Quantitative NanoLC/NSI+-HRMS Method for 1,3-Butadiene Induced bis-N7-guanine DNA-DNA Cross-Links in Urine
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Animal Study
2.3. Sample Processing and bis-N7G-BD Adduct Enrichment
2.4. NanoLC/NSI+-HRMS Analysis of Urinary bis-N7G-BD Adducts
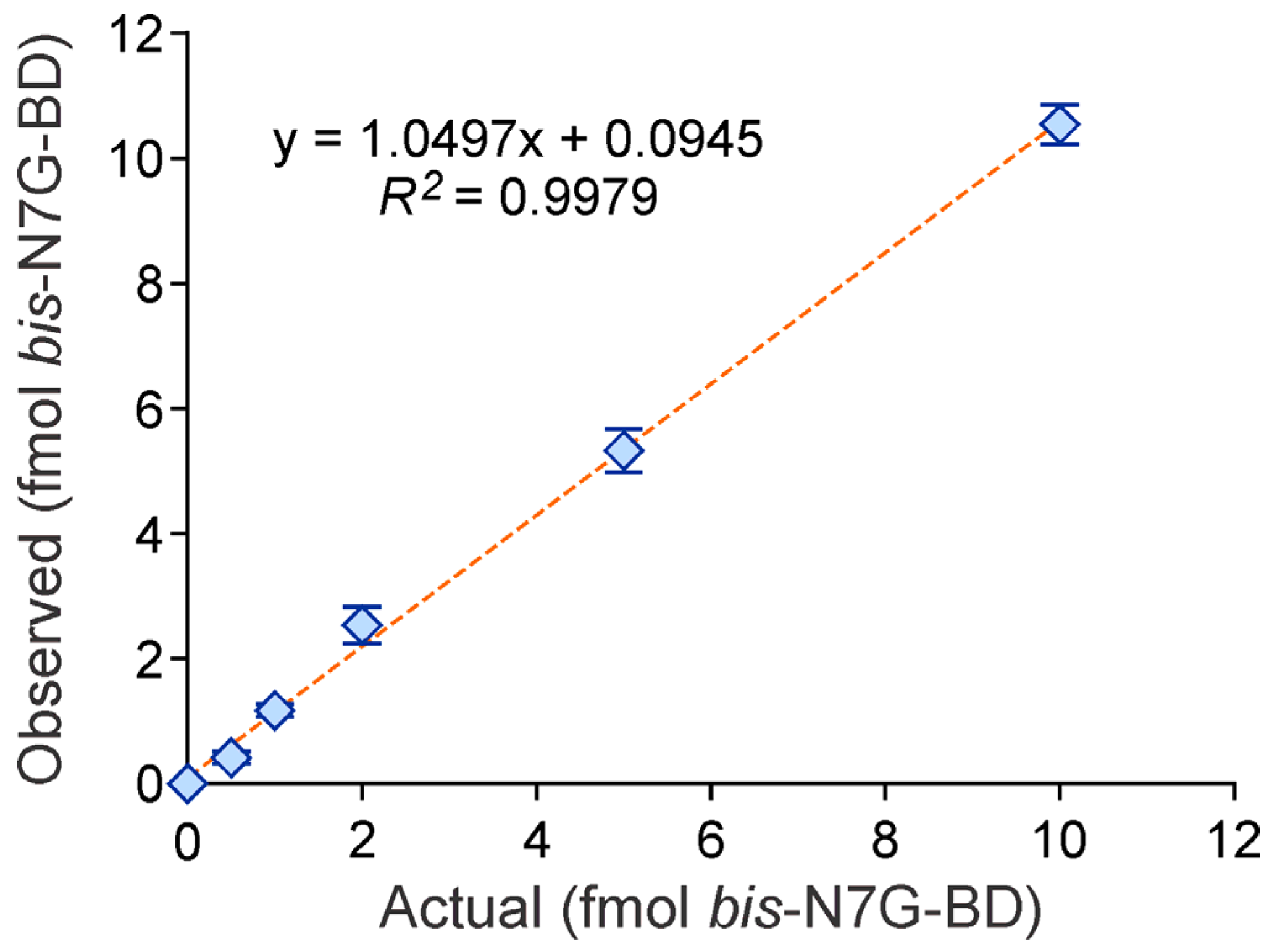
2.5. Method Validation
2.6. Quantitation of bis-BDMA
2.7. Quantitation of MHBMA and DHBMA
2.8. Statistical Analyses
3. Results
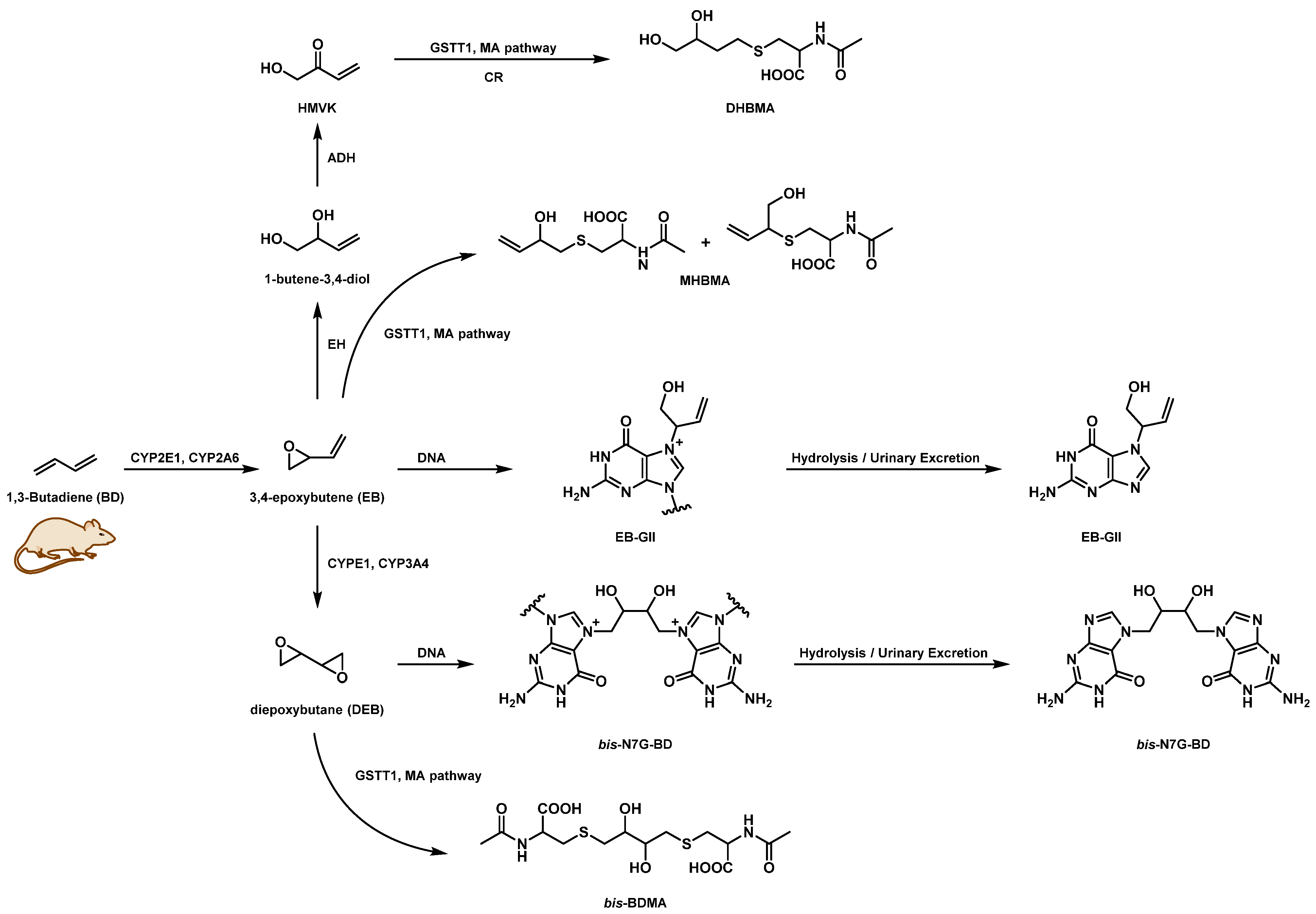
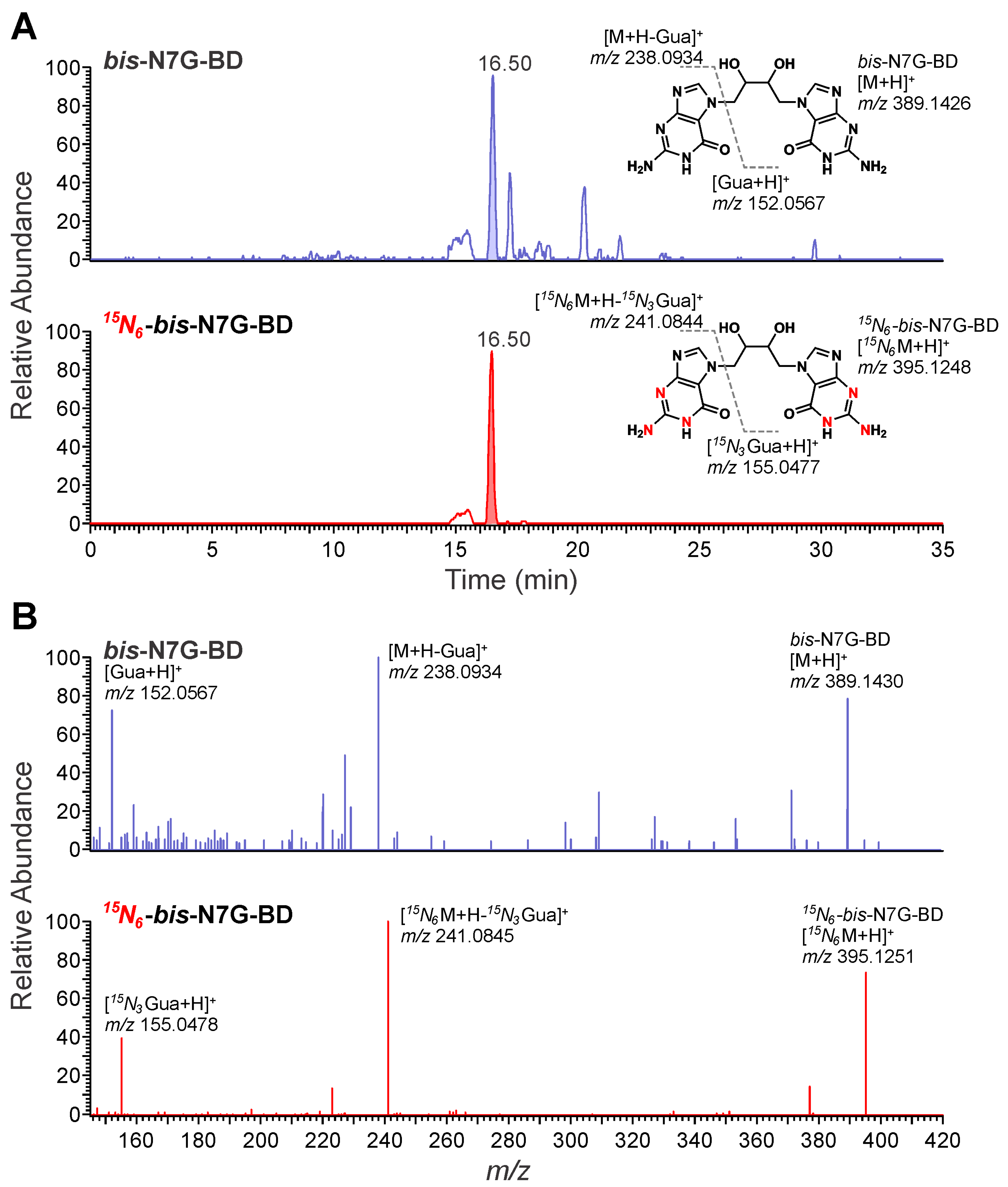
3.1. NanoLC/NSI+-HRMS Method Development for bis-N7G-BD in Mouse Urine
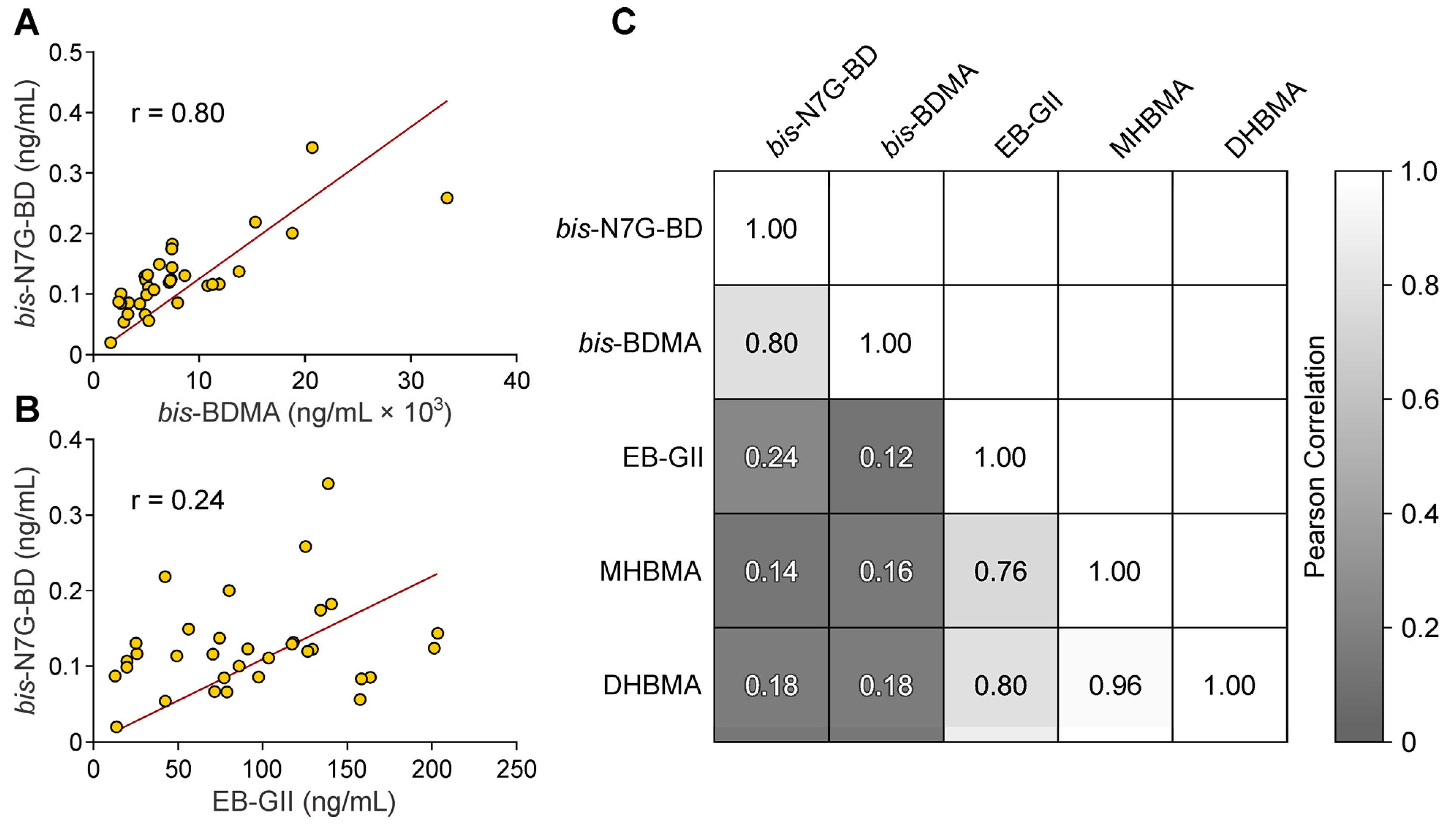
3.2. Quantitation of bis-N7G-BD in Urine of Mice Exposed to BD by Inhalation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef]
- Pelz, N.; Dempster, N.M.; Shore, P.R. Analysis of low molecular weight hydrocarbons including 1,3-butadiene in engine exhaust gases using an aluminum oxide porous-layer open-tubular fused-silica column. J. Chromatogr. Sci. 1990, 28, 230–235. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monogr. Eval. Carcinog. Risks Hum. 2008, 97, 3–471. [Google Scholar]
- Melnick, R.L.; Huff, J.; Chou, B.J.; Miller, R.A. Carcinogenicity of 1,3-butadiene in C57BL/6 x C3H F1 mice at low exposure concentrations. Cancer Res. 1990, 50, 6592–6599. [Google Scholar]
