Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health
Abstract
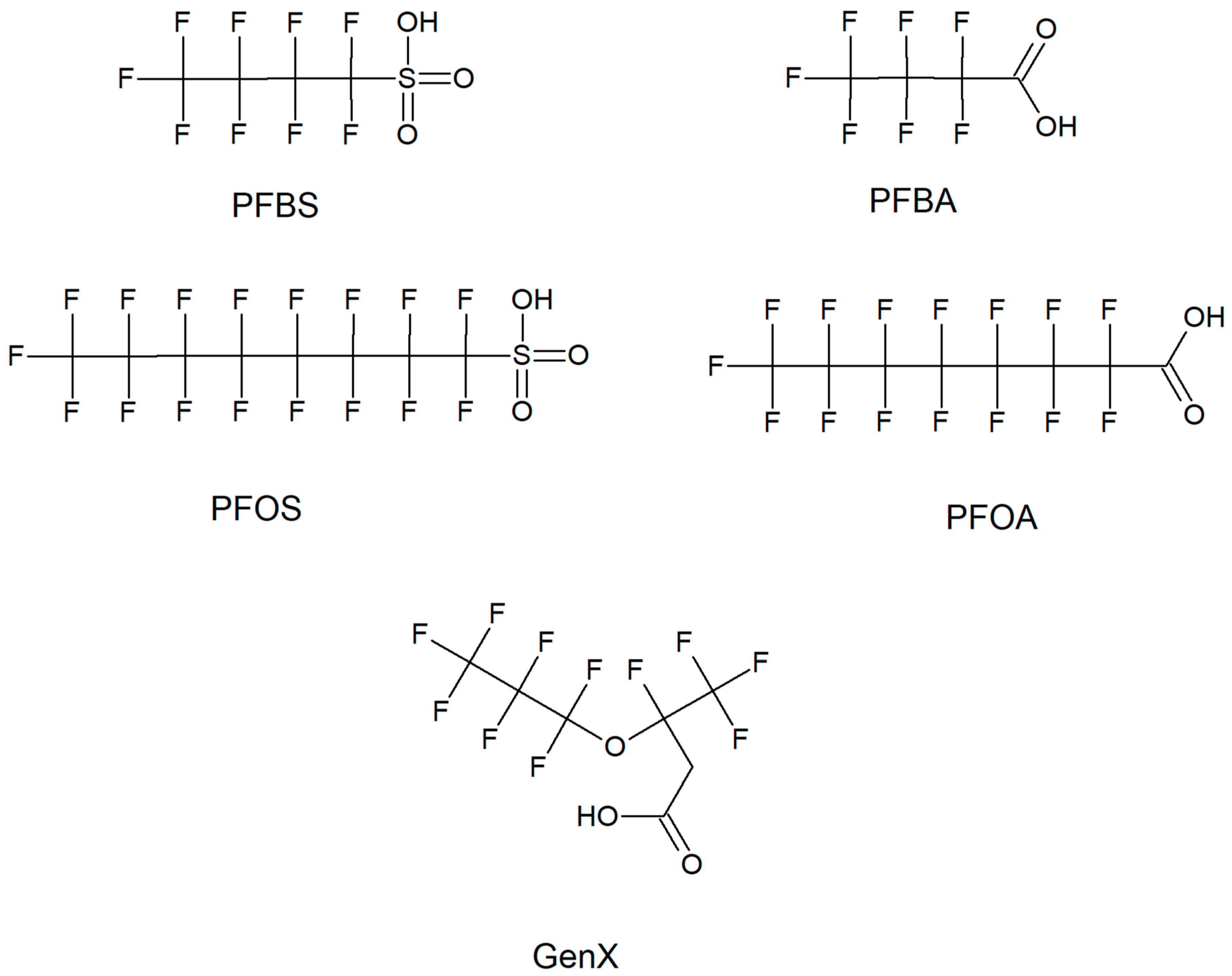
:1. Introduction
2. Qualities of Good Sentinel Species
3. Invertebrate Species Used to Study Neurotoxicity of PFAS
3.1. Caenorhabditis elegans
3.2. Dugesia japonica
4. Vertebrate Species Used to Study Neurotoxicity of PFAS
4.1. Danio rerio
4.2. Oryzias melastigma
4.3. Frogs
4.4. Ursus maritimus
5. Critical Analyses of the Use of Sentinels in PFAS Toxicity: Integration of Findings across Sentinel and Non-Traditional Models and Potential to Predict Adverse Outcomes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brendel, S.; Fetter, E.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Hebert, G.N.; Strauss, S.H.; Field, J.A. Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. J. Environ. Monit. 2003, 5, 341–345. [Google Scholar] [CrossRef]
- Stahl, T.; Falk, S.; Failing, K.; Berger, J.; Georgii, S.; Brunn, H. Perfluorooctanoic acid and perfluorooctane sulfonate in liver and muscle tissue from wild boar in Hesse, Germany. Arch. Environ. Contam. Toxicol. 2012, 62, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; van Leeuwen, S.P.J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands. Environ. Int. 2020, 137, 105583. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.M.J.; Munoz, G.; Bottos, E.M.; Duy, S.V.; Sauve, S.; Liu, J.; Van Hamme, J.D. Degradation and defluorination of 6:2 fluorotelomer sulfonamidoalkyl betaine and 6:2 fluorotelomer sulfonate by Gordonia sp. strain NB4-1Y under sulfur-limiting conditions. Sci. Total Environ. 2019, 647, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F. Emerging poly- and perfluoroalkyl substances in the aquatic environment: A review of current literature. Water Res. 2017, 124, 482–495. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- McCord, J.; Strynar, M. Identification of per- and polyfluoroalkyl substances in the cape fear river by high resolution mass spectrometry and nontargeted screening. Environ. Sci. Technol. 2019, 53, 4717–4727. [Google Scholar] [CrossRef]
- Sun, M.; Arevalo, E.; Strynar, M.; Lindstrom, A.; Richardson, M.; Kearns, B.; Pickett, A.; Smith, C.; Knappe, D.R.U. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the cape fear river watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3, 415–419. [Google Scholar] [CrossRef]
- Gebbink, W.A.; van Asseldonk, L.; van Leeuwen, S.P.J. Presence of emerging per- and polyfluoroalkyl substances (PFASs) in river and drinking water near a fluorochemical production plant in the Netherlands. Environ. Sci. Technol. 2017, 51, 11057–11065. [Google Scholar] [CrossRef] [Green Version]
- Cannon, R.E.; Richards, A.C.; Trexler, A.W.; Juberg, C.T.; Sinha, B.; Knudsen, G.A.; Birnbaum, L.S. Effect of GenX on P-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 at the blood-brain barrier. Environ. Health Perspect. 2020, 128, 37002. [Google Scholar] [CrossRef] [PubMed]
- Lien, N.P.H.; Fujii, S.; Tanaka, S.; Nozoe, M.; Tanaka, H. Contamination of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in surface water of the Yodo River basin (Japan). Desalination 2008, 226, 338–347. [Google Scholar] [CrossRef]
