Association Between Volatile Organic Compounds and Circadian Syndrome Among Pre- and Postmenopausal Women
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Menopausal Status
2.3. Measurement of Urinary VOC Metabolites
2.4. Measurement of Circadian Syndrome
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics
3.2. Distribution and Correlation of Urinary VOC Metabolites
3.3. Associations Between Single Urinary VOC Metabolites and CircS Revealed by the Weighted Multiple Linear Regression Model
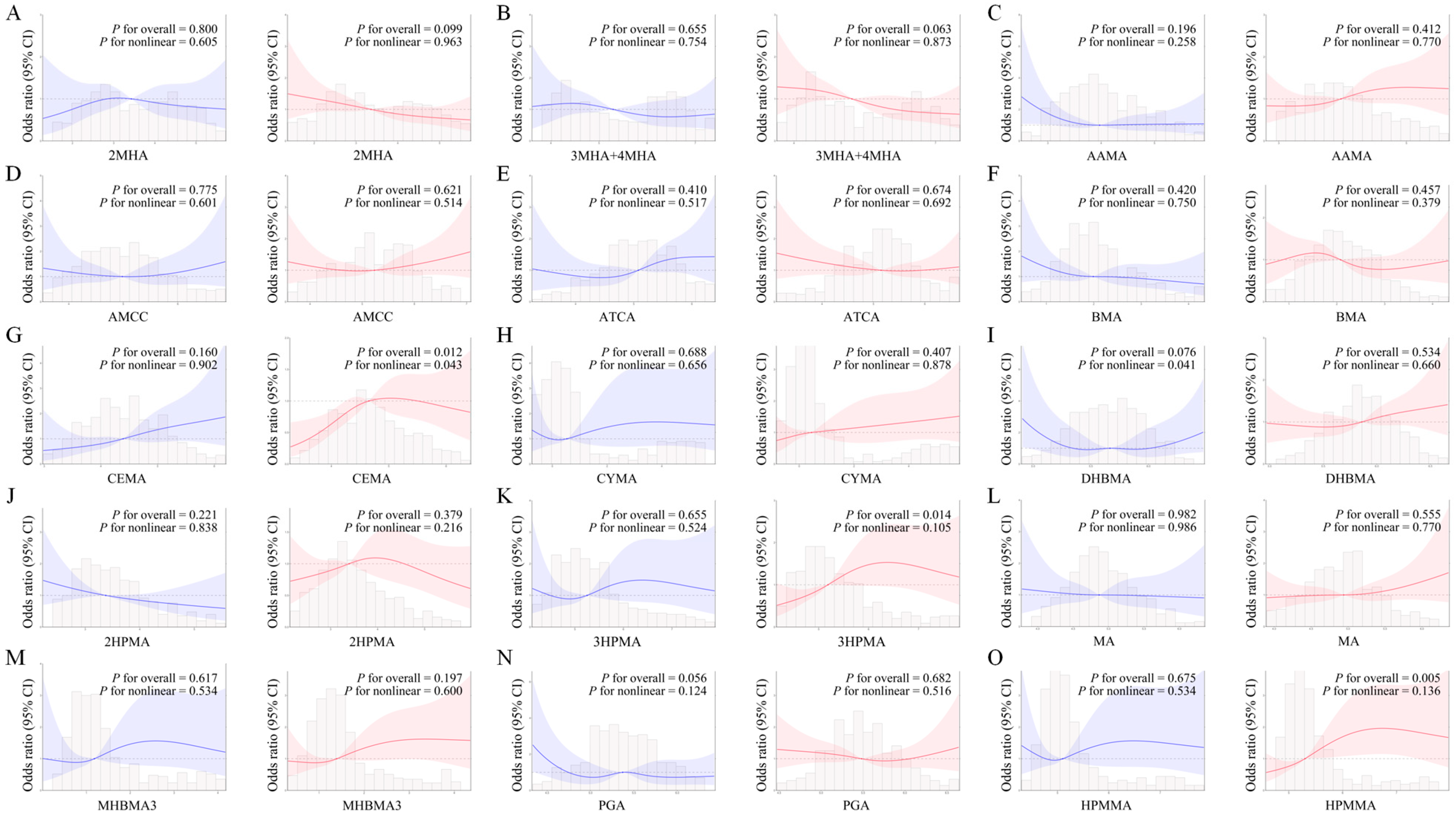
3.4. Dose–Response Relationship Between Single Urinary VOC Metabolites and CircS Assessed by the RCS
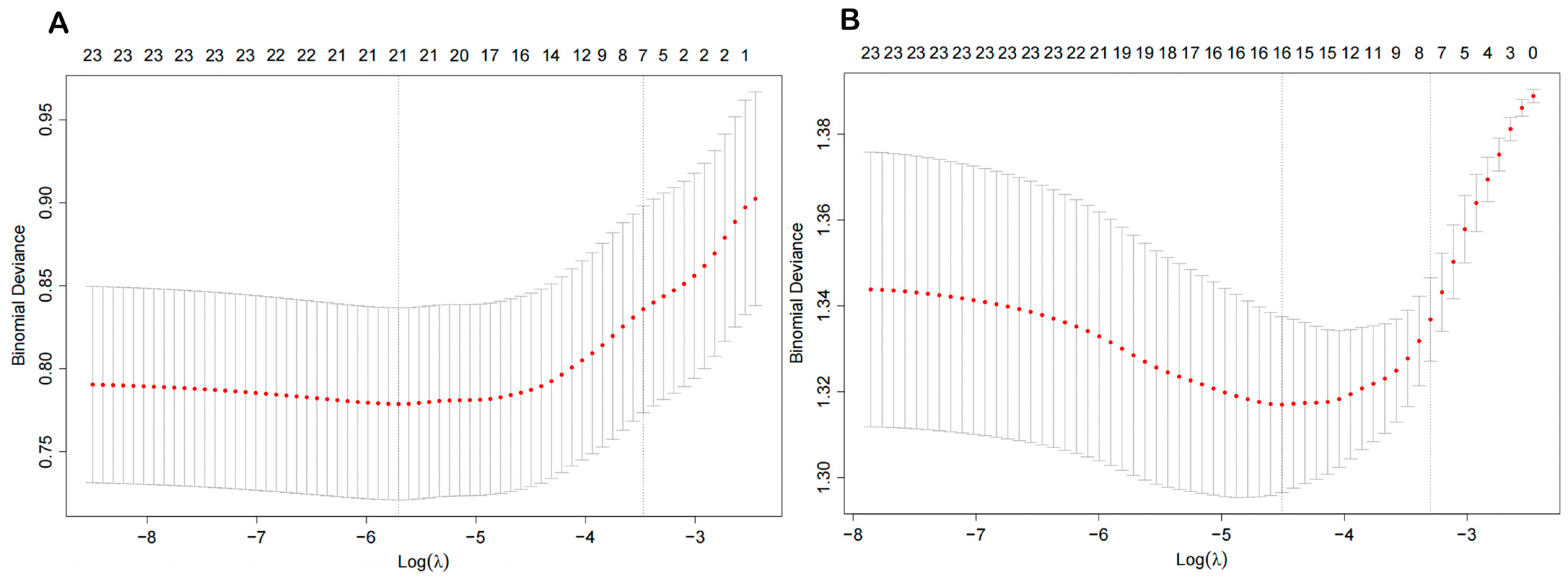
3.5. The Key VOC Metabolites Screened by the LASSO Regression Model
3.6. Associations of Key Urinary VOC Metabolites with CircS Evaluated by the WQS Model
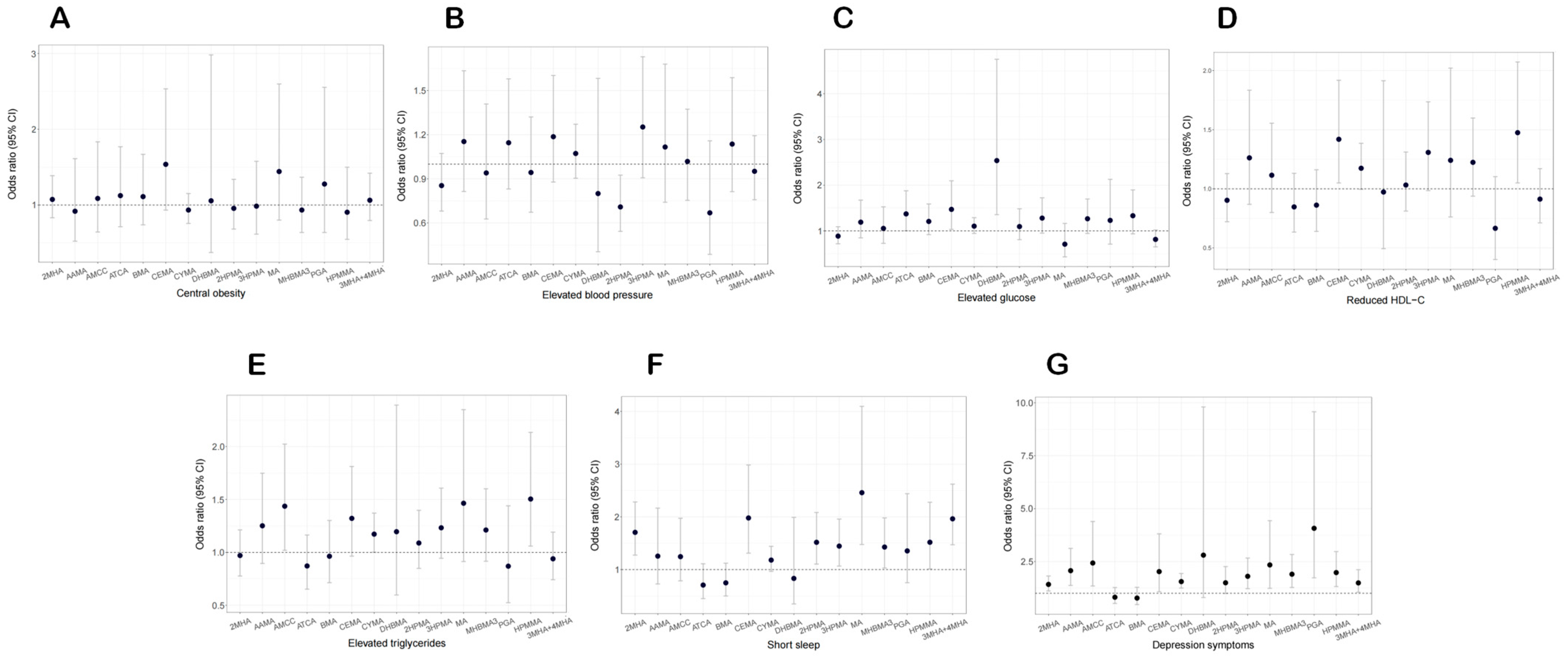
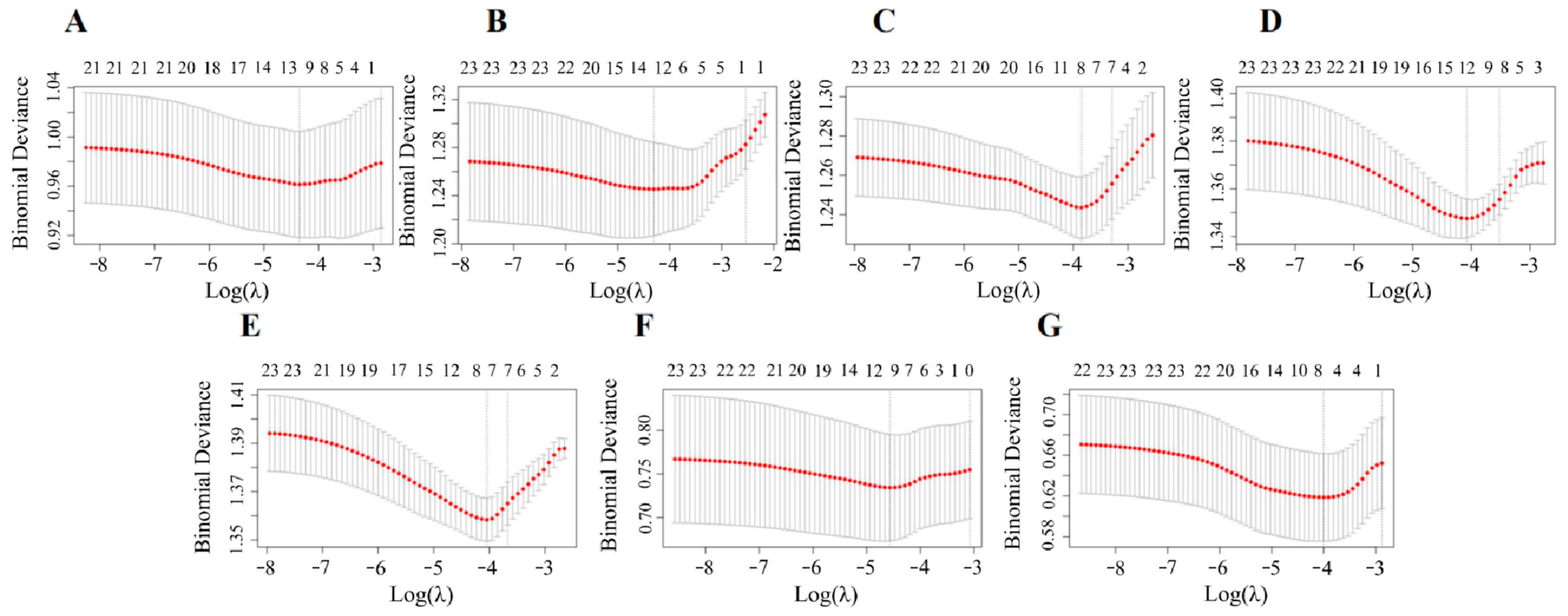
3.7. Relationship of Urinary VOC Metabolites with CircS Component
3.8. Subgroup Analysis
3.9. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome—What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26 (Suppl. S2), 33–46. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A Compass for Global Action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Staels, B. When the Clock stops ticking, metabolic syndrome explodes. Nat. Med. 2006, 12, 54–55, discussion 55. [Google Scholar] [CrossRef]
- Bray, M.S.; Young, M.E. Circadian rhythms in the development of obesity: Potential role for the circadian clock within the adipocyte. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2007, 8, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.K.; Stern, N.; El-Osta, A.; Bilu, C.; Einat, H.; Zimmet, P. The circadian syndrome predicts cardiovascular disease better than metabolic syndrome in Chinese adults. J. Intern. Med. 2021, 289, 851–860. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhong, Q.; Zhang, Y.; Liu, Z.; Wang, X. The association between circadian syndrome and chronic kidney disease in an aging population: A 4-year follow-up study. Front. Endocrinol. 2024, 15, 1338110. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Zhang, Y.; Liu, J. Relationship between circadian syndrome and stroke: A cross-sectional study of the national health and nutrition examination survey. Front. Neurol. 2022, 13, 946172. [Google Scholar] [CrossRef]
