Nanosilver Environmental Safety in Marine Organisms: Ecotoxicological Assessment of a Commercial Nano-Enabled Product vs an Eco-Design Formulation
Abstract
1. Introduction
2. Materials and Method
2.1. Nanosilver Characterization
2.2. Nanosilver Dissolution in Exposure Media
2.3. Microalgal Growth Inhibition Test
2.4. A. franciscana Immobilization Test
2.5. M. galloprovincialis In Vivo Exposure
2.6. Statistical Analysis
3. Results and Discussion
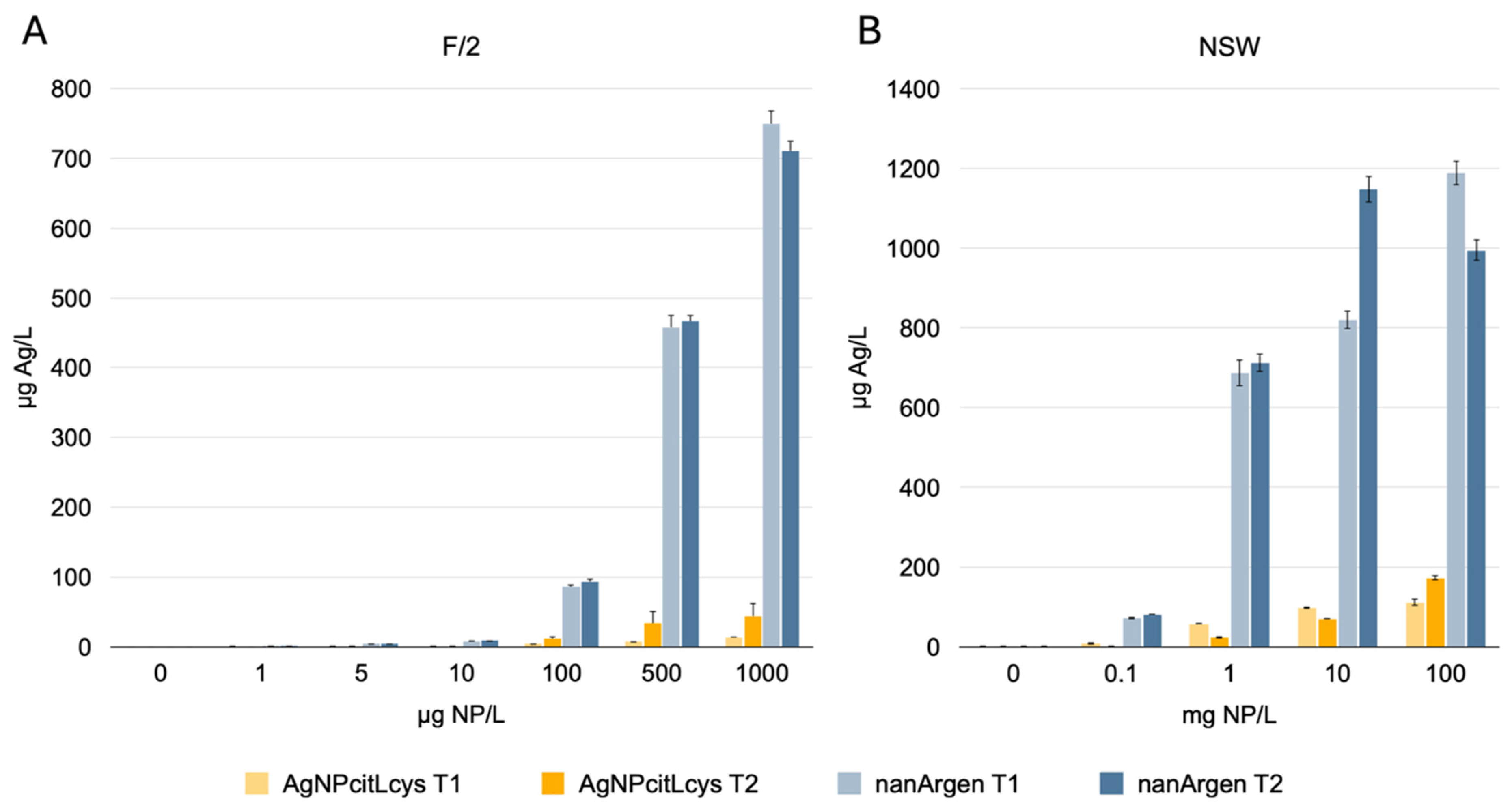
3.1. Behavior of Nanosilver Formulations in Exposure Media
3.2. Ecotoxicity Tests
3.2.1. Microalgal 72 h Growth Inhibition Test with P. tricornutum
3.2.2. A. franciscana Immobilization Test
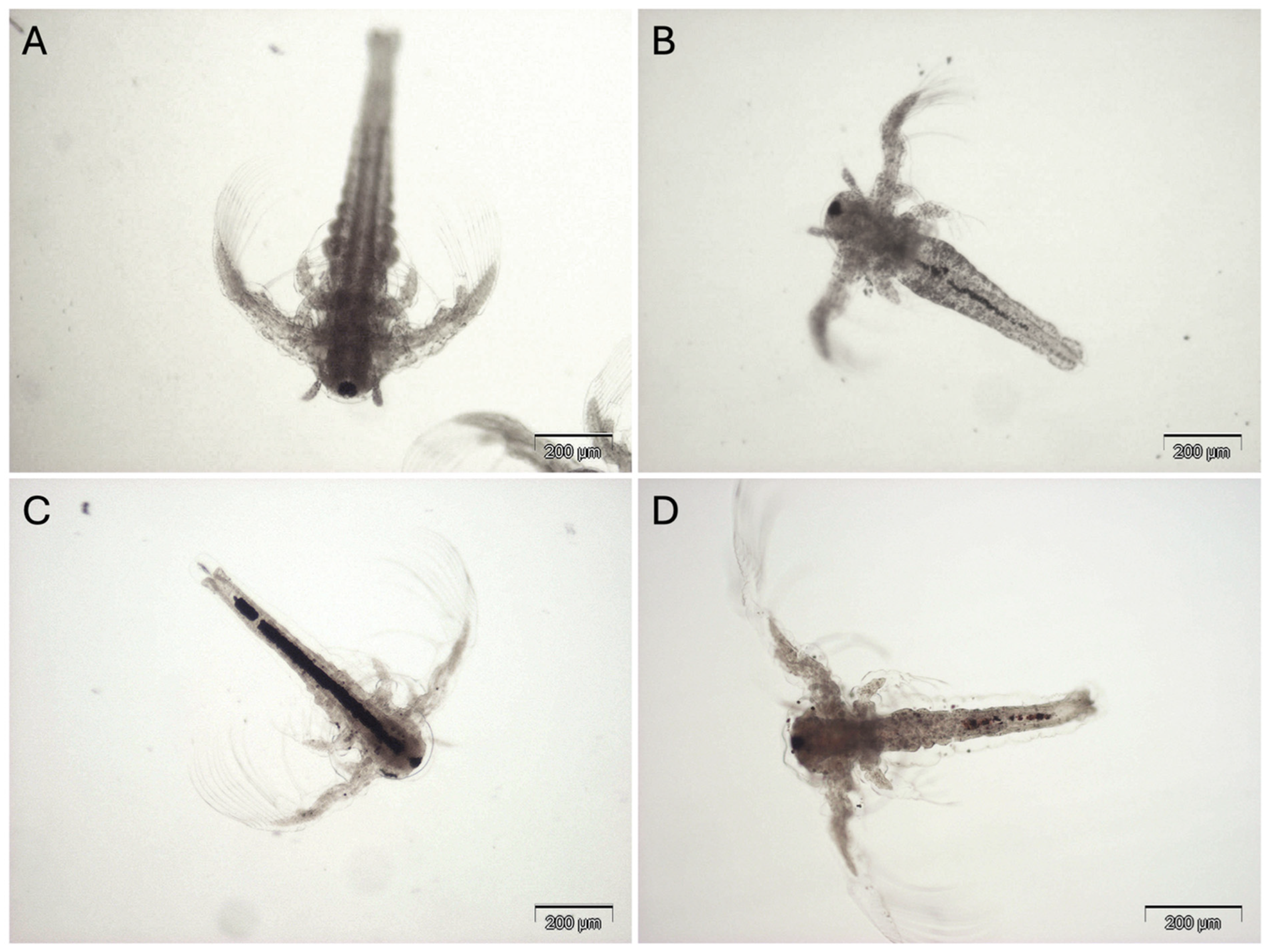
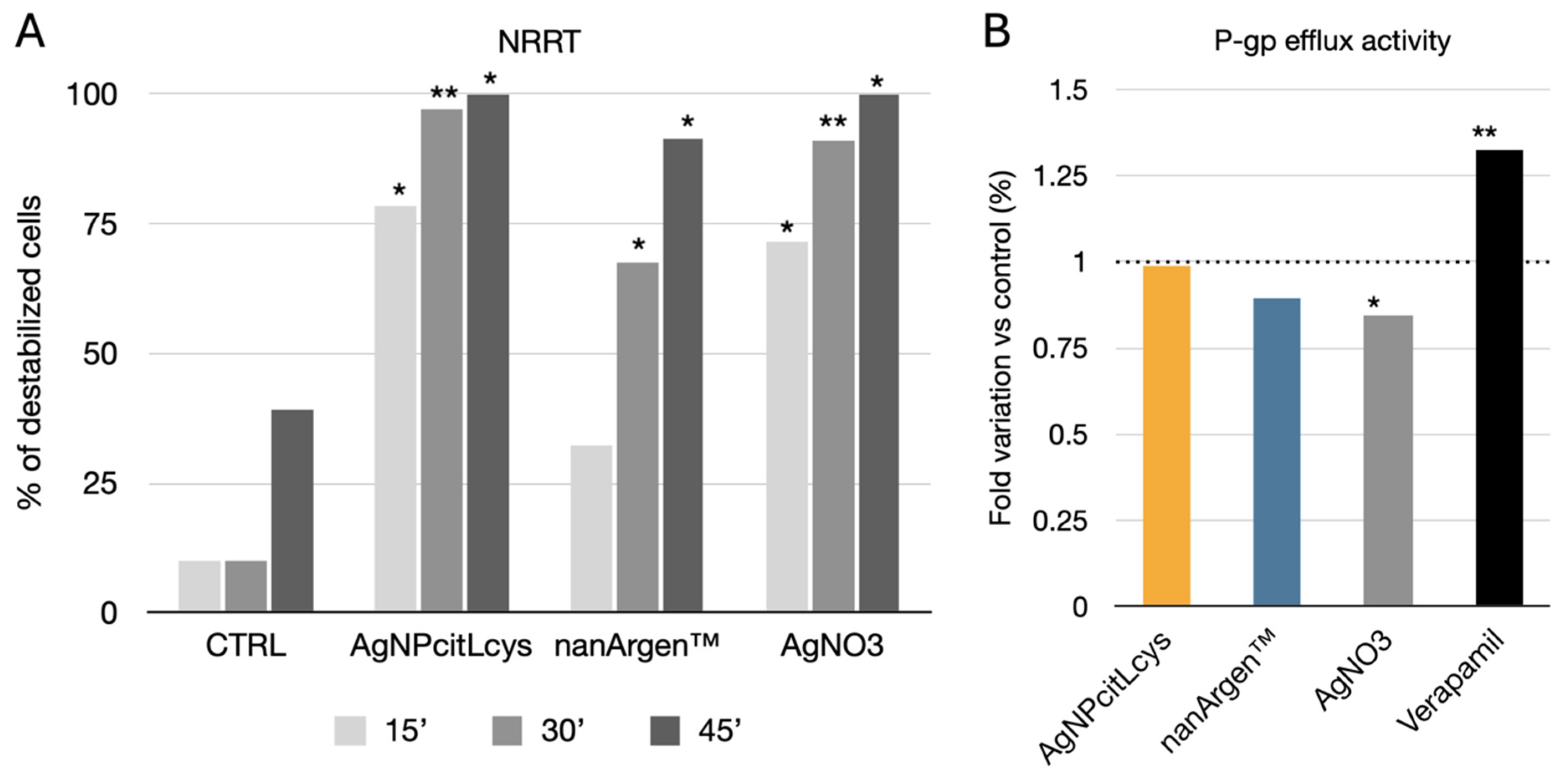
3.2.3. M. galloprovincialis In Vivo Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; von Gleich, A.; Gottschalk, F. Risks, release and concentrations of engineered nanomaterial in the environment. Sci. Rep. 2018, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Keller, A.A.; Cervantes-Avilés, P.; Nelson, J. Fast multielement quantification of nanoparticles in wastewater and sludge using single-particle ICP-MS. ACS ES&T Water 2020, 1, 205–213. [Google Scholar]
- Azimzada, A.; Jreije, I.; Hadioui, M.; Shaw, P.; Farner, J.M.; Wilkinson, K.J. Quantification and characterization of Ti-, Ce-, and Ag-nanoparticles in global surface waters and precipitation. Environ. Sci. Technol. 2021, 55, 9836–9844. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Avilés, P.; Keller, A.A. Incidence of metal-based nanoparticles in the conventional wastewater treatment process. Water Res. 2021, 189, 116603. [Google Scholar] [CrossRef]
- Ale, A.; Andrade, V.S.; Gutierrez, M.F.; Ayech, A.; Monserrat, J.M.; Desimone, M.F.; Cazenave, J. Metal-based nanomaterials in aquatic environments: What do we know so far about their ecotoxicity? Aquat. Toxicol. 2024, 275, 107069. [Google Scholar] [CrossRef]
