A Review of Biomonitoring of Phthalate Exposures
Abstract
:1. Introduction
2. Sources of Phthalates
3. Biomonitoring of Phthalates
3.1. Phthalate Metabolites in Urine
3.2. Phthalate Metabolites in Serum
3.3. Phthalate Metabolites in Amniotic Fluid, Breast Milk, Semen, and Saliva
4. Select Epidemiological Studies Linking Phthalate Exposure and Health Outcomes
4.1. Diabetes
4.2. Overweight and Obesity
4.3. Allergy and Asthma
4.4. Reproductive Health
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Latini, G. Monitoring phthalate exposure in humans. Clin. Chim. Acta 2005, 361, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Skakkebaek, N.E.; Andersson, A.M. Metabolism of phthalates in humans. Mol. Nutr. Food Res. 2007, 51, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.H.; Breindahl, T. Plasticizers in total diet samples, baby food and infant formulae. Food Addit. Contam. 2000, 17, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbuhler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.R. Phthalate ester plasticizers—Why and how they are used. Environ. Health Perspect. 1973, 3, 3–12. [Google Scholar] [PubMed]
- Mackintosh, C.E.; Maldonado, J.A.; Ikonomou, M.G.; Gobas, F.A.P.C. Sorption of phthalate esters and PCBs in a marine ecosystem. Environ. Sci. Technol. 2006, 40, 3481–3488. [Google Scholar] [CrossRef]
- Sirivarasai, J.; Wananukul, W.; Kaojarern, S.; Chanprasertyothin, S.; Thongmung, N.; Ratanachaiwong, W.; Sura, T.; Sritara, P. Association between inflammatory marker, environmental lead exposure and glutathione S-transferase gene. Toxicol. Lett. 2013, 221, 61. [Google Scholar] [CrossRef]
- Net, S.; Sempere, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, fate, behavior and ecotoxicological state of phthalates in different environmental matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Gimeno, P.; Thomas, S.; Bousquet, C.; Maggio, A.F.; Civade, C.; Brenier, C.; Bonnet, P.A. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC–MS. J. Chromatogr. B 2014, 949–950, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef]
- Talsness, C.E.; Andrade, A.J.M.; Kuriyama, S.N.; Taylor, J.A.; vom Saal, F.S. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.L.E.; Ostby, J.; Furr, J.; Wolf, C.J.; Lambright, C.; Parks, L.; Veeramachaneni, D.N.; Wilson, V.; Price, M.; Hotchkiss, A.; et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update 2001, 7, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.; Mohamed, Z.; El-Haleem, M.; Samak, M. Effects of exposure to plasticizers di-(2-ethylhexyl) phthalate and trioctyltrimellitate on the histological structure of adult male albino rats’ liver. J. Clin. Toxicol. 2013, 3, 169–178. [Google Scholar]
- Rusyn, I.; Peters, J.M.; Cunningham, M.L. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 2006, 36, 459–479. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Song, L.; Wei, J.; Chen, T.; Chen, J.; Lin, Y.; Xia, W.; Xu, B.; Li, X.; Chen, X.; et al. Maternal exposure to di-(2-ethylhexyl)phthalate alters kidney development through the renin–angiotensin system in offspring. Toxicol. Lett. 2012, 212, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Crocker, J.F.S.; Safe, S.H.; Acott, P. Effects of chronic phthalate exposure on the kidney. J. Toxicol. Environ. Health 1988, 23, 433–444. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, L.; Kannan, K. Phthalates and parabens in personal care products from China: Concentrations and human exposure. Arch. Environ. Contam. Toxicol. 2014, 66, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Specht, I.O.; Toft, G.; Hougaard, K.S.; Lindh, C.H.; Lenters, V.; Jonsson, B.A.G.; Heederik, D.; Giwercman, A.; Bonde, J.P.E. Associations between serum phthalates and biomarkers of reproductive function in 589 adult men. Environ. Int. 2014, 66, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.H.; Zhou, T.; Zhao, S.Z.; Zhang, H.; Zhang, M.R.; Chen, J.S.; Wang, M.; Wu, M.; Li, S.G.; Chen, B. Food consumption survey of Shanghai adults in 2012 and its associations with phthalate metabolites in urine. Environ. Int. 2017, 101, 80–88. [Google Scholar] [CrossRef]
- Silva, M.J.; Samandar, E.; Preau, J.L., Jr.; Reidy, J.A.; Needham, L.L.; Calafat, A.M. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 860, 106–112. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, H.; Zhou, N.Y.; Sun, L.; Bao, H.Q.; Tan, L.; Chen, H.Q.; Ling, X.; Zhang, G.W.; Huang, L.P.; et al. Phthalate exposure, even below US EPA reference doses, was associated with semen quality and reproductive hormones: Prospective MARHCS study in general population. Environ. Int. 2017, 104, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Coull, B.A.; Skakkebaek, N.E.; Williams, M.A.; Dadd, R.; Minguez-Alarcon, L.; Krawetz, S.A.; Hait, E.J.; Korzenik, J.R.; Moss, A.C.; et al. A crossover-crossback prospective study of dibutyl-phthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environ. Int. 2016, 95, 120–130. [Google Scholar] [CrossRef]
- Högberg, J.; Hanberg, A.; Berglund, M.; Skerfving, S.; Remberger, M.; Calafat, A.M.; Filipsson, A.F.; Jansson, B.; Johansson, N.; Appelgren, M.; et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ. Health Perspect. 2008, 116, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Main, K.M.; Mortensen, G.K.; Kaleva, M.M.; Boisen, K.A.; Damgaard, I.N.; Chellakooty, M.; Schmidt, I.M.; Suomi, A.-M.; Virtanen, H.E.; Petersen, J.H.; et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ. Health Perspect. 2006, 114, 270–276. [Google Scholar] [CrossRef]
- Asimakopoulos, A.G.; Xue, J.; De Carvalho, B.P.; Iyer, A.; Abualnaja, K.O.; Yaghmoor, S.S.; Kumosani, T.A.; Kannan, K. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res. 2016, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Chen, Y.-H.; Mukherjee, B.; Meeker, J.D. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: A repeated measures analysis. Environ. Health Perspect. 2015, 123, 210–216. [Google Scholar] [CrossRef]
- Colón, I.; Caro, D.; Bourdony, C.J.; Rosario, O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ. Health Perspect. 2000, 108, 895–900. [Google Scholar] [PubMed]
- Buck Louis, G.M.; Gray, L.E.; Marcus, M.; Ojeda, S.R.; Pescovitz, O.H.; Witchel, S.F.; Sippell, W.; Abbott, D.H.; Soto, A.; Tyl, R.W.; et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics 2008, 121, 192–207. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Latini, G.; Felice, C.D.; Razzi, S.; Paris, I.; Ruggieri, F.; Mazzeo, P.; Petraglia, F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum. Reprod. 2003, 18, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Rozati, R.; Reddy, B.V.R.; Raman, N. General gynaecology: Association of phthalate esters with endometriosis in Indian women. Int. J. Gynaecol. Obstet. 2006, 113, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.M.; Silva, M.J.; Barr, D.B.; Brock, J.W.; Ryan, L.; Chen, Z.; Herrick, R.F.; Christiani, D.C.; Hauser, R. Phthalate exposure and human semen parameters. Epidemiology 2003, 14, 269–277. [Google Scholar] [CrossRef]
- Joensen Ulla, N.; Frederiksen, H.; Jensen Martin, B.; Lauritsen Mette, P.; Olesen Inge, A.; Lassen Tina, H.; Andersson, A.M.; Jørgensen, N. Phthalate excretion pattern and testicular function: A study of 881 healthy danish men. Environ. Health Perspect. 2012, 120, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Yaghjyan, L.; Sites, S.; Ruan, Y.; Chang, S.H. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999–2004. Int. J. Obstet. 2015, 36, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to phthalates and breast cancer risk in Northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Monographs on the Evaluation of Carcinogenic Risks to Humans. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono77.pdf (accessed on 5 April 2019).
- Le Moal, J.; Sharpe, R.M.; Jϕrgensen, N.; Levine, H.; Jurewicz, J.; Mendiola, J.; Swan, S.H.; Virtanen, H.; Christin-Maître, S.; Cordier, S.; et al. Toward a multi-country monitoring system of reproductive health in the context of endocrine disrupting chemical exposure. Eur. J. Public Health. 2016, 26, 76–83. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Skakkebaek, N.E. Testicular dysgenesis syndrome: Mechanistic insights and potential new downstream effects. Fertil. Steril. 2008, 89, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Moon, K.; Thayer, K.A.; Navas-Acien, A. Environmental chemicals and type 2 diabetes: An updated systematic review of the epidemiologic evidence. Curr. Diabetes Rep. 2013, 13, 831–849. [Google Scholar] [CrossRef]
- Swan, S.H. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008, 108, 177–184. [Google Scholar] [CrossRef]
- Goen, T.; Dobler, L.; Koschorreck, J.; Muller, J.; Wiesmuller, G.A.; Drexler, H.; Kolossa-Gehring, M. Trends of the internal phthalate exposure of young adults in Germany—Follow-up of a retrospective human biomonitoring study. Int. J. Hyg. Environ. Health 2011, 215, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tranfo, G.; Caporossi, L.; Pigini, D.; Capanna, S.; Papaleo, B.; Paci, E. Temporal trends of urinary phthalate concentrations in two populations: Effects of REACH authorization after five years. Int. J. Environ. Res. Public Health 2018, 15, 1950. [Google Scholar] [CrossRef]
- North, M.L.; Takaro, T.K.; Diamond, M.L.; Ellis, A.K. Effects of phthalates on the development and expression of allergic disease and asthma. Ann. Allergy Asthma Immunol. 2014, 112, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Wittassek, M.; Koch, H.M.; Angerer, J.; Bruning, T. Assessing exposure to phthalates—The human biomonitoring approach. Mol. Nutr. Food Res. 2011, 55, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Sapozhnikova, Y.; Hoh, E. Suspect screening of chemicals in food packaging film by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. LCGC N. Am. 2019, 37, 52–60. [Google Scholar]
- Tran, T.M.; Kannan, K. Occurrence of phthalate diesters in particulate and vapor phases in indoor air and implications for human exposure in Albany, New York, USA. Arch. Environ. Contam. Toxicol. 2015, 68, 489–499. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environ. Sci. Technol. 2011, 45, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Moreta, C.; Tena, M.T.; Kannan, K. Analytical method for the determination and a survey of parabens and its derivatives in pharmaceuticals. Environ. Res. 2015, 142, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Duty, S.; Godfrey-Bailey, L.; Calafat, A.M. Medications as a source of human exposure to phthalates. Environ. Health Perspect. 2004, 112, 751–753. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Safety Assessment of Di(2-ethylhexyl)phthalate (DEHP) Released from PVC Medical Devices, Rockville, MD 20852. Available online: https://noharm-global.org/documents/safety-assessment-dehp-released-pvc-medical-devices (accessed on 5 April 2019).