- Owen, P.E.; Glaister, J.R.; Gaunt, I.F.; Pullinger, D.H. Inhalation toxicity studies with 1,3-butadiene. 3. Two year toxicity/carcinogenicity study in rats. Am. Ind. Hyg. Assoc. J. 1987, 48, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Himmelstein, M.W.; Acquavella, J.F.; Recio, L.; Medinsky, M.A.; Bond, J.A. Toxicology and epidemiology of 1,3-butadiene. Crit. Rev. Toxicol. 1997, 27, 1–108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Sathiakumar, N.; Graff, J.; Matthews, R.; Delzell, E. 1,3-Butadiene and leukemia among synthetic rubber industry workers: Exposure-response relationships. Chem. Biol. Interact. 2007, 166, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Delzell, E.; Sathiakumar, N.; Hovinga, M.; Macaluso, M.; Julian, J.; Larson, R.; Cole, P.; Muir, D.C. A follow-up study of synthetic rubber workers. Toxicology 1996, 113, 182–189. [Google Scholar] [CrossRef]
- Sathiakumar, N.; Graff, J.; Macaluso, M.; Maldonado, G.; Matthews, R.; Delzell, E. An updated study of mortality among North American synthetic rubber industry workers. Occup. Environ. Med. 2005, 62, 822–829. [Google Scholar] [CrossRef]
- Csanady, G.A.; Guengerich, F.P.; Bond, J.A. Comparison of the biotransformation of 1,3-butadiene and its metabolite, butadiene monoepoxide, by hepatic and pulmonary tissues from humans, rats and mice. Carcinogenesis 1992, 13, 1143–1153. [Google Scholar] [CrossRef]
- Seaton, M.J.; Follansbee, M.H.; Bond, J.A. Oxidation of 1,2-epoxy-3-butene to 1,2:3,4-diepoxybutane by cDNA-expressed human cytochromes P450 2E1 and 3A4 and human, mouse and rat liver microsomes. Carcinogenesis 1995, 16, 2287–2293. [Google Scholar] [CrossRef]
