Effects of Polystyrene Nanoplastics on Oxidative Stress, Blood Biochemistry, and Digestive Enzyme Activity in Goldfish (Carassius auratus)
Abstract
1. Introduction
2. Materials and Methods
2.1. NPs and Characterization
2.2. NP-Diet Preparation
2.3. Fish Maintenance, Bioassay and Sampling
2.4. The Oxidative Stress Biomarkers
2.5. Integrated Biomarker Response (IBR)
2.6. The Blood Biochemical Parameters
2.7. The Digestive Enzyme Activities in Intestinal Tissue
2.8. Data Analysis
3. Results
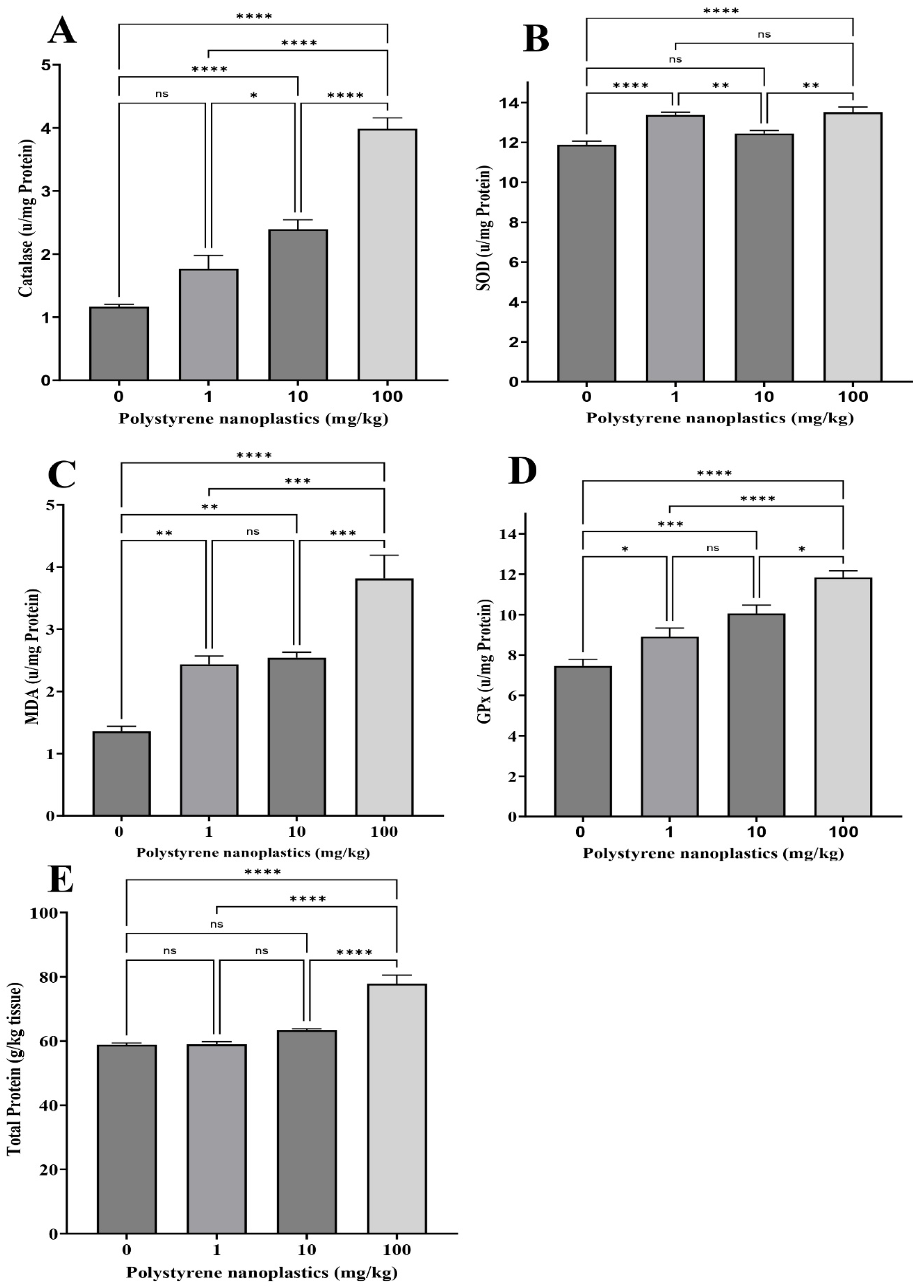
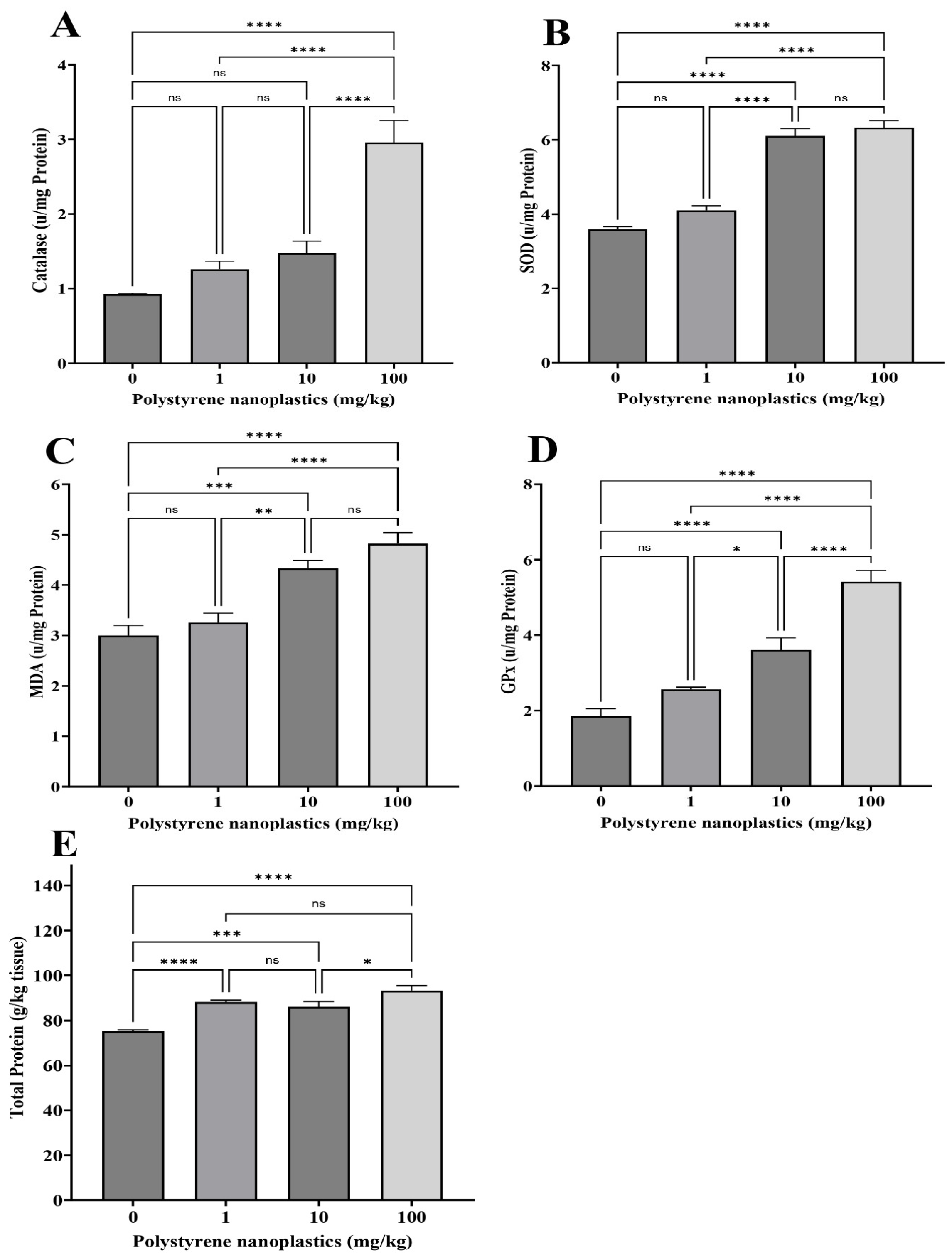
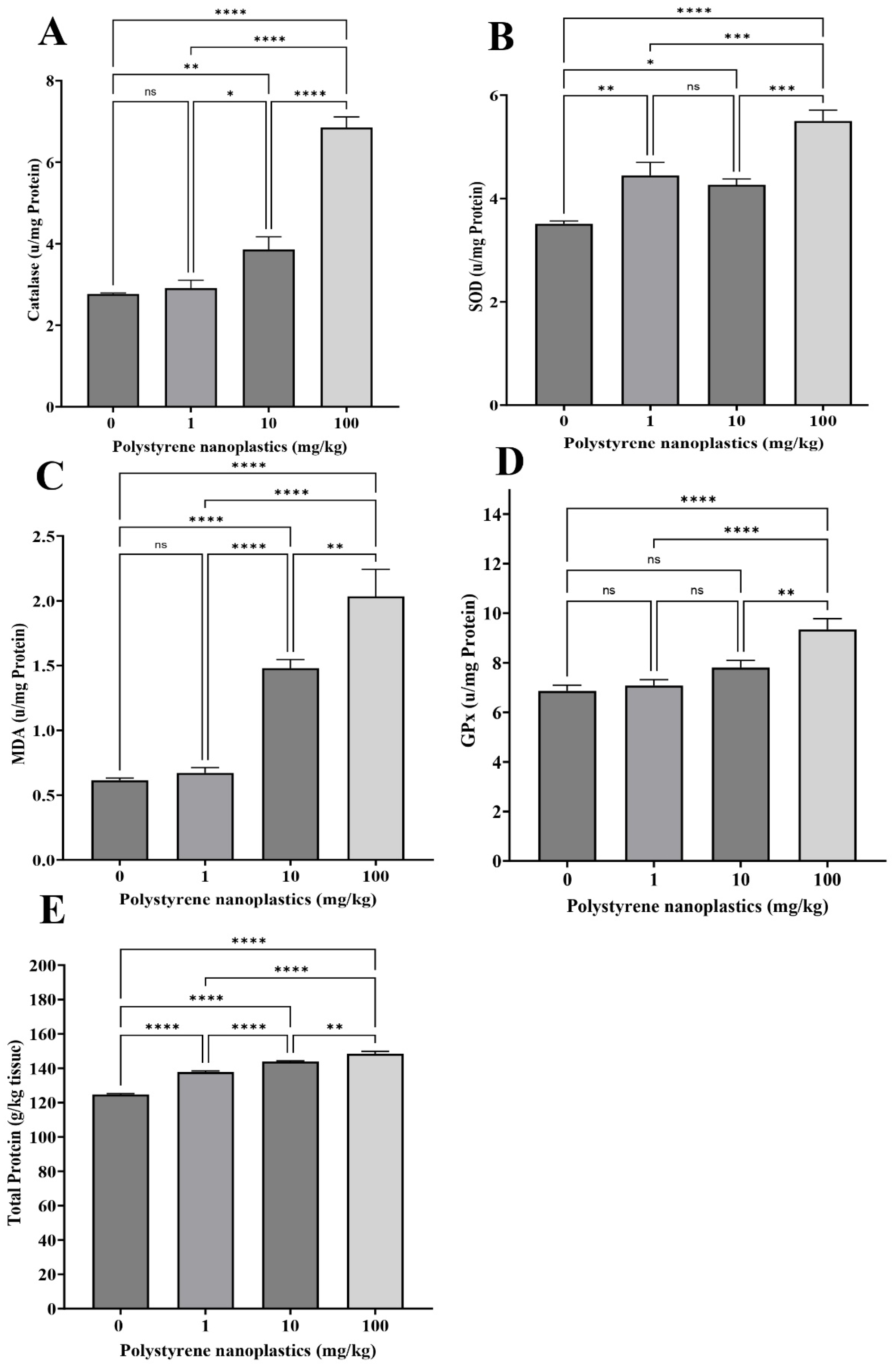
3.1. Changes in Antioxidant Biomarkers in Gill Tissue
3.2. Changes in Antioxidant Biomarkers in Kidney Tissue
3.3. Changes in Antioxidant Biomarkers in the Liver Tissue
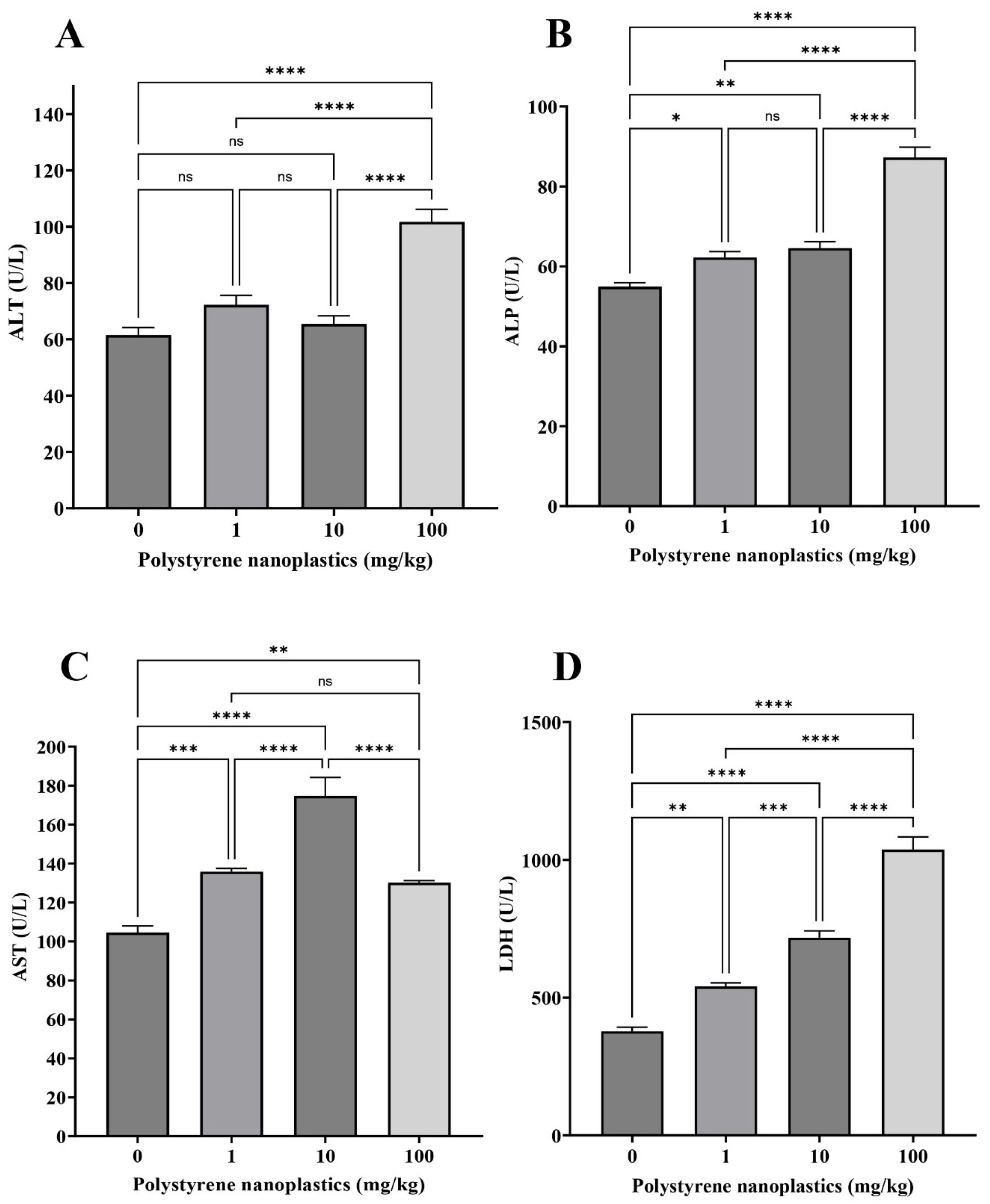
3.4. Changes in the Plasma Biochemical Parameters
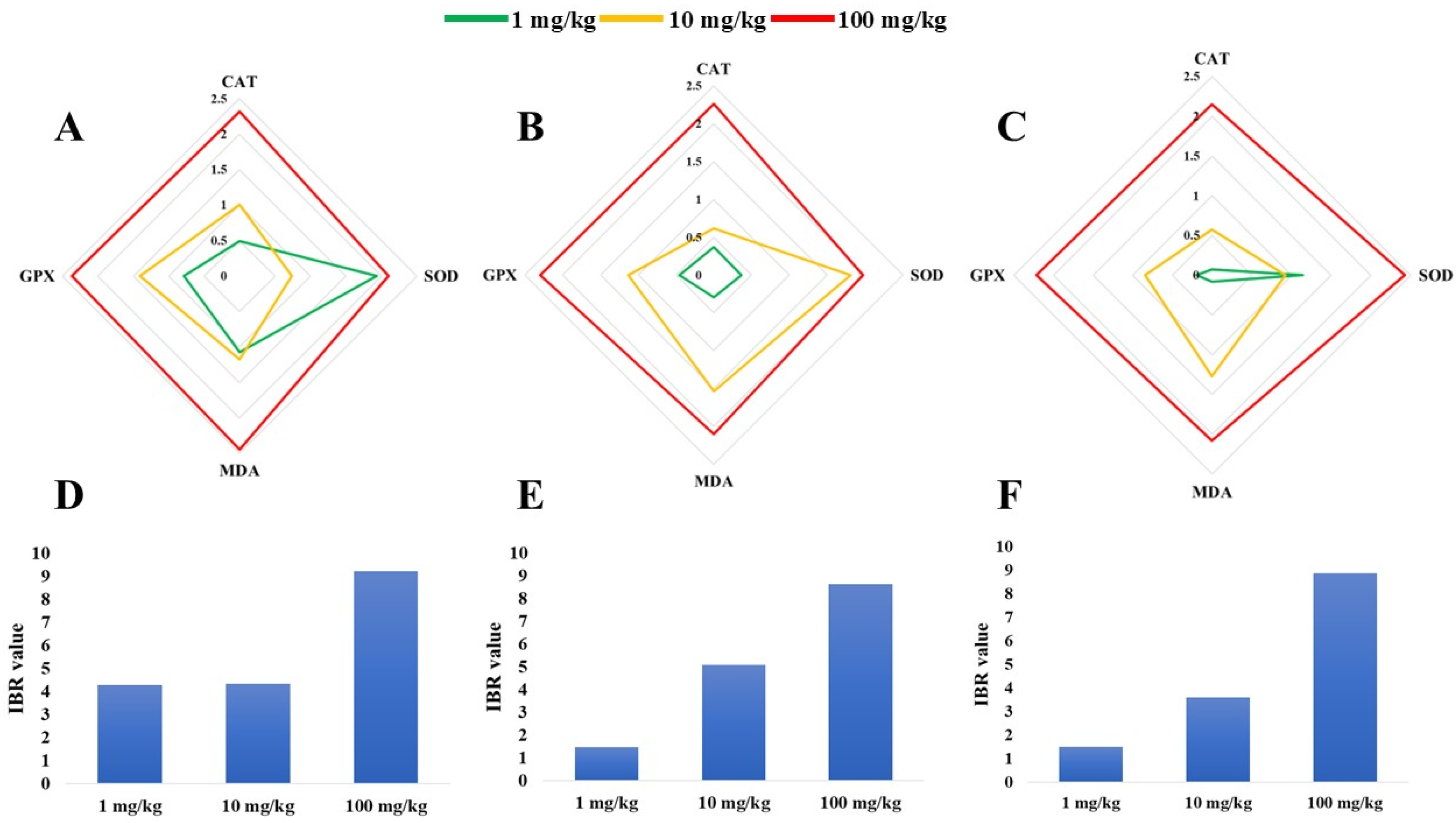
3.5. IBR Index for Antioxidant Biomarkers in Liver, Kidney, and Gill Tissues
3.6. Changes in Digestive Enzyme Activities in the Intestinal Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NP | Nanoplastic |
| MP | Microplastic |
| PS | Polystyrene |
| C. auratus | Carassius auratus |
| T0 | Treatment 0 mg/kg |
| T1 | Treatment 1 mg/kg |
| T10 | Treatment 10 mg/kg |
| T100 | Treatment 100 mg/kg |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| ALP | alkaline phosphatase |
| LDH | lactate dehydrogenase |
| GPx | glutathione peroxidase |
| CAT | catalase |
| SOD | superoxide dismutase |
| GSH | glutathione |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| IBR | Integrated Biomarker Response |
| EDTA | Ethylene Diamine Tetraacetic Acid |
| μ-FT-IR | Micro Fourier Transform InfraRed spectroscopy |
| DLS | dynamic light scattering |
| PBS | Phosphate-Buffered Saline |
References
- Da Costa, J.P.; Santos, P.S.; Duarte, A.C.; Rocha-Santos, T. (Nano)plastics in the environment—Sources, fates and effects. Sci. Total Environ. 2016, 566, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Stoett, P.; Scrich, V.M.; Elliff, C.I.; Andrade, M.M.; Grilli, N.D.M.; Turra, A. Global plastic pollution, sustainable development, and plastic justice. World Dev. 2024, 184, 106756. [Google Scholar] [CrossRef]
- Banaee, M.; Multisanti, C.R.; Impellitteri, F.; Piccione, G.; Faggio, C. Environmental toxicology of microplastic particles on fish: A review. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 287, 110042. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Wagner, M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Shim, W.J. Nanoplastics in the Aquatic Environment. Critical Review. In Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 325–340. [Google Scholar] [CrossRef]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.Á.; Duarte, C.M. Plastic accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef]
- Mattsson, K.; Jocic, S.; Doverbratt, I.; Hansson, L.A. Nanoplastics in the Aquatic Environment. In Microplastic Contamination in Aquatic Environments; Elsevier: Amsterdam, The Netherlands, 2018; pp. 379–399. [Google Scholar] [CrossRef]
