Eco-Friendly Synthesis of Zirconia Nanoparticles Using Sonchus asper Extract: A Sustainable Approach to Enhancing Chinese Cabbage Growth and Remediating Chromium-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Plant-Fabricated Zirconia Nanoparticles (PF-ZrO2 NPs)
2.3. Characterization of PF-ZrO2 Nanoparticles
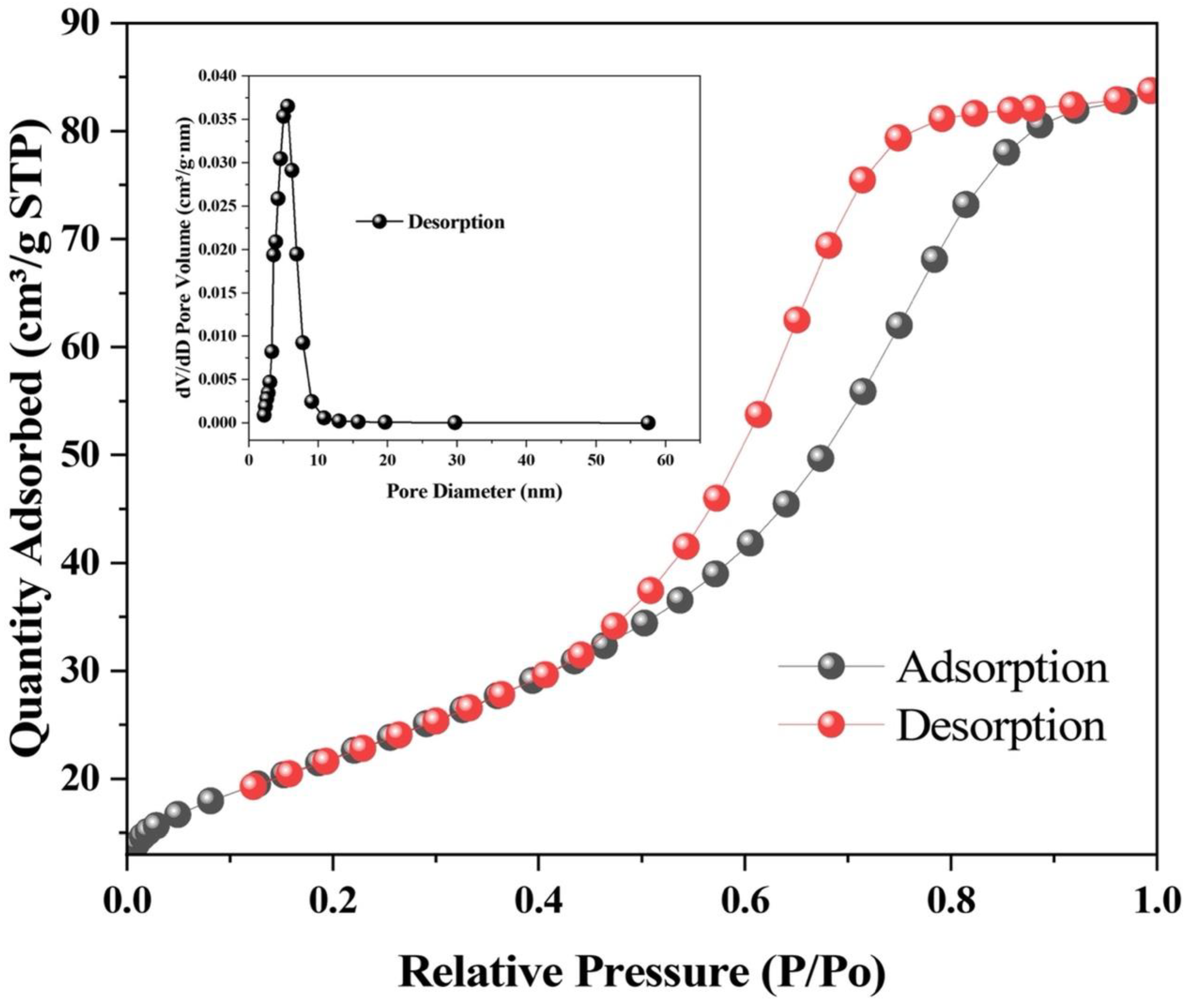
2.4. Pore Size Distribution and Pore Volume Analysis
2.5. Plant Growth Experiment
2.5.1. Experimental Design and Setup
2.5.2. Soil Collection and Preparation
2.5.3. Seed Sowing and Nanoparticle Application
2.6. Sample Collections
2.7. Methods of Analysis
2.8. Soil Physicochemical Properties After the Experiment
2.9. Data Analysis
3. Results and Discussion
3.1. Morphological and Structural Characterization of PF-ZrO2 Nanoparticles
3.2. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.3. X-Ray Diffraction (XRD) Analysis
3.4. Energy-Dispersive X-Ray Spectroscopy (EDS) and Elemental Mapping Analysis
3.5. Surface Area and Pore Structure Analysis of PF-ZrO2 Nanoparticles
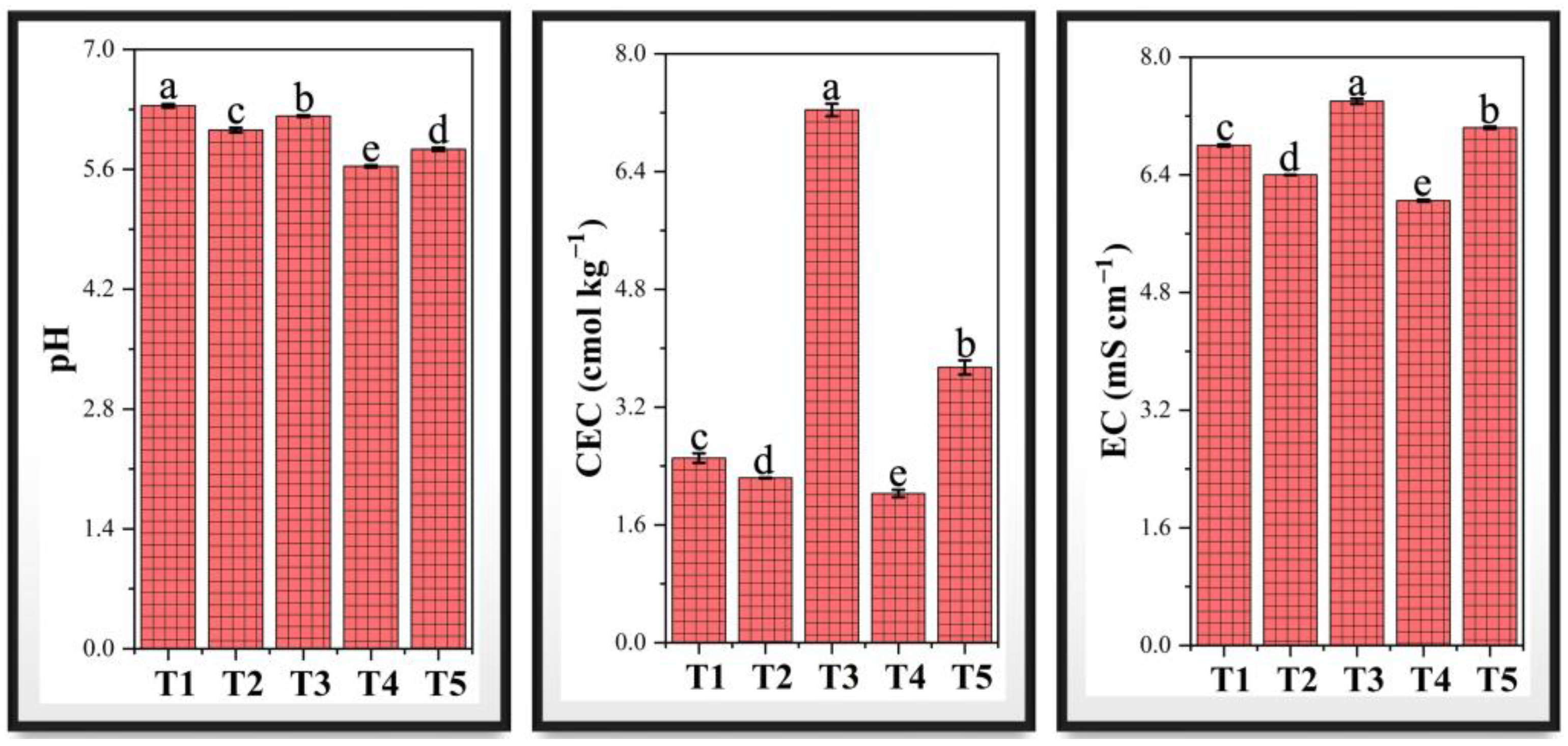
3.6. Soil Physicochemical Properties Under Chromium Stress and PF-ZrO2 NP Application
3.7. Macro and Micronutrient Availability in Soil Under Chromium Stress and PF-ZrO2 NP Application
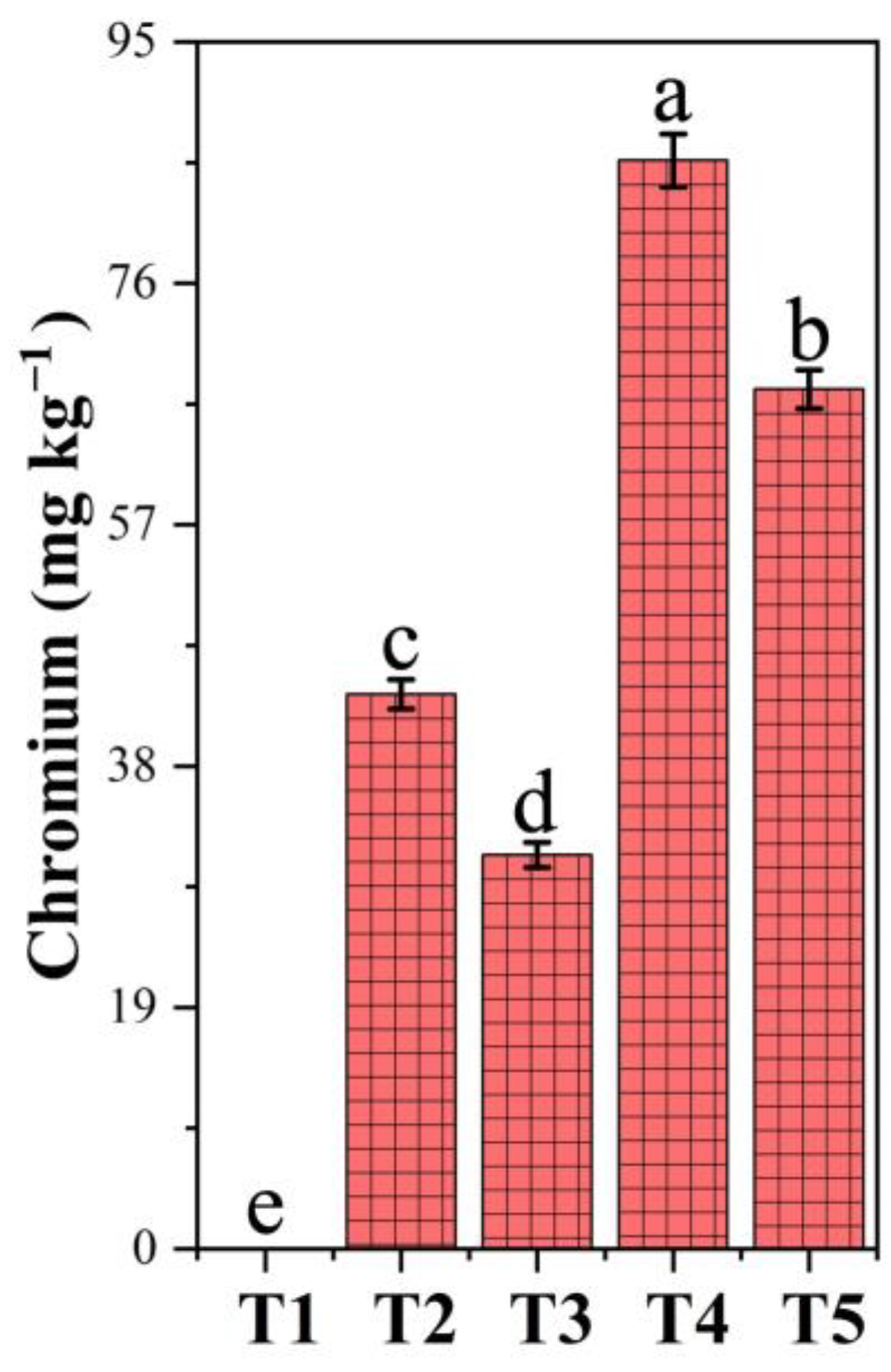
3.8. Chromium Accumulation in Soil Under PF-ZrO2 NP Application
3.9. Photosynthetic Pigments Under Chromium Stress and PF-ZrO2 NP Application
3.10. Biochemical Responses of Chinese Cabbage Under Chromium Stress and PF-ZrO2 NP Application
3.11. Antioxidant Enzyme Activities Under Chromium Stress and PF-ZrO2 NP Application
3.12. Plant Growth Parameters Under Chromium Stress and PF-ZrO2 NP Application
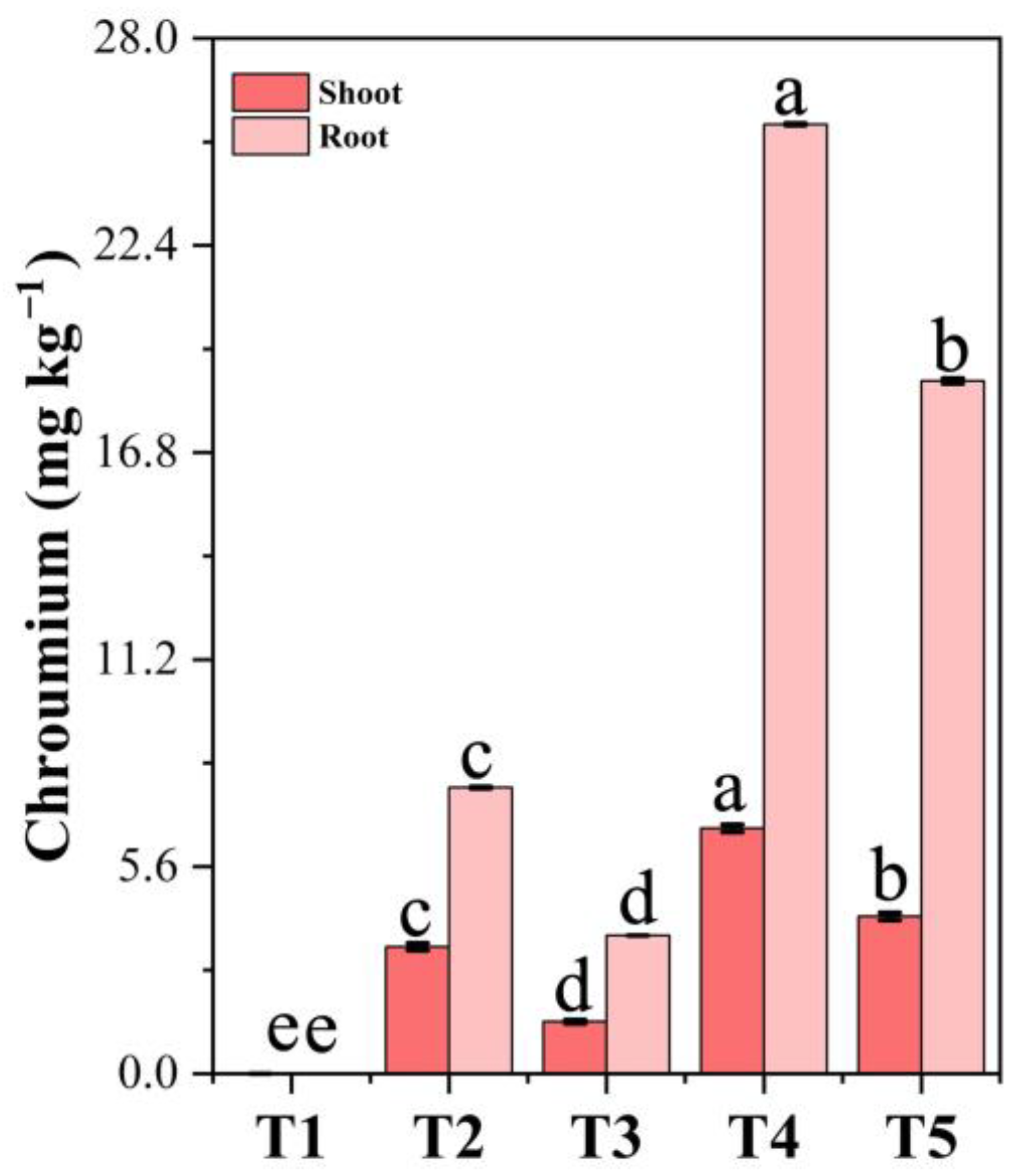
3.13. Chromium Accumulation in Plant Tissues Under PF-ZrO2 NP Application
3.14. Macro and Micronutrient Uptake Under Chromium Stress and PF-ZrO2 NP Application
3.15. Oxidative Stress Indicators Under Chromium Stress and PF-ZrO2 NP Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, S.; Liu, Q.; Wang, S.; Jan, A.U.; Sharif, H.M.A.; Ditta, A.; Wang, G.; Cheng, H. Sources, impacts, factors affecting Cr Uptake in plants, and mechanisms behind phytoremediation of Cr-contaminated soils. Sci. Total Environ. 2023, 899, 165726. [Google Scholar] [CrossRef] [PubMed]
- Pushkar, B.; Sevak, P.; Parab, S.; Nilkanth, N. Chromium pollution and its bioremediation mechanisms in bacteria: A review. J. Environ. Manag. 2021, 287, 112279. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium toxicity in plants: Signaling, mitigation, and future perspectives. Plants 2023, 12, 1502. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Sharma, N.; Sodhi, K.K.; Kumar, M.; Singh, D.K. Heavy metal pollution: Insights into chromium eco-toxicity and recent advancement in its remediation. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100388. [Google Scholar] [CrossRef]
- Park, J.H. Contrasting Effects of Cr(III) and Cr(VI) on Lettuce Grown in Hydroponics and Soil: Chromium and Manganese Speciation. Environ. Pollut. 2020, 266, 115073. [Google Scholar] [CrossRef]
- Nikolaou, K.E.; Chatzistathis, T.; Theocharis, S.; Argiriou, A.; Koundouras, S.; Zioziou, E. Effects of chromium toxicity on physiological performance and nutrient uptake in two grapevine cultivars (Vitis Vinifera L.) growing on own roots or grafted onto different rootstocks. Horticulturae 2022, 8, 493. [Google Scholar] [CrossRef]