- Azimzada, A.; Tufenkji, N.; Wilkinson, K.J. Transformations of silver nanoparticles in wastewater effluents: Links to Ag bioavailability. Environ. Sci. Nano 2017, 4, 1339–1349. [Google Scholar] [CrossRef]
- Costa, A.L.; Blosi, M.; Brigliadori, A.; Zanoni, I.; Ortelli, S.; Simeone, F.C.; Delbue, S.; D’Alessandro, S.; Parapini, S.; Vineis, C. Eco design for Ag-based solutions against SARS-CoV-2 and E. coli. Environ. Sci. Nano 2022, 9, 4295–4304. [Google Scholar] [CrossRef]
- Keller, A.A.; Ehrens, A.; Zheng, Y.; Nowack, B. Developing trends in nanomaterials and their environmental implications. Nat. Nanotechnol. 2023, 18, 834–837. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles–A material of the future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Bellingeri, A.; Protano, G.; Faleri, C.; Corsi, I.; Battocchio, C.; Iucci, G.; Tortora, L.; Secchi, V. Bifunctionalized Silver Nanoparticles as Hg2+ Plasmonic Sensor in Water: Synthesis, Characterizations, and Ecosafety. Nanomaterials 2019, 9, 1353. [Google Scholar] [CrossRef]
- Ivanišević, I. The role of silver nanoparticles in electrochemical sensors for aquatic environmental analysis. Sensors 2023, 23, 3692. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.-H.; Azzem, M.A. Recent advances in silver nanoparticle-based electrochemical sensors for determining organic pollutants in water: A review. Mater. Adv. 2021, 2, 7350–7365. [Google Scholar] [CrossRef]
- De, A.; Kalita, D. Bio-fabricated gold and silver nanoparticle based plasmonic sensors for detection of environmental pollutants: An overview. Crit. Rev. Anal. Chem. 2023, 53, 672–688. [Google Scholar] [CrossRef] [PubMed]