- Cole, M.; Galloway, T.S. Ingestion of nanoplastics and microplastics by Pacific oyster larvae. Environ. Sci. Technol. 2015, 49, 14625–14632. [Google Scholar] [CrossRef]
- Kashiwada, S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ. Health Perspect. 2006, 114, 1697–1702. [Google Scholar] [CrossRef]
- Von Moos, N.; Burkhardt-Holm, P.; Köhler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Della Torre, C.; Bergami, E.; Salvati, A.; Faleri, C.; Cirino, P.; Dawson, K.A.; Corsi, I. Accumulation and embryotoxicity of polystyrene nanoparticles at early stage of development of sea urchin embryos Paracentrotus lividus. Environ. Sci. Technol. 2014, 48, 12302–12311. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Kim, D.; Kim, S.W.; An, Y.J. Trophic transfer and individual impact of nano-sized polystyrene in a four-species freshwater food chain. Sci. Rep. 2018, 8, 284. [Google Scholar] [CrossRef]
- Cedervall, T.; Hansson, L.A.; Lard, M.; Frohm, B.; Linse, S. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS ONE 2012, 7, e32254. [Google Scholar] [CrossRef]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- Van Pomeren, M.; Brun, N.R.; Peijnenburg, W.J.G.M.; Vijver, M.G. Exploring uptake and biodistribution of polystyrene (nano)particles in zebrafish embryos at different developmental stages. Aquat. Toxicol. 2017, 190, 40–45. [Google Scholar] [CrossRef]
- Clark, N.J.; Khan, F.R.; Mitrano, D.M.; Boyle, D.; Thompson, R.C. Demonstrating the translocation of nanoplastics across the fish intestine using palladium-doped polystyrene in a salmon gut-sac. Environ. Int. 2022, 159, 106994. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Q.; Li, J.; Li, B.; Liang, W.; Su, L.; Shi, H. Distribution and translocation of micro- and nanoplastics in fish. Crit. Rev. Toxicol. 2022, 51, 740–753. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Bergami, E.; Monopoli, M.P.; Dawson, K.A.; Papa, S.; Corsi, I. Evidence for immunomodulation and apoptotic processes induced by cationic polystyrene nanoparticles in the hemocytes of the marine bivalve Mytilus. Mar. Environ. Res. 2015, 111, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Lee, J.S. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the monogonont rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef] [PubMed]
- Greven, A.C.; Merk, T.; Karagöz, F.; Mohr, K.; Klapper, M.; Jovanović, B.; Palić, D. Polycarbonate and polystyrene nanoplastic particles act as stressors to the innate immune system of fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2016, 35, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Wang, B.; Lurling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. Magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef]
- Pinsino, A.; Bergami, E.; Della Torre, C.; Vannuccini, M.L.; Addis, P.; Secci, M.; Corsi, I. Amino-modified polystyrene nanoparticles affect signaling pathways of the sea urchin (Paracentrotus lividus) embryos. Nanotoxicology 2017, 11, 201–209. [Google Scholar] [CrossRef]
