Long-Term Exposure to Microplastics Promotes Early-Stage Hepatocarcinogenesis Induced by Diethylnitrosamine in Rats by Modulation of Their Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
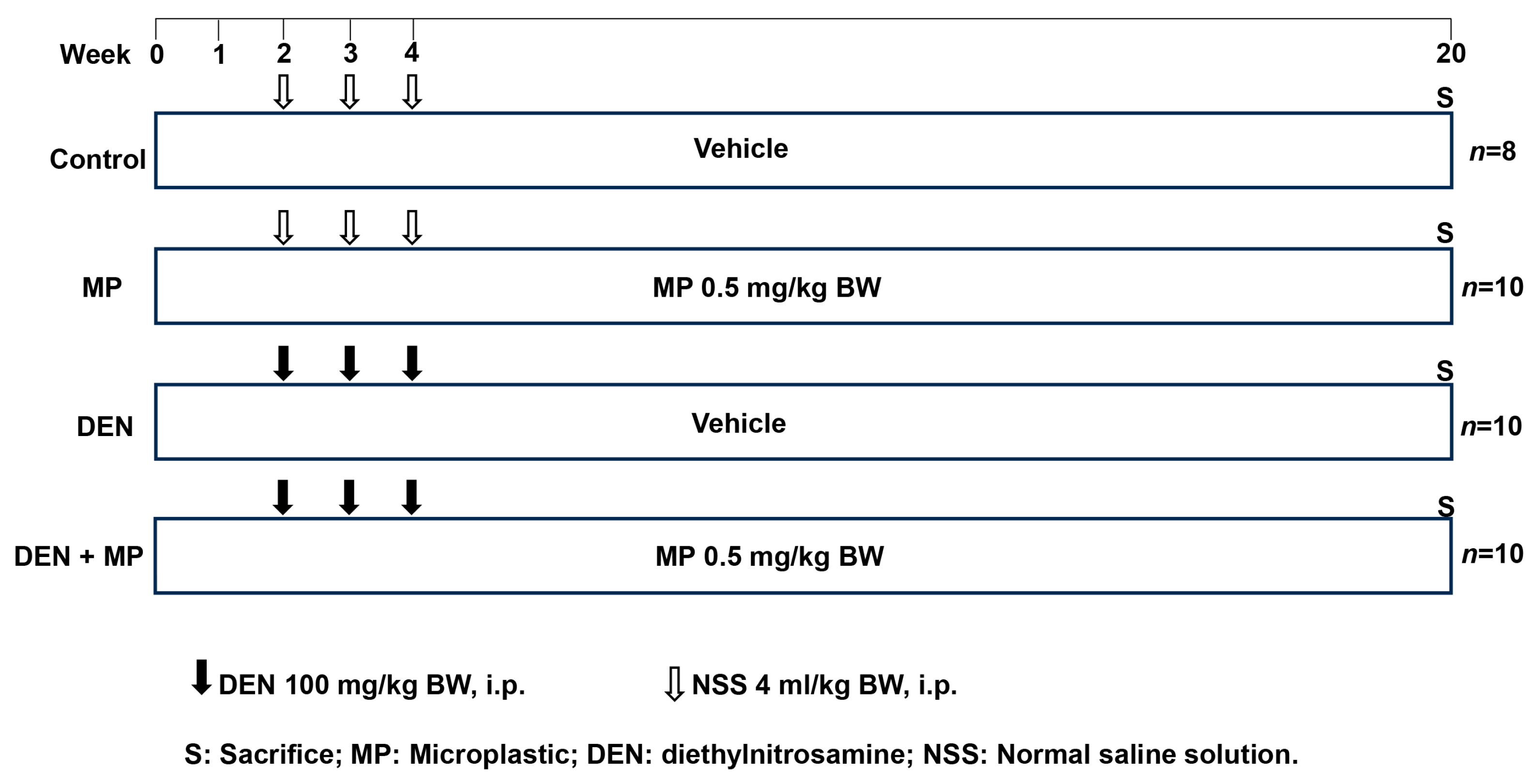
2.1. Animals and Experimental Protocol
2.1.1. Animals and Exposure
2.1.2. Experimental Design
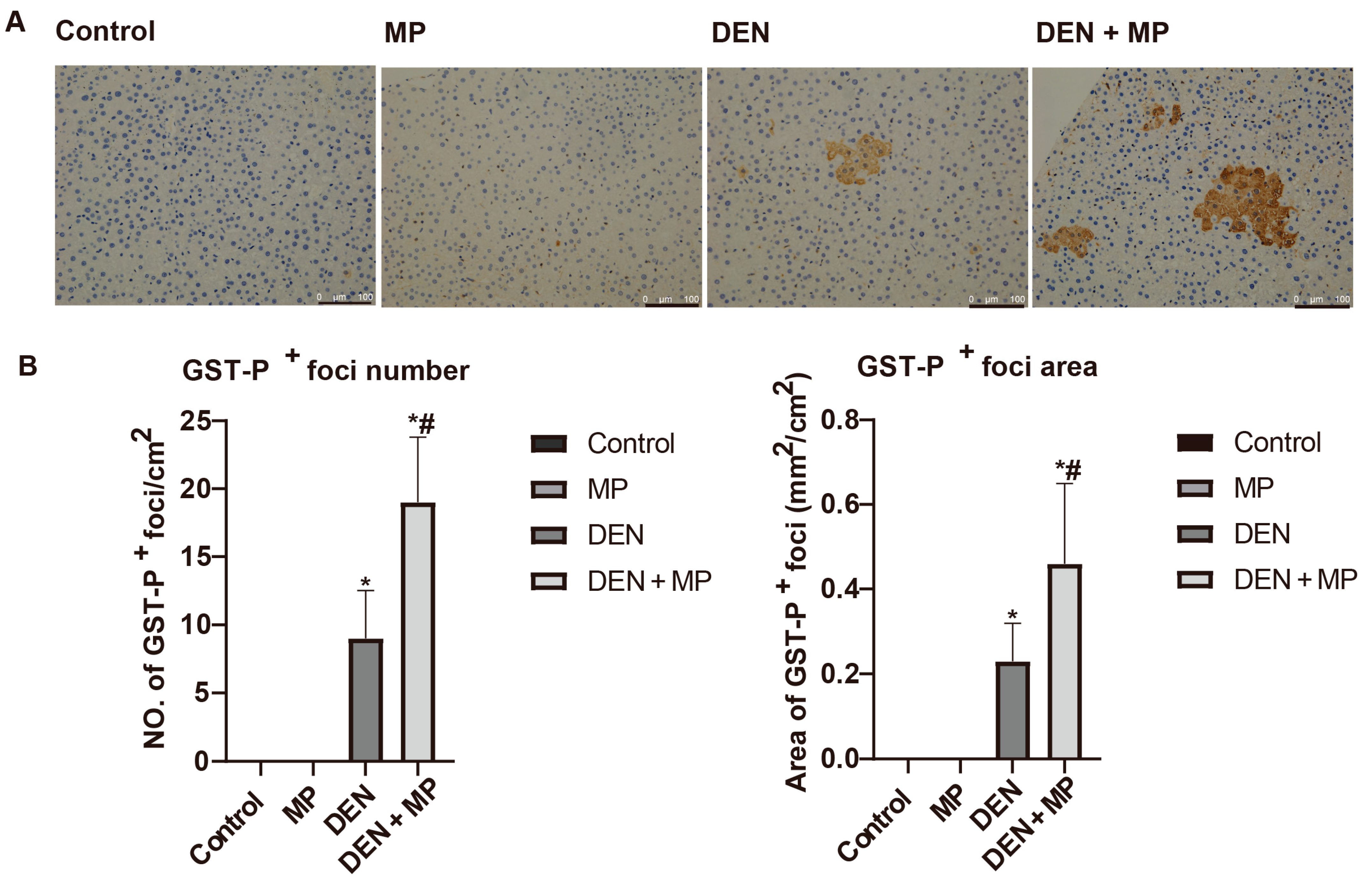
2.2. Determination of Liver Preneoplastic Lesions and Cell Proliferation
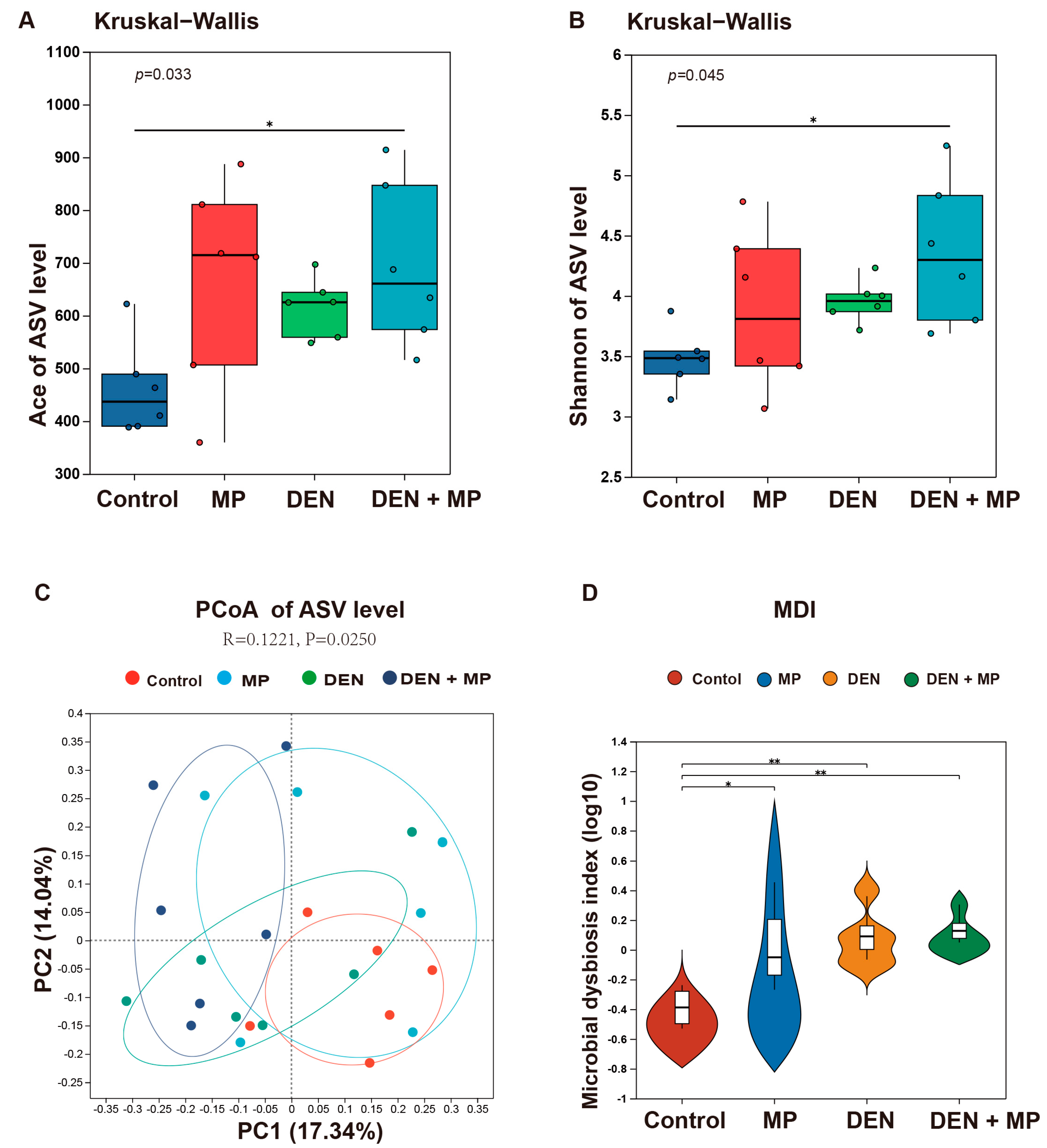
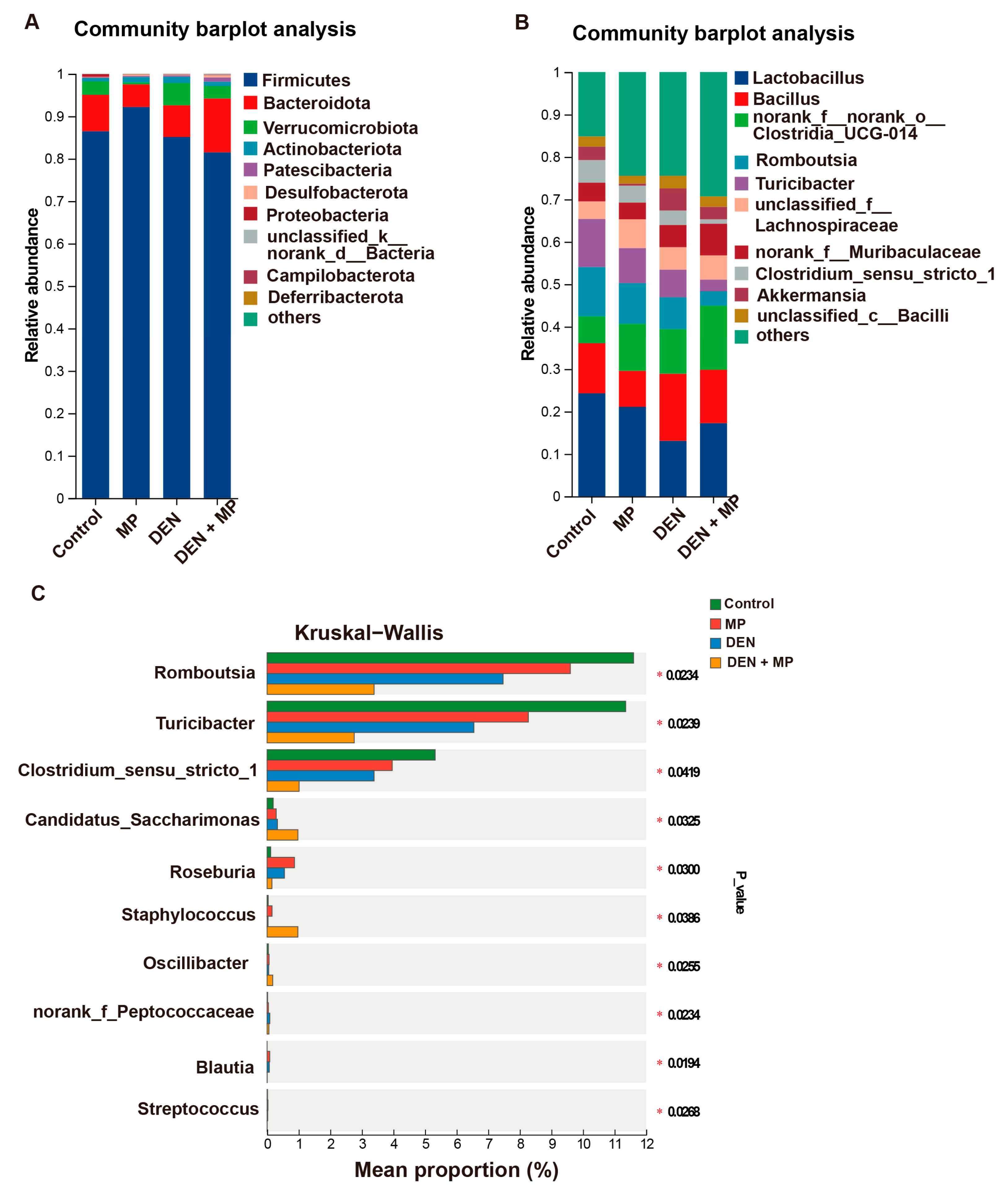
2.3. Investigation of Fecal Intestinal Microbiota in Rats
2.4. Measurement of SCFAs in Rat Feces
2.5. Statistical Analysis
3. Results
3.1. MP Altered the Growth Phenotypes and Liver Enzyme Levels in DEN-Induced Rats
3.2. MP Promoted Preneoplastic Lesion Formation in the Livers of DEN-Induced Rats
3.3. MP Enhanced Cellular Proliferation in the Livers of DEN-Induced Rats
3.4. MPs Changed the Gut Microbiota Composition of DEN-Induced Rats
3.5. MPs Reshaped the Composition of SCFAs in DEN-Induced Rats
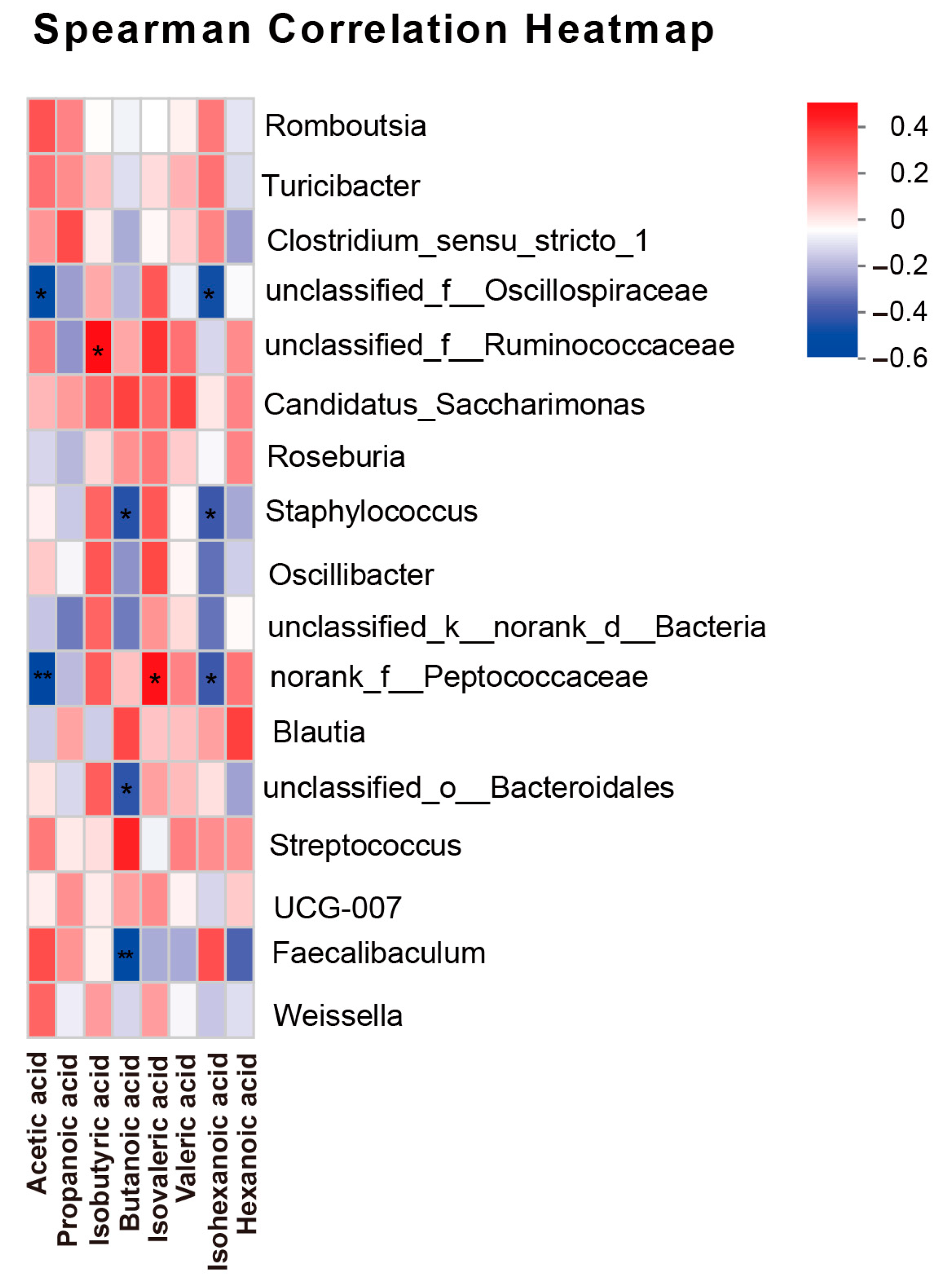
3.6. Correlation Analysis Between SCFAs and Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid physicochemical changes in microplastic induced by biofilm formation. Front. Bioeng. Biotechnol. 2020, 8, 205. [Google Scholar] [CrossRef]
- Traversa, A.; Mari, E.; Pontecorvi, P.; Gerini, G.; Romano, E.; Megiorni, F.; Amedei, A.; Marchese, C.; Ranieri, D.; Ceccarelli, S. Polyethylene micro/nanoplastics exposure induces epithelial-mesenchymal transition in human bronchial and alveolar epithelial cells. Int. J. Mol. Sci. 2024, 25, 10168. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, N.; Bousserrhine, N.; Belbekhouche, S.; Missawi, O.; Alphonse, V.; Boughatass, I.; Banni, M. First report on the presence of small microplastics (≤3 μm) in tissue of the commercial fish Serranus scriba (Linnaeus. 1758) from Tunisian coasts and associated cellular alterations. Environ. Pollut. 