- Palani, G.; Trilaksana, H.; Sujatha, R.M.; Kannan, K.; Rajendran, S.; Korniejenko, K.; Nykiel, M.; Uthayakumar, M. Silver nanoparticles for waste water management. Molecules 2023, 28, 3520. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Shrivastava, C.; Chaubey, K.K.; Tyagi, S.; Kushwah, M.; Rajput, P.; Pramanik, A.; Hariharan, S.; Pandey, S.D.; Pant, G. Role of silver nanoparticles on wastewater treatment, environmental implications, and challenges. In Nanomaterials for Environmental and Agricultural Sectors; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–27. [Google Scholar]
- Xiang, Q.-Q.; Li, Q.-Q.; Wang, P.; Yang, H.-C.; Fu, Z.-H.; Liang, X.; Chen, L.-Q. Metabolomics reveals the mechanism of persistent toxicity of AgNPs at environmentally relevant concentrations to Daphnia magna. Environ. Sci. Nano 2025, 12, 563–575. [Google Scholar] [CrossRef]
- Andrade, V.S.; Ale, A.; Antezana, P.E.; Desimone, M.F.; Cazenave, J.; Gutierrez, M.F. Ecotoxicity of nanosilver on cladocerans and the role of algae provision. Environ. Sci. Pollut. Res. 2023, 30, 27137–27149. [Google Scholar] [CrossRef]
- Babaei, M.; Tayemeh, M.B.; Jo, M.S.; Yu, I.J.; Johari, S.A. Trophic transfer and toxicity of silver nanoparticles along a phytoplankton-zooplankton-fish food chain. Sci. Total Environ. 2022, 842, 156807. [Google Scholar] [CrossRef]
- Dang, F.; Huang, Y.; Wang, Y.; Zhou, D.; Xing, B. Transfer and toxicity of silver nanoparticles in the food chain. Environ. Sci. Nano 2021, 8, 1519–1535. [Google Scholar] [CrossRef]
- Lekamge, S.; Miranda, A.F.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of coated silver nanoparticles to Daphnia carinata and trophic transfer from alga Raphidocelis subcapitata. PLoS ONE 2019, 14, e0214398. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Differential protein expression in mussels Mytilus galloprovincialis exposed to nano and ionic Ag. Aquat. Toxicol. 2013, 136, 79–90. [Google Scholar] [CrossRef]