- Ferreira, C.S.; Venâncio, C.; Oliveira, M. Nanoplastics and Biota Behaviour: Known Effects, Environmental Relevance, and Research Needs. TrAC Trends Anal. Chem. 2023, 165, 117129. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, D.; Yu, Y.; Tong, D.; Zhou, W.; Yu, Y.; Shi, W. Micro/nanoplastics impair the feeding of goldfish by disrupting the complicated peripheral and central regulation of appetite. Sci. Total Environ. 2024, 946, 174112. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Gu, H.X.; Wang, S.X.; Wang, X.H.; Yu, X.; Hu, M.H.; Huang, W.; Wang, Y.J. Nanoplastics impair the intestinal health of the juvenile large yellow croaker Larimichthys crocea. J. Hazard. Mater. 2020, 397, 122773. [Google Scholar] [CrossRef]
- Chen, Q.; Lackmann, C.; Wang, W.; Seiler, T.B.; Hollert, H.; Shi, H. Microplastics lead to hyperactive swimming behaviour in adult zebrafish. Aquat. Toxicol. 2020, 224, 105521. [Google Scholar] [CrossRef] [PubMed]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.H.; Liang, S.T.; Chen, J.R.; Chen, K.H.; Hsiao, C.D. Nanoplastics cause neurobehavioral impairments, reproductive and oxidative damages, and biomarker responses in zebrafish: Throwing up alarms of wide spread health risk of exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, H.; Chang, X.; Huang, W.; Sokolova, I.M.; Wei, S.; Sun, L.; Li, S.; Wang, X.; Hu, M.; et al. Oxidative stress induced by nanoplastics in the liver of juvenile large yellow croaker Larimichthys crocea. Mar. Pollut. Bull. 2021, 170, 112661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, F.Q.; Liang, K.S.; Niu, W.B.; Duan, X.Z.; Jia, X.Z.; Wu, X.F.; Xu, P.; Zhou, L. Polystyrene nanoplastics affect digestive function and growth in juvenile groupers. Sci. Total Environ. 2022, 808, 152098. [Google Scholar] [CrossRef]
- Tallec, K.; Huvet, A.; Di Poi, C.; Gonzalez-Fernandez, C.; Lambert, C.; Petton, B.; Le Goïc, N.; Berchel, M.; Soudant, P.; Paul-Pont, I. Nanoplastics impaired oyster free living stages, gametes and embryos. Environ. Pollut. 2018, 242, 1226–1235. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Y.; Zhao, H.; Wang, D.; Guo, M.; Mu, M.; Liu, Y.; Nie, X.; Li, B.; Li, J.; et al. A comparative review of microplastics and nanoplastics: Toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 2021, 774, 145758. [Google Scholar] [CrossRef]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Rabei, A.; Hichami, A.; Beldi, H.; Bellenger, S.; Khan, N.A.; Soltani, N. Fatty acid composition, enzyme activities and metallothioneins in Donax trunculus (Mollusca, Bivalvia) from polluted and reference sites in the Gulf of Annaba (Algeria): Pattern of recovery during transplantation. Environ. Pollut. 2018, 237, 900–907. [Google Scholar] [CrossRef]
- Gu, H.; Hu, M.; Wei, S.; Kong, H.; Huang, X.; Bao, Y.; Wang, Y. Combined effects of toxic Microcystis aeruginosa and hypoxia on the digestive enzyme activities of the triangle sail mussel Hyriopsis cumingii. Aquat. Toxicol. 2019, 212, 241–246. [Google Scholar] [CrossRef]
- Kong, H.; Wu, F.; Jiang, X.; Wang, T.; Hu, M.; Chen, J.; Huang, W.; Bao, Y.; Wang, Y. Nano-TiO2 impairs digestive enzyme activities of marine mussels under ocean acidification. Chemosphere 2019, 237, 124561. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Sadegh, T.H.; Dawood, M.A.O.O.; Dadar, M.; Sheikhzadeh, N. The effects of dietary Pediococcus pentosaceus on growth performance, hemato-immunological parameters and digestive enzyme activities of common carp (Cyprinus carpio). Aquaculture 2020, 516, 734656. [Google Scholar] [CrossRef]