2020, 263, 114576. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty years of microplastic pollution research-what have we learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, L.J.W.; Bosker, T.; Peijnenburg, W. Deciphering potential implications of dietary microplastics for human health. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Huang, H.; Hou, J.; Yu, C.; Wei, F.; Xi, B. Microplastics exacerbate tissue damage and promote carcinogenesis following liver infection in mice. Ecotoxicol. Environ. Saf. 2024, 286, 117217. [Google Scholar] [CrossRef] [PubMed]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K.J.E. Microplastics detected in cirrhotic liver tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Pontecorvi, P.; Ceccarelli, S.; Cece, F.; Camero, S.; Lotti, L.V.; Niccolai, E.; Nannini, G.; Gerini, G.; Anastasiadou, E.; Scialis, E.S.; et al. Assessing the impact of polyethylene nano/microplastic exposure on human vaginal keratinocytes. Int. J. Mol. Sci. 2023, 24, 11379. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, J.; Chen, L.; He, J.; Qu, D.; Zhang, Z.; Liu, Y.; Li, X.; Liu, J.; Li, J.; et al. Gut microbiota participates in polystyrene microplastics-induced hepatic injuries by modulating the gut-liver axis. ACS Nano 2023, 17, 15125–15145. [Google Scholar] [CrossRef]
- Djouina, M.; Waxin, C.; Dubuquoy, L.; Launay, D.; Vignal, C.; Body-Malapel, M. Oral exposure to polyethylene microplastics induces inflammatory and metabolic changes and promotes fibrosis in mouse liver. Ecotoxicol. Environ. Saf. 2023, 264, 115417. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Xu, F.; Meng, S.; Li, H.; Song, Y. Polystyrene microplastics postpone APAP-induced liver injury through impeding macrophage polarization. Toxics 2022, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Zhao, Y.; Liu, S.; Chen, Y.; Yuan, H.; Xu, H. Polystyrene microplastics exacerbated liver injury from cyclophosphamide in mice: Insight into gut microbiota. Sci. Total Environ. 2022, 840, 156668. [Google Scholar] [CrossRef]