- Ding, L.; Duan, J.; Yang, T.; Jin, C.; Lv, S.; Ma, A.; Qin, Y. Association between circadian syndrome and chronic diarrhea: A cross-sectional study of NHANES 2005–2010 data. Front. Physiol. 2024, 15, 1301450. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, W.; Huang, Y. Association between serum uric acid levels and depressive symptoms according to menopausal status. J. Affect. Disord. 2024, 350, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, L. Association between sleep duration and depression in menopausal women: A population-based study. Front. Endocrinol. 2024, 15, 1301775. [Google Scholar] [CrossRef]
- Cybulska, A.M.; Schneider-Matyka, D.; Bosiacki, M.; Chlubek, D.; Panczyk, M.; Grochans, E. The Levels of Bioelements in Postmenopausal Women with Metabolic Syndrome. Nutrients 2022, 14, 4102. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.Y.; Chiu, C.J.; Shea, J.L.; Wang, C.L.; Tang, H.H.; Kuo, P.C.; Yang, Y.C.; Wu, C.H. Role of age, menopausal status, and symptoms in midlife women: Examination of sleep patterns and rest-activity circadian rhythms. Sleep Med. 2024, 113, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Ahmed, S.; Dipti, R.K.; Siddiquee, R.E.; Hawlader, M.D.H. The prevalence and associated factors of depression during pre-, peri-, and post-menopausal period among the middle-aged women of Dhaka city. Asian J. Psychiatry 2020, 54, 102312. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Orru, H.; Ebi, K.L.; Forsberg, B. The Interplay of Climate Change and Air Pollution on Health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, L.; Yao, X.; Yao, Z. Recent advances in the chemical oxidation of gaseous volatile organic compounds (VOCs) in liquid phase. Chemosphere 2022, 295, 133868. [Google Scholar] [CrossRef]
- Chang, M.; Lee, D.; Park, H.; Ha, M.; Hong, Y.C.; Kim, Y.; Kim, B.N.; Kim, Y.; Lim, Y.H.; Ha, E.H. Prenatal TVOCs exposure negatively influences postnatal neurobehavioral development. Sci. Total Environ. 2018, 618, 977–981. [Google Scholar] [CrossRef]
- Villeneuve, P.J.; Jerrett, M.; Brenner, D.; Su, J.; Chen, H.; McLaughlin, J.R. A case-control study of long-term exposure to ambient volatile organic compounds and lung cancer in Toronto, Ontario, Canada. Am. J. Epidemiol. 2014, 179, 443–451. [Google Scholar] [CrossRef]
- Wang, B.; Yu, L.; Liu, W.; Yang, M.; Fan, L.; Zhou, M.; Ma, J.; Wang, X.; Nie, X.; Cheng, M.; et al. Cross-sectional and longitudinal associations of acrolein exposure with pulmonary function alteration: Assessing the potential roles of oxidative DNA damage, inflammation, and pulmonary epithelium injury in a general adult population. Environ. Int. 2022, 167, 107401. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Y.; Zeb, R.; Chen, F.; Chen, H.; Wang, K.J. The intergenerational toxic effects on offspring of medaka fish Oryzias melastigma from parental benzo[a]pyrene exposure via interference of the circadian rhythm. Environ. Pollut. 2020, 267, 115437. [Google Scholar] [CrossRef]
