Bioaccumulation, Biotransformation and Oxidative Stress of 6:2 Fluorotelomer Sulfonamidoalkyl Betaine (6:2 FTAB) in Earthworms (Eisenia fetida)
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. In Vivo Exposure Experiment
2.3. In Vitro Exposure Experiment
2.4. Enzyme Assays
2.5. Biodegradation of 6:2 FTAB by Earthworm Gut Microbes
2.6. Chemical Extraction and Instrumental Analysis
2.7. Quality Assurance and Quality Control
2.8. Data Analysis
2.9. Statistical Analysis
3. Results and Discussion
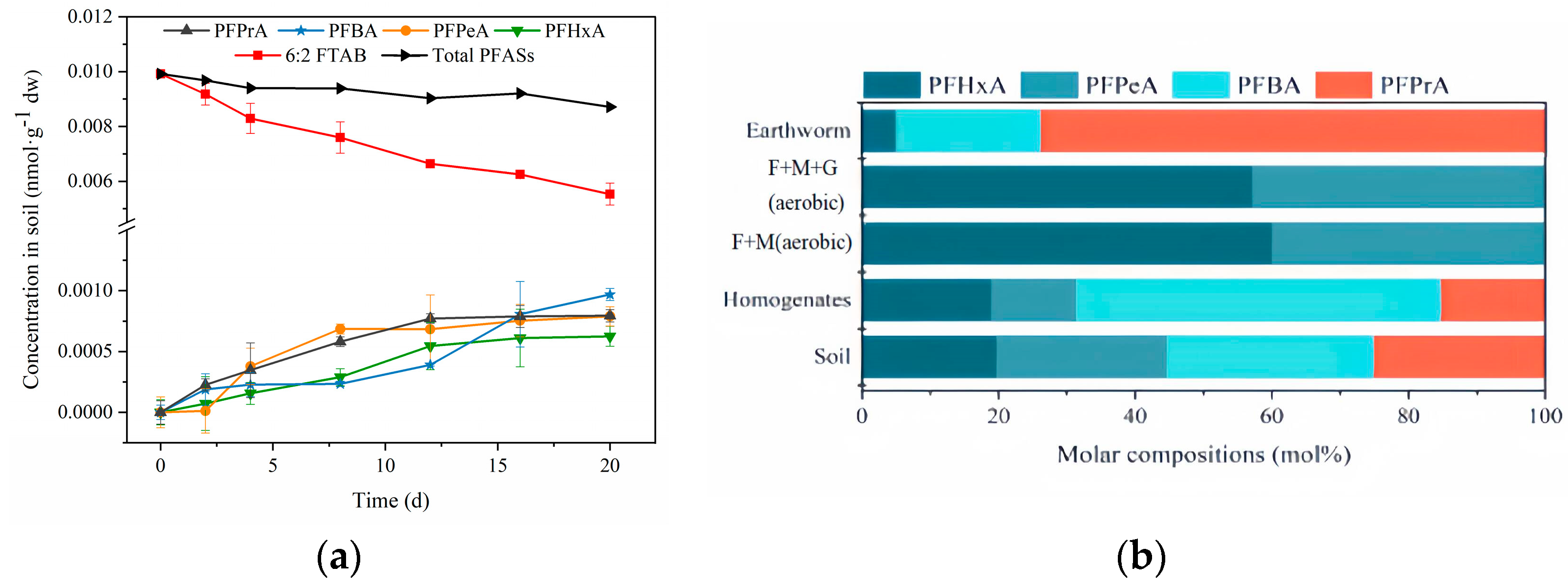
3.1. Degradation of 6:2 FTAB in Soil
3.2. Uptake and Elimination of 6:2 FTAB in Earthworms
3.3. In Vitro Biotransformation of 6:2 FTAB in Earthworm Homogenate
3.4. Biodegradation of 6:2 FTAB by Earthworm Gut Microorganisms
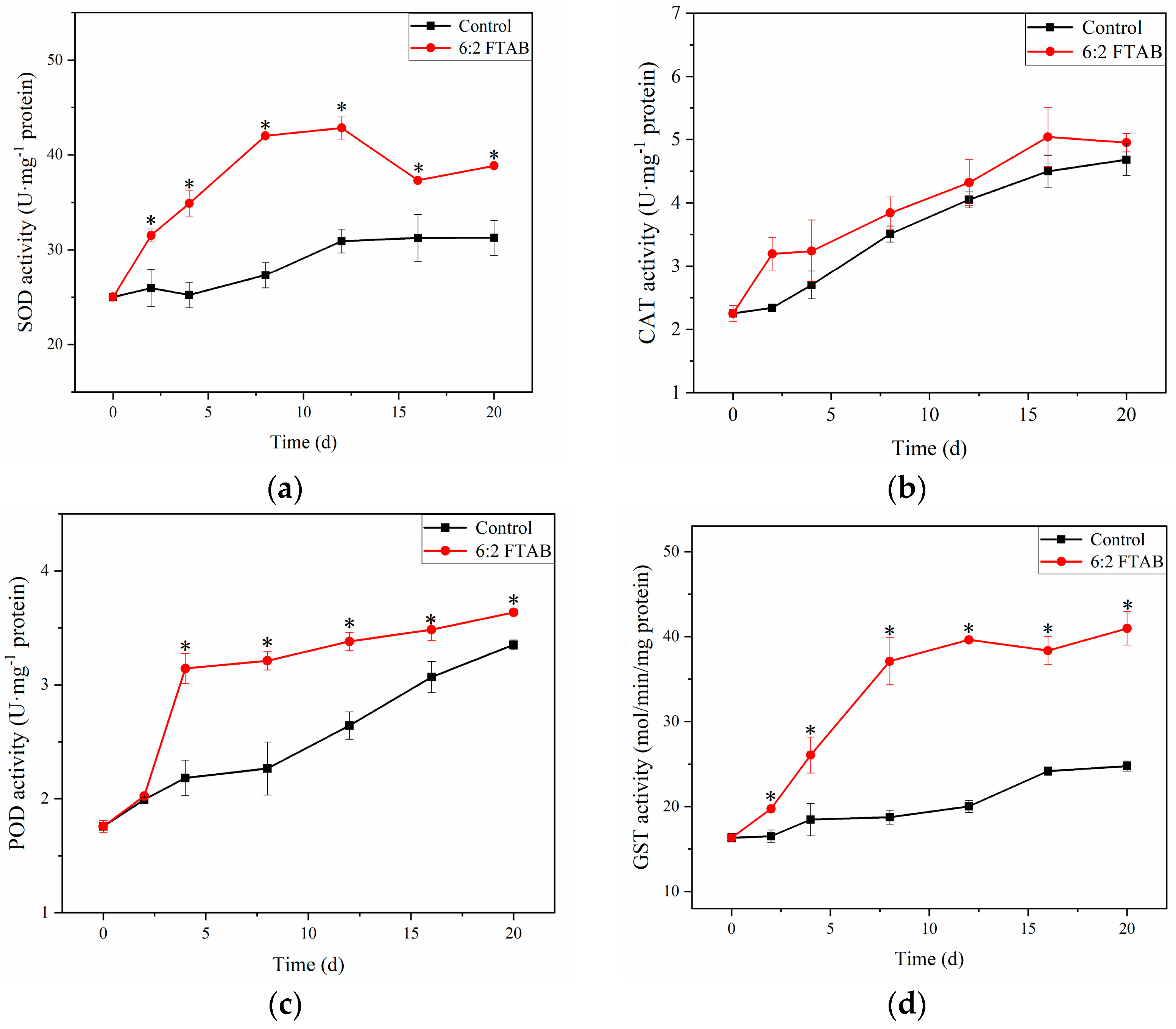
3.5. Oxidative Stress of Earthworms in Response to 6:2 FTAB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; Van Leeuwen, S.P.J. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Wu, Y.M.; Wang, S.J.; Chen, M.; Liu, Q.; Zhu, L.Y. Accumulation and transformation of perfluorooctane sulfonamide in wheat and earthworms. Chin. J. Environ. Sci. 2021, 41, 2434–2440. [Google Scholar]