- van Sittert, N.J.; Megens, H.J.; Watson, W.P.; Boogaard, P.J. Biomarkers of exposure to 1,3-butadiene as a basis for cancer risk assessment. Toxicol. Sci. 2000, 56, 189–202. [Google Scholar] [CrossRef]
- Boysen, G.; Arora, R.; Degner, A.; Vevang, K.R.; Chao, C.; Rodriguez, F.; Walmsley, S.J.; Erber, L.; Tretyakova, N.Y.; Peterson, L.A. Effects of GSTT1 Genotype on the Detoxification of 1,3-Butadiene Derived Diepoxide and Formation of Promutagenic DNA-DNA Cross-Links in Human Hapmap Cell Lines. Chem. Res. Toxicol. 2021, 34, 119–131. [Google Scholar] [CrossRef]
- Park, S.L.; Kotapati, S.; Wilkens, L.R.; Tiirikainen, M.; Murphy, S.E.; Tretyakova, N.; Le Marchand, L. 1,3-Butadiene exposure and metabolism among Japanese American, Native Hawaiian, and White smokers. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2240–2249. [Google Scholar] [CrossRef]
- Krause, R.J.; Sharer, J.E.; Elfarra, A.A. Epoxide hydrolase-dependent metabolism of butadiene monoxide to 3-butene-1,2-diol in mouse, rat, and human liver. Drug Metab. Dispos. 1997, 25, 1013–1015. [Google Scholar]
- Kotapati, S.; Sangaraju, D.; Esades, A.; Hallberg, L.; Walker, V.E.; Swenberg, J.A.; Tretyakova, N.Y. Bis-butanediol-mercapturic acid (bis-BDMA) as a urinary biomarker of metabolic activation of butadiene to its ultimate carcinogenic species. Carcinogenesis 2014, 35, 1371–1378. [Google Scholar] [CrossRef]