- Guo, Y.; Wu, Q.; Kannan, K. Phthalate metabolites in urine from China, and implications for human exposures. Environ. Int. 2011, 37, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.M.; Roos, V.; Ronn, M.; Johansson, L.; Ahlstrom, H.; Kullberg, J.; Lind, L. Serum concentrations of phthalate metabolites are related to abdominal fat distribution two years later in elderly women. Environ. Health 2012, 11, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Silva, M.J.; Brock, J.W.; Reidy, J.A.; Malek, N.A.; Hodge, C.C.; Nakazawa, H.; Needham, L.L.; Barr, D.B. Quantitative Detection of Nine Phthalate Metabolites in Human Serum Using Reversed-Phase High-Performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. J. Anal. Toxicol. 2003, 27, 284–289. [Google Scholar] [CrossRef]
- Hauser, R.; Meeker, J.D.; Duty, S.; Silva, M.J.; Calafat, A.M. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 2006, 17, 682–691. [Google Scholar] [CrossRef]
- Zhu, J.P.; Phillips, S.P.; Feng, Y.L.; Yang, X.F. Phthalate esters in human milk: Concentration variations over a 6-month postpartum time. Environ. Sci. Technol. 2006, 40, 5276–5281. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, I.N.; Skakkebæk, N.E.; Toppari, J.; Virtanen, H.E.; Shen, H.; Schramm, K.W.; Petersen, J.H.; Jensen, T.K.; Main, K.M.; the Nordic Cryptorchidism Study, G. Persistent pesticides in human breast milk and cryptorchidism. Environ. Health Perspect. 2006, 114, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Reidy, J.A.; Samandar, E.; Herbert, A.R.; Needham, L.L.; Calafat, A.M. Detection of phthalate metabolites in human saliva. Arch. Toxicol. 2005, 79, 647–652. [Google Scholar] [CrossRef]
- Fennell, T.R.; Krol, W.L.; Sumner, S.C.J.; Snyder, R.W. Pharmacokinetics of dibutylphthalate in pregnant rats. Toxicol. Sci. 2004, 82, 407–418. [Google Scholar] [CrossRef]
- Silva, M.J.; Reidy, J.A.; Herbert, A.R.; Preau, J.L.; Needham, L.L.; Calafat, A.M. Detection of phthalate metabolites in human amniotic fluid. Bull. Environ. Contam. Toxicol. 2004, 72, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Knudsen, L.E.; Mizrak, S.; Joas, A. Identification of exposure to environmental chemicals in children and older adults using human biomonitoring data sorted by age: Results from a literature review. Int. J. Hyg. Environ. Health 2017, 220, 282–298. [Google Scholar] [CrossRef]
- Heffernan, A.L.; Thompson, K.; Eaglesham, G.; Vijayasarathy, S.; Mueller, J.F.; Sly, P.D.; Gomez, M.J. Rapid, automated online SPE-LC-QTRAP-MS/MS method for the simultaneous analysis of 14 phthalate metabolites and 5 bisphenol analogues in human urine. Talanta 2016, 151, 224–233. [Google Scholar] [CrossRef]
- Hartmann, C.; Uhl, M.; Weiss, S.; Koch, H.M.; Scharf, S.; Konig, J. Human biomonitoring of phthalate exposure in Austrian children and adults and cumulative risk assessment. Int. J. Hyg. Environ. Health 2015, 218, 489–499. [Google Scholar] [CrossRef]
- Dewalque, L.; Charlier, C.; Pirard, C. Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population. Toxicol. Lett. 2014, 231, 161–168. [Google Scholar] [CrossRef]
- Geens, T.; Bruckers, L.; Covaci, A.; Schoeters, G.; Fierens, T.; Sioen, I.; Vanermen, G.; Baeyens, W.; Morrens, B.; Loots, I.; et al. Determinants of bisphenol A and phthalate metabolites in urine of Flemish adolescents. Environ. Res. 2014, 134, 110–117. [Google Scholar] [CrossRef]
- Dewalque, L.; Pirard, C.; Charlier, C. Measurement of urinary biomarkers of parabens, benzophenone-3, and phthalates in a Belgian population. Biomed. Res. Int. 2014, 2014, 649314–649327. [Google Scholar] [CrossRef]
- Dewalque, L.; Pirard, C.; Dubois, N.; Charlier, C. Simultaneous determination of some phthalate metabolites, parabens and benzophenone-3 in urine by ultra high pressure liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 949–950, 37–47. [Google Scholar] [CrossRef]
- Rocha, B.A.; Asimakopoulos, A.G.; Barbosa, F., Jr.; Kannan, K. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ. 2017, 586, 152–162. [Google Scholar] [CrossRef]
- Saravanabhavan, G.; Guay, M.; Langlois, E.; Giroux, S.; Murray, J.; Haines, D. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007–2009). Int. J. Hyg. Environ. Health 2013, 216, 652–661. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Davis, K.; Marro, L.; Fisher, M.; Legrand, M.; LeBlanc, A.; Gaudreau, E.; Foster, W.G.; Choeurng, V.; Fraser, W.D.; et al. Phthalate and bisphenol A exposure among pregnant women in Canada—Results from the MIREC study. Environ. Int. 2014, 68, 55–65. [Google Scholar] [CrossRef]
- Arbuckle, T.E.; Fisher, M.; MacPherson, S.; Lang, C.; Provencher, G.; LeBlanc, A.; Hauser, R.; Feeley, M.; Ayotte, P.; Neisa, A.; et al. Maternal and early life exposure to phthalates: The plastics and personal-care products use in pregnancy (P4) study. Sci. Total Environ. 2016, 551–552, 344–356. [Google Scholar] [CrossRef]
- Gao, C.-J.; Liu, L.-Y.; Ma, W.-L.; Ren, N.-Q.; Guo, Y.; Zhu, N.-Z.; Jiang, L.; Li, Y.-F.; Kannan, K. Phthalate metabolites in urine of Chinese young adults: Concentration, profile, exposure and cumulative risk assessment. Sci. Total Environ. 2016, 543, 19–27. [Google Scholar] [CrossRef]
- Guo, Y.; Alomirah, H.; Cho, H.-S.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Kannan, K. Occurrence of phthalate metabolites in human urine from several Asian countries. Environ. Sci. Technol. 2011, 45, 3138–3144. [Google Scholar] [CrossRef]
- Shen, Q.; Shi, H.J.; Zhang, Y.H.; Cao, Y. Dietary intake and phthalates body burden in boys and girls. Arch. Public Health 2015, 73, 5–10. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Wang, X.; Huang, Q.; Tian, M.; Shen, H. Low-level environmental phthalate exposure associates with urine metabolome alteration in a Chinese male cohort. Environ. Sci. Technol. 2016, 50, 5953–5960. [Google Scholar] [CrossRef]
- Gong, M.; Weschler, C.J.; Liu, L.; Shen, H.; Huang, L.; Sundell, J.; Zhang, Y. Phthalate metabolites in urine samples from Beijing children and correlations with phthalate levels in their handwipes. Indoor Air 2015, 25, 572–581. [Google Scholar] [CrossRef]
- Černá, M.; Malý, M.; Rudnai, P.; Középesy, S.; Náray, M.; Halzlová, K.; Jajcaj, M.; Grafnetterová, A.; Krsková, A.; Antošová, D.; et al. Case study: Possible differences in phthalates exposure among the Czech, Hungarian, and Slovak populations identified based on the DEMOCOPHES pilot study results. Environ. Res. 2015, 141, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Jorgensen, N.; Andersson, A.M. Correlations between phthalate metabolites in urine, serum, and seminal plasma from young Danish men determined by isotope dilution liquid chromatography tandem mass spectrometry. J. Anal. Toxicol. 2010, 34, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Nielsen, J.K.S.; Mørck, T.A.; Hansen, P.W.; Jensen, J.F.; Nielsen, O.; Andersson, A.-M.; Knudsen, L.E. Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother–child pairs. Int. J. Hyg. Environ. Health 2013, 216, 772–783. [Google Scholar] [CrossRef]
- Frederiksen, H.; Aksglaede, L.; Sorensen, K.; Skakkebaek, N.E.; Juul, A.; Andersson, A.-M. Urinary excretion of phthalate metabolites in 129 healthy Danish children and adolescents: Estimation of daily phthalate intake. Environ. Res. 2011, 111, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.; Beko, G.; Weschler, C.J.; Brive, L.M.; Toftum, J.; Callesen, M.; Clausen, G. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int. J. Hyg. Environ. Health 2014, 217, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Schwedler, G.; Seiwert, M.; Fiddicke, U.; Issleb, S.; Holzer, J.; Nendza, J.; Wilhelm, M.; Wittsiepe, J.; Koch, H.M.; Schindler, B.K.; et al. Human biomonitoring pilot study DEMOCOPHES in Germany: Contribution to a harmonized European approach. Int. J. Hyg. Environ. Health 2017, 220, 686–696. [Google Scholar] [CrossRef]