- Wakeel, A.; Ali, I.; Wu, M.; Raza Kkan, A.; Jan, M.; Ali, A.; Liu, Y.; Ge, S.; Wu, J.; Liu, B.; et al. Ethylene mediates dichromate-induced oxidative stress and regulation of the enzymatic antioxidant system-related transcriptome in Arabidopsis thaliana. Environ. Exp. Bot. 2019, 161, 166–179. [Google Scholar] [CrossRef]
- Ali, H.H.; Ilyas, M.; Zaheer, M.S.; Hameed, A.; Ikram, K.; Khan, W.; Ud, D.; Iqbal, R.; Awan, T.H.; Rizwan, M.; et al. Alleviation of chromium toxicity in mung bean (Vigna radiata L.) using salicylic acid and azospirillum brasilense. BMC Plant Biol. 2023, 23, 1–10. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed]
- Baby, R.; Hussein, M.Z.; Abdullah, A.H.; Zainal, Z. Nanomaterials for the treatment of heavy metal contaminated water. Polymers 2022, 14, 583. [Google Scholar] [CrossRef]
- Pan, S.; Shen, J.; Deng, Z.; Zhang, X.; Pan, B. Metastable nano-zirconium phosphate inside gel-type ion exchanger for enhanced removal of heavy metals. J. Hazard. Mater. 2022, 423, 127158. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Azeem, M.; Li, R.; Xing, L.; Li, Y.; Zhang, Y.; Guo, Z.; Wang, Q.; Ngo, H.H.; Qu, G.; et al. Zirconium hydroxide nanoparticle encapsulated magnetic biochar composite derived from rice residue: Application for As(III) and As(V) polluted water purification. J. Hazard. Mater. 2022, 423, 127081. [Google Scholar] [CrossRef]
- Pirilä, M. Adsorption and Photocatalysis in Water Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2015; ISBN 9789526207612. [Google Scholar]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the restoration of polluted soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, G.; Sharma, M.; Mandzhieva, S.; Minkina, T.; Rajput, V.D. Sustainable use of nano-assisted remediation for mitigation of heavy metals and mine spills. Water 2022, 14, 3972. [Google Scholar] [CrossRef]
- Hsueh, T.J.; Ding, R.Y. A room temperature ZnO-NPs/MEMS ammonia gas sensor. Nanomaterials 2022, 12, 3287. [Google Scholar] [CrossRef]
- Mahesh, S.; Narasaiah, B.P.; Himabindu, B.; Balaji, G.L.; Pradeepkiran, J.A.; Padhy, H. Sunflower-assisted bio-derived ZnO-NPs as an efficient nanocatalyst for the synthesis of novel quinazolines with highly antioxidant activities. Antioxidants 2022, 11, 688. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.U.; Shah, A.; Haleem, A.; Shah, S.M.; Shah, I. Eucalyptus Globulus mediated green synthesis of environmentally benign metal based nanostructures: A review. Nanomaterials 2023, 13, 2019. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Sareen, S.; Verma, M.; Sharma, S.; Sharma, A.; Sohal, H.S.; Mehta, S.K.; Park, J.; Mutreja, V. Zirconia-based nanomaterials: Recent developments in synthesis and applications. Nanoscale Adv. 2022, 4, 4210–4236. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Ahmed, M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus Asper (L.) Hill. Chem. Cent. J. 2012, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.; Khan, M.R.; Sahreen, S. Brain antioxidant markers, cognitive performance and acetylcholinesterase activity of rats: Efficiency of sonchus asper. Behav. Brain Funct. 2012, 8, 1–7. [Google Scholar] [CrossRef]