- Li, J.G. Perfluorinated organic compounds: Emerging environmental pollutants with potential health risks. Chin. J. Prev. Med. 2015, 49, 467–469. [Google Scholar]
- Wang, Z.Y.; Cousins, I.T.; Scheringer, M.; Hungerbühler, K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2015, 60, 242–248. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.A.; Shi, G.; Liu, C.; Hao, Q.; Wu, L. Occurrence and risk assessment of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in surface water, groundwater, and sediments of the Jin River Basin, Southeastern China. Bull. Environ. Contam. Toxicol. 2022, 108, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Yvonne, R.; Michael, T.S.; Lisa, T.; Robyn, L.T. Review of the zebrafish as a model to investigate per- and polyfluoroalkyl substance toxicity. Toxicol. Sci. 2023, 194, 138–152. [Google Scholar]
- Boiteux, V.; Dauchy, X.; Bach, C.; Colin, A.; Hemard, J.; Sagres, V.; Rosin, C.; Munoz, J.-F. Concentrations and patterns of perfluoroalkyl and polyfluoroalkyl substances in a river and three drinking water treatment plants near and far from a major production source. Sci. Total Environ. 2017, 583, 393–400. [Google Scholar] [CrossRef]
- Dauchy, X.; Boiteux, V.; Bach, C.; Colin, A.; Hemard, J.; Rosin, C.; Munoz, J.-F. Mass flows and fate of per- and polyfluoroalkyl substances (PFASs) in the wastewater treatment plant of a fluorochemical manufacturing facility. Sci. Total Environ. 2017, 576, 549–558. [Google Scholar] [CrossRef]
- Shi, G.; Xie, Y.; Guo, Y.; Dai, J. 6:2 Fluorotelomer sulfonamide alkylbetaine (6:2 FTAB), a novel perfluorooctane sulfonate alternative, induced developmental toxicity in zebrafish embryos. Aquat. Toxicol. 2018, 195, 24–32. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, L.; Yang, X.; Zhao, S. Behaviors of 6:2 fluorotelomer sulfonamide alkylbetaine (6:2 FTAB) in wheat seedlings: Bioaccumulation, biotransformation and ecotoxicity. Ecotoxicol. Environ. Saf. 2022, 238, 113585. [Google Scholar] [CrossRef]
- OECD Test No. 207: Earthworm, Acute Toxicity Tests. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1984.