- Acharya, C.; Sahingur, S.E.; Bajaj, J.S. Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight 2017, 2, e94416. [Google Scholar] [CrossRef]
- Zhou, A.; Tang, L.; Zeng, S.; Lei, Y.; Yang, S.; Tang, B. Gut microbiota: A new piece in understanding hepatocarcinogenesis. Cancer Lett. 2020, 474, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Ni, J.; Huang, R.; Zhou, H.; Xu, X.; Li, Y.; Cao, P.; Zhong, K.; Ge, M.; Chen, X.; Hou, B.; et al. Analysis of the relationship between the degree of dysbiosis in gut microbiota and prognosis at different stages of primary hepatocellular carcinoma. Front. Microbiol. 2019, 10, 1458. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; El-Nezami, H. Targeting gut microbiota in hepatocellular carcinoma: Probiotics as a novel therapy. Hepatobiliary Surg. Nutr. 2018, 7, 11–20. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. The role of short-chain fatty acids in cancer prevention and cancer treatment. Arch. Biochem. Biophys. 2024, 761, 110172. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Limtrakul Dejkriengkraikul, P.; Wongpoomchai, R. Protective effects of defatted sticky rice bran extracts on the early stages of hepatocarcinogenesis in rats. Molecules 2019, 24, 2142. [Google Scholar] [CrossRef]
- Zhao, B.; Rehati, P.; Yang, Z.; Cai, Z.; Guo, C.; Li, Y. The potential toxicity of microplastics on human health. Sci. Total Environ. 2024, 912, 168946. [Google Scholar] [CrossRef]
- Gouin, T.; Ellis-Hutchings, R.; Thornton Hampton, L.M.; Lemieux, C.L.; Wright, S.L. Screening and prioritization of nano- and microplastic particle toxicity studies for evaluating human health risks—Development and application of a toxicity study assessment tool. Microplastics Nanoplastics 2022, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Khuanphram, N.; Taya, S.; Kongtawelert, P.; Wongpoomchai, R. Sesame extract promotes chemopreventive effect of hesperidin on early phase of diethylnitrosamine-initiated hepatocarcinogenesis in rats. Pharmaceutics 2021, 13, 1687. [Google Scholar] [CrossRef]