- Fu, X.; Mehmood, S.; Ahmed, W.; Ou, W.; Suo, P.; Zhang, Q.; Fu, X.; Sun, Z.; Li, W. Reducing chromium toxicity in chinese cabbage through synergistic effects of silicon and selenium: A study of plant growth, chromium content, and biochemical parameters. Sustainability 2023, 15, 5361. [Google Scholar] [CrossRef]
- Zou, C.; Lu, T.; Wang, R.; Xu, P.; Jing, Y.; Wang, R.; Xu, J.; Wan, J. Comparative physiological and metabolomic analyses reveal that Fe3O4 and ZnO nanoparticles alleviate Cd toxicity in tobacco. J. Nanobiotech. 2022, 20, 1–22. [Google Scholar] [CrossRef]
- Kazemi, F.; Arianpour, F.; Taheri, M.; Saberi, A.; Rezaie, H.R. Effects of chelating agents on the sol-gel synthesis of nano-zirconia: Comparison of the pechini and sugar-based methods. Int. J. Miner. Metall. Mater. 2020, 27, 693–702. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, H.; Wei, L.; Peng, H.; Ma, D.; Leng, Y. Influence of B4C particle size on the microstructure and mechanical properties of B4C/Al composites fabricated by pressureless infiltration. Metals 2023, 13, 1358. [Google Scholar] [CrossRef]
- Moja, M.M.; Mapossa, A.B.; Chirwa, E.M.N.; Tichapondwa, S. Photocatalytic degradation of 2,4-dichlorophenol using nanomaterials silver halide catalysts. Environ. Sci. Pollut. Res. 2024, 31, 11857–11872. [Google Scholar] [CrossRef]
- Antoniadis, V.; Zanni, A.A.; Levizou, E.; Shaheen, S.M.; Dimirkou, A.; Bolan, N.; Rinklebe, J. Modulation of hexavalent chromium toxicity on οriganum vulgare in an acidic soil amended with peat, lime, and zeolite. Chemosphere 2018, 195, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jing, Z.Q.; Cheng, L.L.; Shen, W.; Kong, Y. Adsorption of Cr (VI) by attapulgite-zeolite composite ceramisite from aqueous solution. Res. J. Chem. Environ. 2009, 13, 825–834. [Google Scholar]
- Nanda, A.; Mohapatra, D.B.B.; Mahapatra, A.P.K.; Mahapatra, A.P.K.; Mahapatra, A.P.K. Multiple comparison test by Tukey’s honestly significant difference (HSD): Do the confident level control Type I Error. Int. J. Stat. Appl. Math. 2021, 6, 59–65. [Google Scholar] [CrossRef]
- Shaafi, F.B.; Motavalizadehkakhky, A.; Zhiani, R.; Nouri, S.M.M.; Hosseiny, M. Sulfated zirconium oxide-decorated magnetite KCC-1 as a durable and recyclable adsorbent for the efficient removal of asphaltene from crude oil. RSC Adv. 2021, 11, 26174–26187. [Google Scholar] [CrossRef]
- Shi, W.; Li, Z.; Gong, Z.; Liang, Z.; Liu, H.; Han, Y.C.; Niu, H.; Song, B.; Chi, X.; Zhou, J.; et al. Transient and general synthesis of high-density and ultrasmall nanoparticles on two-dimensional porous carbon via coordinated carbothermal shock. Nat. Commun. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Pérez-Alvarez, M.; Cadenas-Pliego, G.; Pérez-Camacho, O.; Comparán-Padilla, V.E.; Cabello-Alvarado, C.J.; Saucedo-Salazar, E. Green synthesis of copper nanoparticles using cotton. Polymers 2021, 13, 1906. [Google Scholar] [CrossRef]
- Senthamarai Kannan, M.; Hari Haran, P.S.; Sundar, K.; Kunjiappan, S.; Balakrishnan, V. Fabrication of anti-bacterial cotton bandage using biologically synthesized nanoparticles for medical applications. Prog. Biomater. 2022, 11, 229–241. [Google Scholar] [CrossRef]
