Response to Oxidative Stress Induced by Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in Differentiated PC12 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Counting Kit-8 Assay
2.4. Mitochondrial Membrane Potential Assay
2.5. Measurements of Intracellular Free Calcium Ion (Ca2+)
2.6. Western Blot
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. RNA Sequencing
2.9. Statistical Analysis
3. Results
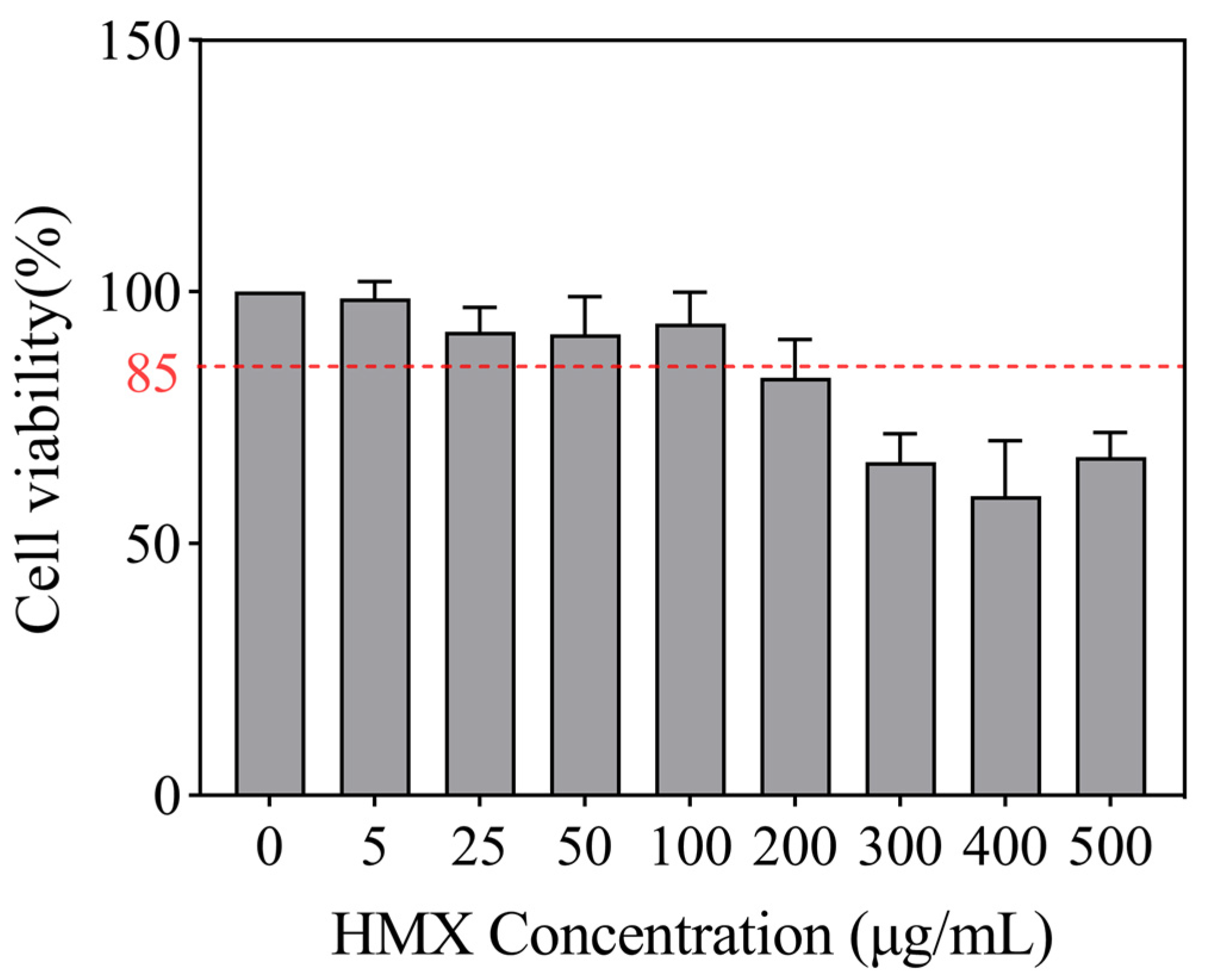
3.1. HMX Induced Cytotoxicity in Differentiated PC12 Cells
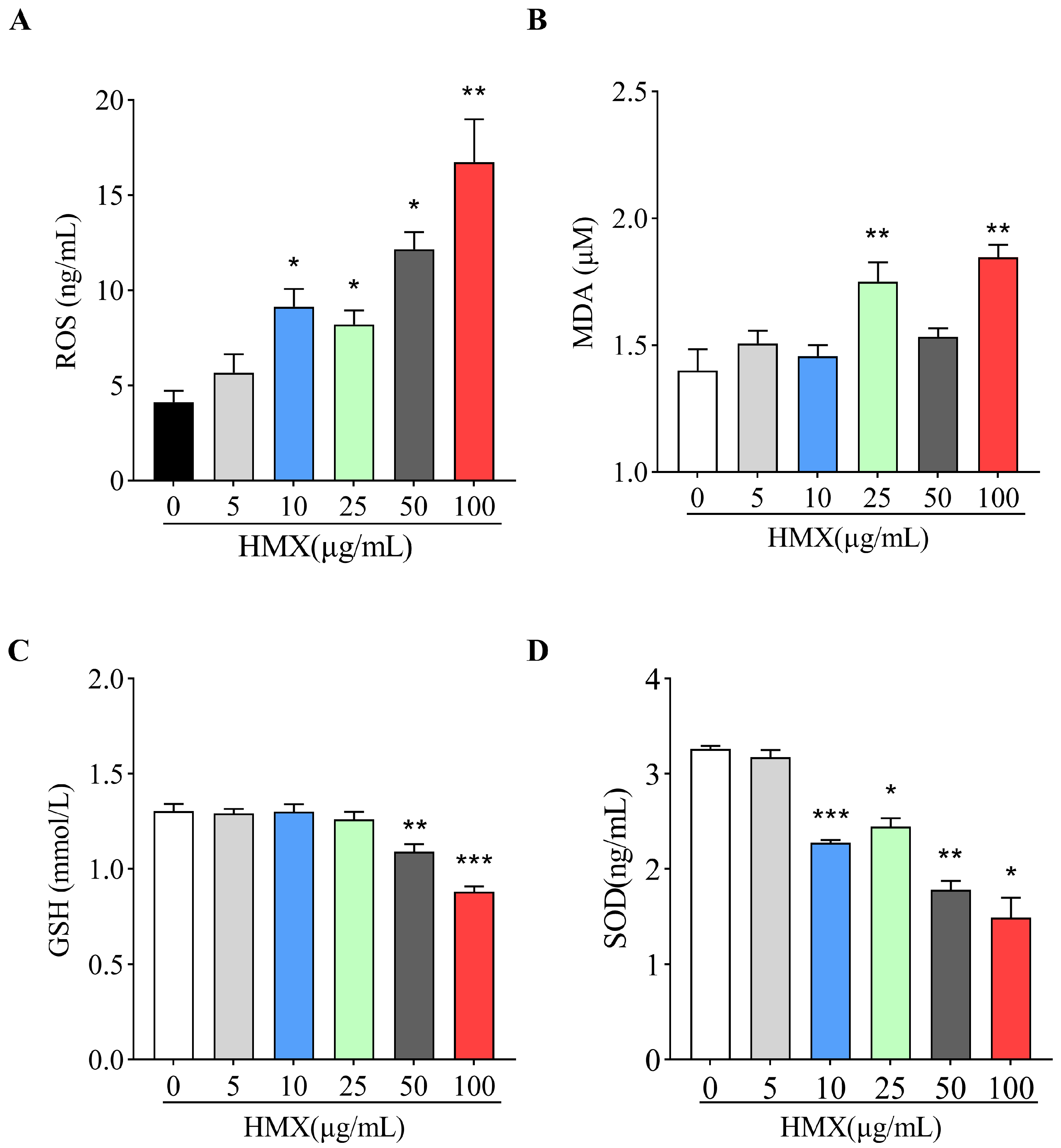
3.2. HMX Increased Oxidative Stress in Differentiated PC12 Cells
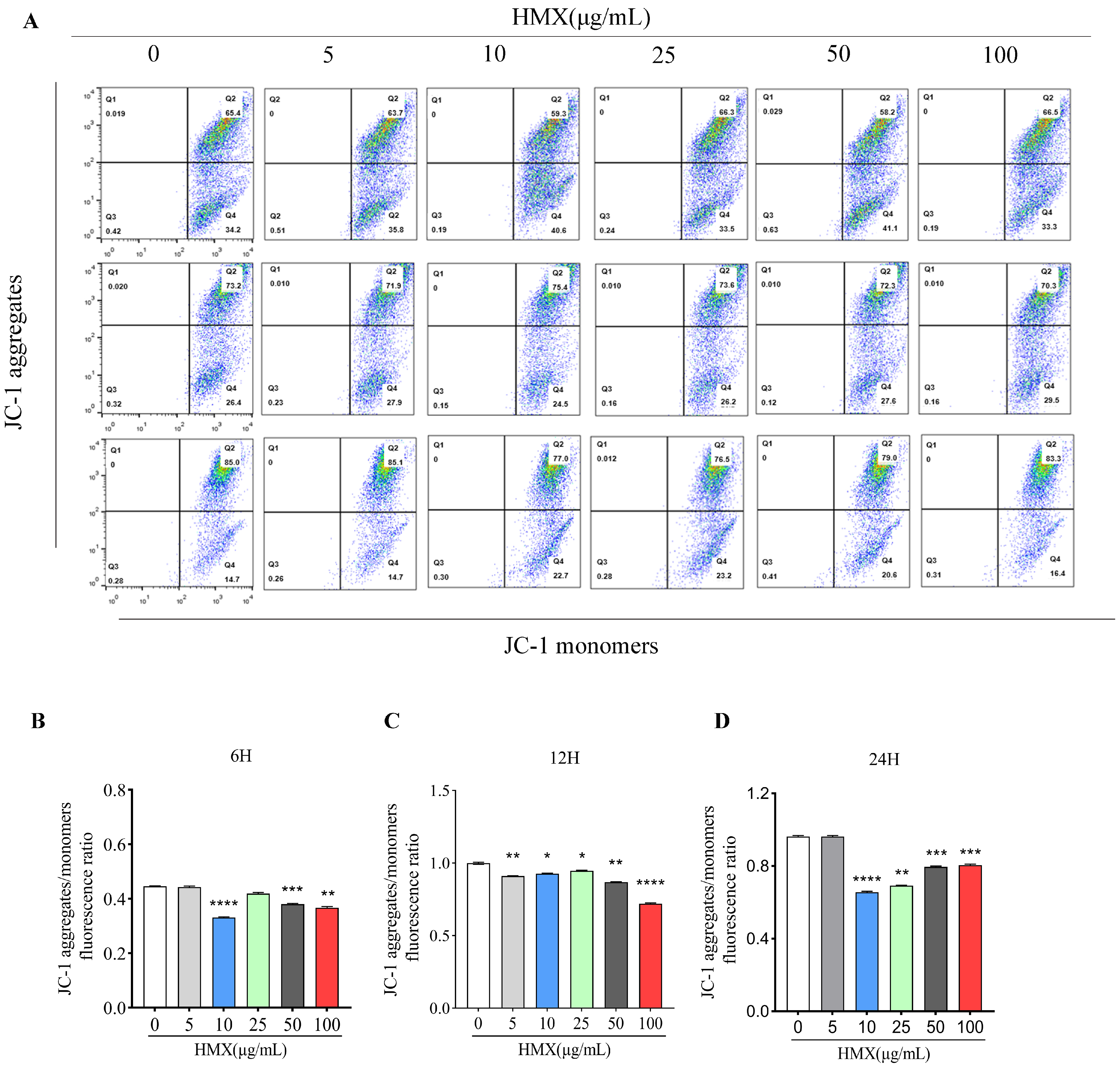
3.3. HMX Resulted in Lower Mitochondrial Membrane Potential (MMP) in PC12 Cells
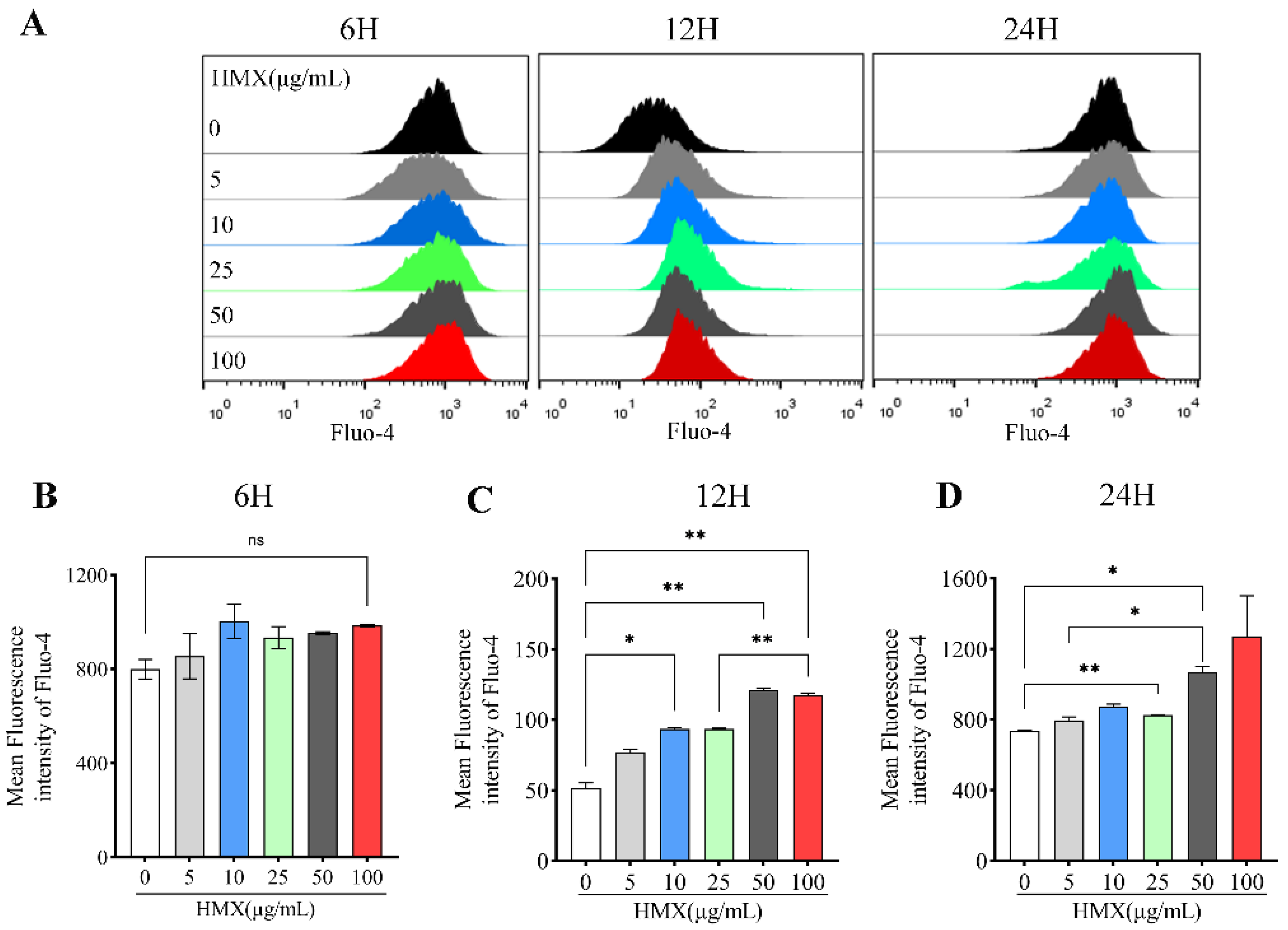
3.4. HMX Increased Free Calcium Ion (Ca2+) in Differentiated PC12 Cells
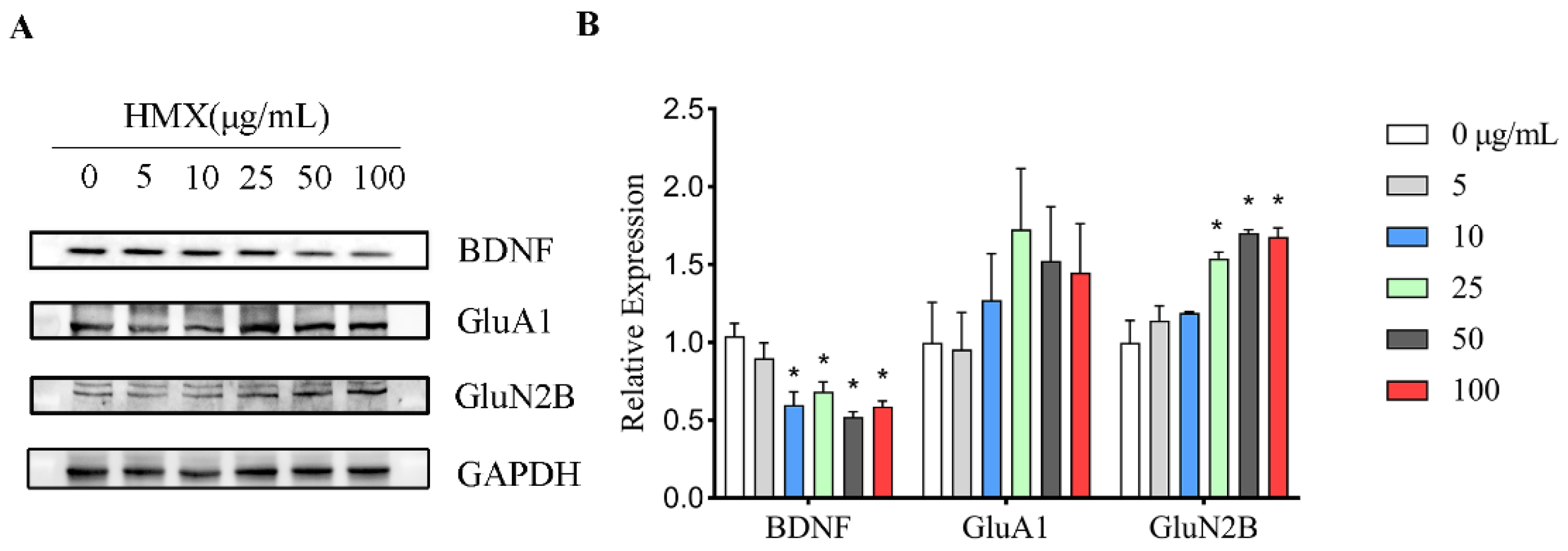
3.5. HMX Reduced BDNF Expression but Increased GluN2B Expression in Differentiated PC12 Cells
3.6. HMX Caused Abnormal Transcription Profile in Differentiated PC12 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, E.; Pulham, C.R.; Morrison, C.A. Structure and properties of nitrocellulose: Approaching 200 years of research. RSC Adv. 2023, 13, 32321–32333. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.T.; Adams, A.A.; Deschamps, J.R.; Veitch, S.; Hanson, A.; Kusterbeck, A.W. Detection of explosives in a dynamic marine environment using a moored TNT immunosensor. Sensors 2014, 14, 4074–4085. [Google Scholar] [CrossRef]
- Homma-Takeda, S.; Hiraku, Y.; Ohkuma, Y.; Oikawa, S.; Murata, M.; Ogawa, K.; Iwamuro, T.; Li, S.; Sun, G.F.; Kumagai, Y.; et al. 2,4,6-trinitrotoluene-induced reproductive toxicity via oxidative DNA damage by its metabolite. Free Radic. Res. 2002, 36, 555–566. [Google Scholar] [CrossRef]
