± 0.05 cd | 1.10 ± 0.03 bc | 1.06 ± 0.02 bc |
| Alanine Transaminase [ALT] (unit/L) | 78.40 ± 4.40 c | 147.80 ± 5.5 a | 103.20 ± 6.31 b | 82 ± 4.77 c | 70.20 ± 4.74 c | 72 ± 9.92 c |
| Aspartate Transaminase [AST] (unit/L) | 56.80 ± 3.67 d | 222.80 ± 4.4 a | 85.80 ± 4.52 c | 114 ± 4.51 b | 36 ± 3.24 e | 37 ± 3.0 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheweita, S.A.; Al-Qahtani, S.M.; Ahmed, R.M.; Sheweita, M.S.; Atta, A. Molecular Mechanisms Contributing to the Impairment of Steroid Hormones, Sperm Characteristics, and Testicular Architecture in Male Rabbits After Chronic Exposure to Cadmium: Role of Gallic Acid and Selenium as Antioxidants. Toxics 2025, 13, 323. https://doi.org/10.3390/toxics13040323
Sheweita SA, Al-Qahtani SM, Ahmed RM, Sheweita MS, Atta A. Molecular Mechanisms Contributing to the Impairment of Steroid Hormones, Sperm Characteristics, and Testicular Architecture in Male Rabbits After Chronic Exposure to Cadmium: Role of Gallic Acid and Selenium as Antioxidants. Toxics. 2025; 13(4):323. https://doi.org/10.3390/toxics13040323
Chicago/Turabian StyleSheweita, Salah A., Saleh M. Al-Qahtani, Rofida M. Ahmed, Mohamed S. Sheweita, and Ahmed Atta. 2025. "Molecular Mechanisms Contributing to the Impairment of Steroid Hormones, Sperm Characteristics, and Testicular Architecture in Male Rabbits After Chronic Exposure to Cadmium: Role of Gallic Acid and Selenium as Antioxidants" Toxics 13, no. 4: 323. https://doi.org/10.3390/toxics13040323
APA StyleSheweita, S. A., Al-Qahtani, S. M., Ahmed, R. M., Sheweita, M. S., & Atta, A. (2025). Molecular Mechanisms Contributing to the Impairment of Steroid Hormones, Sperm Characteristics, and Testicular Architecture in Male Rabbits After Chronic Exposure to Cadmium: Role of Gallic Acid and Selenium as Antioxidants. Toxics, 13(4), 323. https://doi.org/10.3390/toxics13040323