- Siragam, S.; Dubey, R.S.; Pappula, L.; Satheesh Babu, G. Synthesis and Investigation of dielectric ceramic nanoparticles for microstrip patch antenna applications. Sci. Rep. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Hasan, I.M.A.; Salah El-Din, H.; AbdElRaady, A.A. Peppermint-mediated green synthesis of nano ZrO2 and its adsorptive removal of cobalt from water. Inorganics 2022, 10, 257. [Google Scholar] [CrossRef]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green synthesis of nanoparticles and their energy storage, environmental, and biomedical applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Qasim, M.; Clarkson, A.N.; Hinkley, S.F.R. Green synthesis of carbon nanoparticles (CNPs) from biomass for biomedical applications. Int. J. Mol. Sci. 2023, 24, 1023. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Zhang, Y.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Rani, N.U.; Pavani, P.; Rao, P.T.S.R.K.P. Facile green synthesis and characterization of titanium dioxide nanoparticles using Kigelia Africana (Lam) benth., aqueous leaf extract and its antioxidant and antibacterial activity. Asian J. Chem. 2022, 34, 409–414. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- White, R.L. A temperature perturbation infrared spectroscopy comparison of HY and NaY zeolite dehydration/rehydration. Minerals 2024, 14, 104. [Google Scholar] [CrossRef]
- Matias, M.L.; Carlos, E.; Branquinho, R.; do Valle, H.; Marcelino, J.; Morais, M.; Pimentel, A.; Rodrigues, J.; Monteiro, T.; Fortunato, E.; et al. A comparison between solution-based synthesis methods of ZrO2 nanomaterials for energy storage applications. Energies 2022, 15, 6452. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef]
- Vera, J.; Herrera, W.; Hermosilla, E.; Díaz, M.; Parada, J.; Seabra, A.B.; Tortella, G.; Pesenti, H.; Ciudad, G.; Rubilar, O. Antioxidant activity as an indicator of the efficiency of plant extract-mediated synthesis of zinc oxide nanoparticles. Antioxidants 2023, 12, 784. [Google Scholar] [CrossRef]
- Sivaperumal, V.R.; Mani, R.; Polisetti, V.; Aruchamy, K.; Oh, T. Synthesis of hydroxyapatite (HAp)-zirconia nanocomposite powder and evaluation of its biocompatibility: An in vitro study. Appl. Sci. 2022, 12, 11056. [Google Scholar] [CrossRef]
- Safdar, A.; Mohamed, H.E.A.; Hkiri, K.; Muhaymin, A.; Maaza, M. Green synthesis of cobalt oxide nanoparticles using hyphaene thebaica fruit extract and their photocatalytic application. Appl. Sci. 2023, 13, 9082. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Fatimah, I. One-pot hydrothermal synthesis and characterization of zirconium oxide nanoparticles. Sci. Technol. Indones. 2023, 8, 585–593. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, K.; Kaushal, S.; Badru, R. Selective N-alkylation of amines with DMC over biogenic Cu-Zr bimetallic nanoparticles. ACS Omega 2021, 6, 15300–15307. [Google Scholar] [CrossRef] [PubMed]
- Aneggi, E.; Campagnolo, F.; Segato, J.; Zuccaccia, D.; Baratta, W.; Llorca, J.; Trovarelli, A. Solvent-free selective oxidation of benzyl alcohol using ru loaded ceria-zirconia catalysts. Mol. Catal. 2023, 540, 113049. [Google Scholar] [CrossRef]