- Woody, R.C.; Kearns, G.L.; Brewster, M.A.; Turley, C.P.; Sharp, G.B.; Lake, R.S. The neurotoxicity of cyclotrimethylenetrinitramine (RDX) in a child: A clinical and pharmacokinetic evaluation. J. Toxicol. Clin. Toxicol. 1986, 24, 305–319. [Google Scholar] [CrossRef]
- Davies, J.O.; Roberts, D.M.; Hittarage, A.; Buckley, N.A. Oral C-4 plastic explosive in humans—A case series. Clin. Toxicol. 2007, 45, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Bolt, H.M.; Degen, G.H.; Dorn, S.B.; Plöttner, S.; Harth, V. Genotoxicity and potential carcinogenicity of 2,4,6-TNT trinitrotoluene: Structural and toxicological considerations. Rev. Environ. Health 2006, 21, 217–228. [Google Scholar] [CrossRef]
- Tan, B.; Liao, L.; Zhou, Y.; Long, X.; Li, J. Toward the toxicology of some nitro-compounds. Mod. Org. Chem. Res. 2018, 3, 111–121. [Google Scholar] [CrossRef]
- Sarwat, S.; Akhtar, A.; Dayan, S.F.A.; Shaheen, N.; Durrani, H.S.; Usman, T.; Afghani, T. Ring-shaped cataract and urinary metabolites among 2,4,6-trinitrotoluene exposed population of Pakistan. Int. Ophthalmol. 2022, 42, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.J., Jr.; Crouse, L.C.; McFarland, C.A.; LaFiandra, E.M.; Johnson, M.S. Reproductive and developmental effects and physical and chemical properties of pentaerythritol tetranitrate (PETN) in the rat. Birth Defects Res. Part B-Dev. Reprod. Toxicol. 2009, 86, 65–71. [Google Scholar] [CrossRef]
- Yang, C.S.; Zhang, S.H. Investigation of dislocation and twinning behavior in HMX under high-velocity impact employing molecular dynamics simulations. J. Mol. Graph. Model. 2024, 30, 50. [Google Scholar] [CrossRef]