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharmacal Res. 2005, 28, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Ding, J.; Xiong, C.; Zhu, D.; Li, G.; Jia, X.-Y.; Zhu, Y.-G.; Xue, X.-M. Exposure to microplastics lowers arsenic accumulation and alters gut bacterial communities of earthworm Metaphire californica. Environ. Pollut. 2019, 251, 110–116. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, T.; Zhu, L.; Yang, L.; Zong, Y.; Zhao, H.; Hu, L.; Zhan, J. Formation of perfluorocarboxylic acids (PFCAs) during the exposure of earthworms to 6:2 fluorotelomer sulfonic acid (6:2 FTSA). Sci. Total Environ. 2021, 760, 143356. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Zhong, Z.; Liu, T.; Liang, T.; Zhan, J. Contributions of enzymes and gut microbes to biotransformation of perfluorooctane sulfonamide in earthworms (Eisenia fetida). Chemosphere 2020, 238, 124619. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, F.; Kärrman, A.; Eriksson, U.; Yeung, L.W.Y. Occurrence and fate of fluoroalkyl sulfonamide-based copolymers in earthworms–bioavailability, transformation, and potential impact of sludge application. Environ. Sci. Technol. 2024, 58, 18304–18312. [Google Scholar] [CrossRef] [PubMed]
- Loewen, M.; Halldorson, T.; Wang, F.Y.; Tomy, G. Fluorotelomer carboxylic acids and PFOS in rainwater from an urban center in Canada. Environ. Sci. Technol. 2005, 39, 2944–2951. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, T.; Zhang, J.; Wang, J.; Wang, J.; Zhu, L.; Yang, J. Biochemical and genetic toxicity of the ionic liquid 1-octyl-3-methylimidazolium chloride on earthworms (Eisenia fetida). Environ. Toxicol. Chem. 2015, 35, 411–418. [Google Scholar] [CrossRef]
- Moe, M.K.; Huber, S.; Svenson, J.; Hagenaars, A.; Pabon, M.; Trümper, M.; Berger, U.; Knapen, D.; Herzke, D. The structure of the fire fighting foam surfactant Forafac1157 and its biological and photolytic transformation products. Chemosphere 2012, 89, 869–875. [Google Scholar] [CrossRef]
- Li, R.; Munoz, G.; Liu, Y.; Sauvé, S.; Ghoshal, S.; Liu, J. Transformation of novel polyfluoroalkyl substances (PFASs) as co-contaminants during biopile remediation of petroleum hydrocarbons. J. Hazard. Mater. 2019, 362, 140–147. [Google Scholar] [CrossRef]
- Fang, B.; Zhang, Y.; Chen, H.; Qiao, B.; Yu, H.; Zhao, M.; Gao, M.; Li, X.; Yao, Y.; Zhu, L.; et al. Stability and biotransformation of 6:2 fluorotelomer sulfonic acid, sulfonamide amine oxide, and sulfonamide alkylbetaine in aerobic sludge. Environ. Sci. Technol. 2024, 58, 2446–2457. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.; Munoz, G.; Bottos, E.M.; Duy, S.V.; Sauvé, S.; Liu, J.; Van Hamme, J.D. Degradation and defluorination of 6:2 fluorotelomer sulfonamidoalkyl betaine and 6:2 fluorotelomer sulfonate by Gordonia sp. strain NB4-1Y under sulfur-limiting conditions. Sci. Total Environ. 2018, 647, 690–698. [Google Scholar] [CrossRef]
- Liang, J.; Xia, X.; Zaman, W.Q.; Zhang, W.; Lin, K.; Hu, S.; Lin, Z. Bioaccumulation and toxic effects of decabromodiphenyl ether in the presence of nanoscale zero-valent iron in an earthworm–soil system. Chemosphere 2017, 169, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhu, L.; Liu, L.; Liu, Z.; Zhang, Y. Bioaccumulation of perfluoroalkyl carboxylates (PFCAs) and perfluoroalkane sulfonates (PFSAs) by earthworms (Eisenia fetida) in soil. Environ. Pollut. 2013, 179, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, L.; Corvini, P.F.; Ji, R. Accumulation and transformation of 2,2′,4,4′-tetrabrominated diphenyl ether (BDE47) by the earthworm Metaphire vulgaris in soil. Bull. Environ. Contam. Toxicol. 2020, 104, 701–706. [Google Scholar] [CrossRef]