- Mak, Y.L.; Taniyasu, S.; Yeung, L.W.; Lu, G.; Jin, L.; Yang, Y.; Lam, P.K.; Kannan, K.; Yamashita, N. Perfluorinated compounds in tap water from China and several other countries. Environ. Sci. Technol. 2009, 43, 4824–4829. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Zushi, Y.; Masunaga, S.; Gilligan, M.; Pride, C.; Sajwan, K.S. Perfluorinated organic contaminants in sediment and aquatic wildlife, including sharks, from Georgia, USA. Mar. Pollut. Bull. 2009, 58, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Tully, J.S.; Needham, L.L. Serum concentrations of 11 polyfluoroalkyl compounds in the US population: Data from the national health and nutrition examination survey (NHANES). Environ. Sci. Technol. 2007, 41, 2237–2242. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Riker, C.D.; Lu, S.E.; Fan, Z.T. Biomonitoring of emerging contaminants, perfluoroalkyl and polyfluoroalkyl substances (PFAS), in New Jersey adults in 2016–2018. Int. J. Hyg. Environ. Health 2020, 223, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Aimuzi, R.; Luo, K.; Chen, Q.; Wang, H.; Feng, L.; Ouyang, F.; Zhang, J. Perfluoroalkyl and polyfluoroalkyl substances and fetal thyroid hormone levels in umbilical cord blood among newborns by prelabor caesarean delivery. Environ. Int. 2019, 130, 104929. [Google Scholar] [CrossRef]
- Karrman, A.; Ericson, I.; van Bavel, B.; Darnerud, P.O.; Aune, M.; Glynn, A.; Lignell, S.; Lindstrom, G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Perspect. 2007, 115, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Mamsen, L.S.; Bjorvang, R.D.; Mucs, D.; Vinnars, M.T.; Papadogiannakis, N.; Lindh, C.H.; Andersen, C.Y.; Damdimopoulou, P. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ. Int. 2019, 124, 482–492. [Google Scholar] [CrossRef]
- Olsen, G.W.; Butenhoff, J.L.; Zobel, L.R. Perfluoroalkyl chemicals and human fetal development: An epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol. 2009, 27, 212–230. [Google Scholar] [CrossRef]
- So, M.K.; Yamashita, N.; Taniyasu, S.; Jiang, Q.; Giesy, J.P.; Chen, K.; Lam, P.K. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ. Sci. Technol. 2006, 40, 2924–2929. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.R.; Wolff, M.S.; Calafat, A.M.; Kato, K.; Engel, S.M. Comparison of polyfluoroalkyl compound concentrations in maternal serum and amniotic fluid: A pilot study. Reprod. Toxicol. 2012, 34, 312–316. [Google Scholar] [CrossRef] [Green Version]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 38. [Google Scholar] [CrossRef] [Green Version]
- Johansson, N.; Fredriksson, A.; Eriksson, P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 2008, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Goulding, D.R.; White, S.S.; McBride, S.J.; Fenton, S.E.; Harry, G.J. Gestational exposure to perfluorooctanoic acid (PFOA): Alterations in motor related behaviors. Neurotoxicology 2017, 58, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Zhou, Q.F.; Liao, C.Y.; Fu, J.J.; Jiang, G.B. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch. Environ. Contam. Toxicol. 2009, 56, 338–349. [Google Scholar] [CrossRef]