- Yen, P.L.; Lin, T.A.; Chang, C.H.; Yu, C.W.; Kuo, Y.H.; Chang, T.T.; Liao, V.H. Di(2-ethylhexyl) phthalate disrupts circadian rhythm associated with changes in metabolites and cytochrome P450 gene expression in Caenorhabditis elegans. Environ. Pollut. 2024, 363 Pt 1, 125062. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Qian, H.; Yang, J.; Hu, Y. The association analysis between exposure to volatile organic chemicals and obesity in the general USA population: A cross-sectional study from NHANES program. Chemosphere 2023, 315, 137738. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.E.; Konkle, S.L.; Riggs, D.W.; Rai, S.N.; DeJarnett, N.; Xie, Z.; Keith, R.J.; Oshunbade, A.; Hall, M.E.; Shimbo, D.; et al. Exposure to Volatile Organic Compounds Is Associated with Hypertension in Black Adults: The Jackson Heart Study. Environ. Res. 2023, 223, 115384. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, W.; Wu, X.; Song, X.; Yang, X.; Zhang, G.; Niu, P.; Chen, T. Exposure to volatile organic compounds is a risk factor for diabetes: A cross-sectional study. Chemosphere 2023, 338, 139424. [Google Scholar] [CrossRef]
- Tang, L.; Liu, M.; Tian, J. Volatile organic compounds exposure associated with depression among U.S. adults: Results from NHANES 2011–2020. Chemosphere 2024, 349, 140690. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, M.; Pang, Y.; Liu, Q.; Liu, Y.; Jin, X.; Tang, J.; Bao, L.; Niu, Y.; Zheng, Y.; et al. Interior decorative volatile organic compounds exposure induces sleep disorders through aberrant branched chain amino acid transaminase 2 mediated glutamatergic signaling resulting from a neuroinflammatory cascade. Sci. Total Environ. 2024, 934, 173254. [Google Scholar] [CrossRef]
- Lee, K.A. Alterations in sleep during pregnancy and postpartum: A review of 30 years of research. Sleep Med. Rev. 1998, 2, 231–242. [Google Scholar] [CrossRef]
- Azarmanesh, D.; Bertone-Johnson, E.R.; Pearlman, J.; Liu, Z.; Carbone, E.T. Association of the Dietary Inflammatory Index with Depressive Symptoms among Pre- and Post-Menopausal Women: Findings from the National Health and Nutrition Examination Survey (NHANES) 2005–2010. Nutrients 2022, 14, 1980. [Google Scholar] [CrossRef]
- Alwis, K.U.; Blount, B.C.; Britt, A.S.; Patel, D.; Ashley, D.L. Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS). Anal. Chim. Acta 2012, 750, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Akbar, Z.; Shi, Z. Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016. Nutrients 2023, 15, 3396. [Google Scholar] [CrossRef]