- Haug, L.S.; Sakhi, A.K.; Cequier, E.; Casas, M.; Maitre, L.; Basagana, X.; Andrusaityte, S.; Chalkiadaki, G.; Chatzi, L.; Coen, M.; et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ. Int. 2018, 121, 751–763. [Google Scholar] [CrossRef]
- Zeman, F.A.; Boudet, C.; Tack, K.; Floch Barneaud, A.; Brochot, C.; Pery, A.R.; Oleko, A.; Vandentorren, S. Exposure assessment of phthalates in French pregnant women: Results of the ELFE pilot study. Int. J. Hyg. Environ. Health 2013, 216, 271–279. [Google Scholar] [CrossRef]
- Wittassek, M.; Wiesmuller, G.A.; Koch, H.M.; Eckard, R.; Dobler, L.; Muller, J.; Angerer, J.; Schluter, C. Internal phthalate exposure over the last two decades—A retrospective human biomonitoring study. Int. J. Hyg. Environ. Health 2007, 210, 319–333. [Google Scholar] [CrossRef]
- Becker, K.; Seiwert, M.; Angerer, J.; Heger, W.; Koch, H.M.; Nagorka, R.; Roßkamp, E.; Schlüter, C.; Seifert, B.; Ullrich, D. DEHP metabolites in urine of children and DEHP in house dust. Int. J. Hyg. Environ. Health 2004, 207, 409–417. [Google Scholar] [CrossRef]
- Koch, H.M.; Drexler, H.; Angerer, J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int. J. Hyg. Environ. Health 2003, 206, 77–83. [Google Scholar] [CrossRef]
- Fromme, H.; Bolte, G.; Koch, H.M.; Angerer, J.; Boehmer, S.; Drexler, H.; Mayer, R.; Liebl, B. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int. J. Hyg. Environ. Health 2007, 210, 21–33. [Google Scholar] [CrossRef]
- Koch, H.M.; Ruther, M.; Schutze, A.; Conrad, A.; Palmke, C.; Apel, P.; Bruning, T.; Kolossa-Gehring, M. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220, 130–141. [Google Scholar] [CrossRef]
- Koch, H.M.; Wittassek, M.; Bruning, T.; Angerer, J.; Heudorf, U. Exposure to phthalates in 5–6 years old primary school starters in Germany—A human biomonitoring study and a cumulative risk assessment. Int. J. Hyg. Environ. Health 2011, 214, 188–195. [Google Scholar] [CrossRef]
- Kasper-Sonnenberg, M.; Koch, H.M.; Wittsiepe, J.; Bruning, T.; Wilhelm, M. Phthalate metabolites and bisphenol A in urines from German school-aged children: Results of the Duisburg birth cohort and Bochum cohort studies. Int. J. Hyg. Environ. Health 2014, 217, 830–838. [Google Scholar] [CrossRef]
- Becker, K.; Goen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Muller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef]
- Volkel, W.; Kiranoglu, M.; Schuster, R.; Fromme, H. Hbmnet Phthalate intake by infants calculated from biomonitoring data. Toxicol. Lett. 2014, 225, 222–229. [Google Scholar] [CrossRef]
- Kasper-Sonnenberg, M.; Koch, H.M.; Wittsiepe, J.; Wilhelm, M. Levels of phthalate metabolites in urine among mother-child-pairs—Results from the Duisburg birth cohort study, Germany. Int. J. Hyg. Environ. Health 2012, 215, 373–382. [Google Scholar] [CrossRef]
- Myridakis, A.; Fthenou, E.; Balaska, E.; Vakinti, M.; Kogevinas, M.; Stephanou, E.G. Phthalate esters, parabens and bisphenol-A exposure among mothers and their children in Greece (Rhea cohort). Environ. Int. 2015, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, M.Z.; Gorji, M.E.; Rietjens, I.M.C.M.; Louisse, J.; Bruinen de Bruin, Y.; Liska, R. Biomonitoring and subsequent risk assessment of combined exposure to phthalates in Iranian children and adolescents. Int. J. Environ. Res. Public Health 2018, 15, 2336. [Google Scholar] [CrossRef]
- Cullen, E.; Evans, D.; Griffin, C.; Burke, P.; Mannion, R.; Burns, D.; Flanagan, A.; Kellegher, A.; Schoeters, G.; Govarts, E.; et al. Urinary phthalate concentrations in mothers and their children in Ireland: Results of the DEMOCOPHES human biomonitoring study. Int. J. Environ. Res. Public Health 2017, 14, 1456. [Google Scholar] [CrossRef] [PubMed]
- Berman, T.; Goldsmith, R.; Goen, T.; Spungen, J.; Novack, L.; Levine, H.; Amitai, Y.; Shohat, T.; Grotto, I. Urinary concentrations of environmental contaminants and phytoestrogens in adults in Israel. Environ. Int. 2013, 59, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Tranfo, G.; Papaleo, B.; Caporossi, L.; Capanna, S.; De Rosa, M.; Pigini, D.; Corsetti, F.; Paci, E. Urinary metabolite concentrations of phthalate metabolites in Central Italy healthy volunteers determined by a validated HPLC/MS/MS analytical method. Int. J. Hyg. Environ. Health 2013, 216, 481–485. [Google Scholar] [CrossRef]
- Itoh, H.; Iwasaki, M.; Hanaoka, T.; Sasaki, H.; Tanaka, T.; Tsugane, S. Urinary phthalate monoesters and endometriosis in infertile Japanese women. Sci. Total Environ. 2009, 408, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Yoshida, K.; Masunaga, S. Quantitative identification of unknown exposure pathways of phthalates based on measuring their metabolites in human urine. Environ. Sci. Technol. 2007, 41, 4542–4547. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshinaga, J.; Mizumoto, Y.; Serizawa, S.; Shiraishi, H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int. J. Androl. 2012, 35, 236–244. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.; Lee, G.; Lee, S.; Jo, A.; Kwak, K.; Kim, D.; Koh, D.; Kho, Y.L.; Kim, S.; et al. Urinary phthalate metabolites among elementary school children of Korea: Sources, risks, and their association with oxidative stress marker. Sci. Total Environ. 2014, 472, 49–55. [Google Scholar] [CrossRef]
- Ji, K.; Lim Kho, Y.; Park, Y.; Choi, K. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: A pilot study with “Temple Stay” participants. Environ. Res. 2010, 110, 375–382. [Google Scholar] [CrossRef]
- Jo, A.; Kim, H.; Chung, H.; Chang, N. Associations between dietary intake and urinary bisphenol A and phthalates levels in Korean women of reproductive age. Int. J. Environ. Res. Public Health 2016, 13, 680. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.; Shin, M.Y.; Kim, K.N.; Hong, Y.C. Risk assessment for phthalate exposures in the elderly: A repeated biomonitoring study. Sci. Total Environ. 2018, 618, 690–696. [Google Scholar] [CrossRef]
- Choi, W.; Kim, S.; Baek, Y.W.; Choi, K.; Lee, K.; Kim, S.; Yu, S.D.; Choi, K. Exposure to environmental chemicals among Korean adults-updates from the second Korean National Environmental Health Survey (2012–2014). Int. J. Hyg. Environ. Health 2017, 220, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, W.; Hwang, M.; Lee, Y.; Kim, S.; Yu, S.; Lee, I.; Paek, D.; Choi, K. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population—Korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci. Total Environ. 2017, 584–585, 950–957. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Park, J.; Kim, H.J.; Cho, G.J.; Kim, G.H.; Eun, S.H.; Lee, J.J.; Choi, G.; Suh, E.; et al. Urinary phthalate metabolites over the first 15months of life and risk assessment—CHECK cohort study. Sci. Total Environ. 2017, 607–608, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Song, N.R.; On, J.W.; Lee, J.; Park, J.D.; Kwon, H.J.; Yoon, H.J.; Pyo, H. Biomonitoring of urinary di(2-ethylhexyl) phthalate metabolites of mother and child pairs in South Korea. Environ. Int. 2013, 54, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pierik, F.H.; Hauser, R.; Duty, S.; Angerer, J.; Park, M.M.; Burdorf, A.; Hofman, A.; Jaddoe, V.W.; Mackenbach, J.P.; et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environ. Res. 2008, 108, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pierik, F.H.; Angerer, J.; Meltzer, H.M.; Jaddoe, V.W.V.; Tiemeier, H.; Hoppin, J.A.; Longnecker, M.P. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int. J. Hyg. Environ. Health 2009, 212, 481–491. [Google Scholar] [CrossRef]