- Obrien, D.J.; Kaneene, J.B.; Poppenga, R.H. The use of mammals as sentinels for human exposure to toxic contaminants in the environment. Environ. Health Perspect. 1993, 99, 351–368. [Google Scholar] [CrossRef]
- Reif, J.S. Animal sentinels for environmental and public health. Public Health Rep. 2011, 126 (Suppl. S1), 50–57. [Google Scholar] [CrossRef] [Green Version]
- Schulenburg, H.; Felix, M.A. The natural biotic environment of Caenorhabditis elegans. Genetics 2017, 206, 55–86. [Google Scholar] [CrossRef] [Green Version]
- Sammi, S.R.; Foguth, R.M.; Nieves, C.S.; De Perre, C.; Wipf, P.; McMurray, C.T.; Lee, L.S.; Cannon, J.R. Perfluorooctane sulfonate (PFOS) produces dopaminergic neuropathology in Caenorhabditis elegans. Toxicol. Sci. 2019, 172, 417–434. [Google Scholar] [CrossRef]
- Chen, F.J.; Wei, C.Y.; Chen, Q.Y.; Zhang, J.; Wang, L.; Zhou, Z.; Chen, M.J.; Liang, Y. Internal concentrations of perfluorobutane sulfonate (PFBS) comparable to those of perfluorooctane sulfonate (PFOS) induce reproductive toxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 158, 223–229. [Google Scholar] [CrossRef]
- Xu, T.; Li, P.; Wu, S.; Li, D.; Wu, J.; Raley-Susman, K.M.; He, D. Chronic exposure to perfluorooctane sulfonate reduces lifespan of caenorhabditis elegans through Insulin/IGF-1 signaling. Bull. Environ. Contam. Toxicol. 2016, 97, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Li, J.; Li, D.; Yang, Y.; He, D. Chronic exposure to perfluorooctane sulfonate induces behavior defects and neurotoxicity through oxidative damages, in vivo and in vitro. PLoS ONE 2014, 9, e113453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.M.; Long, N.P.; Yoon, S.J.; Anh, N.H.; Kim, S.J.; Park, J.H.; Kwon, S.W. Omics approach reveals perturbation of metabolism and phenotype in Caenorhabditis elegans triggered by perfluorinated compounds. Sci. Total Environ. 2020, 703, 135500. [Google Scholar] [CrossRef] [PubMed]
- Greaves, A.K.; Letcher, R.J. Linear and branched perfluorooctane sulfonate (PFOS) isomer patterns differ among several tissues and blood of polar bears. Chemosphere 2013, 93, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Conder, J.M.; Hoke, R.A.; De Wolf, W.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Bangma, J.T.; Reiner, J.L.; Botha, H.; Cantu, T.M.; Gouws, M.A.; Guillette, M.P.; Koelmel, J.P.; Luus-Powell, W.J.; Myburgh, J.; Rynders, O.; et al. Tissue distribution of perfluoroalkyl acids and health status in wild Mozambique tilapia (Oreochromis mossambicus) from Loskop Dam, Mpumalanga, South Africa. J. Environ. Sci. 2017, 61, 59–67. [Google Scholar] [CrossRef]
- Spulber, S.; Kilian, P.; Wan Ibrahim, W.N.; Onishchenko, N.; Ulhaq, M.; Norrgren, L.; Negri, S.; Di Tuccio, M.; Ceccatelli, S. PFOS induces behavioral alterations, including spontaneous hyperactivity that is corrected by dexamfetamine in zebrafish larvae. PLoS ONE 2014, 9, e94227. [Google Scholar] [CrossRef] [Green Version]
- Foguth, R.M.; Flynn, R.W.; de Perre, C.; Iacchetta, M.; Lee, L.S.; Sepulveda, M.S.; Cannon, J.R. Developmental exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) selectively decreases brain dopamine levels in Northern leopard frogs. Toxicol. Appl. Pharmacol. 2019, 377, 114623. [Google Scholar] [CrossRef]