- Barría, C.; Brandts, I.; Tort, L.; Oliveira, M.; Teles, M. Effect of nanoplastics on fish health and performance: A review. Mar. Pollut. Bull. 2020, 151, 110791. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Sundarrajan, L.; Bertucci, J.I.; Unniappan, S. Why goldfish? Merits and challenges in employing goldfish as a model organism in comparative endocrinology research. Gen. Comp. Endocrinol. 2018, 257, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Cerra, M.C.; Imbrogno, S. The goldfish Carassius auratus: An emerging animal model for comparative cardiac research. J. Comp. Physiol. B 2021, 192, 27–48. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Estrela, F.N.; de Lima Rodrigues, A.S.; Chagas, T.Q.; Pereira, P.S.; Silva, F.G.; Malafaia, G. Nanopolystyrene particles at environmentally relevant concentrations causes behavioral and biochemical changes in juvenile grass carp (Ctenopharyngodon idella). J. Hazard. Mater. 2021, 403, 123864. [Google Scholar] [CrossRef]
- Marana, M.H.; Poulsen, R.; Thormar, E.A.; Clausen, C.G.; Thit, A.; Mathiessen, H.; von Gersdorff Jørgensen, L. Plastic nanoparticles cause mild inflammation, disrupt metabolic pathways, change the gut microbiota and affect reproduction in zebrafish: A full generation multi-omics study. J. Hazard. Mater. 2022, 424, 127705. [Google Scholar] [CrossRef]
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Llorca, M.; Vega-Herrera, A.; Schirinzi, G.; Savva, K.; Abad, E.; Farré, M. Screening of suspected micro(nano)plastics in the Ebro Delta (Mediterranean Sea). J. Hazard. Mater. 2021, 404, 124022. [Google Scholar] [CrossRef]
- Brandts, I.; Balasch, J.C.; Gonçalves, A.P.; Martins, M.A.; Pereira, M.L.; Tvarijonaviciute, A.; Oliveira, M. Immuno-modulatory effects of nanoplastics and humic acids in the European seabass (Dicentrarchus labrax). J. Hazard. Mater. 2021, 414, 125562. [Google Scholar] [CrossRef]
- Hou, B.L.; Wang, F.Y.; Liu, T.; Wang, Z.P. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible impact on human health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Clark, N.J.; Boyle, D.; Eynon, B.P.; Handy, R.D. Dietary Exposure to Silver Nitrate Compared to Two Forms of Silver Nanoparticles in Rainbow Trout: Bioaccumulation Potential with Minimal Physiological Effects. Environ. Sci. Nano 2019, 6, 1393–1405. [Google Scholar] [CrossRef]
- Clark, N.J.; Khan, F.R.; Crowther, C.; Mitrano, D.M.; Thompson, R.C. Uptake, distribution and elimination of palladium-doped polystyrene nanoplastics in rainbow trout (Oncorhynchus mykiss) following dietary exposure. Sci. Total Environ. 2023, 854, 158765. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, 4th ed.; U.S. Environmental Protection Agency, Office of Water: Washington, DC, USA, 2002; EPA-821-R-02-013.
- Balamurugan, J.; Kumar, T.T.A.; Prakash, S.; Meenakumari, B.; Balasundaram, C.; Harikrishnan, R. Clove extract: A potential source for stress free transport of fish. Aquaculture 2016, 454, 171–175. [Google Scholar] [CrossRef]