- Giovanoulis, G.; Alves, A.; Papadopoulou, E.; Cousins, A.P.; Schutze, A.; Koch, H.M.; Haug, L.S.; Covaci, A.; Magner, J.; Voorspoels, S. Evaluation of exposure to phthalate esters and DINCH in urine and nails from a Norwegian study population. Environ. Res. 2016, 151, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Sabaredzovic, A.; Sakhi, A.K.; Brantsaeter, A.L.; Thomsen, C. Determination of 12 urinary phthalate metabolites in Norwegian pregnant women by core-shell high performance liquid chromatography with on-line solid-phase extraction, column switching and tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1002, 343–352. [Google Scholar] [CrossRef]
- Correia-Sa, L.; Kasper-Sonnenberg, M.; Palmke, C.; Schutze, A.; Norberto, S.; Calhau, C.; Domingues, V.F.; Koch, H.M. Obesity or diet? Levels and determinants of phthalate body burden—A case study on Portuguese children. Int. J. Hyg. Environ. Health 2018, 221, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Petrovicova, I.; Kolena, B.; Sidlovska, M.; Pilka, T.; Wimmerova, S.; Trnovec, T. Occupational exposure to phthalates in relation to gender, consumer practices and body composition. Environ. Sci. Pollut. Res. Int. 2016, 23, 24125–24134. [Google Scholar] [CrossRef] [PubMed]
- Kolena, B.; Petrovicova, I.; Sidlovska, M.; Pilka, T.; Neuschlova, M.; Valentova, I.; Rybansky, L.; Trnovec, T. Occupational phthalate exposure and health outcomes among hairdressing apprentices. Hum. Exp. Toxicol. 2017, 36, 1100–1112. [Google Scholar] [CrossRef]
- Pilka, T.; Petrovicova, I.; Kolena, B.; Zatko, T.; Trnovec, T. Relationship between variation of seasonal temperature and extent of occupational exposure to phthalates. Environ. Sci. Pollut. Res. Int. 2015, 22, 434–440. [Google Scholar] [CrossRef]
- Valvi, D.; Monfort, N.; Ventura, R.; Casas, M.; Casas, L.; Sunyer, J.; Vrijheid, M. Variability and predictors of urinary phthalate metabolites in Spanish pregnant women. Int. J. Hyg. Environ. Health 2015, 218, 220–231. [Google Scholar] [CrossRef]
- Cutanda, F.; Koch, H.M.; Esteban, M.; Sanchez, J.; Angerer, J.; Castano, A. Urinary levels of eight phthalate metabolites and bisphenol A in mother-child pairs from two Spanish locations. Int. J. Hyg. Environ. Health 2015, 218, 47–57. [Google Scholar] [CrossRef]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Jönsson, B.A.G.; Lindh, C.H.; Giwercman, A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ. Int. 2015, 85, 54–60. [Google Scholar] [CrossRef]
- Chen, M.L.; Chen, J.S.; Tang, C.L.; Mao, I.F. The internal exposure of Taiwanese to phthalate—An evidence of intensive use of plastic materials. Environ. Int. 2008, 34, 79–85. [Google Scholar] [CrossRef]
- Wirth, J.J.; Rossano, M.G.; Potter, R.; Puscheck, E.; Daly, D.C.; Paneth, N.; Krawetz, S.A.; Protas, B.M.; Diamond, M.P. A pilot study associating urinary concentrations of phthalate metabolites and semen quality. Syst. Biol. Reprod. Med. 2008, 54, 143–154. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Britton, J.A.; Calafat, A.M.; Ye, X.; Silva, M.J.; Reidy, J.A.; Galvez, M.P.; Brenner, B.L.; Wolff, M.S. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 2008, 106, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; Loch-Caruso, R.; Meeker, J.D. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999–2006. Environ. Res. 2011, 111, 718–726. [Google Scholar] [CrossRef]
- Brock, J.W.; Caudill, S.P.; Silva, M.J.; Needham, L.L.; Hilborn, E.D. Phthalate monoesters levels in the urine of young children. Bull. Environ. Contam. Toxicol. 2002, 68, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Just, A.C.; Adibi, J.J.; Rundle, A.G.; Calafat, A.M.; Camann, D.E.; Hauser, R.; Silva, M.J.; Whyatt, R.M. Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York city. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Polinski, K.J.; Dabelea, D.; Hamman, R.F.; Adgate, J.L.; Calafat, A.M.; Ye, X.; Starling, A.P. Distribution and predictors of urinary concentrations of phthalate metabolites and phenols among pregnant women in the Healthy Start Study. Environ. Res. 2018, 162, 308–317. [Google Scholar] [CrossRef]
- Wenzel, A.G.; Brock, J.W.; Cruze, L.; Newman, R.B.; Unal, E.R.; Wolf, B.J.; Somerville, S.E.; Kucklick, J.R. Prevalence and predictors of phthalate exposure in pregnant women in Charleston, SC. Chemosphere 2018, 193, 394–402. [Google Scholar] [CrossRef]
- Calafat, A.M.; McKee, R.H. Integrating biomonitoring exposure data into the risk assessment process: Phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environ. Health Perspect. 2006, 114, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Colacino Justin, A.; Harris, T.R.; Schecter, A. Dietary intake Is associated with phthalate body burden in a nationally representative sample. Environ. Health Perspect. 2010, 118, 998–1003. [Google Scholar] [CrossRef]
- Hines, E.P.; Calafat, A.M.; Silva, M.J.; Mendola, P.; Fenton, S.E. Concentrations of phthalate metabolites in milk, urine, saliva, and Serum of lactating North Carolina women. Environ. Health Perspect. 2009, 117, 86–92. [Google Scholar] [CrossRef]
- Buckley, J.P.; Palmieri, R.T.; Matuszewski, J.M.; Herring, A.H.; Baird, D.D.; Hartmann, K.E.; Hoppin, J.A. Consumer product exposures associated with urinary phthalate levels in pregnant women. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 468–475. [Google Scholar] [CrossRef]
- Duty, S.M.; Ackerman, R.M.; Calafat, A.M.; Hauser, R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect. 2005, 113, 1530–1535. [Google Scholar] [CrossRef]
- Schlumpf, M.; Kypke, K.; Wittassek, M.; Angerer, J.; Mascher, H.; Mascher, D.; Vökt, C.; Birchler, M.; Lichtensteiger, W. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: Correlation of UV filters with use of cosmetics. Chemosphere 2010, 81, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Vanermen, G.; Covaci, A.; Voorspoels, S. Ultrasound assisted extraction combined with dispersive liquid-liquid microextraction (US-DLLME)—A fast new approach to measure phthalate metabolites in nails. Anal. Bioanal. Chem. 2016, 408, 6169–6180. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.S.; Rozati, R.; Reddy, S.; Kodampur, S.; Reddy, P.; Reddy, R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: A prospective case control study. Fertil. Steril. 2006, 85, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Specht, I.O.; Bonde, J.P.; Toft, G.; Lindh, C.H.; Jonsson, B.A.G.; Jorgensen, K.T. Serum phthalate levels and time to pregnancy in couples from Greenland, Poland and Ukraine. PLoS ONE 2015, 10, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Slakman, A.R.; Silva, M.J.; Herbert, A.R.; Needham, L.L. Automated solid phase extraction and quantitative analysis of human milk for 13 phthalate metabolites. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 805, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Malek, N.A.; Hodge, C.C.; Reidy, J.A.; Kato, K.; Barr, D.B.; Needham, L.L.; Brock, J.W. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry. J. Chromatogr. B 2003, 789, 393–404. [Google Scholar] [CrossRef]
- Calafat, A.M.; Wong, L.Y.; Silva, M.J.; Samandar, E.; Preau, J.L., Jr.; Jia, L.T.; Needham, L.L. Selecting adequate exposure biomarkers of diisononyl and diisodecyl phthalates: Data from the 2005-2006 National Health and Nutrition Examination Survey. Environ. Health Perspect. 2011, 119, 50–55. [Google Scholar] [CrossRef]
- Zota, A.R.; Calafat, A.M.; Woodruff, T.J. Temporal trends in phthalate exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. 2014, 122, 235–241. [Google Scholar] [CrossRef]
- CDC. NHANES Fourth Annual Report. 2012. Available online: https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf (accessed on 5 April 2019).