- Soloff, A.C.; Wolf, B.J.; White, N.D.; Muir, D.; Courtney, S.; Hardiman, G.; Bossart, G.D.; Fair, P.A. Environmental perfluorooctane sulfonate exposure drives T cell activation in bottlenose dolphins. J. Appl. Toxicol. 2017, 37, 1108–1116. [Google Scholar] [CrossRef]
- Levin, M.; Gebhard, E.; Jasperse, L.; Desforges, J.P.; Dietz, R.; Sonne, C.; Eulaers, I.; Covaci, A.; Bossi, R.; De Guise, S. Immunomodulatory effects of exposure to polychlorinated biphenyls and perfluoroalkyl acids in East Greenland ringed seals (Pusa hispida). Environ. Res. 2016, 151, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, G.; Sonne, C.; Riget, F.F.; Dietz, R.; Letcher, R.J. Bioaccumulation and biomagnification of perfluoroalkyl acids and precursors in East Greenland polar bears and their ringed seal prey. Environ. Pollut. 2019, 252, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Custer, C.M.; Custer, T.W.; Schoenfuss, H.L.; Poganski, B.H.; Solem, L. Exposure and effects of perfluoroalkyl compounds on tree swallows nesting at Lake Johanna in east central Minnesota, USA. Reprod. Toxicol. 2012, 33, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.X.; Kunisue, T.; Tao, L.; Kannan, K.; Subramanian, A.; Tanabe, S.; Iwata, H. Dioxin-like and perfluorinated compounds in pigs in an Indian open waste dumping site: Toxicokinetics and effects on hepatic cytochrome P450 and blood plasma hormones. Environ. Toxicol. Chem. 2010, 29, 1551–1560. [Google Scholar] [CrossRef]
- Yuan, Z.; Shao, X.; Miao, Z.; Zhao, B.; Zheng, Z.; Zhang, J. Perfluorooctane sulfonate induced neurotoxicity responses associated with neural genes expression, neurotransmitter levels and acetylcholinesterase activity in planarians Dugesia japonica. Chemosphere 2018, 206, 150–156. [Google Scholar] [CrossRef]
- Huang, H.; Huang, C.; Wang, L.; Ye, X.; Bai, C.; Simonich, M.T.; Tanguay, R.L.; Dong, Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat. Toxicol. 2010, 98, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Jantzen, C.E.; Annunziato, K.A.; Bugel, S.M.; Cooper, K.R. PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat. Toxicol. 2016, 175, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Jantzen, C.E.; Annunziato, K.M.; Cooper, K.R. Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquat. Toxicol. 2016, 180, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulhaq, M.; Orn, S.; Carlsson, G.; Morrison, D.A.; Norrgren, L. Locomotor behavior in zebrafish (Danio rerio) larvae exposed to perfluoroalkyl acids. Aquat. Toxicol. 2013, 144, 332–340. [Google Scholar] [CrossRef]
- Chen, L.; Tsui, M.M.P.; Shi, Q.; Hu, C.; Wang, Q.; Zhou, B.; Lam, P.K.S.; Lam, J.C.W. Accumulation of perfluorobutane sulfonate (PFBS) and impairment of visual function in the eyes of marine medaka after a life-cycle exposure. Aquat. Toxicol. 2018, 201, 1–10. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Lu, S.; Zheng, B.; Xie, P.; Chen, J.; Li, G.; Liu, C.; Wu, Q.; Cheng, H.; et al. Perfluorododecanoic acid exposure induced developmental neurotoxicity in zebrafish embryos. Environ. Pollut. 2018, 241, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Eggers Pedersen, K.; Basu, N.; Letcher, R.; Greaves, A.K.; Sonne, C.; Dietz, R.; Styrishave, B. Brain region-specific perfluoroalkylated sulfonate (PFSA) and carboxylic acid (PFCA) accumulation and neurochemical biomarker responses in east Greenland polar bears (Ursus maritimus). Environ. Res. 2015, 138, 22–31. [Google Scholar] [CrossRef]