- Shi, Z.; Stern, N.; Liu, J.; Tuomilehto, J.; Kronfeld-Schor, N.; El-Osta, A.; Alberti, G.; Chai, Z.; Bilu, C.; Einat, H.; et al. The circadian syndrome is a predictor for cognition impairment in middle-aged adults: Comparison with the metabolic syndrome. Diabetes/Metab. Res. Rev. 2024, 40, e3827. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Arabi, A.; Nasrallah, D.; Mohsen, S.; Abugharbieh, L.; Al-Hashimi, D.; AlMass, S.; Albasti, S.; Al-Ajmi, S.A.; Khan, M.N.; Zughaier, S.M. Association between Serum Vitamin D Status and Circadian Syndrome: A Cross-Sectional Study. Nutrients 2024, 16, 2111. [Google Scholar] [CrossRef] [PubMed]
- Akbar, Z.; Shi, Z. Unfavorable Mealtime, Meal Skipping, and Shiftwork Are Associated with Circadian Syndrome in Adults Participating in NHANES 2005–2016. Nutrients 2024, 16, 1581. [Google Scholar] [CrossRef]
- Hu, X.; Nie, Z.; Ou, Y.; Lin, L.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; Zhou, Y.; Wu, Y.; Dong, G.; et al. Long-term exposure to ambient air pollution, circadian syndrome and cardiovascular disease: A nationwide study in China. Sci. Total Environ. 2023, 868, 161696. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Lv, X.; Wang, R.; Li, X.; Xu, W.; Wang, N.; Ma, S.; Huang, H.; Niu, Y.; Kong, X. Association of marine PUFAs intakes with cardiovascular disease, all-cause mortality, and cardiovascular mortality in American adult male patients with dyslipidemia: The U.S. National Health and Nutrition Examination Survey, 2001 to 2016. Nutr. J. 2023, 22, 48. [Google Scholar] [CrossRef]
- Bai, T.; Li, X.; Zhang, H.; Yang, W.; Lv, C.; Du, X.; Xu, S.; Zhao, A.; Xi, Y. The association between brominated flame retardants exposure with bone mineral density in US adults: A cross-sectional study of the national health and nutrition examination survey (NHANES) 2005–2014. Environ. Res. 2024, 251 Pt 1, 118580. [Google Scholar] [CrossRef]
- Zhao, L.; Ogden, C.L.; Yang, Q.; Jackson, S.L.; Loria, C.M.; Galuska, D.A.; Wiltz, J.L.; Merritt, R.; Cogswell, M.E. Association of Usual Sodium Intake with Obesity Among US Children and Adolescents, NHANES 2009–2016. Obesity 2021, 29, 587–594. [Google Scholar] [CrossRef]
- Cucinella, L.; Tiranini, L.; Nappi, R.E. Impact of climate and environmental change on the menopause. Maturitas 2023, 178, 107825. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Pan, M.; Zhang, K.; Zhou, F.; Tong, J.; Chen, Z.; Xiang, H. Exposure to air pollution and prevalence of metabolic syndrome: A nationwide study in China from 2011 to 2015. Sci. Total Environ. 2023, 855, 158596. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, F.; Zhang, K.; Wu, T.; Pan, M.; Wang, X.; Tong, J.; Chen, Z.; Xiang, H. Effects of air pollution and residential greenness on sleep disorder: A 8-year nationwide cohort study. Environ. Res. 2023, 220, 115177. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Yu, Z.; Zhang, X.; Wu, M.; Wang, J.; Shui, L.; Lin, H.; Jin, M.; Tang, M.; Chen, K. Long-term exposure to ambient air pollution and incidence of depression: A population-based cohort study in China. Sci. Total Environ. 2022, 804, 149986. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Cheng, D.; Cao, Y.; Xie, X.; Zhou, J.; Wu, Y.; Li, X.; Yu, J.; Yang, B. Association between urinary metabolites of volatile organic compounds and cardiovascular disease in the general population from NHANES 2011–2018. Ecotoxicol. Environ. Saf. 2023, 264, 115412. [Google Scholar] [CrossRef]
- Wu, M.; Liu, M.; Zhang, Y.; Wu, J.; Gao, M.; Huang, F.; Chen, H.; Zhu, Z. Serum HDL partially mediates the association between exposure to volatile organic compounds and kidney stones: A nationally representative cross-sectional study from NHANES. Sci. Total Environ. 2024, 907, 167915. [Google Scholar] [CrossRef]
- Hallajzadeh, J.; Khoramdad, M.; Izadi, N.; Karamzad, N.; Almasi-Hashiani, A.; Ayubi, E.; Qorbani, M.; Pakzad, R.; Hasanzadeh, A.; Sullman, M.J.M.; et al. Metabolic syndrome and its components in premenopausal and postmenopausal women: A comprehensive systematic review and meta-analysis on observational studies. Menopause 2018, 25, 1155–1164. [Google Scholar] [CrossRef]
- Zhu, B.; Grandner, M.A.; Jackson, N.J.; Pien, G.W.; Srimoragot, M.; Knutson, K.L.; Izci-Balserak, B. Associations between Diet and Sleep Duration in Different Menopausal Stages. West. J. Nurs. Res. 2021, 43, 984–994. [Google Scholar] [CrossRef]
- Dong, R.; Chang, D.; Shen, C.; Shen, Y.; Shen, Z.; Tian, T.; Wang, J. Association of volatile organic compound exposure with metabolic syndrome and its components: A nationwide cross-sectional study. BMC Public Health 2024, 24, 671. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gui, C.; Xiao, Y.; Ma, R.; Liu, C.; He, L.; Zhao, H.; Luo, B. Association between Exposure to Volatile Organic Compounds and the Prevalence of Sleep Problems in US Adults. Toxics 2024, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Tello-Palencia, M.A.; Yang, T.; Sularz, O.; Demers, L.E.; Ma, Y.; Boycott, C.; Zhang, H.A.; Lubecka-Gajewska, K.; Kumar, S.; Ramsey, B.S.; et al. Pterostilbene Targets Hallmarks of Aging in the Gene Expression Landscape in Blood of Healthy Rats. Mol. Nutr. Food Res. 2024, 68, e2400662. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Zhang, X.; Zhang, H.; Zhang, Y.; Wang, S.; Yin, L. Combined association of urinary volatile organic compounds with chronic bronchitis and emphysema among adults in NHANES 2011–2014: The mediating role of inflammation. Chemosphere 2024, 361, 141485. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; He, D.; Zhang, D.; Liu, X. Association between different triglyceride glucose index-related indicators and depression in premenopausal and postmenopausal women: NHANES, 2013–2016. J. Affect. Disord. 2024, 360, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 143, 242–255. [Google Scholar] [CrossRef]
- Jaszczak, E.; Polkowska, Ż.; Narkowicz, S.; Namieśnik, J. Cyanides in the environment-analysis-problems and challenges. Environ. Sci. Pollut. Res. Int. 2017, 24, 15929–15948. [Google Scholar] [CrossRef]
- Kang, D.; Lee, E.S.; Kim, T.K.; Kim, Y.J.; Lee, S.; Lee, W.; Sim, H.; Kim, S.Y. Association with Combined Occupational Hazards Exposure and Risk of Metabolic Syndrome: A Workers’ Health Examination Cohort 2012–2021. Saf. Health Work. 2023, 14, 279–286. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, H.; Luo, H.; Zhang, T.; Sun, H.; Kannan, K. Daily Exposure to Environmental Volatile Organic Compounds Triggers Oxidative Damage: Evidence from a Large-Scale Survey in China. Environ. Sci. Technol. 2023, 57, 20501–20509. [Google Scholar] [CrossRef]
- Wang, F.; Liu, F.; Liu, H.; Chen, W.; Si, X.; Ma, X. Effects of immunological and hematological parameter in mice exposed to mixture of volatile organic compounds. Inhal. Toxicol. 2016, 28, 164–169. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, N.L.; McEwen, B.S. Insulin resistance-a missing link no more. Mol. Psychiatry 2016, 21, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Falco, A.; Patrono, C. Lipid peroxidation in diabetes mellitus. Antioxid. Redox Signal. 2005, 7, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Moriel, P.; Plavnik, F.L.; Zanella, M.T.; Bertolami, M.C.; Abdalla, D.S. Lipid peroxidation and antioxidants in hyperlipidemia and hypertension. Biol. Res. 2000, 33, 105–112. [Google Scholar] [CrossRef]
- de Souza, J.F.T.; Dáttilo, M.; de Mello, M.T.; Tufik, S.; Antunes, H.K.M. High-Intensity Interval Training Attenuates Insulin Resistance Induced by Sleep Deprivation in Healthy Males. Front. Physiol. 2017, 8, 992. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.K.; Ryoo, J.H.; Oh, C.M.; Mansur, R.B.; Alfonsi, J.E.; Cha, D.S.; Lee, Y.; McIntyre, R.S.; Jung, J.Y. The association between insulin resistance and depression in the Korean general population. J. Affect. Disord. 2017, 208, 553–559. [Google Scholar] [CrossRef]
- Syed, A.A.; Reza, M.I.; Singh, P.; Husain, A.; Dadge, S.; Gayen, J.R. Polyphenolic-rich Cissus quadrangularis extract ameliorates insulin resistance by activating AdipoR1 in peri-/post-menopausal rats. Exp. Gerontol. 2022, 159, 111681. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]