- Johns, L.E.; Cooper, G.S.; Galizia, A.; Meeker, J.D. Exposure assessment issues in epidemiology studies of phthalates. Environ. Int. 2015, 85, 27–39. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.; Lee, J.; Cho, G.; Choi, S.; Choi, G.; Kim, S.Y.; Eun, S.H.; Suh, E.; Kim, S.K.; et al. Association of diethylhexyl phthalate with obesity-related markers and body mass change from birth to 3 months of age. J. Epidemiol. Community Health 2016, 70, 466–472. [Google Scholar] [CrossRef]
- Koch, H.M.; Drexler, H.; Angerer, J. Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl) phthalate (DEHP). Int. J. Hyg. Environ. Health 2004, 207, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.M.; The AAP Committee on Environmental Health. Pediatric exposure and potential toxicity of phthalate plasticizers. Pediatrics 2003, 111, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ku, H.-Y.; Su, P.-H.; Chen, J.-W.; Huang, P.-C.; Angerer, J.; Wang, S.-L. Phthalate exposure in pregnant women and their children in central Taiwan. Chemosphere 2011, 82, 947–955. [Google Scholar] [CrossRef]
- Calafat, A.M.; Weuve, J.; Ye, X.; Jia, L.T.; Hu, H.; Ringer, S.; Huttner, K.; Hauser, R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ. Health Perspect. 2009, 117, 639–644. [Google Scholar] [CrossRef]
- Koch, H.M.; Preuss, R.; Angerer, J. Di(2-ethylhexyl)phthalate (DEHP): Human metabolism and internal exposure—An update and latest results. Int. J. Androl. 2006, 29, 155–165, discussion 181–185. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Hays, S.M.; Gagne, M.; Krishnan, K. Derivation of Biomonitoring Equivalents for di-n-butyl phthalate (DBP), benzylbutyl phthalate (BzBP), and diethyl phthalate (DEP). Regul. Toxicol. Pharmacol. 2009, 55, 259–267. [Google Scholar] [CrossRef]
- Hays, S.M.; Aylward, L.L.; Kirman, C.R.; Krishnan, K.; Nong, A. Biomonitoring Equivalents for di-isononyl phthalate (DINP). Regul. Toxicol. Pharmacol. 2011, 60, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Kato, K.; Malek, N.A.; Hodge, C.C.; Hurtz, D.; Calafat, A.M.; Needham, L.L.; Brock, J.W. Glucuronidation patterns of common urinary and serum monoester phthalate metabolites. Arch. Toxicol. 2003, 77, 561–567. [Google Scholar] [CrossRef]
- Koch, H.M.; Bolt, H.M.; Preuss, R.; Angerer, J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch. Toxicol. 2005, 79, 367–376. [Google Scholar] [CrossRef]
- Mettang, T.; Alscher, D.M.; Pauli-Magnus, C.; Dunst, R.; Kuhlmann, U.; Rettenmeier, A.W. Phthalic acid is the main metabolite of the plasticizer di(2-ethylhexyl) phthalate in peritoneal dialysis patients. Adv. Perit. Dial. 1999, 15, 229–233. [Google Scholar] [PubMed]
- Pollack, G.M.; Buchanan, J.F.; Slaughter, R.L.; Kohli, R.K.; Shen, D.D. Circulating concentrations of di(2-ethylhexyl) phthalate and its de-esterified phthalic acid products following plasticizer exposure in patients receiving hemodialysis. Toxicol. Appl. Pharmacol. 1985, 79, 257–267. [Google Scholar] [CrossRef]
- Choi, J.; Eom, J.; Kim, J.; Lee, S.; Kim, Y. Association between some endocrine-disrupting chemicals and childhood obesity in biological samples of young girls: A cross-sectional study. Environ. Toxicol. Pharmacol. 2014, 38, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kardas, F.; Bayram, A.K.; Demirci, E.; Akin, L.; Ozmen, S.; Kendirci, M.; Canpolat, M.; Oztop, D.B.; Narin, F.; Gumus, H.; et al. Increased serum phthalates (MEHP, DEHP) and bisphenol A concentrations in children with autism spectrum disorder: The role of endocrine disruptors in autism etiopathogenesis. J. Child Neurol. 2016, 31, 629–635. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Park, J.; Kim, H.J.; Cho, G.; Kim, G.H.; Eun, S.H.; Lee, J.J.; Choi, G.; Suh, E.; et al. Concentrations of phthalate metabolites in breast milk in Korea: Estimating exposure to phthalates and potential risks among breast-fed infants. Sci. Total Environ. 2015, 508, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, G.K.; Main, K.M.; Andersson, A.-M.; Leffers, H.; Skakkebæk, N.E. Determination of phthalate monoesters in human milk, consumer milk, and infant formula by tandem mass spectrometry (LC–MS–MS). Anal. Bioanal. Chem. 2005, 382, 1084–1092. [Google Scholar] [CrossRef]
- Kato, K.; Silva, M.J.; Needham, L.L.; Calafat, A.M. Quantifying phthalate metabolites in human meconium and semen using automated off-line solid-phase extraction coupled with on-line SPE and isotope-dilution high-performance liquid chromatography-tandem mass spectrometry. Anal. Chem. 2006, 78, 6651–6655. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.; zur Nieden, A.; Koch, H.M.; Schuppe, H.-C.; Fieber, C.; Angerer, J.; Eikmann, T.; Stilianakis, N.I. Urinary di(2-ethylhexyl)phthalate (DEHP)—Metabolites and male human markers of reproductive function. Int. J. Hyg. Environ. Health 2009, 212, 648–653. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Smarr, M.M.; Sun, L.; Chen, Z.; Honda, M.; Wang, W.; Karthikraj, R.; Weck, J.; Kannan, K. Endocrine disrupting chemicals in seminal plasma and couple fecundity. Environ. Res. 2018, 163, 64–70. [Google Scholar] [CrossRef]
- Smarr, M.M.; Kannan, K.; Sun, L.; Honda, M.; Wang, W.; Karthikraj, R.; Chen, Z.; Weck, J.; Buck Louis, G.M. Preconception seminal plasma concentrations of endocrine disrupting chemicals in relation to semen quality parameters among male partners planning for pregnancy. Environ. Res. 2018, 167, 78–86. [Google Scholar] [CrossRef]
- Fabjan, E.; Hulzebos, E.; Mennes, W.; Piersma, A.H. A category approach for reproductive effects of phthalates. Crit. Rev. Toxicol. 2006, 36, 695–726. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Gutleb, A.C.; Bergman, A.; Eriksen, G.S.; Murk, A.J.; Ropstad, E.; Saunders, M.; Skaare, J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 225–249. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Boberg, J.; Axelstad, M.; Dalgaard, M.; Vinggaard, A.M.; Metzdorff, S.B.; Hass, U. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod. Toxicol. 2010, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.J.M.; Grande, S.W.; Talsness, C.E.; Grote, K.; Golombiewski, A.; Sterner-Kock, A.; Chahoud, I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl) phthalate (DEHP): Effects on androgenic status, developmental landmarks and testicular histology in male offspring rats. Toxicology 2006, 225, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Culty, M.; Thuillier, R.; Li, W.P.; Wang, Y.; Martinez-Arguelles, D.B.; Benjamin, C.G.; Triantafilou, K.M.; Zirkin, B.R.; Papadopoulos, V. In utero exposure to di-(2-ethylhexyl) phthalate exerts both short-term and long-lasting suppressive effects on testosterone production in the rat. Biol. Reprod. 2008, 78, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.E.; Barlow, N.J.; Howdeshell, K.L.; Ostby, J.S.; Furr, J.R.; Gray, C.L. Transgenerational Effects of Di (2-Ethylhexyl) Phthalate in the Male CRL:CD(SD) Rat: Added Value of Assessing Multiple Offspring per Litter. Toxicol. Sci. 2009, 110, 411–425. [Google Scholar] [CrossRef]
- Jarfelt, K.; Dalgaard, M.; Hass, U.; Borch, J.; Jacobsen, H.; Ladefoged, O. Antiandrogenic effects in male rats perinatally exposed to a mixture of di(2-ethylhexyl) phthalate and di(2-ethylhexyl) adipate. Reprod. Toxicol. 2005, 19, 505–515. [Google Scholar] [CrossRef]