- Saili, K.S.; Zurlinden, T.J.; Schwab, A.J.; Silvin, A.; Baker, N.C.; Hunter, E.S., 3rd; Ginhoux, F.; Knudsen, T.B. Blood-brain barrier development: Systems modeling and predictive toxicology. Birth Defects Res. 2017, 109, 1680–1710. [Google Scholar] [CrossRef]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef]
- Li, Y.; Men, B.; He, Y.; Xu, H.; Liu, M.; Wang, D. Effect of single-wall carbon nanotubes on bioconcentration and toxicity of perfluorooctane sulfonate in zebrafish (Danio rerio). Sci. Total Environ. 2017, 607, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Cui, Q.; Zhang, H.; Cui, R.; Guo, Y.; Dai, J. Accumulation, biotransformation, and endocrine disruption effects of fluorotelomer surfactant mixtures on Zebrafish. Chem. Res. Toxicol. 2019, 32, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, S.; Swank, A.; Sobus, J.R.; Howey, X.M.; Schmid, J.; Catron, T.; McCord, J.; Hines, E.; Strynar, M.; Tal, T. Evaluation of Developmental toxicity, developmental neurotoxicity, and tissue dose in Zebrafish exposed to GenX and Other PFAS. Environ. Health Perspect. 2020, 128, 47005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menger, F.; Pohl, J.; Ahrens, L.; Carlsson, G.; Orn, S. Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos. Chemosphere 2020, 245, 125573. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef]
- de Abreu, M.S.; Giacomini, A.; Genario, R.; Rech, N.; Carboni, J.; Lakstygal, A.M.; Amstislavskaya, T.G.; Demin, K.A.; Leonard, B.E.; Vlok, M.; et al. Non-pharmacological and pharmacological approaches for psychiatric disorders: Re-appraisal and insights from zebrafish models. Pharmacol. Biochem. Behav. 2020, 193, 172928. [Google Scholar] [CrossRef]
- Gawel, K.; Banono, N.S.; Michalak, A.; Esguerra, C.V. A critical review of zebrafish schizophrenia models: Time for validation? Neurosci. Biobehav. Rev. 2019, 107, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Kasap, M.; Rajani, V.; Rajani, J.; Dwyer, D.S. Surprising conservation of schizophrenia risk genes in lower organisms reflects their essential function and the evolution of genetic liability. Schizophr. Res. 2018, 202, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.Y.; Chen, T.; Xia, W.; Zhou, Y.; Wan, Y.J.; Lv, Z.Q.; Li, G.Q.; Xu, S.Q. Abnormal development of motor neurons in perfluorooctane sulphonate exposed zebrafish embryos. Ecotoxicology 2011, 20, 643–652. [Google Scholar] [CrossRef]
- Cannatella, D.C.; de Sá, R.O. Xenopus laevis as a Model Organism. Syst. Biol. 1993, 42. [Google Scholar] [CrossRef]
- Lindquist, N.G.; Larsson, B.S.; Lyden-Sokolowski, A. Autoradiography of [14C]paraquat or [14C]diquat in frogs and mice: Accumulation in neuromelanin. Neurosci. Lett. 1988, 93, 1–6. [Google Scholar] [CrossRef]
- Flynn, R.W.; Chislock, M.F.; Gannon, M.E.; Bauer, S.J.; Tornabene, B.J.; Hoverman, J.T.; Sepulveda, M.S. Acute and chronic effects of perfluoroalkyl substance mixtures on larval American bullfrogs (Rana catesbeiana). Chemosphere 2019, 236, 124350. [Google Scholar] [CrossRef]