- Mosbacher, M.; Holzinger, M.; Galetz, M.; Glatzel, U. The influence of oxide color on the surface characteristics of zirconium alloy ZrNb7 (Wt%) after different heat treatments. Oxid. Met. 2021, 95, 377–388. [Google Scholar] [CrossRef]
- Garg, R.; Rani, P.; Garg, R.; Eddy, N.O. Study on potential applications and toxicity analysis of green synthesized nanoparticles. Turkish J. Chem. 2021, 45, 1690–1706. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, S.C.; An, T.; Lee, D.; Hwang, H.; Choi, S.Q.; Park, J. Synthesis of aluminum-based metal–organic framework (MOF)-derived carbon nanomaterials and their water adsorption isotherm. Nanomaterials 2023, 13, 2351. [Google Scholar] [CrossRef]
- Morishige, K. Revisiting the nature of adsorption and desorption branches: Temperature dependence of adsorption hysteresis in ordered mesoporous silica. ACS Omega 2021, 6, 15964–15974. [Google Scholar] [CrossRef]
- Naghdi, S.; Cherevan, A.; Giesriegl, A.; Guillet-Nicolas, R.; Biswas, S.; Gupta, T.; Wang, J.; Haunold, T.; Bayer, B.C.; Rupprechter, G.; et al. Selective ligand removal to improve accessibility of active sites in hierarchical MOFs for heterogeneous photocatalysis. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Katsayal, B.S.; Sallau, A.B.; Muhammad, A. Kinetics and thermodynamics of Cr (VI) reduction by Tamarindus Indica methanol leaves extract under optimized reaction conditions. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Pinto, A.P.; Teixeira, D.M.; Barrulas, P.; Brito, I.; Carvalho, M. Subcellular element distribution in shoots of wheat grown in an acidic soil with native AMF extraradical mycelium. Agronomy 2022, 12, 2173. [Google Scholar] [CrossRef]
- Alharby, H.F.; Ali, S. Combined role of Fe nanoparticles (Fe NPs) and Staphylococcus Aureus L. in the alleviation of chromium stress in rice plants. Life 2022, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, F.; Salehi, A.; Dehnavi, M.M.; Mirshekari, A.; Hamidian, M.; Hazrati, S. Correction to: Biochemical response and nutrient uptake of two Arbuscular Mycorrhiza-inoculated chamomile varieties under different osmotic stresses. Bot. Stud. 2021, 62, 22. [Google Scholar] [CrossRef] [PubMed]
- Sári, D.; Ferroudj, A.; Abdalla, N.; El-Ramady, H.; Dobránszki, J.; Prokisch, J. Nano-management approaches for Salt tolerance in plants under field and in vitro conditions. Agronomy 2023, 13, 2695. [Google Scholar] [CrossRef]
- Rosa, M.I.G.; Boga, G.A.; Cruz, S.S.V.; de Andrade, F.R.D.; Furquim, S.A.C.; Shinzato, M.C. Mechanisms of chromium(VI) removal from solution by zeolite and vermiculite modified with iron(II). Environ. Sci. Pollut. Res. 2022, 29, 49724–49738. [Google Scholar] [CrossRef]
- Mahmoud, E.; El-shahawy, A.; Ibrahim, M.; Abd El-Halim, A.E.H.A.; Abo-Ogiala, A.; Shokr, M.S.; Mohamed, E.S.; Rebouh, N.Y.; Ismail, S.M. Enhancing maize yield and soil health through the residual impact of nanomaterials in contaminated soils to sustain food. Nanomaterials 2024, 14, 369. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Hang, H.; Chen, S.; Liu, H.; Su, J.; Lv, H.; Jia, H.; Zhao, G. Effects of balancing exchangeable cations Ca, Mg, and K on the growth of tomato seedlings (Solanum Lycopersicum L.) based on increased soil cation exchange capacity. Agronomy 2024, 14, 629. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, Y.; Jin, L.; Ren, X.; He, C.; Liu, Q. Responses of mineral nutrient contents and transport in red clover under aluminum stress. Legum. Sci. 2021, 3, e94. [Google Scholar] [CrossRef]