- Vo, T.T.B.; Jung, E.M.; Dang, V.H.; Jung, K.; Baek, J.; Choi, K.C.; Jeung, E.B. Differential effects of flutamide and di-(2-ethylhexyl) phthalate on male reproductive organs in a rat model. J. Reprod. Dev. 2009, 55, 400–411. [Google Scholar] [CrossRef]
- Wilson, V.S.; Howdeshell, K.L.; Lambright, C.S.; Furr, J.; Gray, L.E. Differential expression of the phthalate syndrome in male Sprague-Dawley and Wistar rats after in utero DEHP exposure. Toxicol. Lett. 2007, 170, 177–184. [Google Scholar] [CrossRef]
- Boberg, J.; Metzdorff, S.; Wortziger, R.; Axelstad, M.; Brokken, L.; Vinggaard, A.M.; Dalgaard, M.; Nellemann, C. Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology 2008, 250, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Borch, J.; Axelstad, M.; Vinggaard, A.M.; Dalgaard, M. Diisobutyl phthalate has comparable anti-androgenic effects to di-n-butyl phthalate in fetal rat testis. Toxicol. Lett. 2006, 163, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Saillenfait, A.M.; Sabate, J.P.; Gallissot, F. Developmental toxic effects of diisobutyl phthalate, the methyl-branched analogue of di-n-butyl phthalate, administered by gavage to rats. Toxicol. Lett. 2006, 165, 39–46. [Google Scholar] [CrossRef]
- Saillenfait, A.M.; Sabate, J.P.; Gallissot, F. Diisobutyl phthalate impairs the androgen-dependent reproductive development of the male rat. Reprod. Toxicol. 2008, 26, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.M.; Hutchison, G.R.; Jobling, M.S.; McKinnell, C.; Drake, A.J.; Sharpe, R.M. Relationship between androgen action in the “Male Programming Window,” fetal sertoli cell number, and adult testis size in the rat. Endocrinology 2008, 149, 5280–5287. [Google Scholar] [CrossRef] [PubMed]
- Struve, M.F.; Gaido, K.W.; Hensley, J.B.; Lehmann, K.P.; Ross, S.M.; Sochaski, M.A.; Willson, G.A.; Dorman, D.C. Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Jung, K.K.; Kim, S.S.; Kang, I.H.; Baek, J.H.; Nam, H.S.; Hong, S.K.; Lee, B.M.; Hong, J.T.; Oh, K.W.; et al. Effects of in utero exposure to di(n-butyl) phthalate on development of male reproductive tracts in Sprague-Dawley rats. J. Toxicol. Environ. Health A Curr. Issues 2010, 73, 1544–1559. [Google Scholar] [CrossRef]
- MacLeod, D.J.; Sharpe, R.M.; Welsh, M.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Drake, A.J.; van den Driesche, S. Androgen action in the masculinization programming window and development of male reproductive organs. Int. J. Androl. 2010, 33, 279–286. [Google Scholar] [CrossRef]
- Jiang, J.T.; Sun, W.L.; Jing, Y.F.; Liu, S.B.; Ma, Z.; Hong, Y.; Ma, L.; Qin, C.; Liu, Q.; Stratton, H.J.; et al. Prenatal exposure to di-n-butyl phthalate induces anorectal malformations in male rat offspring. Toxicology 2011, 290, 322–326. [Google Scholar] [CrossRef]
- Howarth, J.A.; Price, S.C.; Dobrota, M.; Kentish, P.A.; Hinton, R.H. Effects on male rats of di-(2-ethylhexyl) phthalate and di-n-hexylphthalate administered alone or in combination. Toxicol. Lett. 2001, 121, 35–43. [Google Scholar] [CrossRef]
- Zhai, W.H.; Huang, Z.G.; Chen, L.; Feng, C.; Li, B.; Li, T.S. Thyroid endocrine disruption in Zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PLoS ONE 2014, 9, 92465–92471. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Frame, S.R.; Ladics, G.S. Evaluation of a 15-day screening assay using intact male rats for identifying antiandrogens. Toxicol. Sci. 2002, 69, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.T.; Hansen, J.S.; Hansen, E.W.; Clausen, P.A.; Nielsen, G.D. Airway inflammation and adjuvant effect after repeated airborne exposures to di-(2-ethylhexyl)phthalate and ovalbumin in BALB/c mice. Toxicology 2007, 235, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Dearman, R.J.; Beresford, L.; Bailey, L.; Caddick, H.T.; Betts, C.J.; Kimber, I. Di-(2-ethylhexyl) phthalate is without adjuvant effect in mice on ovalbumin. Toxicology 2008, 244, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Zhuang, M.Z.; Li, T.; Shi, N. Neurobehavioral toxicity study of dibutyl phthalate on rats following in utero and lactational exposure. J. Appl. Toxicol. 2009, 29, 603–611. [Google Scholar] [CrossRef]
- Li, X.J.; Jiang, L.; Chen, L.; Chen, H.S.; Li, X. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: A study in rats. Environ. Toxicol. Pharmacol. 2013, 36, 392–402. [Google Scholar] [CrossRef]
- Tanaka, T. Reproductive and neurobehavioural toxicity study of bis(2-ethylhexyl) phthalate (DEHP) administered to mice in the diet. Food Chem. Toxicol. 2002, 40, 1499–1506. [Google Scholar] [CrossRef]
- Hoshi, H.; Ohtsuka, T. Adult rats exposed to low-doses of di-n-butyl phthalate during gestation exhibit decreased grooming behavior. Bull. Environ. Contam. Toxicol. 2009, 83, 62–66. [Google Scholar] [CrossRef]
- Sun, Q.; Cornelis, M.C.; Townsend, M.K.; Tobias, D.K.; Heather Eliassen, A.; Franke, A.A.; Hauser, R.; Hu, F.B. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: A prospective investigation in the nurses’ health study (NHS) and NHSII cohorts. Environ. Health Perspect. 2014, 122, 616–623. [Google Scholar] [CrossRef]
- Stahlhut Richard, W.; van Wijngaarden, E.; Dye Timothy, D.; Cook, S.; Swan Shanna, H. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect. 2007, 115, 876–882. [Google Scholar] [CrossRef]
- Trasande, L.; Sathyanarayana, S.; Spanier, A.J.; Trachtman, H.; Attina, T.M.; Urbina, E.M. Urinary phthalates are associated with higher blood pressure in childhood. J. Pediatr. 2013, 163, 747–753. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Tang, C.; He, Y.; Wu, J.; Chen, Y.; Jiang, Q. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS ONE 2013, 8, 56800–56809. [Google Scholar] [CrossRef] [PubMed]
- Buser, M.C.; Murray, H.E.; Scinicariello, F. Age and sex differences in childhood and adulthood obesity association with phthalates: Analyses of NHANES 2007–2010. Int. J. Hyg. Environ. Health 2014, 217, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, X.; Chen, L.; Li, D.; Zhao, L.; Zhao, Y.; Li, L.; Shi, H. Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty. PLoS ONE 2014, 9, e104852. [Google Scholar] [CrossRef] [PubMed]
- Hatch, E.; Nelson, J.; Qureshi, M. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A crosssectional study of NHANES data 1999–2002. Environ. Health 2008, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dirtu, A.C.; Geens, T.; Dirinck, E.; Malarvannan, G.; Neels, H.; Van Gaal, L.; Jorens, P.G.; Covaci, A. Phthalate metabolites in obese individuals undergoing weight loss: Urinary levels and estimation of the phthalates daily intake. Environ. Int. 2013, 59, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Sathyanarayana, S.; Hauser, R. Phthalate exposure and children’s health. Curr. Opin. Pediatr. 2013, 25, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hoppin, J.A.; Jaramillo, R.; London, S.J.; Bertelsen, R.J.; Salo, P.M.; Sandler, D.P.; Zeldin, D.C. Phthalate exposure and allergy in the U.S. population: Results from NHANES 2005-2006. Environ. Health Perspect. 2013, 121, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Ait Bamai, Y.; Shibata, E.; Saito, I.; Araki, A.; Kanazawa, A.; Morimoto, K.; Nakayama, K.; Tanaka, M.; Takigawa, T.; Yoshimura, T.; et al. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Sci. Total Environ. 2014, 485–486, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Karmaus, W.J.; Chen, S.L.; Holloway, J.W.; Ewart, S. Effects ofphthalate exposure on asthma may be mediated through alterations in DNAmethylation. Clin. Epigenet. 2015, 7, 27–36. [Google Scholar] [CrossRef]