- Ahmadi-Noorbakhsh, S.; Mirabzadeh Ardakani, E.; Sadighi, J.; Aldavood, S.J.; Farajli Abbasi, M.; Farzad-Mohajeri, S.; Shamsi Gooshki, E. Guideline for the care and use of laboratory animals in Iran. Lab. Anim. 2021, 50, 303–305. [Google Scholar] [CrossRef]
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322. [Google Scholar] [CrossRef]
- Resende, A.C.; Pereira, D.M.C. IBRtools: An R package for calculating integrated biomarker indexes. Ecol. Evol. 2024, 14, e10864. [Google Scholar] [CrossRef]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO: Rome, Italy, 2017. [Google Scholar]
- Jacob, H.; Besson, M.; Swarzenski, P.W.; Lecchini, D.; Metian, M. Effects of virgin micro- and nanoplastics on fish: Trends, meta-analysis, and perspectives. Environ. Sci. Technol. 2020, 54, 4733–4745. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- Guerrera, M.C.; Aragona, M.; Porcino, C.; Fazio, F.; Laurà, R.; Levanti, M.; Germanà, A. Micro and nano plastics distribution in fish as model organisms: Histopathology, blood response and bioaccumulation in different organs. Appl. Sci. 2021, 11, 5768. [Google Scholar] [CrossRef]
- Bao, S.; Yi, J.; Xian, B.; Rao, C.; Xiang, D.; Tang, W.; Fang, T. Global analysis of the adverse effects of micro- and nanoplastics on intestinal health and microbiota of fish. J. Hazard. Mater. 2024, 470, 134157. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Ghosal, I.; Das, D.; Chakraborty, S.B. Melatonin ameliorates H2O2-induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol. Res. 2018, 51, 17. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yue, Y.; Bao, X.; Feng, X.; Ou, Z.; Qiu, Y.; Yu, H. Effects of Polystyrene Nanoplastics on Oxidative Stress, Histopathology and Intestinal Microbiota in Largemouth Bass (Micropterus salmoides). Aquacult. Rep. 2022, 27, 101423. [Google Scholar] [CrossRef]
- Abele, D.; Vazquez-Medina, J.P.; Zenteno-Savin, T. (Eds.) Oxidative Stress in Aquatic Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Chainy, G.B.N.; Paital, B.; Dandapat, J. An overview of seasonal changes in oxidative stress and antioxidant defense parameters in some invertebrate and vertebrate species. Scientifica 2016, 2016, 6126570. [Google Scholar] [CrossRef]
- Romano, N.; Ashikin, M.; Teh, J.C.; Syukri, F.; Karami, A. Effects of pristine polyvinyl chloride fragments on whole body histology and protease activity in silver barb Barbodes gonionotus fry. Environ. Pollut. 2018, 237, 1106–1111. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, X.; Ren, A.; Shi, D.K.; Shi, L.; Zhu, J.; Yu, H.S.; Zhao, M.W. Functional Analysis of the Role of Glutathione Peroxidase (GPx) in the ROS Signaling Pathway, Hyphal Branching and the Regulation of Ganoderic Acid Biosynthesis in Ganoderma lucidum. Microbiol. Res. 2018, 209, 43–54. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, J.C.; Kim, J.H. Toxic effects of microplastic (polyethylene) on fish: Accumulation, hematological parameters and antioxidant responses in Korean bullhead, Pseudobagrus fulvidraco. Sci. Total Environ. 2023, 877, 162874. [Google Scholar] [CrossRef]