- Karunakaran, A.; Fathima, Y.; Singh, P.; Beniwal, R.; Singh, J.; Ramakrishna, W. Next-generation biofertilizers: Nanoparticle-coated plant growth-promoting bacteria biofertilizers for enhancing nutrient uptake and wheat growth. Agriculture 2024, 14, 517. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, Z.M.; Holatko, J.; Hammerschmiedt, T.; Głuchowski, A.; Mizerski, A.; Jaskulska, I.; Baltazar, T.; Kintl, A.; Jaskulski, D.; et al. Nano zero valent iron (NZVI) as an amendment for phytostabilization of highly multi-PTE contaminated soil. Materials 2021, 14, 2559. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, D.; Wang, L. Synthesis of CoFe2O4/graphene oxide-grafted tetraethylenepentamine for removal of Cr (VI) from aqueous solution. Adv. Condens. Matter Phys. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, M.; Fahad, S.; Qayyum, A.; Chen, Y.; Zhu, G. Chromium induces toxicity at different phenotypic, physiological, biochemical, and ultrastructural levels in sweet potato (Ipomoea Batatas L.) plants. Int. J. Mol. Sci. 2022, 23, 13496. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Babu, S.; Krishnan, P.; Kaur, B.; Bana, R.S.; Chakraborty, D.; Kumar, V.; Joshi, B.; Lal, S.K. Zinc oxide and ferric oxide nanoparticles combination increase plant growth, yield, and quality of soybean under semiarid region. Chemosphere 2024, 352, 141432. [Google Scholar] [CrossRef]
- Singh, A.; Sengar, R.S.; Rajput, V.D.; Minkina, T.; Singh, R.K. Zinc oxide nanoparticles improve salt tolerance in rice seedlings by improving physiological and biochemical indices. Agriculture 2022, 12, 1014. [Google Scholar] [CrossRef]
- Francia, V.; Reker-Smit, C.; Salvati, A. Mechanisms of uptake and membrane curvature generation for the internalization of silica nanoparticles by cells. Nano Lett. 2022, 22, 3118–3124. [Google Scholar] [CrossRef] [PubMed]
- Gautam, V.; Kohli, S.K.; Kapoor, D.; Bakshi, P.; Sharma, P.; Arora, S.; Bhardwaj, R.; Ahmad, P. Stress protective effect of Rhododendron arboreum leaves (MEL) on chromium-treated Vigna radiata plants. J. Plant Growth Regul. 2021, 40, 423–435. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Arabloo, M. Iron nanoparticle regulate succinate dehydrogenase activity in canola plants under drought stress. Sci. Rep. 2023, 13, 1–14. [Google Scholar] [CrossRef]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-wide analysis and expression profile of superoxide dismutase (sod) gene family in rapeseed (Brassica Napus l.) under different hormones and abiotic stress conditions. Antioxidants 2021, 10, 1182. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, J.; Wang, X.; Zhang, N.; Si, H. Potato Stu-MiR398b-3p negatively regulates Cu/Zn-SOD response to drought tolerance. Int. J. Mol. Sci. 2023, 24, 2525. [Google Scholar] [CrossRef]
- Skliri, E.; Vamvasakis, I.; Papadas, I.T.; Choulis, S.A.; Armatas, G.S. Mesoporous composite networks of linked MnFe2O4 and ZnFe2O4 nanoparticles as efficient photocatalysts for the reduction of Cr(VI). Catalysts 2021, 11, 199. [Google Scholar] [CrossRef]