- Duan, B.; Lu, X.; Mo, H.; Tan, B.; Wang, B.; Liu, N. Fabrication of CL-20/HMX Cocrystal@Melamine-Formaldehyde Resin Core-Shell Composites Featuring Enhanced Thermal and Safety Performance via In Situ Polymerization. Int. J. Mol. Sci. 2022, 23, 6710. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, R.J.; McDonald, P. HMX: 14-Day Toxicity Study in Mice by Dietary Administration; Research and Development Command; Army Medical Bioengineering Research and Development Laboratory: Frederick, MD, USA, 1985. [Google Scholar]
- Cuthbert, J.A.; Burt, D.; Carr, S.M. HMX: Acute Toxicity Tests in Laboratory Animals; Report No 2051; US. Army Medical Research and Development Command: Fort Detrick, MD, USA, 1985. [Google Scholar]
- Wilson, A.B. Determination of the Acute and Subchronic Mammalian Toxicity of HMX; AD A173743; US Army Medical Research and Development Command: Ft. Detrick, MD, USA, 1985. [Google Scholar]
- Nagar, S.; Anand, S.; Chatterjee, S.; Rawat, C.D.; Lamba, J.; Rai, P.K. A review of toxicity and biodegradation of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) in the environment. Environ. Technol. Innov. 2021, 23, 101750. [Google Scholar] [CrossRef]
- Everett, D.J.; Maddock, S.M. HMX: 13-Week Toxicity Study in Mice by Dietary Administration; AD A171602; United States Army Medical Research and Development Command: Frederick, MD, USA, 1985. [Google Scholar]
- Johnson, M.S.; McFarland, C.A.; Bazar, M.A.; Quinn, M.J., Jr.; LaFiandra, E.M.; Talent, L.G. Toxicity of octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) in three vertebrate species. Arch. Environ. Contam. Toxicol. 2010, 58, 836–843. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.K.L.; Hu, W.W.H.; Zheng, Z.X.; Huang, M.; Lin, Y.X.; Wang, C.Y.; Gong, G.W.; Yang, X.Y.; Tsim, W.K.; Dong, T.X. Quercetin Potentiates the NGF-Induced Effects in Cultured PC 12 Cells: Identification by HerboChips Showing a Binding with NGF. Evid.-Based Complement. Altern. Med. 2018, 2018, 1502457. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef]
- Shafer, T.J.; Atchison, W.D. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: A model for neurotoxicological studies. Neurotoxicology 1991, 12, 473–492. [Google Scholar]
- Westerink, R.H.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, S.; Wei, F.; Liang, G.; Yang, X.; Huang, Y.; Wang, J.; Zou, Y. Pivotal role of cAMP-PKA-CREB signaling pathway in manganese-induced neurotoxicity in PC12 cells. Environ. Toxicol. 2019, 34, 1052–1062. [Google Scholar] [CrossRef]
- Ahmadian, R.; Heidari, M.R.; Razavi, B.M.; Hosseinzadeh, H. Alpha-mangostin Protects PC12 Cells Against Neurotoxicity Induced by Cadmium and Arsenic. Biol. Trace Elem. Res. 2023, 201, 4008–4021. [Google Scholar] [CrossRef]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Sharma, N.; Kumar, V.; Sharma, D.; Devi, B.; Kapil, L.; Singh, C.; Singh, A. The Role of Mitophagy in Various Neurological Diseases as a Therapeutic Approach. Cell. Mol. Neurobiol. 2023, 43, 1849–1865. [Google Scholar] [CrossRef]
- Yang, K.; Yan, Y.; Yu, A.; Zhang, R.; Zhang, Y.; Qiu, Z.; Li, Z.; Zhang, Q.; Wu, S.; Li, F. Mitophagy in neurodegenerative disease pathogenesis. Neural Regen. Res. 2024, 19, 998–1005. [Google Scholar] [CrossRef]