- Pei, X.; Heng, X.; Chu, W. Polystyrene Nano/Microplastics Induce Microbiota Dysbiosis, Oxidative Damage, and Innate Immune Disruption in Zebrafish. Microb. Pathog. 2022, 163, 105387. [Google Scholar] [CrossRef]
- Kang, H.M.; Byeon, E.; Jeong, H.; Kim, M.S.; Chen, Q.; Lee, J.S. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka, Oryzias melastigma. J. Hazard. Mater. 2021, 405, 124207. [Google Scholar] [CrossRef]
- Rios-Fuster, B.; Arechavala-Lopez, P.; García-Marcos, K.; Alomar, C.; Compa, M.; Álvarez, E.; Deudero, S. Experimental Evidence of Physiological and Behavioral Effects of Microplastic Ingestion in Sparus aurata. Aquat. Toxicol. 2021, 231, 105737. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Chen, X.; Jiang, H.; Wang, M.; Zhang, T.; Zhang, W. Effects of Acute Exposure to Polystyrene Nanoplastics on the Channel Catfish Larvae: Insights from Energy Metabolism and Transcriptomic Analysis. Front. Physiol. 2022, 13, 923278. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Li, D.; Pan, B.; Han, X.; Lu, Y.; Wang, X. Toxicity risks associated with trace metals call for conservation of threatened fish species in heavily sediment-laden Yellow River. J. Hazard. Mater. 2023, 448, 130928. [Google Scholar] [CrossRef]
- Zhou, Y.; Gui, L.; Wei, W.; Xu, E.G.; Zhou, W.; Sokolova, I.M.; Wang, Y. Low Particle Concentrations of Nanoplastics Impair the Gut Health of Medaka. Aquat. Toxicol. 2023, 256, 106422. [Google Scholar] [CrossRef]
- Yang, H.; Ju, J.; Wang, Y.; Zhu, Z.; Lu, W.; Zhang, Y. Micro- and Nano-Plastics Induce Kidney Damage and Suppression of Innate Immune Function in Zebrafish (Danio rerio) Larvae. Sci. Total Environ. 2024, 931, 172952. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary Administration of PVC and PE Microplastics Produces Histological Damage, Oxidative Stress and Immunoregulation in European Sea Bass (Dicentrarchus labrax L.). Fish. Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Choi, J.S.; Jung, Y.J.; Hong, N.H.; Hong, S.H.; Park, J.W. Toxicological effects of irregularly shaped and spherical microplastics in a marine teleost, the sheepshead minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 2018, 129, 231–240. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, X.; Sui, Q.; Sun, X.; Zhu, L.; Booth, A.M.; Xia, B. Polystyrene nanoplastics affected the nutritional quality of Chlamys farreri through disturbing the function of gills and physiological metabolism: Comparison with microplastics. Sci. Total Environ. 2024, 910, 168457. [Google Scholar] [CrossRef]
- Singh, Z.; Karthigesu, I.P.; Singh, P.; Rupinder, K.A.U.R. Use of malondialdehyde as a biomarker for assessing oxidative stress in different disease pathologies: A review. Iran. J. Public Health 2014, 43 (Suppl. S3), 7–16. [Google Scholar]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.U.; Chen, H.; Gu, H.; Wang, T.; Dupont, S.; Kong, H.; Wang, Y. Antioxidant responses of the mussel Mytilus coruscus co-exposed to ocean acidification, hypoxia and warming. Mar. Pollut. Bull. 2021, 162, 111869. [Google Scholar] [CrossRef]