- Shi, C.; Han, X.; Guo, W.; Wu, Q.; Yang, X.; Wang, Y.; Tang, G.; Wang, S.; Wang, Z.; Liu, Y.; et al. Disturbed gut-liver axis indicating oral exposure to polystyrene microplastic potentially increases the risk of insulin resistance. Environ. Int. 2022, 164, 107273. [Google Scholar] [CrossRef]
- Rafiee, M.; Dargahi, L.; Eslami, A.; Beirami, E.; Jahangiri-Rad, M.; Sabour, S.; Amereh, F. Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere 2018, 193, 745–753. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, H.; Huang, Y.; Zhang, Y.; Ren, H.; Fang, M.; Wang, Q.; Chen, W.; Hale, R.C.; Galloway, T.S.; et al. Long-term exposure to environmentally relevant doses of large polystyrene microplastics disturbs lipid homeostasis via bowel function interference. Environ. Sci. Technol. 2022, 56, 15805–15817. [Google Scholar] [CrossRef]
- Guo, H.; Punvittayagul, C.; Vachiraarunwong, A.; Phannasorn, W.; Wongpoomchai, R. Cancer chemopreventive potential of cooked glutinous purple rice on the early stages of hepatocarcinogenesis in rats. Front. Nutr. 2022, 9, 1032771. [Google Scholar] [CrossRef]
- Zeng, H.; He, S.; Xiong, Z.; Su, J.; Wang, Y.; Zheng, B.; Zhang, Y. Gut microbiota-metabolic axis insight into the hyperlipidemic effect of lotus seed resistant starch in hyperlipidemic mice. Carbohydr. Polym. 2023, 314, 120939. [Google Scholar] [CrossRef] [PubMed]
- Phannasorn, W.; Pharapirom, A.; Thiennimitr, P.; Guo, H.; Ketnawa, S.; Wongpoomchai, R. Enriched riceberry bran oil exerts chemopreventive properties through anti-inflammation and alteration of gut microbiota in carcinogen-induced liver and colon carcinogenesis in rats. Cancers 2022, 14, 4358. [Google Scholar] [CrossRef] [PubMed]