- Shen, C.; Chen, C.; Wang, T.; Gao, T.-Y.; Zeng, M.; Lu, Y.-B.; Zhang, W.-P. The Depletion of NAMPT Disturbs Mitochondrial Homeostasis and Causes Neuronal Degeneration in Mouse Hippocampus. Mol. Neurobiol. 2023, 60, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.D.; Akerman, K.E.; Nicholls, D.G. Calcium-ion transport by intact synaptosomes. Intrasynaptosomal compartmentation and the role of the mitochondrial membrane potential. Biochem. J. 1980, 192, 873–880. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Thomas, C.; Ristow, M. Interrelation between ROS and Ca2+ in aging and age-related diseases. Redox Biol. 2020, 36, 101678. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.E.; Trimmer, J.S. Endoplasmic Reticulum-Plasma Membrane Junctions as Sites of Depolarization-Induced Ca2+ Signaling in Excitable Cells. Annu. Rev. Physiol. 2023, 85, 217–243. [Google Scholar] [CrossRef]
- Bading, H. Nuclear calcium signaling in the regulation of brain function. Nat. Rev. Neurosci. 2013, 14, 593–608. [Google Scholar] [CrossRef]
- Borowiec, A.S.; Bidaux, G.; Pigat, N.; Goffin, V.; Bernichtein, S.; Capiod, T. Calcium channels, external calcium concentration and cell proliferation. Eur. J. Pharmacol. 2014, 739, 19–25. [Google Scholar] [CrossRef]
- Ton, T.T.; Kovi, R.C.; Peddada, T.N.; Chhabria, R.M.; Shockley, K.R.; Flagler, N.D.; Gerrish, K.E.; Herbert, R.A.; Behl, M.; Hoenerhoff, M.J.; et al. Cobalt-induced oxidative stress contributes to alveolar/bronchiolar carcinogenesis in B6C3F1/N mice. Arch. Toxicol. 2021, 95, 3171–3190. [Google Scholar] [CrossRef]
- Shaghayeghi Toosi, F.; Jadidoleslami, M. An Efficient and Solvent-Free Method to Synthesize HMX by Nitrolysis of DPT Catalyzed by Reusable Solid Acid Catalysts. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2016, 46, 159–162. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Toxicological Profile for HMX; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 1997.