- Gomez-Zubeldia, M.A.; Hernandez, R.; Viguera, J.; Arbues, J.J.; Aparicio, A.; Millan, J.C. Effect of bilateral ovariectomy and ovarian steroid hormones on the antioxidant systems and plasma malondialdehyde activity in Wistar rats. Endocr. Res. 2000, 26, 97–107. [Google Scholar] [CrossRef]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Devin, S.; Burgeot, T.; Giambérini, L.; Minguez, L.; Pain-Devin, S. The integrated biomarker response revisited: Optimization to avoid misuse. Environ. Sci. Pollut. Res. 2014, 21, 2448–2454. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Meng, L.J.; Li, X.X.; Wang, M.H.; Gao, J.Z.; Chen, Z.Z. Integrated response of growth, antioxidant defense and isotopic composition to microplastics in juvenile guppy (Poecilia reticulata). J. Hazard. Mater. 2020, 399, 123044. [Google Scholar] [CrossRef]
- Banaei, M.; Forouzanfar, M.; Jafarinia, M. Toxic effects of polyethylene microplastics on transcriptional changes, biochemical response, and oxidative stress in common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 261, 109423. [Google Scholar] [CrossRef]
- Banaee, M.; Akhlaghi, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Rakhshaninejad, M. Combined effects of exposure to sub-lethal concentration of the insecticide chlorpyrifos and the herbicide glyphosate on the biochemical changes in the freshwater crayfish Pontastacus leptodactylus. Ecotoxicology 2020, 29, 1500–1515. [Google Scholar] [CrossRef]
- Balabanova, L.; Bondarev, G.; Seitkalieva, A.; Son, O.; Tekutyeva, L. Insights into alkaline phosphatase anti-inflammatory mechanisms. Biomedicines 2024, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.; Osman, A.G.; Sayed, A.E.D.H. Assessment the effect of exposure to microplastics in Nile tilapia (Oreochromis niloticus) early juvenile: I. Blood biomarkers. Chemosphere 2019, 228, 345–350. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Bhattacharjee, M.; Kadirvelu, K.; Ramesh, M. Exposure to polystyrene microplastics induced gene modulated biological responses in zebrafish (Danio rerio). Chemosphere 2021, 281, 128592. [Google Scholar] [CrossRef] [PubMed]
- Nematdoost Haghi, B.; Banaee, M. Effects of micro-plastic particles on paraquat toxicity to common carp (Cyprinus carpio): Biochemical changes. Int. J. Environ. Sci. Technol. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Banaee, M.; Soltanian, S.; Sureda, A.; Gholamhosseini, A.; Haghi, B.N.; Akhlaghi, M.; Derikvandy, A. Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 2019, 236, 124335. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.A.; Salaah, S.M.; Hamed, M.; Sayed, A.E.D.H. Toxicity of Co-Exposure of Microplastics and Lead in African Catfish (Clarias gariepinus). Front. Vet. Sci. 2023, 10, 1279382. [Google Scholar] [CrossRef]
- Lai, W.; Xu, D.; Li, J.; Wang, Z.; Ding, Y.; Wang, X.; Ai, Q. Dietary Polystyrene Nanoplastics Exposure Alters Liver Lipid Metabolism and Muscle Nutritional Quality in Carnivorous Marine Fish Large Yellow Croaker (Larimichthys crocea). J. Hazard. Mater. 2021, 419, 126454. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.R.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.N.; Liu, J.H.; Feng, X.S. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus). Environ. Pollut. 2018, 243, 462–471. [Google Scholar] [CrossRef]