- Zuri, G.; Karanasiou, A.; Lacorte, S. Microplastics: Human exposure assessment through air, water, and food. Environ. Int. 2023, 179, 108150. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, J.; Pan, X.; Meng, D.; Zhu, Y.; Bai, Y.; Shi, C.; Duan, Y.; Wang, T.; Li, X.; et al. Gut microbiome mediates the protective effects of exercise after myocardial infarction. Microbiome 2022, 10, 82. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Yang, J.; Liu, J.; Li, J.; Liu, Y.; Li, X.; Chen, L.; Hsu, C.; Zeng, J.; et al. Gut microbiota-derived short-chain fatty acids ameliorate methamphetamine-induced depression- and anxiety-like behaviors in a Sigmar-1 receptor-dependent manner. Acta. Pharm. Sin. B. 2023, 13, 4801–4822. [Google Scholar] [CrossRef]
- Taya, S.; Dissook, S.; Ruangsuriya, J.; Yodkeeree, S.; Boonyapranai, K.; Chewonarin, T.; Wongpoomchai, R. Thai fermented soybean (Thua-Nao) prevents early stages of colorectal carcinogenesis induced by diethylnitrosamine and 1,2-dimethylhydrazine through modulations of cell proliferation and gut microbiota in rats. Nutrients 2024, 16, 3506. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D.; Li, R.; Li, Z.; Wang, D. Health risk of human exposure to microplastics: A review. Environ. Chem. Lett. 2024, 22, 1155–1183. [Google Scholar] [CrossRef]
- Park, J.E.; Seo, J.E.; Lee, J.Y.; Kwon, H. Distribution of seven N-nitrosamines in food. Toxicol. Res. 2015, 31, 279–288. [Google Scholar] [CrossRef]
- Sato, K. Glutathione transferases as markers of preneoplasia and neoplasia. Adv. Cancer Res. 1989, 52, 205–255. [Google Scholar] [CrossRef]
- Sun, W.; Yan, S.; Meng, Z.; Tian, S.; Jia, M.; Huang, S.; Wang, Y.; Zhou, Z.; Diao, J.; Zhu, W. Combined ingestion of polystyrene microplastics and epoxiconazole increases health risk to mice: Based on their synergistic bioaccumulation in vivo. Environ. Int. 2022, 166, 107391. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Ballesteros, S.; Rubio, L.; Marcos, R.; Hernández, A. Long-term exposure to nanoplastics alters molecular and functional traits related to the carcinogenic process. J. Hazard. Mater. 2022, 438, 129470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lamba, M.; Pachar, A.K.; Yadav, S.; Acharya, A. Microplastics—A growing concern as carcinogens in cancer etiology: Emphasis on biochemical and molecular mechanisms. Cell Biochem. Biophys. 2024, 82, 3109–3121. [Google Scholar] [CrossRef]
- Dzierżyński, E.; Gawlik, P.J.; Puźniak, D.; Flieger, W.; Jóźwik, K.; Teresiński, G.; Forma, A.; Wdowiak, P.; Baj, J.; Flieger, J. Microplastics in the human body: Exposure, detection, and risk of carcinogenesis: A state-of-the-art review. Cancers 2024, 16, 3703. [Google Scholar] [CrossRef] [PubMed]
- Verna, L.; Whysner, J.; Williams, G.M. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 1996, 71, 57–81. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Molina-Frechero, N.; Mori-Estevez, A.D.; Sánchez-Acuña, G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e174–e179. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Libertucci, J.; Dutta, U.; Kaur, S.; Jury, J.; Rossi, L.; Fontes, M.E.; Shajib, M.S.; Khan, W.I.; Surette, M.G.; Verdu, E.F.; et al. Inflammation-related differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn’s disease. Am. J. Physiol. 2018, 315, G420–G431. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Yun, Y.; Kim, H.N.; Lee, E.J.; Ryu, S.; Chang, Y.; Shin, H.; Kim, H.L.; Kim, T.H.; Yoo, K.; Kim, H.Y. Fecal and blood microbiota profiles and presence of nonalcoholic fatty liver disease in obese versus lean subjects. PLoS ONE 2019, 14, e0213692. [Google Scholar] [CrossRef] [PubMed]