| Variable | Overall (n = 1051) | Premenopausal (n = 520) | Postmenopausal (n = 531) | p-Value |
|---|---|---|---|---|
| Age (years), median (Q1, Q3) | 50.0 (34.0, 62.0) | 34.5 (27.0, 43.8) | 62.0 (55.0, 70.0) | <0.001 |
| Race, n (%) | 0.008 | |||
| Mexican American | 119 (11.3) | 61 (11.7) | 58 (10.9) | |
| Other Hispanic | 114 (10.8) | 56 (10.8) | 58 (10.9) | |
| Non-Hispanic White | 406 (38.6) | 178 (34.2) | 228 (42.9) | |
| Non-Hispanic Black | 253 (24.1) | 128 (24.6) | 125 (23.5) | |
| Other | 159 (15.1) | 97 (18.7) | 62 (11.7) | |
| Educational level, n (%) | <0.001 | |||
| Below high school | 183 (17.4) | 75 (14.4) | 108 (20.3) | |
| High school | 226 (21.5) | 96 (18.5) | 130 (24.5) | |
| Above high school | 642 (61.1) | 349 (67.1) | 293 (55.2) | |
| Marital status, n (%) | 0.006 | |||
| Married/living with partner | 560 (53.3) | 300 (57.7) | 260 (49.0) | |
| Widowed/divorced/separated | 317 (30.2) | 134 (25.8) | 183 (34.5) | |
| Never married | 174 (16.6) | 86 (16.5) | 88 (16.6) | |
| Physical activity, n (%) | <0.001 | |||
| Active | 772 (73.5) | 411 (79.0) | 361 (68.0) | |
| Inactive | 279 (26.5) | 109 (21.0) | 170 (32.0) | |
| Drinker, n (%) | <0.001 | |||
| Yes | 598 (56.9) | 338 (65.0) | 260 (43.5) | |
| No | 453 (43.1) | 182 (35.0) | 271 (51.0) | |
| Smoker, n (%) | 0.002 | |||
| Yes | 377 (35.9) | 163 (31.3) | 214 (40.3) | |
| No | 674 (64.1) | 357 (68.7) | 317 (59.7) | |
| Poverty income ratio, n (%) | 0.002 | |||
| <1.0 | 243 (23.1) | 144 (27.7) | 99 (18.6) | |
| 1.0–3.0 | 424 (40.3) | 197 (37.9) | 227 (42.7) | |
| >3.0 | 384 (36.5) | 179 (34.4) | 205 (38.6) | |
| Central obesity, n (%) | <0.001 | |||
| Yes | 757 (72.0) | 327 (62.9) | 430 (81.0) | |
| No | 294 (28.0) | 193 (37.1) | 101 (19.0) | |
| Elevated glucose, n (%) | <0.001 | |||
| Yes | 527 (50.1) | 175 (33.7) | 352 (66.3) | |
| No | 524 (49.9) | 345 (66.3) | 179 (33.7) | |
| Elevated triglycerides, n (%) | <0.001 | |||
| Yes | 362 (34.4) | 82 (15.8) | 280 (52.7) | |
| No | 689 (65.6) | 438 (84.2) | 251 (47.3) | |
| Reduced HDL-C, n (%) | <0.001 | |||
| Yes | 487 (46.3) | 185 (35.6) | 302 (56.9) | |
| No | 564 (53.7) | 335 (64.4) | 229 (43.1) | |
| Elevated blood pressure, n (%) | <0.001 | |||
| Yes | 446 (42.4) | 106 (20.4) | 340 (64.0) | |
| No | 605 (57.6) | 414 (79.6) | 191 (36.0) | |
| Depression symptoms, n (%) | 0.750 | |||
| Yes | 108 (10.3) | 55 (10.6) | 53 (10.0) | |
| No | 943 (89.7) | 465 (89.4) | 478 (90.0) | |
| Short sleep, n (%) | 0.753 | |||
| Yes | 134 (12.7) | 68 (13.1) | 66 (12.4) | |