- Tortella, G.; Rubilar, O.; Durán, N.; Diez, M.; Martínez, M.; Parada, J.; Seabra, A. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Dobias, J.; Bernier-Latmani, R. Silver release from silver nanoparticles in natural waters. Environ. Sci. Technol. 2013, 47, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Diendorf, J.; Sengstock, C.; Ruiz-Gonzalez, L.; Gonzalez-Calbet, J.; Vallet-Regi, M.; Köller, M.; Epple, M. The dissolution and biological effects of silver nanoparticles in biological media. J. Mater. Chem. B 2014, 2, 1634–1643. [Google Scholar] [CrossRef]
- Smith, J.N.; Thomas, D.G.; Jolley, H.; Kodali, V.K.; Littke, M.H.; Munusamy, P.; Baer, D.R.; Gaffrey, M.J.; Thrall, B.D.; Teeguarden, J.G. All that is silver is not toxic: Silver ion and particle kinetics reveals the role of silver ion aging and dosimetry on the toxicity of silver nanoparticles. Part. Fibre Toxicol. 2018, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, B.; Fang, T. Chemical transformation of silver nanoparticles in aquatic environments: Mechanism, morphology and toxicity. Chemosphere 2018, 191, 324–334. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.u.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Janah, I.M.; Roto, R.; Konishi, K.; Siswanta, D. EDTA-capped silver nanoparticles as a probe for highly sensitive and selective colorimetric sensing of creatinine and optimization using response surface methodology-Box Behnken Design. Talanta Open 2022, 6, 100170. [Google Scholar] [CrossRef]
- Bellingeri, A.; Scattoni, M.; Venditti, I.; Battocchio, C.; Protano, G.; Corsi, I. Ecologically based methods for promoting safer nanosilver for environmental applications. J. Hazard. Mater. 2022, 438, 129523. [Google Scholar] [CrossRef]