- Sanapareddy, N.; Legge, R.M.; Jovov, B.; McCoy, A.; Burcal, L.; Araujo-Perez, F.; Randall, T.A.; Galanko, J.; Benson, A.; Sandler, R.S.; et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012, 6, 1858–1868. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Lynch, D.B.; Brown, J.M.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Duncan, S.H.; Garcia-Gil, L.J.; Martinez-Medina, M. Faecalibacterium prausnitzii: From microbiology to diagnostics and prognostics. ISME J. 2017, 11, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Deleu, S.; Arnauts, K.; Deprez, L.; Machiels, K.; Ferrante, M.; Huys, G.R.B.; Thevelein, J.M.; Raes, J.; Vermeire, S. High acetate concentration protects intestinal barrier and exerts anti-inflammatory effects in organoid-derived epithelial monolayer cultures from patients with Ulcerative Colitis. Int. J. Mol. Sci. 2023, 24, 768. [Google Scholar] [CrossRef]
- Hodgkinson, K.; El Abbar, F.; Dobranowski, P.; Manoogian, J.; Butcher, J.; Figeys, D.; Mack, D.; Stintzi, A. Butyrate’s role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin. Nutr. 2023, 42, 61–75. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Medhat, A.; Spector, I. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis Rev. 2023, 42, 677–698. [Google Scholar] [CrossRef]

| Group | Initial Body Weight (g) | Final Body Weight (g) | Consumption (Per Rat Per Day) | |
|---|---|---|---|---|
| Food (g) | Water (mL) | |||
| Control | 101 ± 10 | 565 ± 43 | 27.4 ± 3.5 | 21.7 ± 5.9 |
| MP | 102 ± 7 | 543 ± 47 | 28.5 ± 1.6 | 22.2 ± 1.8 |
| DEN | 102 ± 14 | 558 ± 55 | 27.7 ± 2.0 | 21.2 ± 2.6 |
| DEN + MP | 101 ± 12 | 506 ± 75 * | 25.4 ± 1.1 | 18.0 ± 0.9 |
| Group | Relative Organ Weight (%) | Liver Function Test (Unit/L) | |||
|---|---|---|---|---|---|
| Liver | Spleen | Kidney | ALT | AST | |
| Control | 3.39 ± 0.30 | 0.18 ± 0.02 | 0.61 ± 0.08 | 54.3 ± 1.5 | 95.0 ± 4.2 |
| MP | 3.16 ± 0.10 * | 0.18 ± 0.01 | 0.59 ± 0.07 | 63.3 ± 7.5 | 121.1 ± 31.9 |
| DEN | 3.24 ± 0.30 | 0.20 ± 0.03 | 0.57 ± 0.06 | 62.9 ± 9.2 | 101.7 ± 24.5 |
| DEN + MP | 3.06 ± 0.14 * | 0.21 ± 0.04 * | 0.55 ± 0.06 | 71.4 ± 9.1 *# | 123.3 ± 21.3 |
| SCFA | Group | |||
|---|---|---|---|---|