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef]
- Buhlman, L.M. Parkin loss-of-function pathology: Premature neuronal senescence induced by high levels of reactive oxygen species? Mech. Ageing Dev. 2017, 161, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Teleanu, D.M.; Niculescu, A.-G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Videla, L.A.; Marimán, A.; Ramos, B.; José Silva, M.; del Campo, A. Standpoints in mitochondrial dysfunction: Underlying mechanisms in search of therapeutic strategies. Mitochondrion 2022, 63, 9–22. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10 (Suppl. 7), S18–S25. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, C.; Guo, X.; Hu, G.; Li, G.; Zhuang, Y.; Cao, H.; Li, L.; Xing, C.; Zhang, C.; et al. Exposed to Mercury-Induced Oxidative Stress, Changes of Intestinal Microflora, and Association between them in Mice. Biol. Trace Elem. Res. 2021, 199, 1900–1907. [Google Scholar] [CrossRef]
- Vieira, H.C.; Bordalo, M.D.; Rodrigues, A.C.M.; Pires, S.; Rocha, R.; Soares, A.; Osten, J.R.-V.; Abreu, S.; Morgado, F. Water temperature modulates mercury accumulation and oxidative stress status of common goby (Pomatoschistus microps). Environ. Res. 2021, 193, 110585. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Q.; Yue, C.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 13491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gill, K.D. Oxidative stress and mitochondrial dysfunction in aluminium neurotoxicity and its amelioration: A review. Neurotoxicology 2014, 41, 154–166. [Google Scholar] [CrossRef]
- Li, C.; Deng, H.; Liu, Z.; Lv, X.; Gao, W.; Gao, Y.; Gao, J.; Hu, L. Salidroside protect Chinese hamster V79 cells from genotoxicity and oxidative stress induced by CL-20. Toxicol. Res. 2023, 12, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Jacobsen, J.; Maser, E. Metabolic activation of 2,4,6-trinitrotoluene; a case for ROS-induced cell damage. Redox Biol. 2024, 72, 103082. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, calcium and mitochondria: A triad in synaptic neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Oh, B.-C. Phosphoinositides and intracellular calcium signaling: Novel insights into phosphoinositides and calcium coupling as negative regulators of cellular signaling. Exp. Mol. Med. 2023, 55, 1702–1712. [Google Scholar] [CrossRef]
- Khodorov, B.; Pinelis, V.; Vergun, O.; Storozhevykh, T.; Vinskaya, N. Mitochondrial deenergization underlies neuronal calcium overload following a prolonged glutamate challenge. FEBS Lett. 1996, 397, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Khodorov, B.I.; Storozhevykh, T.P.; Surin, A.M.; Yuryavichyus, A.I.; Sorokina, E.G.; Borodin, A.V.; Vinskaya, N.P.; Khaspekov, L.G.; Pinelis, V.G. The leading role of mitochondrial depolarization in the mechanism of glutamate-induced disruptions in Ca2+ homeostasis. Neurosci. Behav. Physiol. 2002, 32, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Hayashi, Y.; Hell, J.W. CaMKII: A central molecular organizer of synaptic plasticity, learning and memory. Nat. Rev. Neurosci. 2022, 23, 666–682. [Google Scholar] [CrossRef]
- Lv, D.; Xiao, B.; Liu, H.; Wang, L.; Li, Y.; Zhang, Y.H.; Jin, Q. Enhanced NMDA receptor pathway and glutamate transmission in the hippocampal dentate gyrus mediate the spatial learning and memory impairment of obese rats. Pflug. Arch.-Eur. J. Physiol. 2024, 476, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, M.; Zhang, S.; Zhao, C.; Kang, W.; Wang, K. Roles of mtDNA damage and disordered Ca2+ homeostasis in the joint toxicities of cadmium and BDE209. Ecotoxicol. Environ. Saf. 2019, 186, 109767. [Google Scholar] [CrossRef]