- Carmical, J.R.; Nechev, L.V.; Harris, C.M.; Harris, T.M.; Lloyd, R.S. Mutagenic potential of adenine N(6) adducts of monoepoxide and diolepoxide derivatives of butadiene. Environ. Mol. Mutagen. 2000, 35, 48–56. [Google Scholar] [CrossRef]
- Kotapati, S.; Esades, A.; Matter, B.; Le, C.; Tretyakova, N. High throughput HPLC-ESI−-MS/MS methodology for mercapturic acid metabolites of 1,3-butadiene: Biomarkers of exposure and bioactivation. Chem. Biol. Interact. 2015, 241, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Rajski, S.R.; Williams, R.M. DNA Cross-Linking Agents as Antitumor Drugs. Chem. Rev. 1998, 98, 2723–2796. [Google Scholar] [CrossRef] [PubMed]
- Lawley, P.D.; Brookes, P. Interstrand cross-linking of DNA by difunctional alkylating agents. J. Mol. Biol. 1967, 25, 143–160. [Google Scholar] [CrossRef]
- Cochrane, J.E.; Skopek, T.R. Mutagenicity of butadiene and its epoxide metabolites: II. Mutational spectra of butadiene, 1,2-epoxybutene and diepoxybutane at the hprt locus in splenic T cells from exposed B6C3F1 mice. Carcinogenesis 1994, 15, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Henderson, R.F.; Walker, D.M.; Bauer, M.J.; Reilly, A.A.; Walker, V.E. Mutagenicity of the racemic mixtures of butadiene monoepoxide and butadiene diepoxide at the Hprt locus of T-lymphocytes following inhalation exposures of female mice and rats. Mutat. Res. 1999, 429, 127–140. [Google Scholar] [CrossRef]