- Verma, K.; Agrawal, N.; Farooq, M.; Misra, R.; Hans, R. Endosulfan degradation by a Rhodococcus strain isolated from earthworm gut. Ecotoxicol. Environ. Saf. 2006, 64, 377–381. [Google Scholar] [CrossRef]
- Ramteke, P.W.; Hans, R.K. Isolation of hexachlorocyclohexane (HCH) degrading microorganisms from earthworm gut. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1992, 27, 2113–2122. [Google Scholar]
- Valavanidis, A.; Vlahogianni, T.; Dassenakis, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, T.; Wang, B.; Fu, J.; Liang, T.; Zhong, Z.; Zhan, J.; Liu, L. Accumulation, biodegradation and toxicological effects of N-ethyl perfluorooctane sulfonamidoethanol on the earthworms Eisenia fetida exposed to quartz sands. Ecotoxicol. Environ. Saf. 2019, 181, 138–145. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Q.; Xie, X.; Lin, D.; Dong, L. Oxidative stress and DNA damage in the earthworm Eisenia fetida induced by toluene, ethylbenzene and xylene. Ecotoxicology 2010, 19, 1551–1559. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Wen, Y.; Liu, W. Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ. Pollut. 2013, 174, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wu, Q.; Kania-Korwel, I.; Tharappel, J.C.; Telu, S.; Coleman, M.C.; Glauert, H.P.; Kannan, K.; Mariappan, S.V.S.; Spitz, D.R.; et al. Subacute exposure to N-ethyl perfluorooctanesulfonamidoethanol results in the formation of perfluorooctanesulfonate and alters superoxide dismutase activity in female rats. Arch. Toxicol. 2009, 83, 909–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, B.; Hu, X.; Wu, Y.; Pan, Y.; Huang, H.; Liu, L.; Zhang, S. Uptake, translocation, and metabolism of 8:2 fluorotelomer alcohol in soybean (Glycine max L. Merrill). Environ. Sci. Technol. 2016, 50, 13309–13317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Fang, S.; Zhu, L.; Liu, L.; Liu, Z.; Zhang, Y. Mutual impacts of wheat (Triticum aestivum L.) and earthworms (Eisenia fetida) on the bioavailability of perfluoroalkyl substances (PFASs) in soil. Environ. Pollut. 2014, 184, 495–501. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Zhu, L.; Liang, T.; Meng, C.; Yang, L.; Lv, J.; Liu, L. Uptake, elimination and biotransformation of N-ethyl perfluorooctane sulfonamide (N-EtFOSA) by the earthworms (Eisenia fetida) after in vivo and in vitro exposure. Environ. Pollut. 2018, 241, 19. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L. Uptake and metabolism of 10:2 fluorotelomer alcohol in soil-earthworm (Eisenia fetida) and soil-wheat (Triticum aestivum L.) systems. Environ. Pollut. 2017, 220, 124–131. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Fang, M.; Bai, Z.; Zong, Y.; Zhao, S.; Zhan, J. Bioaccumulation, Biotransformation and Oxidative Stress of 6:2 Fluorotelomer Sulfonamidoalkyl Betaine (6:2 FTAB) in Earthworms (Eisenia fetida). Toxics 2025, 13, 337. https://doi.org/10.3390/toxics13050337
Zhang X, Fang M, Bai Z, Zong Y, Zhao S, Zhan J. Bioaccumulation, Biotransformation and Oxidative Stress of 6:2 Fluorotelomer Sulfonamidoalkyl Betaine (6:2 FTAB) in Earthworms (Eisenia fetida). Toxics. 2025; 13(5):337. https://doi.org/10.3390/toxics13050337
Chicago/Turabian StyleZhang, Xinlei, Mengyao Fang, Zhiyuan Bai, Yulu Zong, Shuyan Zhao, and Jingjing Zhan. 2025. "Bioaccumulation, Biotransformation and Oxidative Stress of 6:2 Fluorotelomer Sulfonamidoalkyl Betaine (6:2 FTAB) in Earthworms (Eisenia fetida)" Toxics 13, no. 5: 337. https://doi.org/10.3390/toxics13050337
APA StyleZhang, X., Fang, M., Bai, Z., Zong, Y., Zhao, S., & Zhan, J. (2025). Bioaccumulation, Biotransformation and Oxidative Stress of 6:2 Fluorotelomer Sulfonamidoalkyl Betaine (6:2 FTAB) in Earthworms (Eisenia fetida). Toxics, 13(5), 337. https://doi.org/10.3390/toxics13050337