- Gascon, M.; Casas, M.; Morales, E.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Monfort, N.; Ventura, R.; Martínez, D.; et al. Prenatal exposure to bisphenol A and phthalates and childhood respiratory tract infections and allergy. J. Allergy Clin. Immunol. 2015, 135, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, H.; Sørensen, K.; Mouritsen, A.; Aksglaede, L.; Hagen, C.P.; Petersen, J.H.; Skakkebaek, N.E.; Andersson, A.M.; Juul, A. High urinary phthalate concentration associated with delayed pubarche in girls. Int. J. Androl. 2012, 35, 216–226. [Google Scholar] [CrossRef]
- Upson, K.; Sathyanarayana, S.; De Roos, A.J.; Thompson, M.L.; Scholes, D.; Dills, R.; Holt, V.L. Phthalates and risk of endometriosis. Environ. Res. 2013, 126, 91–97. [Google Scholar] [CrossRef]
- Durmaz, E.; Özmert, E.N.; Erkekoğlu, P.; Giray, B.; Derman, O.; Hıncal, F.; Yurdakök, K. Plasma phthalate levels in pubertal gynecomastia. Pediatrics 2010, 125, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate exposure and male reproductive outcomes: A systematic review of human epidemiological evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef] [PubMed]
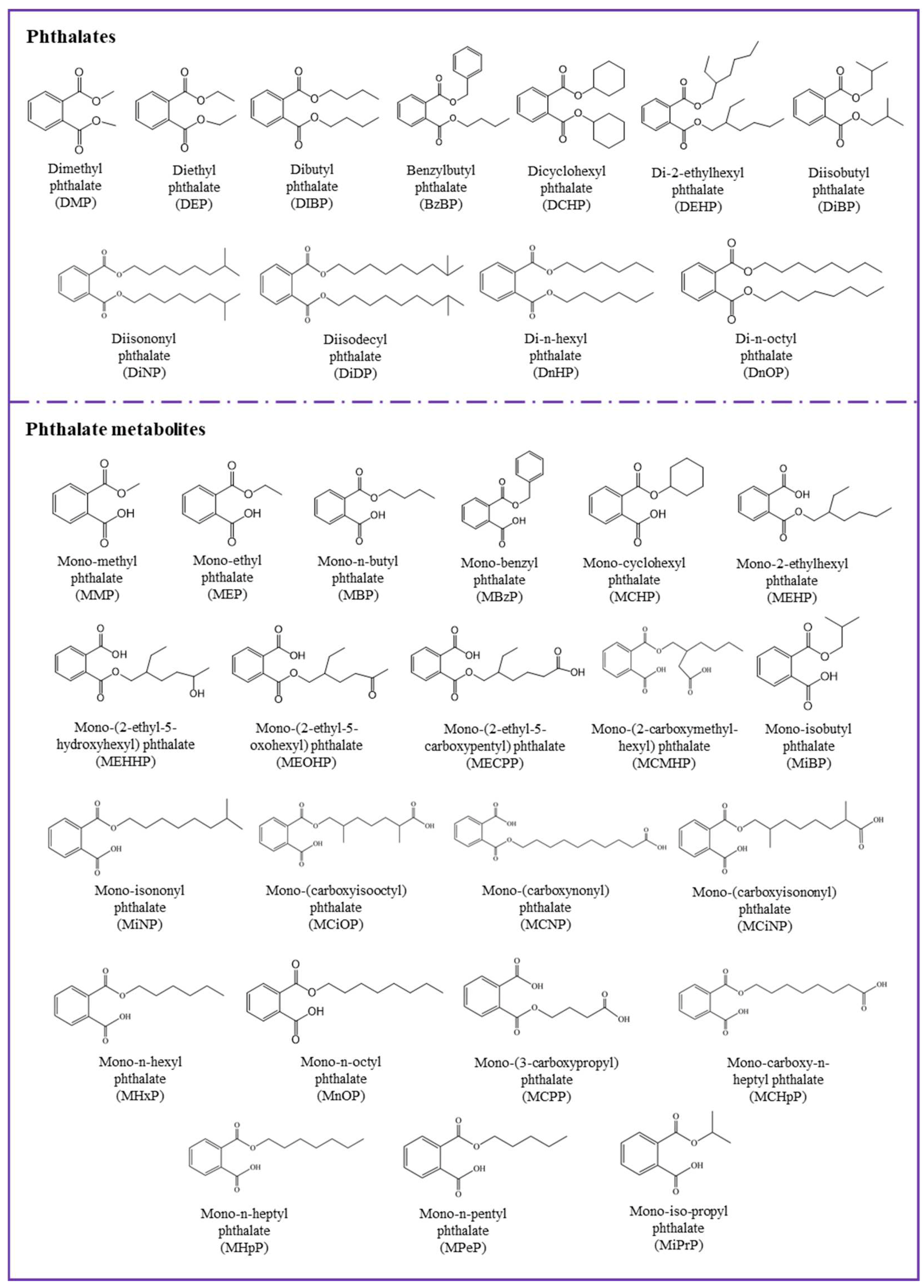



| Parent Compounds | Abb. | Major Metabolites | Abb. |
|---|---|---|---|
| Dimethyl phthalate | DMP | Mono-methyl phthalate | MMP |
| Diethyl phthalate | DEP | Mono-ethyl phthalate | MEP |
| Dibutyl phthalate | DBP | Mono-n-butyl phthalate | MBP |
| Benzylbutyl phthalate | BzBP | Mono-benzyl phthalate (some mono-n-butyl phthalate) | MBzP |
| Dicyclohexyl phthalate | DCHP | Mono-cyclohexyl phthalate | MCHP |
| Di-2-ethylhexyl phthalate | DEHP | Mono-2-ethylhexyl phthalate | MEHP |
| Mono-(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP (5OH-MEHP) | ||
| Mono-(2-ethyl-5-oxohexyl) phthalate | MEOHP (5oxo-MEHP) | ||
| Mono-(2-ethyl-5-carboxypentyl) phthalate | MECPP (5cx-MEPP) | ||
| Mono-(2-carboxymethyl-hexyl) phthalate | MCMHP (2cx-MMHP) | ||
| Diisobutyl phthalate | DiBP | Mono-isobutyl phthalate | MiBP |
| Diisononyl phthalate | DiNP | Mono-isononyl phthalate | MiNP |
| Mono-(carboxyisooctyl) phthalate | MCiOP | ||
| Diisodecyl phthalate | DiDP | Mono-(carboxynonyl) phthalate | MCNP |
| Mono-(carboxyisononyl) phthalate | MCiNP | ||
| Di-n-hexyl phthalate | DnHP | Mono-n-hexyl phthalate | MHxP |
| Di-n-octyl phthalate | DnOP | Mono-n-octyl phthalate | MnOP |
| Mono-(3-carboxypropyl) phthalate | MCPP | ||
| Mono-carboxy-n-heptyl phthalate | MCHpP | ||
| Mono-n-heptyl phthalate | MHpP | ||
| Mono-n-pentyl phthalate | MPeP | ||
| Mono-iso-propyl phthalate | MiPrP |
| Exposure Route | Dust Ingestion | Dust Dermal Absorption | Personal Care Products (Dermal) | Diet | Indoor Air Inhalation |
|---|---|---|---|---|---|
| Infants (<1 y *) | 1.12 | 0.001 | 0.0095 | - | 0.845 |
| Toddlers (1–3 y) | 1.7 | 0.0008 | 0.0059 | - | 0.423 |
| Children (3–11 y) | 0.468 | 0.0006 | - | 4.68 | 0.203 |
| Teenagers (11–18 y) | 0.291 | 0.0005 | - | - | 0.089 |
| Adults (>18 y) | 0.233 | 0.0002 | 0.013–0.49 | 1.03 | 0.07 |
| Matrix | Country/Region | Studied Population | Concentration | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| MMP | MEP | MBP | MiBP | MDEHP | Unit | ||||
| Urine | Australia | 30 non-occupational exposure | 18.5 | 11.8 | 7.3 | 25.2 | μg/L; median | [61] | |
| Urine | Austria | 251 children/adolescents; 272 adults; 72 senior citizens | 25 | 10 | 28 | 15.5 | μg/L; median | [62] | |
| Urine | Belgium | 261 persons | 34.3 | 33.3 | 24.3 | 11.7 | μg/L; median | [63] | |
| Urine | Belgium | 210 adolescents | 38.5 | 52.7 | μg/L; median | [64] | |||
| Urine | Belgium | 123 men 138 women | 37.6 | 31.3 | 26.2 | 17.1 | μg/L; median | [65] | |
| Urine | Belgium | 25 persons | 20.4 | 15.6 | 15.9 | 12.01 | μg/L; median | [66] | |
| Urine | Brazil | 300 children (6–14 years old). | 8.3 | 57.3 | 42.4 | 43.8 | 109 | μg/L; median | [67] |
| Urine | Canada | 3236 persons (6–49 years old) | 49.1 | 23.8 | 40.9 | μg/L; median | [68] | ||
| Urine | Canada | 2000 women (first trimester) | 32.02 | 11.59 | μg/L; GM | [69] | |||
| Urine | Canada | 80 infants | 7.01 | 10.63 | μg/L; median | [70] | |||
| Urine | China | 108 young adults | 31.8 | 37.5 | 67 | 57.2 | 65.3 | μg/L; median | [71] |
| Urine | China | 21 women 19 men | 16.5 | 20.7 | 49.6 | 44 | 44.2 | μg/L; median | [72] |
| Urine | China | 430 children (208 girls and 222 boys) | 15.7 | 4.14 | 21.9 | 14.3 | μg/L; median | [73] | |
| Urine | China | 183 samples | 14.6 | 22.1 | 63.5 | 57.1 | 76.1 | μg/L; median | [51] |
| Urine | China | 364 males (19–44 years old) | 28.2 | 47.1 | 42 | μg/L; median | [74] | ||
| Urine | China | 39 children (5–9 years) | 28.5 | 232 | 81.3 | 79.1 | μg/L; median | [75] | |
| Urine | Czech | 117 women | ND | 56.7 | 32.2 | μg/L; median | [76] | ||
| Urine | Czech | 120 children | ND | 31.6 | 61.9 | μg/L; median | [76] | ||
| Urine | Denmark | 60 men | 54.5 | 36.8 | 47.3 | 68.1 | μg/L; median | [77] | |
| Urine | Denmark | 145 women | 74 | 26 | 48 | 67 | μg/L; GM | [78] | |
| Urine | Denmark | 143 children | 28 | 39 | 74 | 99 | μg/L; GM | [78] | |
| Urine | Denmark | 129 children | 29 | 111 | 107 | μg/L; median | [79] | ||
| Urine | Denmark | 441 children | 16.6 | 80.1 | 72.2 | 89.8 | μg/L; median | [80] | |
| Urine | Europe | 171 individuals | 49.9 | 0 | 4.5 | µg/g CR; median | [42] | ||
| Urine | Europe | 1335 children | 34.4 | 38.4 | 45.4 | 47.6 | μg/L; median | [81] | |
| Urine | Europe | 1347 mother | 48.2 | 23.9 | 30.1 | 29.2 | μg/L; median | [81] | |
| Urine | Europe | 1301 mother | 72 | 18.3 | 23.3 | 22.4 | μg/L; median | [82] | |
| Urine | France | 279 mothers | 43.5 | 35.7 | 53.7 | 84.6 | μg/L; median | [83] | |
| Urine | Germany | 634 individuals | 109 | 35.4 | 45.3 | μg/L; median | [84] | ||
| Urine | Germany | 254 children | 99.9 | μg/L; median | [85] | ||||
| Urine | Germany | 53 women 32 men | 90.2 | 181 | 83.3 | μg/L; median | [86] | ||
| Urine | Germany | 399 individuals | 49.6 | 44.9 | 38.8 | μg/L; median | [87] | ||
| Urine | Germany | 120 females and 120 males | 19.6 | 25.5 | 19.3 | μg/L; median | [41] | ||
| Urine | Germany | 30 males and 30 females (2015) | 2.8 | 13.5 | 8.0 | 9.8 | 12.3 | μg/L; median | [88] |
| Urine | Germany | 30 males and 30 females (2007) | 8.0 | 53.6 | 16.4 | 19.3 | 33.4 | μg/L; median | [88] |
| Urine | Germany | 111 children (48 girls and 63 boys) | 53.6 | 74.9 | 130.1 | μg/L; median | [89] | ||
| Urine | Germany | 465 children (8–10 years old) | 52.5 | 62.8 | 75.7 | μg/L; median | [90] | ||
| Urine | Germany | 599 children | 95.6 | 94.3 | 174.6 | μg/L; median | [91] | ||
| Urine | Germany | 600 children (3–14 years old) | 96 | 85 | μg/L; median | [91] | |||
| Urine | Germany | 207 infants (1–5 month) | 12.1 | 1.1 | μg/L; median | [92] | |||
| Urine | Germany | 104 mothers | 50.5 | 66.6 | 28.9 | μg/L; median | [93] | ||