- Goggin, M.; Seneviratne, U.; Swenberg, J.A.; Walker, V.E.; Tretyakova, N. Column switching HPLC-ESI+-MS/MS methods for quantitative analysis of exocyclic dA adducts in the DNA of laboratory animals exposed to 1,3-butadiene. Chem. Res. Toxicol. 2010, 23, 808–812. [Google Scholar] [CrossRef]
- Goggin, M.; Swenberg, J.A.; Walker, V.E.; Tretyakova, N. Molecular dosimetry of 1,2,3,4-diepoxybutane-induced DNA-DNA cross-links in B6C3F1 mice and F344 rats exposed to 1,3-butadiene by inhalation. Cancer Res. 2009, 69, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Tretyakova, N. Structural characterization of the major DNA-DNA cross-link of 1,2,3,4-diepoxybutane. Chem. Res. Toxicol. 2004, 17, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Vacek, P.M.; Albertini, R.J.; Sram, R.J.; Upton, P.; Swenberg, J.A. Hemoglobin adducts in 1,3-butadiene exposed Czech workers: Female-male comparisons. Chem. Biol. Interact. 2010, 188, 668–676. [Google Scholar] [CrossRef]
- Georgieva, N.I.; Boysen, G.; Bordeerat, N.; Walker, V.E.; Swenberg, J.A. Exposure-response of 1,2:3,4-diepoxybutane-specific N-terminal valine adducts in mice and rats after inhalation exposure to 1,3-butadiene. Toxicol. Sci. 2010, 115, 322–329. [Google Scholar] [CrossRef]
- Boysen, G.; Georgieva, N.I.; Bordeerat, N.K.; Sram, R.J.; Vacek, P.; Albertini, R.J.; Swenberg, J.A. Formation of 1,2:3,4-diepoxybutane-specific hemoglobin adducts in 1,3-butadiene exposed workers. Toxicol. Sci. 2012, 125, 30–40. [Google Scholar] [CrossRef]