- Sebastian, S.; Hoffmann, M.K.; Howard, D.; Young, C.; Washington, J.; Unterweger, H.; Alexiou, C.; Turnbull, T.; D’Andrea, R.; Hoffmann, P.; et al. Kinetic effects of transferrin-conjugated gold nanoparticles on the antioxidant glutathione-thioredoxin pathway. Antioxidants 2023, 12, 1617. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alyemeni, M.N.; Moustakas, M.; Ahmad, P. 5-Aminolevulinic acid induces chromium [Cr(VI)] tolerance in tomatoes by alleviating oxidative damage and protecting photosystem II: A mechanistic approach. Plants 2023, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gao, L.; Xu, L.; Zheng, Q.; Sun, S.; Liu, X.; Zhang, Z.; Tian, Z.; Dai, T.; Sun, J. Melatonin alleviates chromium toxicity by altering chromium subcellular distribution and enhancing antioxidant metabolism in wheat seedlings. Environ. Sci. Pollut. Res. 2023, 30, 50743–50758. [Google Scholar] [CrossRef] [PubMed]
- Răcuciu, M.; Tecucianu, A.; Oancea, S. Impact of magnetite nanoparticles coated with aspartic acid on the growth, antioxidant enzymes activity and chlorophyll content of maize. Antioxidants 2022, 11, 1193. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Q. Cross-Talk between oxidative stress and M6A RNA methylation in cancer. Oxid. Med. Cell. Longev. 2021, 2021, 6545728. [Google Scholar] [CrossRef]
- Pang, Q.Q.; Kim, J.H.; Kim, H.Y.; Kim, J.H.; Cho, E.J. Protective effects and mechanisms of Pectolinarin against H2O2-Induced oxidative stress in SH-SY5Y neuronal cells. Molecules 2023, 28, 5826. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Q.; Liu, W.; Zhang, J. 24-epibrassinolide confers zinc stress tolerance in watermelon seedlings through modulating antioxidative capacities and lignin accumulation. PeerJ. 2023, 11, e15330. [Google Scholar] [CrossRef]
- Sutulienė, R.; Brazaitytė, A.; Małek, S.; Jasik, M.; Samuolienė, G. Response of oxidative stress and antioxidant system in pea plants exposed to drought and boron nanoparticles. Antioxidants 2023, 12, 528. [Google Scholar] [CrossRef]
- Alam, P.; Azzam, M.A.; Balawi, T.A.; Raja, V.; Bhat, J.A.; Ahmad, P. Mitigation of negative effects of chromium (VI) toxicity in faba bean (Vicia Faba) plants through the supplementation of kinetin (KN) and gibberellic acid (GA3). Plants 2022, 11, 3302. [Google Scholar] [CrossRef]
- Saber, N.E.; Abdel-Rahman, M.M.; Mabrouk, M.E.M.; Eldebawy, E.M.M.; Ismail, G.S.M. Silicon alleviates cadmium toxicity in Triticum Aestivum L. Plants by modulating antioxidants, nutrient uptake, and gene expression. Egypt. J. Bot. 2022, 62, 319–336. [Google Scholar] [CrossRef]
- Basit, F.; Nazir, M.M.; Shahid, M.; Abbas, S.; Javed, M.T.; Naqqash, T.; Liu, Y.; Yajing, G. Application of zinc oxide nanoparticles immobilizes the chromium uptake in rice plants by regulating the physiological, biochemical and cellular attributes. Physiol. Mol. Biol. Plants 2022, 28, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hua, J.; Li, R.; Zhang, Y.; Jin, H.; Wang, S.; Chen, G. Application of magnetic nanocomposites in water treatment: Core–shell Fe3O4 material for efficient adsorption of Cr(VI). Water 2023, 15, 2827. [Google Scholar] [CrossRef]