- Canettieri, G.; Morantte, I.; Guzmán, E.; Asahara, H.; Herzig, S.; Anderson, S.D.; Yates, J.R.; Montminy, M. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat. Struct. Mol. Biol. 2003, 10, 175–181. [Google Scholar] [CrossRef]
- Ahsas Goyal Kumari, R.; Verma, A.; Dubey, N.; Agrawal, A. Matrix Metalloproteinase-9 and Its Involvement in Parkinson’s Disease. Neurochem. J. 2023, 17, 236–242. [Google Scholar] [CrossRef]
- Marie, P.J.; Haÿ, E.; Modrowski, D.; Revollo, L.; Mbalaviele, G.; Civitelli, R. Cadherin-mediated cell-cell adhesion and signaling in the skeleton. Calcif. Tissue Int. 2014, 94, 46–54. [Google Scholar] [CrossRef]
- Chiesa, A.; Crisafulli, C.; Porcelli, S.; Han, C.; Patkar, A.A.; Lee, S.J.; Park, M.H.; Jun, T.Y.; Serretti, A.; Pae, C.U. Influence of GRIA1, GRIA2 and GRIA4 polymorphisms on diagnosis and response to treatment in patients with major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 305–311. [Google Scholar] [CrossRef]
- Umemori, H.; Linhoff, M.W.; Ornitz, D.M.; Sanes, J.R. FGF22 and its close relatives are presynaptic organizing molecules in the mammalian brain. Cell 2004, 118, 257–270. [Google Scholar] [CrossRef]
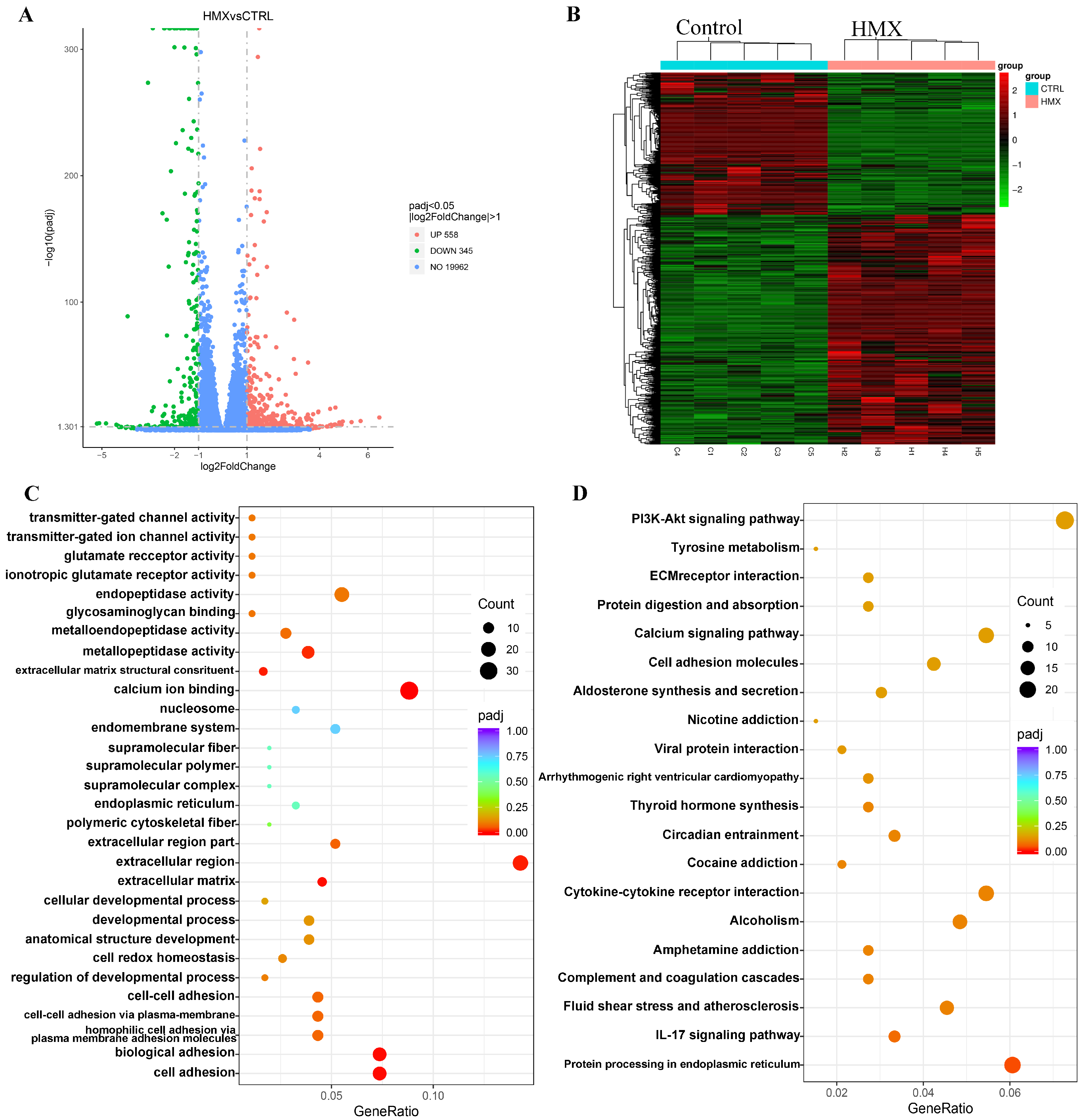
| GO Pathway | Gene Name |
|---|---|
| Cell adhesion | Lama4/Lama2/Ncan/Thbs2/Cdh5/Hapln1/Sned1/Pcdhb10/Hapln2/LOC108348201/Cdh16/Dsc3/Fat2/Pcdhb7/Pcdhb11/Fat3/Cdh26 |
| Biological adhesion | Lama4/Lama2/Ncan/Thbs2/Cdh5/Hapln1/Sned1/Pcdhb10/Hapln2/LOC108348201/Cdh16/Dsc3/Fat2/Pcdhb7/Pcdhb11/Fat3/Cdh26 |
| Homophilic cell adhesion via plasma membrane adhesion molecules | Cdh5/Pcdhb10/LOC108348201/Cdh16/Dsc3/Fat2/Pcdhb7/Pcdhb11/Fat3/Cdh26 |
| Cell–cell adhesion via plasma-membrane adhesion molecules | Cdh5/Pcdhb10/LOC108348201/Cdh16/Dsc3/Fat2/Pcdhb7/Pcdhb11/Fat3/Cdh26 |
| Cell–cell adhesion | Cdh5/Pcdhb10/LOC108348201/Cdh16/Dsc3/Fat2/Pcdhb7/Pcdhb11/Fat3/Cdh26 |
| Regulation of developmental process | Lama4/Lama2/Palmd/Csf3 |
| Cell redox homeostasis | Pdia6/Pdia3/Pdia4/Txndc11/Glrx/Pdia5 |
| Anatomical structure development | Lama4/Lama2/Spry1/Palmd/Siah3/Csf3/Draxin/Prm2/Crx |
| Developmental process | Lama4/Lama2/Spry1/Palmd/Siah3/Csf3/Draxin/Prm2/Crx |
| Cellular developmental process | Palmd/Csf3/Draxin/Prm2 |
| Extracellular matrix | Mmp19/Mmp15/Mmp11/Mmp3/Mmp10/Mmp13/Mmp9 |
| Extracellular region | Mmp19/Stc2/Mmp15/Ccl2/Igfbp7/Mmp11/Nppb/Mmp3/Plat/Mmp10/Thbs2/Pinlyp/Umodl1/Mmp13/Mmp9/Csf2/Esm1/Draxin/Igfbp3/C3/Htra3/Chit1 |
| Extracellular region part | Mmp19/Mmp15/Mmp11/Mmp3/Mmp10/Mmp13/Mmp9/C3 |
| Polymeric cytoskeletal fiber | Des/Nefm/LOC102555739 |
| Endoplasmic reticulum | Calr/Canx/P4ha1/Selenos/Calr4 |
| Supramolecular complex | Des/Nefm/LOC102555739 |
| Supramolecular polymer | Des/Nefm/LOC102555739 |
| Supramolecular fiber | Des/Nefm/LOC102555739 |
| Endomembrane system | Calr/Canx/P4ha1/Uso1/Csgalnact2/Selenos/Calr4/Spesp1 |
| Nucleosome | Hist3h2ba/H2ac25/Hist1h3b/ENSRNOG00000064540/H2bc18 |
| Calcium ion binding | Calr/Canx/Creld2/LOC100910088/Nucb2/Sparc/Ltbp4/Notch4/Efhd1/Lcp1/Thbs2/Ppef1/Umodl1/Padi3/Cdh5/Hmcn2/Pcdhb10/Calm2/LOC108348201/Calml4/Cdh16/Calr4/Tescl/Plcb2/Dsc3/Fat2/Pcdhb7/Pcdhb11/RGD1559821/Fat3/Cdh26 |
| Extracellular matrix structural constituent | Col5a2/Col5a3/Col3a1/Col1a2/Col4a3/Col11a1 |
| Metallopeptidase activity | Mmp19/Mmp15/Mmp11/Adamts4/Mmp3/Mmp10/Cpm/Mmp13/Mmp9/Ace/Aebp1/Adam11/Adamts5/Cpz |
| Metalloendopeptidase activity | Mmp19/Mmp15/Mmp11/Adamts4/Mmp3/Mmp10/Mmp13/Mmp9/Adam11/Adamts5 |
| Glycosaminoglycan binding | App/Ncan/Hapln1/Hapln2 |
| Endopeptidase activity | Mmp19/Mmp15/Mmp11/Prss22/Adamts4/Mmp3/Plat/Masp1/Mmp10/Mmp13/Ctrb1/Pcsk2/Mmp9/RGD1559662/Adam11/Klk13/Rhbdl2/Adamts5/Cym/Plg |