| (µg/mg Feces) | Control | MP | DEN | DEN + MP |
| Acetic acid | 5.958 ± 0.682 | 5.731 ± 0.403 # | 4.570 ± 0.656 * | 4.631 ± 0.942 * |
| Propanoic acid | 1.438 ± 0.585 | 0.981 ± 0.294 | 0.944 ± 0.410 | 0.778 ± 0.273 * |
| Isobutyric acid | 0.077 ± 0.013 | 0.084 ± 0.010 | 0.071 ± 0.020 | 0.101 ± 0.031 # |
| Butanoic acid | 1.485 ± 0.833 | 2.169 ± 1.153 | 1.806 ± 0.937 | 1.468 ± 0.837 |
| Isovaleric acid | 0.053 ± 0.010 | 0.065 ± 0.011 | 0.060 ± 0.015 | 0.075 ± 0.026 * |
| Valeric acid | 0.106 ± 0.039 | 0.105 ± 0.016 | 0.091 ± 0.020 | 0.106 ± 0.037 |
| Isohexanoic acid | 0.005 ± 0.004 | 0.003 ± 0.001 | 0.002 ± 0.001 * | 0.002 ± 0.001 * |
| Hexanoic acid | 0.196 ± 0.098 | 0.294 ± 0.164 | 0.215 ± 0.136 | 0.260 ± 0.150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Wang, J.; Huang, S.; Sooranna, S.R.; Shu, F.; Li, G. Long-Term Exposure to Microplastics Promotes Early-Stage Hepatocarcinogenesis Induced by Diethylnitrosamine in Rats by Modulation of Their Gut Microbiota. Toxics 2025, 13, 353. https://doi.org/10.3390/toxics13050353
Guo H, Wang J, Huang S, Sooranna SR, Shu F, Li G. Long-Term Exposure to Microplastics Promotes Early-Stage Hepatocarcinogenesis Induced by Diethylnitrosamine in Rats by Modulation of Their Gut Microbiota. Toxics. 2025; 13(5):353. https://doi.org/10.3390/toxics13050353
Chicago/Turabian StyleGuo, Huina, Jianan Wang, Shaowen Huang, Suren Rao Sooranna, Fangyi Shu, and Genliang Li. 2025. "Long-Term Exposure to Microplastics Promotes Early-Stage Hepatocarcinogenesis Induced by Diethylnitrosamine in Rats by Modulation of Their Gut Microbiota" Toxics 13, no. 5: 353. https://doi.org/10.3390/toxics13050353
APA StyleGuo, H., Wang, J., Huang, S., Sooranna, S. R., Shu, F., & Li, G. (2025). Long-Term Exposure to Microplastics Promotes Early-Stage Hepatocarcinogenesis Induced by Diethylnitrosamine in Rats by Modulation of Their Gut Microbiota. Toxics, 13(5), 353. https://doi.org/10.3390/toxics13050353