- Boysen, G.; Georgieva, N.I.; Upton, P.B.; Jayaraj, K.; Li, Y.; Walker, V.E.; Swenberg, J.A. Analysis of diepoxide-specific cyclic N-terminal globin adducts in mice and rats after inhalation exposure to 1,3-butadiene. Cancer Res. 2004, 64, 8517–8520. [Google Scholar] [CrossRef]
- Hecht, S.S. Human urinary carcinogen metabolites: Biomarkers for investigating tobacco and cancer. Carcinogenesis 2002, 23, 907–922. [Google Scholar] [CrossRef]
- Urban, M.; Gilch, G.; Schepers, G.; van Miert, E.; Scherer, G. Determination of the major mercapturic acids of 1,3-butadiene in human and rat urine using liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2003, 796, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Carmella, S.G.; Chen, M.; Han, S.; Briggs, A.; Jensen, J.; Hatsukami, D.K.; Hecht, S.S. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem. Res. Toxicol. 2009, 22, 734–741. [Google Scholar] [CrossRef]
- Jokipii Krueger, C.C.; Park, S.L.; Madugundu, G.; Patel, Y.; Le Marchand, L.; Stram, D.O.; Tretyakova, N. Ethnic differences in excretion of butadiene-DNA adducts by current smokers. Carcinogenesis 2021. [Google Scholar] [CrossRef]
- Jokipii Krueger, C.C.; Madugundu, G.; Degner, A.; Patel, Y.; Stram, D.O.; Church, T.R.; Tretyakova, N. Urinary N7-(1-hydroxy-3-buten-2-yl) guanine adducts in humans: Temporal stability and association with smoking. Mutagenesis 2020, 35, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Sangaraju, D.; Boldry, E.J.; Patel, Y.M.; Walker, V.; Stepanov, I.; Stram, D.; Hatsukami, D.; Tretyakova, N. Isotope