- Beltifa, A.; Feriani, A.; Macherki, M.; Ghorbel, A.; Ghazouani, L.; Di Bella, G.; Mansour, H.B. Persistent plasticizers and bisphenol in the cheese of Tunisian markets induced biochemical and histopathological alterations in male BALB/c mice. Environ. Sci. Pollut. Res. 2018, 25, 6545–6557. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Zhu, J.G.; Zhang, Y.S.; Gao, J.Z.; Chen, Z.Z. Exposure to microplastics impairs digestive performance, stimulates immune response and induces microbiota dysbiosis in the gut of juvenile guppy (Poecilia reticulata). Sci. Total Environ. 2020, 733, 138929. [Google Scholar] [CrossRef]
- Frank, Y.A.; Interesova, E.A.; Solovyev, M.M.; Xu, J.; Vorobiev, D.S. Effect of microplastics on the activity of digestive and oxidative-stress-related enzymes in peled whitefish (Coregonus peled Gmelin) larvae. Int. J. Mol. Sci. 2023, 24, 10998. [Google Scholar] [CrossRef]
- Wu, W.; Qiu, J.; Lin, Y.; Li, X.; Li, W.; Ma, K.; Fu, Y. Enzymatic stress responses of Coreius guichenoti to microplastics with different particle sizes. Toxics 2023, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Barus, B.S.; Purwiyanto, A.I.S.; Suteja, Y.; Dwinanti, S.H. The effect of single and combined microplastics with heavy metals Cu and Pb on digestive enzymes in Paphia undulata. Res. Sq. 2023; submitted. [Google Scholar] [CrossRef]
- Trestrail, C.; Walpitagama, M.; Miranda, A.; Nugegoda, D.; Shimeta, J. Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021, 779, 146418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, L.; Xu, H.; Xu, E.G.; Li, M.; Wang, Y. Long-term exposure to polystyrene nanoplastics impairs the liver health of medaka. Water 2022, 14, 2767. [Google Scholar] [CrossRef]
- Xiao, K.; Song, L.; Li, Y.; Li, C.; Zhang, S. Dietary intake of microplastics impairs digestive performance, induces hepatic dysfunction, and shortens lifespan in the annual fish Nothobranchius guentheri. Biogerontology 2023, 24, 207–223. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azarm-Karnagh, S.; Sattari, M.; Banaee, M.; Shirkavand Hadavand, B.; Falco, F. Effects of Polystyrene Nanoplastics on Oxidative Stress, Blood Biochemistry, and Digestive Enzyme Activity in Goldfish (Carassius auratus). Toxics 2025, 13, 336. https://doi.org/10.3390/toxics13050336
Azarm-Karnagh S, Sattari M, Banaee M, Shirkavand Hadavand B, Falco F. Effects of Polystyrene Nanoplastics on Oxidative Stress, Blood Biochemistry, and Digestive Enzyme Activity in Goldfish (Carassius auratus). Toxics. 2025; 13(5):336. https://doi.org/10.3390/toxics13050336
Chicago/Turabian StyleAzarm-Karnagh, Sasan, Masoud Sattari, Mahdi Banaee, Behzad Shirkavand Hadavand, and Francesca Falco. 2025. "Effects of Polystyrene Nanoplastics on Oxidative Stress, Blood Biochemistry, and Digestive Enzyme Activity in Goldfish (Carassius auratus)" Toxics 13, no. 5: 336. https://doi.org/10.3390/toxics13050336
APA StyleAzarm-Karnagh, S., Sattari, M., Banaee, M., Shirkavand Hadavand, B., & Falco, F. (2025). Effects of Polystyrene Nanoplastics on Oxidative Stress, Blood Biochemistry, and Digestive Enzyme Activity in Goldfish (Carassius auratus). Toxics, 13(5), 336. https://doi.org/10.3390/toxics13050336