- El-Ballat, E.M.; Elsilk, S.E.; Ali, H.M.; Ali, H.E.; Hano, C.; El-Esawi, M.A. Metal-resistant PGPR strain Azospirillum Brasilense EMCC1454 enhances growth and chromium stress tolerance of chickpea (Cicer Arietinum L.) by modulating redox potential, osmolytes, antioxidants, and stress-related gene expression. Plants 2023, 12, 2110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xin, X.; Cao, Y.; Su, D.; Ji, P.; Zhu, Z.; He, Z. Use of Carbon nanoparticles to improve soil fertility, crop growth and nutrient uptake by corn (Zea Mays L.). Nanomaterials 2021, 11, 2717. [Google Scholar] [CrossRef]
- Ur Rahim, H.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-enable materials promoting sustainability and resilience in modern agriculture. Nanomaterials 2021, 11, 2068. [Google Scholar] [CrossRef]
- Liu, K.; Hua, S.; Song, L. PM2.5 exposure and asthma development: The key role of oxidative stress. Oxid. Med. Cell. Longev. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Ceci, R.; Maldini, M.; Olson, M.E.; Crognale, D.; Horner, K.; Dimauro, I.; Sabatini, S.; Duranti, G. Moringa oleifera leaf extract protects C2C12 myotubes against H2O2-induced oxidative stress. Antioxidants 2022, 11, 1435. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Application of nanoparticles for enhanced UV-B stress tolerance in plants. Plant Nano Biol. 2022, 2, 100014. [Google Scholar] [CrossRef]
- Ou, Q.; Zhang, S.; Fu, C.; Yu, L.; Xin, P.; Gu, Z.; Cao, Z.; Wu, J.; Wang, Y. More natural more better: Triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J. Nanobiotech. 2021, 19, 1–12. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Z.; Zhang, M.; Li, S.; Sun, M.; Song, Z. Hollow manganese dioxide-chitosan hydrogel for the treatment of atopic dermatitis through inflammation-suppression and ros scavenging. J. Nanobiotech. 2023, 21, 1–12. [Google Scholar] [CrossRef]
- Nag, O.K.; Naciri, J.; Lee, K.; Oh, E.; Almeida, B.; Delehanty, J.B. Liquid crystal nanoparticle conjugates for scavenging reactive oxygen species in live cells. Pharmaceuticals 2022, 15, 604. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, G.; Li, W.; Qin, F.; Dong, M.; Yue, S.; Mehmood, S.; Wang, X. Eco-Friendly Synthesis of Zirconia Nanoparticles Using Sonchus asper Extract: A Sustainable Approach to Enhancing Chinese Cabbage Growth and Remediating Chromium-Contaminated Soil. Toxics 2025, 13, 324. https://doi.org/10.3390/toxics13050324
Weng G, Li W, Qin F, Dong M, Yue S, Mehmood S, Wang X. Eco-Friendly Synthesis of Zirconia Nanoparticles Using Sonchus asper Extract: A Sustainable Approach to Enhancing Chinese Cabbage Growth and Remediating Chromium-Contaminated Soil. Toxics. 2025; 13(5):324. https://doi.org/10.3390/toxics13050324
Chicago/Turabian StyleWeng, Guojie, Weidong Li, Fengyue Qin, Menglu Dong, Shuangqi Yue, Sajid Mehmood, and Xu Wang. 2025. "Eco-Friendly Synthesis of Zirconia Nanoparticles Using Sonchus asper Extract: A Sustainable Approach to Enhancing Chinese Cabbage Growth and Remediating Chromium-Contaminated Soil" Toxics 13, no. 5: 324. https://doi.org/10.3390/toxics13050324
APA StyleWeng, G., Li, W., Qin, F., Dong, M., Yue, S., Mehmood, S., & Wang, X. (2025). Eco-Friendly Synthesis of Zirconia Nanoparticles Using Sonchus asper Extract: A Sustainable Approach to Enhancing Chinese Cabbage Growth and Remediating Chromium-Contaminated Soil. Toxics, 13(5), 324. https://doi.org/10.3390/toxics13050324