- Bossart, G.D. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Bytingsvik, J.; van Leeuwen, S.P.; Hamers, T.; Swart, K.; Aars, J.; Lie, E.; Nilsen, E.M.; Wiig, O.; Derocher, A.E.; Jenssen, B.M. Perfluoroalkyl substances in polar bear mother-cub pairs: A comparative study based on plasma levels from 1998 and 2008. Environ. Int. 2012, 49, 92–99. [Google Scholar] [CrossRef]
- Onishchenko, N.; Fischer, C.; Wan Ibrahim, W.N.; Negri, S.; Spulber, S.; Cottica, D.; Ceccatelli, S. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox. Res. 2011, 19, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Koskela, A.; Koponen, J.; Lehenkari, P.; Viluksela, M.; Korkalainen, M.; Tuukkanen, J. Perfluoroalkyl substances in human bone: Concentrations in bones and effects on bone cell differentiation. Sci. Rep. 2017, 7, 6841. [Google Scholar] [CrossRef]
| Species | Sample Type | PFOS (µg/mg) | Exposure | Exposure Time | Reference |
|---|---|---|---|---|---|
| C. elegans | Whole body | 13.06 | 1 mg/L | 72 h | Sammi, 2019 [30] |
| Oreochromis mossambicus | Whole body | 0.0000416 | n/a | n/a | Bangma, 2017 [37] |
| D. rerio | Whole body | 0.000021.6 | 1 mg/L | 6 days | Spulber, 2014 [38] |
| R. pipiens | Whole body | 0.0045 | 1 mg/L | 30 days | Foguth, 2019 [39] |
| Tursiops truncatus | Plasma | 0.000571 | n/a | n/a | Soloff, 2017 [40] |
| Pusa hispida | Serum | 57.3 ng/mL | n/a | n/a | Levin, 2016 [41] |
| U. maritimus | Liver | 0.00002882 | n/a | n/a | Biosvert, 2019 [42] |
| Tachycineta bicolor | Serum | 137 ng/mL | n/a | n/a | Custer, 2012 [43] |
| Sus scrofa | Liver | 0.000040 | n/a | n/a | Watanabe, 2010 [44] |
| (A) | ||||||
| Organism | Concentration (µM) | Length of Exposure | Neurobehavior | Neurotransmitters | Neuropathology | Reference |
| C. elegans | 40–400 | 72 h | ↑Repulsion time | NT | ↓Dopaminergic neurons | Sammi, 2019 [30] |
| C. elegans | 20 | 48 h | ↑Forward movement and thrashing | NT | ↓Dopaminergic and cholinergic neurons | Chen, 2014 [33] |
| D. japonica | 1–20 | 5–7 d | NT | ↑Dopamine Δ Serotonin Δ GABA | Δ Acetylcholinesterase activity Δ Neurodevelopmental genes | Yuan, 2018 [45] |
| D. rerio | 2 | 6 d | ↓Bouts ↑Distance during bout ↑Reaction to light changes ↑Startle | NT | NT | Spulber, 2014 [38] |
| D. rerio | 1–8 | 1–114 h | Δ Speed | NT | NT | Huang, 2010 [46] |
| D. rerio | 0.02–2 | 14 d | ↑Distance Speed | NT | NT | Jantzen, 2016 [47] |
| D. rerio | 2 µM | 117 h, 6 m depuration | ↓Hitting glass-males | NT | NT | Jantzen, 2016 [48] |
| D. rerio | 0.06–20 | 144 h | Δ Activity ΔTime active | NT | NT | Ulhaq, 2013 [49] |
| R. pipiens | 0.2–2 | 30 d | NT | ↓Dopamine ↑Dopamine turnover | NT | Foguth, 2019 [39] |
| (B) | ||||||
| Organism | Concentration (µM) | Length of Exposure | Neurobehavior | Neurotransmitters | Neuropathology | Reference |
| D. rerio | 0.02–1 | 14 d | ↓Distance ↓Speed | NT | NT | Jantzen, 2016 [47] |
| D. rerio | 2 | 117 h, 6 m depuration | ↓Distance ↓Time in the middle ↓Time frozen ↑Speed ↑Hitting glass-males ↑Time in light-males | NT | NT | Jantzen, 2016 [48] |