| Urine | Germany | 104 children | 39.1 | 56.5 | 103.9 | 55.7 | μg/L; median | [93] | |
| Urine | Greece | 239 women | 142 | 32.1 | 36.7 | 44.6 | μg/L; median | [94] | |
| Urine | Greece | 239 children | 35.3 | 23.3 | 36 | 45.6 | μg/L; median | [94] | |
| Urine | Hungary | 115 women | ND | 55 | 32.4 | μg/L; median | [76] | ||
| Urine | Hungary | 117 children | ND | 47 | 56.7 | μg/L; median | [76] | ||
| Urine | India | 15 women 7 men | 8.6 | 150 | 13 | 18.3 | 77.9 | μg/L; median | [72] |
| Urine | Iran | 56 children and adolescent (6–18 years) | 17.4 | 28.2 | 42.9 | 44.9 | μg/L; median | [95] | |
| Urine | Ireland | 120 mothers | 50.2 | 18.5 | 23.8 | 17 | μg/g CR; GM | [96] | |
| Urine | Ireland | 120 children | 38.7 | 26.1 | 41.4 | 32.8 | μg/g CR; GM | [96] | |
| Urine | Israel | 205 adults (20–74 years old) | 27.9 | 37.6 | 81.7 | μg/L; median | [97] | ||
| Urine | Italy | 83 women (2011) | 73.1 | 38.8 | 15.6 | μg/g CR; median | [42] | ||
| Urine | Italy | 111 women (2016) | 49.9 | 0 | 4.5 | μg/g CR; median | [42] | ||
| Urine | Italy | 83 females | 61.0 | 32.5 | 10.5 | μg/L; median | [98] | ||
| Urine | Italy | 74 males | 73.2 | 41.2 | 15.2 | μg/L; median | [98] | ||
| Urine | Japan | 8 women 27 men | 18.2 | 16.4 | 17.7 | 7.5 | 35.1 | μg/L; median | [72] |
| Urine | Japan | 80 women (controls) | 21.4 | 84.3 | 72.7 | μg/L; median | [99] | ||
| Urine | Japan | 57 women (cases) | 39.6 | 87.2 | 89.3 | μg/L; median | [99] | ||
| Urine | Japan | 35 adults 1 children | 33 | 18 | 36 | 5 | μg/L; median | [100] | |
| Urine | Japan | 111 pregnant women | 5.70 | 7.75 | 46.6 | 18.5 | μg/L; median | [101] | |
| Urine | Korea | 39 children | 19.2 | 107 | 53.4 | 145.6 | μg/L; median | [102] | |
| Urine | Korea | 60 individuals | 10 | 13.4 | 16.7 | 4.5 | 43.6 | μg/L; median | [72] |
| Urine | Korea | 25 adults | 80 | 134 | 40.4 | 125.8 | μg/L; median | [103] | |
| Urine | Korea | 305 women | 41 | 23.7 | μg/g CR; median | [104] | |||
| Urine | Korea | 1646 elderly people | 39.5 | 44.8 | μg/L; median | [105] | |||
| Urine | Korea | 6478 adults | 44.2 | 88.2 | μg/L; median | [106] | |||
| Urine | Korea | 6003 adults | 24.2 | 52.2 | μg/L; median | [107] | |||
| Urine | Korea | 171 children | 2.71 | 12.4 | 5.25 | 12.3 | μg/L; median | [108] | |
| Urine | Korea | 392 children | 185 | μg/L; median | [109] | ||||
| Urine | Korea | 265 mothers | 67.4 | μg/L; median | [109] | ||||
| Urine | Korea | 297 adults | 55.7 | μg/L; median | [109] | ||||
| Urine | Kuwait | 22 women 24 men | 10.1 | 411 | 113 | 54.1 | 180.4 | μg/L; median | [72] |
| Urine | Malaysia | 19 women 10 men | 6.3 | 18.6 | 10.5 | 10.8 | 27.5 | μg/L; median | [72] |
| Urine | Netherlands | 100 women | ND | 112 | 43.2 | 41.3 | 61.8 | μg/L; median | [110] |
| Urine | Norway | 10 women | 2 | 310 | 41.1 | 57 | 112.3 | μg/L; median | [111] |
| Urine | Norway | 61 adults | 24.2 | 13.4 | 12.8 | μg/L; median | [112] | ||
| Urine | Norway | 116 pregnant women | 55 | 25 | 20 | 26 | μg/L; median | [113] | |
| Urine | Portugal | 112 children (4–18 years) | 59.4 | 12.7 | 16.9 | 40.4 | μg/L; median | [114] | |
| Urine | Saudi Arabia | 130 individuals | 8.65 | 47.5 | 38.5 | 38.5 | 117.1 | μg/L; median | [26] |
| Urine | Slovakia | 129 occupational exposure | 110 | 39.2 | 55.9 | μg/L; median | [115] | ||
| Urine | Slovakia | 68 occupational exposure population | 201 | 103 | 61.4 | 82.7 | μg/L; median | [116] | |
| Urine | Slovakia | 125 women | ND | 54.8 | 36.7 | μg/L; median | [76] | ||
| Urine | Slovakia | 127 children | ND | 39.6 | 82.8 | μg/L; median | [76] | ||
| Urine | Slovakia | 85 occupational exposure | 78.5 | 85.6 | 21.5 | μg/L; median | [117] | ||
| Urine | Slovakia | 70 general population | 78.1 | 96 | 14.7 | μg/L; median | [117] | ||
| Urine | Spain | 391 pregnant women | 246 | 27.1 | 28.4 | 87.8 | μg/L; median | [118] | |
| Urine | Spain | 120 children | 198.9 | 63 | μg/g CR; GM | [119] | |||
| Urine | Spain | 120 mothers | 150.8 | 33.3 | μg/g CR; GM | [119] | |||
| Urine | Sweden | 314 men | 41 | 47 | 48.4 | μg/L; median | [120] | ||
| Urine | Sweden | 38 women | 1.2 | 35 | 46 | 16 | 35 | μg/L; median | [24] |
| Urine | Taiwan | 41 women and 19 men (21–67 years) | 32.3 | 36.5 | 15.9 | μg/L; median | [121] | ||
| Urine | Taiwan | 155 women | 5.7 | 25.3 | 80 | 22.6 | μg/L; median | ||
| Urine | Taiwan | 30 (children, age: 2) | 100.4 | 17.2 | 195.8 | μg/L; median | |||
| Urine | Taiwan | 59 (children, age: 5) | 75.2 | 25.2 | 148.9 | μg/L; median | |||
| Urine | Taiwan | 100 women | 72.3 | 12.5 | 96.8 | μg/L; median | |||
| Urine | U.S. | 45 males (subfertile couples) | 108 | 24.7 | 91.4 | μg/L; median | [122] | ||
| Urine | U.S. | 35 children | 177.7 | 52.4 | 16.6 | 1025.9 | μg/L; median | [123] | |
| Urine | U.S. | 7600–10,031 individuals | 1.4 | 167 | 18.9 | 3.6 | 73.1 | μg/g CR; median | [124] |
| Urine | U.S. | 12–18 months toddlers | 13.2-1388 | 6.6–2540 | <1.7–47.3 | μg/L; median | [125] | ||
| Urine | U.S. | 186 persons in Northern Manhattan | 199 | 36 | μg/L; median | [126] | |||
| Urine | U.S. | 446 pregnant women | 41.1 | µg/g CR; GM | [127] | ||||
| Urine | U.S. | 378 pregnant women | 47 | 13.7 | 9.47 | 14 | μg/L; median | [128] | |
| Urine | U.S. | 482 individuals | 141 | 17.8 | 7.6 | 106.6 | μg/L; median | [27] | |
| Urine | U.S. | 2772 adults | 167 | 35.4 | μg/g CR; median | [129] | |||
| Urine | U.S. | 392 children of 6–11 years old | 96.9 | 69.9 | μg/g CR; median | [129] | |||
| Urine | U.S. | 2350 individuals | 1.8 | 194.4 | 20.7 | 3.7 | 73 | μg/L; median | [130] |
| Urine | U.S. | 33 lactating women | 35.7–45.9 | μg/L; median | [131] | ||||
| Urine | U.S. | 50 pregnant women (18–38) | 61.5 | 18.2 | 31.1 | μg/L; median | [132] | ||
| Urine | U.S. | 406 men | 4.5 | 145 | 14.5 | 5.2 | μg/L; median | [133] | |
| Urine | Vietnam | 16 women 14 men | 8.4 | 7.2 | 19.1 | 13.6 | 56.7 | μg/L; median | [72] |
| Serum | Denmark | 60 men | <LOD | ND | <LOD | 8.4 | μg/L; median | [77] | |
| Serum | Sweden | 36 women | 0.5 | 0.5 | 0.5 | 0.5 | μg/L; median | [24] | |
| Seminal plasma | Denmark | 60 men | <LOD | <LOD | ND | <LOD | μg/L; median | [77] | |
| Breast milk | Denmark | 65 women | 0.1 | 0.9 | 4.3 | 9.5 | μg/L; median | [25] | |
| Breast milk | Finland | 65 women | 0.1 | 1.0 | 12.0 | 13.0 | μg/L; median | [25] | |
| Breast milk | Sweden | 42 women | ND | 0.5 | ND | 0.49) | μg/L; median | [24] | |
| Milk | Switzerland | 54 women | 6.0 | 24.3 | 26.2 | μg/L; median | [134] | ||
| Milk | U.S. | 33 lactating women | 0.3–0.7 | μg/L; median | [131] | ||||
| Nail | Belgian | 10 individuals | 64 | 74 | 138 | µg/g CR; median | [135] | ||
| Nail | Norway | 61 adults | 89.7 | 104.8 | 89.3 | µg/g CR; GM | [112] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. https://doi.org/10.3390/toxics7020021
Wang Y, Zhu H, Kannan K. A Review of Biomonitoring of Phthalate Exposures. Toxics. 2019; 7(2):21. https://doi.org/10.3390/toxics7020021
Chicago/Turabian StyleWang, Yu, Hongkai Zhu, and Kurunthachalam Kannan. 2019. "A Review of Biomonitoring of Phthalate Exposures" Toxics 7, no. 2: 21. https://doi.org/10.3390/toxics7020021
APA StyleWang, Y., Zhu, H., & Kannan, K. (2019). A Review of Biomonitoring of Phthalate Exposures. Toxics, 7(2), 21. https://doi.org/10.3390/toxics7020021