- Bellingeri, A.; Bono, N.; Venditti, I.; Bertelà, F.; Burratti, L.; Faleri, C.; Protano, G.; Paccagnini, E.; Lupetti, P.; Candiani, G. Capping drives the behavior, dissolution and (eco) toxicity of silver nanoparticles towards microorganisms and mammalian cells. Environ. Sci. Nano 2024, 11, 2049–2060. [Google Scholar] [CrossRef]
- Kwok, K.W.; Auffan, M.; Badireddy, A.R.; Nelson, C.M.; Wiesner, M.R.; Chilkoti, A.; Liu, J.; Marinakos, S.M.; Hinton, D.E. Uptake of silver nanoparticles and toxicity to early life stages of Japanese medaka (Oryzias latipes): Effect of coating materials. Aquat. Toxicol. 2012, 120, 59–66. [Google Scholar] [CrossRef]
- Navarro, E.; Wagner, B.; Odzak, N.; Sigg, L.; Behra, R. Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ. Sci. Technol. 2015, 49, 8041–8047. [Google Scholar] [CrossRef] [PubMed]
- Biba, R.; Košpić, K.; Komazec, B.; Markulin, D.; Cvjetko, P.; Pavoković, D.; Peharec Štefanić, P.; Tkalec, M.; Balen, B. Surface coating-modulated phytotoxic responses of silver nanoparticles in plants and freshwater green algae. Nanomaterials 2021, 12, 24. [Google Scholar] [CrossRef]
- Mbanga, O.; Cukrowska, E.; Gulumian, M. Dissolution kinetics of silver nanoparticles: Behaviour in simulated biological fluids and synthetic environmental media. Toxicol. Rep. 2022, 9, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Patinha, C.C.; Farcal, R.; Moretti, C.; Mancini, L.; Rauscher, H.; Rasmussen, K.; Riego, S.J.; Sala, S. Safe and Sustainable by Design Chemicals and Materials Review of Safety and Sustainability Dimensions, Aspects, Methods, Indicators, and Tools. Available online: https://www.researchgate.net/publication/362160148 (accessed on 12 March 2025).
- Cobaleda-Siles, M.; Guillamon, A.; Delpivo, C.; Vázquez-Campos, S.; Puntes, V. Safer by design strategies. J. Phys. Conf. Ser. 2017, 838, 012016. [Google Scholar] [CrossRef]