| D. rerio | 0.06–22 | 144 h | Δ Activity Δ Time active | NT | NT | Ulhaq, 2013 [49] |
| (C) | ||||||
| Organism | Concentration (µM) | Length of Exposure | Neurobehavior | Neurotransmitters | Neuropathology | Reference |
| D. rerio | 0.2 | 14 d | ↑Distance | NT | NT | Jantzen, 2016 [47] |
| D. rerio | 2 | 117 h, 6 m depuration | ↓Time in light-females | NT | NT | Jantzen, 2016 [48] |
| D. rerio | 7.2–2415 | 144 h | Δ activity Δtime active | NT | NT | Ulhaq, 2013 [49] |
| R. pipiens | 2.4 | 30 d | NT | ↓Dopamine ↑Dopamine turnover | NT | Foguth, 2019 [39] |
| Organism | Chemical | Concentration (µM) | Length of Exposure | Neurobehavior | Neurotransmitters | Neuropathology | Reference |
|---|---|---|---|---|---|---|---|
| O. melastigma | PFBS | 0.03 | 6 m | NT | ↑Dopamine Sex-specific Δ norepinephrine Δ Serotonin over time Δ GABA over time ↑Acetylcholine | Δ Transcription factors involved in visual development | Chen, 2018 [50] |
| D. rerio | TFAA, PFBA, PFDA (perfluorodecanoate), or PFBS | 48–14, 33.3–10000, 0.2–58.4, or 33.3–10000 | 144 h | Δ Activity ΔTime active | NT | NT | Ulhaq, 2013 [49] |
| D. rerio | PFDoA | 0.4–10 | 120 h | ↓Speed | ↓Acetylcholine ↑Dopamine | ↓Acetylcholinesterase | Guo, 2018 [51] |
| U. maritimus | PFBS, PFHxS, PFOS, perfluorodecane sulfonate (PFDS), PFHxA, perfluoroheptanoate (PFHpA), PFOA, PFNA, PFDA, perfluoroundecanoate (PFUnDA), perfluorododecanoate (PFDoDA), perfluorotridecanoate (PFTrDA), perfluorotetradecanoate (PFTeDA), and perfluoropentadecanoate (PFPeDA) were quantified. PFAS levels were due to exposure in the wild | PFBS: 0.55 ± 0.08 PFHxS: 1.10 ± 0.10 PFOS: 22.92 ± 0.84 PFDS: 0.66 ± 0.06 PFHxA: 0.13 ± 0.03 PFHpA: not detected PFOA: 1.09 ± 0.13 PFNA: 2.59 ± 0.13 PFDA: 2.63 ± 0.15 PFUnDA: 22.30 ± 1.14 PFDoDA: 8.19 ± 0.46 PFTrDA: 37.87 ± 2.29 PFTeDA: 6.81 ± 0.40 PFPeDA: 4.71 ± 0.42 ng/g wet weight in the whole brain | Unknkown, Ages of bears at sampling were 2–10 years | NT | NT | ↑Glutathione synthase in occipital lobe and frontal cortex ↓Glutathione synthase in hypothalamus ↑Monoamine oxidase activity ↓Dopamine D2 receptors in occipital lobe and cerebellum ↓Muscarinic acetylcholine receptor activity in cerebellum ↑GABA-A receptors ↑Muscarinic acetylcholine receptor activity in frontal cortex ↓Acetylcholinesterase activity in frontal cortex | Eggers Pederson, 2015 [52] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foguth, R.; Sepúlveda, M.S.; Cannon, J. Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health. Toxics 2020, 8, 42. https://doi.org/10.3390/toxics8020042
Foguth R, Sepúlveda MS, Cannon J. Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health. Toxics. 2020; 8(2):42. https://doi.org/10.3390/toxics8020042
Chicago/Turabian StyleFoguth, Rachel, Maria S. Sepúlveda, and Jason Cannon. 2020. "Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health" Toxics 8, no. 2: 42. https://doi.org/10.3390/toxics8020042
APA StyleFoguth, R., Sepúlveda, M. S., & Cannon, J. (2020). Per- and Polyfluoroalkyl Substances (PFAS) Neurotoxicity in Sentinel and Non-Traditional Laboratory Model Systems: Potential Utility in Predicting Adverse Outcomes in Human Health. Toxics, 8(2), 42. https://doi.org/10.3390/toxics8020042