| Ionotropic glutamate receptor activity | Gria2/Grin3b/Grin2d/Grin1 |
| Glutamate receptor activity | Gria2/Grin3b/Grin2d/Grin1 |
| Transmitter-gated ion channel activity | Gria2/Grin3b/Grin2d/Grin1 |
| Transmitter-gated channel activity | Gria2/Grin3b/Grin2d/Grin1 |
| Neurotransmitter receptor activity | Gria2/Grin3b/Grin2d/Grin1 |
| KEGG Pathway | Gene Name |
|---|---|
| Protein processing in endoplasmic reticulum | Hspa5/Hsp90b1/Calr/Pdia6/Canx/Sel1l/Pdia3/Pdia4/Hyou1/Herpud1/Dnajc3/Ero1b/Ddit3/Dnajb11/Atf6/Xbp1/Syvn1/Selenos/Casp12/Calr4 |
| IL-17 signaling pathway | Hsp90b1/Ccl2/Ccl7/Cxcl10/Mmp3/Fosb/Lcn2/Mmp13/Mmp9/Csf2/Csf3 |
| Fluid shear stress and atherosclerosis | Hsp90b1/Sqstm1/Ccl2/Plat/Map2k6/Cdh5/Nos3/Gstm7/Mmp9/Calm2/Calml4/Ncf2/Itgb3/Gstt3 |
| Complement and coagulation cascades | F3/Plat/Masp1/Itgax/Vwf/F13a1/C3/Plg/C1qc |
| Amphetamine addiction | Creb3l1/Gria2/Fosb/Grin3b/Th/Grin2d/Grin1/Calm2/Calml4 |
| Alcoholism | Creb3l1/Fosb/Grin3b/Gng3/Th/Grin2d/Hist3h2ba/Grin1/Calml2/Calml4/LOC102549173/AC130391.1/Hist1h3b/ENSRNOG00000064540/H2bc18 |
| Cytokine–cytokine receptor interaction | Ccl2/Ccl7/Bmp7/Cxcl10/Tnfrsf1b/Il33/Eda2r/Tnfsf18/Ccr7/Csf2/Il5ra/Tnfrsf9/Csf3/Il11ra1/Tslp/Ccl27 |
| Cocaine addiction | Creb3l1/Gria2/Fosb/Grin3b/Th/Grin2d/Grin1 |
| Circadian entrainment | Gria2/Gng3/Adcyap1r1/Grin2d/Cacna1h/Rasd1/Grin1/Calm2/Calml4/Plcb2 |
| Thyroid hormone synthesis | Hspa5/Hsp90b1/Canx/Pdia4/Creb3l1/Gpx1/Atp1a2/Tg/Plcb2 |
| Arrhythmogenic right ventricular cardiomyopathy | Lama2/Atp2a3/Sgcd/Des/Cacng8/Dsp/Itgb7/Itgb3/Sgcg |
| Viral protein interaction with cytokine and cytokine receptor | Ccl2/Ccl7/Cxcl10/Tnfrsf1b/Ccr7/Ccl27 |
| Nicotine addiction | Gria2/Grin3b/Slc17a6/Grin2d/Grin1 |
| Aldosterone synthesis and secretion | Creb3l1/Atp2b4/Atp1a2/Star/Pde2a/Cyp11a1/Cacna1h/Calm2/Calml4/Plcb2 |
| Cell adhesion molecules | Sdc3/Alcam/Siglec1/Cldn4/Cdh5/Spn/Cd80/Cntn2/Ptprf/RT1-M6-2/Itgb7/Esam/Nectin1/Jam2 |
| Calcium signaling pathway | Atp2b4/Atp2a3/Flt1/Fgf22/Plcd4/Nos3/Itpka/Grin2d/Cacna1h/Ptk2b/Grin1/Calm2/Calml4/Fgfr4/Fgf21/Plcb2/Adra1d/Ntrk3 |
| Protein digestion and absorption | Col5a2/Col5a3/Col12a1/Col14a1/Atp1a2/Ctrb1/Col9a3/Col3a1/Col11a1 |
| ECM–receptor interaction | Thbs1/Lama4/Lama2/Lamb2/Thbs2/Col9a3/Itgb7/Vwf/Itgb3 |
| Tyrosine metabolism | Aox1/Aox2/Th/Adh6/RGD1308564 |
| PI3K-Akt signaling pathway | Hsp90b1/Thbs1/Lama4/Lpar1/Creb3l1/Lama2/Lamb2/Pck2/Flt1/Thbs2/Fgf22/Areg/Nos3/Col9a3/Gng3/Csf3/Itgb7/Fgfr4/Tgfa/Fgf21/Vwf/Itgb3/Gys2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Lv, X.; Liu, Z.; Deng, H.; Gao, T.; Li, H.; Peng, X.; Qian, A.; Gao, J.; Hu, L. Response to Oxidative Stress Induced by Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in Differentiated PC12 Cells. Toxics 2025, 13, 347. https://doi.org/10.3390/toxics13050347
Li C, Lv X, Liu Z, Deng H, Gao T, Li H, Peng X, Qian A, Gao J, Hu L. Response to Oxidative Stress Induced by Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in Differentiated PC12 Cells. Toxics. 2025; 13(5):347. https://doi.org/10.3390/toxics13050347
Chicago/Turabian StyleLi, Cunzhi, Xiaoqiang Lv, Zhiyong Liu, Hui Deng, Ting Gao, Huan Li, Xinying Peng, Airong Qian, Junhong Gao, and Lifang Hu. 2025. "Response to Oxidative Stress Induced by Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in Differentiated PC12 Cells" Toxics 13, no. 5: 347. https://doi.org/10.3390/toxics13050347
APA StyleLi, C., Lv, X., Liu, Z., Deng, H., Gao, T., Li, H., Peng, X., Qian, A., Gao, J., & Hu, L. (2025). Response to Oxidative Stress Induced by Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine in Differentiated PC12 Cells. Toxics, 13(5), 347. https://doi.org/10.3390/toxics13050347