- Gottardo, S.; Mech, A.; Drbohlavová, J.; Małyska, A.; Bøwadt, S.; Sintes, J.R.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef]
- Corsi, I.; Venditti, I.; Trotta, F.; Punta, C. Environmental safety of nanotechnologies: The eco-design of manufactured nanomaterials for environmental remediation. Sci. Total Environ. 2023, 864, 161181. [Google Scholar] [CrossRef] [PubMed]
- Ale, A.; Liberatori, G.; Vannuccini, M.L.; Bergami, E.; Ancora, S.; Mariotti, G.; Bianchi, N.; Galdopórpora, J.M.; Desimone, M.F.; Cazenave, J. Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat. Toxicol. 2019, 211, 46–56. [Google Scholar] [CrossRef]
- Rycroft, T.; Larkin, S.; Ganin, A.; Thomas, T.; Matheson, J.; Van Grack, T.; Chen, X.; Plourde, K.; Kennedy, A.; Linkov, I. A framework and pilot tool for the risk-based prioritization and grouping of nano-enabled consumer products. Environ. Sci. Nano 2019, 6, 356–365. [Google Scholar] [CrossRef]
- Corsi, I.; Desimone, M.F.; Cazenave, J. Building the bridge from aquatic nanotoxicology to safety by design silver nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 836742. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Barceló, D. Ethical principles and scientific advancements: In vitro, in silico, and non-vertebrate animal approaches for a green ecotoxicology. Green Anal. Chem. 2024, 8, 100096. [Google Scholar] [CrossRef]
- Skawina, A.; Dąbrowska, A.; Bonk, A.; Paterczyk, B.; Nowakowska, J. Tracking the micro-and nanoplastics in the terrestrial-freshwater food webs. Bivalves as sentinel species. Sci. Total Environ. 2024, 917, 170468. [Google Scholar] [CrossRef]
- ISO/10253; Water Quality—Marine Algal Growth Inhibition Test with Skeletonema costatum and Phaeodactylum tricornutum. ISO: Geneva, Switzerlands, 2006.