| No | 917 (87.3) | 452 (86.9) | 465 (87.6) | |
| Metabolic syndrome, n (%) | <0.001 | |||
| Yes | 481 (45.8) | 141 (27.1) | 340 (64.0) | |
| No | 570 (54.2) | 379 (72.9) | 191 (36.0) | |
| Circadian syndrome, n (%) | <0.001 | |||
| Yes | 356 (33.9) | 86 (16.5) | 270 (50.8) | |
| No | 695 (66.1) | 434 (83.5) | 261 (49.2) |
| VOCs | Premenopausal | Postmenopausal | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| 2MHA | 1.117 (0.882, 1.416) | 0.351 | 0.877 (0.688, 1.119) | 0.284 |
| 3MHA+4MHA | 0.964 (0.749, 1.242) | 0.774 | 0.929 (0.708, 1.220) | 0.589 |
| AAMA | 0.952 (0.540, 1.677) | 0.861 | 1.436 (1.007, 2.050) | 0.046 |
| AMCC | 1.172 (0.731, 1.880) | 0.502 | 1.308 (0.913, 1.876) | 0.140 |
| ATCA | 1.658 (1.011, 2.721) | 0.045 | 0.924 (0.681, 1.253) | 0.603 |
| BMA | 0.820 (0.561, 1.199) | 0.299 | 0.939 (0.710, 1.242) | 0.654 |
| CEMA | 1.693 (1.144, 2.504) | 0.009 | 1.736 (1.269, 2.376) | <0.001 |
| CYMA | 1.153 (0.951, 1.398) | 0.143 | 1.256 (1.048, 1.505) | 0.015 |
| DHBMA | 0.825 (0.297, 2.291) | 0.707 | 1.358 (0.646, 2.856) | 0.412 |
| 2HPMA | 0.818 (0.520, 1.288) | 0.379 | 1.053 (0.819, 1.353) | 0.682 |
| 3HPMA | 1.177 (0.779, 1.776) | 0.431 | 1.528 (1.141, 2.047) | 0.005 |
| HPMMA | 1.245 (0.811, 1.912) | 0.309 | 1.676 (1.172, 2.397) | 0.006 |
| MA | 1.136 (0.716, 1.801) | 0.582 | 2.000 (1.240, 3.224) | 0.005 |
| MHBMA3 | 1.118 (0.788, 1.586) | 0.523 | 1.350 (1.004, 1.814) | 0.047 |
| PGA | 0.603 (0.322, 1.130) | 0.112 | 0.885 (0.512, 1.528) | 0.654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Zhang, Z.; Ren, J.; Pei, H.; Liu, J.; Yin, B.; Zhang, C.; Wen, R.; Qiao, S.; Wang, Z.; et al. Association Between Volatile Organic Compounds and Circadian Syndrome Among Pre- and Postmenopausal Women. Toxics 2025, 13, 328. https://doi.org/10.3390/toxics13050328
Sun X, Zhang Z, Ren J, Pei H, Liu J, Yin B, Zhang C, Wen R, Qiao S, Wang Z, et al. Association Between Volatile Organic Compounds and Circadian Syndrome Among Pre- and Postmenopausal Women. Toxics. 2025; 13(5):328. https://doi.org/10.3390/toxics13050328
Chicago/Turabian StyleSun, Xiaoya, Zhenao Zhang, Jingyi Ren, Huanting Pei, Jie Liu, Bowen Yin, Chongyue Zhang, Rui Wen, Simeng Qiao, Ziyi Wang, and et al. 2025. "Association Between Volatile Organic Compounds and Circadian Syndrome Among Pre- and Postmenopausal Women" Toxics 13, no. 5: 328. https://doi.org/10.3390/toxics13050328
APA StyleSun, X., Zhang, Z., Ren, J., Pei, H., Liu, J., Yin, B., Zhang, C., Wen, R., Qiao, S., Wang, Z., & Ma, Y. (2025). Association Between Volatile Organic Compounds and Circadian Syndrome Among Pre- and Postmenopausal Women. Toxics, 13(5), 328. https://doi.org/10.3390/toxics13050328