- Resgalla, C., Jr.; Poleza, F.; Souza, R.; Máximo, M.; Radetski, C. Evaluation of effectiveness of EDTA and sodium thiosulfate in removing metal toxicity toward sea urchin embryo-larval applying the TIE. Chemosphere 2012, 89, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.P.; Hurd, C.L.; Sander, S.G.; Armstrong, E.; Roleda, M.Y. Copper ecotoxicology of marine algae: A methodological appraisal. Chem. Ecol. 2016, 32, 786–800. [Google Scholar] [CrossRef]
- Lowe, D.M.; Fossato, V.U.; Depledge, M.H. Contaminant-induced lysosomal membrane damage in blood cells of mussels Mytilus galloprovincialis from the Venice Lagoon: An in vitro study. Mar. Ecol. Prog. Ser. 1995, 129, 189–196. [Google Scholar] [CrossRef]
- Neyfakh, A.A. Use of fluorescent dyes as molecular probes for the study of multidrug resistance. Exp. Cell Res. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Luckenbach, T.; Epel, D. Nitromusk and polycyclic musk compounds as long-term inhibitors of cellular xenobiotic defense systems mediated by multidrug transporters. Environ. Health Perspect. 2005, 113, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Nanoparticle aggregation: Principles and modeling. In Nomaterial: Impacts on Cell Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2014; pp. 19–43. [Google Scholar]
- Chu, B.; Biriukov, D.; Bischoff, M.; Předota, M.; Roke, S.; Marchioro, A. Evolution of the electrical double layer with electrolyte concentration probed by second harmonic scattering. Faraday Discuss. 2023, 246, 407–425. [Google Scholar] [CrossRef]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV–Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef]
- Levard, C.; Reinsch, B.C.; Michel, F.M.; Oumahi, C.; Lowry, G.V.; Brown, G.E., Jr. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: Impact on dissolution rate. Environ. Sci. Technol. 2011, 45, 5260–5266. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Colman, B.P.; Dale, A.L.; Truong, L.; Yang, X.; Bone, A.J.; Brown, G.E., Jr.; Tanguay, R.L.; Di Giulio, R.T. Sulfidation of silver nanoparticles: Natural antidote to their toxicity. Environ. Sci. Technol. 2013, 47, 13440–13448. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, S.; Duroudier, N.; Bilbao, E.; Mikolaczyk, M.; Schäfer, J.; Cajaraville, M.; Manzo, S. Effects of PVP/PEI coated and uncoated silver NPs and PVP/PEI coating agent on three species of marine microalgae. Sci. Total Environ. 2017, 577, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tsiola, A.; Pitta, P.; Callol, A.J.; Kagiorgi, M.; Kalantzi, I.; Mylona, K.; Santi, I.; Toncelli, C.; Pergantis, S.; Tsapakis, M. The impact of silver nanoparticles on marine plankton dynamics: Dependence on coating, size and concentration. Sci. Total Environ. 2017, 601, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Angel, B.M.; Batley, G.E.; Jarolimek, C.V.; Rogers, N.J. The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 2013, 93, 359–365. [Google Scholar] [CrossRef]
- An, H.J.; Sarkheil, M.; Park, H.S.; Yu, I.J.; Johari, S.A. Comparative toxicity of silver nanoparticles (AgNPs) and silver nanowires (AgNWs) on saltwater microcrustacean, Artemia salina. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 62–69. [Google Scholar] [CrossRef]
- de Paiva Pinheiro, S.K.; Lima, A.K.M.; Miguel, T.B.A.R.; Souza Filho, A.G.; Ferreira, O.P.; da Silva Pontes, M.; Grillo, R.; de Castro Miguel, E. Assessing toxicity mechanism of silver nanoparticles by using brine shrimp (Artemia salina) as model. Chemosphere 2024, 347, 140673. [Google Scholar] [CrossRef]
- Demarchi, C.A.; da Silva, L.M.; Niedźwiecka, A.; Ślawska-Waniewska, A.; Lewińska, S.; Dal Magro, J.; Calisto, J.F.F.; Martello, R.; Rodrigues, C.A. Nanoecotoxicology study of the response of magnetic O-Carboxymethylchitosan loaded silver nanoparticles on Artemia salina. Environ. Toxicol. Pharmacol. 2020, 74, 103298. [Google Scholar] [CrossRef]
- Efthimiou, I.; Kalamaras, G.; Papavasileiou, K.; Anastasi-Papathanasi, N.; Georgiou, Y.; Dailianis, S.; Deligiannakis, Y.; Vlastos, D. ZnO, Ag and ZnO-Ag nanoparticles exhibit differential modes of toxic and oxidative action in hemocytes of mussel Mytilus galloprovincialis. Sci. Total Environ. 2021, 767, 144699. [Google Scholar] [CrossRef]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of toxicity of Ag nanoparticles in comparison to bulk and ionic Ag on mussel hemocytes and gill cells. PLoS ONE 2015, 10, e0129039. [Google Scholar] [CrossRef]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef]
- Smital, T.; Kurelec, B. Inhibitors of the multixenobiotic resistance mechanism in natural waters: In vivo demonstration of their effects. Environ. Toxicol. Chem. 1997, 16, 2164–2170. [Google Scholar] [CrossRef]
- Shaw, B.J.; Handy, R.D. Physiological effects of nanoparticles on fish: A comparison of nanometals versus metal ions. Environ. Int. 2011, 37, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Beninger, P.G.; St-Jean, S.; Poussart, Y.; Ward, J.E. Gill function and mucocyte distribution in Placopecten magellanicus and Mytilus edulis (Mollusca: Bivalvia): The role of mucus in particle transport. Mar. Ecol. Prog. Ser. 1993, 98, 275–282. [Google Scholar] [CrossRef]
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Porter, A.; Lyons, B.P.; Galloway, T.S.; Lewis, C. Role of marine snows in microplastic fate and bioavailability. Environ. Sci. Technol. 2018, 52, 7111–7119. [Google Scholar] [CrossRef]
- Bouallegui, Y.; Ben Younes, R.; Turki, F.; Mezni, A.; Oueslati, R. Effect of exposure time, particle size and uptake pathways in immune cell lysosomal cytotoxicity of mussels exposed to silver nanoparticles. Drug Chem. Toxicol. 2018, 41, 169–174. [Google Scholar] [CrossRef]

| <2RH> (nm) Intensity | PDI | ζ-Potential (mV) | ||
|---|---|---|---|---|
| AgNPcitLcys | MilliQ | 179 ± 30 | 0.5 | −48 ± 4 |
| F/2 | 560 ± 16 | 0.7 | −8 ± 2 | |
| NSW | 773 ± 61 | 0.29 | −4 ± 1 | |
| nanArgen™ | MilliQ | 226 ± 9 | 0.4 | −14 ± 1 |
| F/2 | 931 ± 34 | 0.28 | −6 ± 3 | |
| NSW | 931 ± 20 | 0.27 | −7 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellingeri, A.; Ale, A.; Rusconi, T.; Scattoni, M.; Lemaire, S.; Protano, G.; Venditti, I.; Corsi, I. Nanosilver Environmental Safety in Marine Organisms: Ecotoxicological Assessment of a Commercial Nano-Enabled Product vs an Eco-Design Formulation. Toxics 2025, 13, 338. https://doi.org/10.3390/toxics13050338
Bellingeri A, Ale A, Rusconi T, Scattoni M, Lemaire S, Protano G, Venditti I, Corsi I. Nanosilver Environmental Safety in Marine Organisms: Ecotoxicological Assessment of a Commercial Nano-Enabled Product vs an Eco-Design Formulation. Toxics. 2025; 13(5):338. https://doi.org/10.3390/toxics13050338
Chicago/Turabian StyleBellingeri, Arianna, Analía Ale, Tatiana Rusconi, Mattia Scattoni, Sofia Lemaire, Giuseppe Protano, Iole Venditti, and Ilaria Corsi. 2025. "Nanosilver Environmental Safety in Marine Organisms: Ecotoxicological Assessment of a Commercial Nano-Enabled Product vs an Eco-Design Formulation" Toxics 13, no. 5: 338. https://doi.org/10.3390/toxics13050338
APA StyleBellingeri, A., Ale, A., Rusconi, T., Scattoni, M., Lemaire, S., Protano, G., Venditti, I., & Corsi, I. (2025). Nanosilver Environmental Safety in Marine Organisms: Ecotoxicological Assessment of a Commercial Nano-Enabled Product vs an Eco-Design Formulation. Toxics, 13(5), 338. https://doi.org/10.3390/toxics13050338