Dilution nanoLC/ESI(+)-HRMS(3) Quantitation of Urinary N7-(1-Hydroxy-3-buten-2-yl) Guanine Adducts in Humans and Their Use as Biomarkers of Exposure to 1,3-Butadiene. Chem. Res. Toxicol. 2017, 30, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Sangaraju, D.; Goggin, M.; Walker, V.; Swenberg, J.; Tretyakova, N. NanoHPLC-nanoESI+-MS/MS quantitation of bis-N7-guanine DNA-DNA cross-links in tissues of B6C3F1 mice exposed to subppm levels of 1,3-butadiene. Anal. Chem. 2012, 84, 1732–1739. [Google Scholar] [CrossRef]
- Goggin, M.; Loeber, R.; Park, S.; Walker, V.; Wickliffe, J.; Tretyakova, N. HPLC-ESI+-MS/MS analysis of N7-guanine-N7-guanine DNA cross-links in tissues of mice exposed to 1,3-butadiene. Chem. Res. Toxicol. 2007, 20, 839–847. [Google Scholar] [CrossRef]
- Park, S.; Anderson, C.; Loeber, R.; Seetharaman, M.; Jones, R.; Tretyakova, N. Interstrand and intrastrand DNA-DNA cross-linking by 1,2,3,4-diepoxybutane: Role of stereochemistry. J. Am. Chem. Soc. 2005, 127, 14355–14365. [Google Scholar] [CrossRef]
- Lewis, L.; Borowa-Mazgaj, B.; de Conti, A.; Chappell, G.A.; Luo, Y.S.; Bodnar, W.; Konganti, K.; Wright, F.A.; Threadgill, D.W.; Chiu, W.A.; et al. Population-Based Analysis of DNA Damage and Epigenetic Effects of 1,3-Butadiene in the Mouse. Chem. Res. Toxicol. 2019, 32, 887–898. [Google Scholar] [CrossRef]
- Koturbash, I.; Scherhag, A.; Sorrentino, J.; Sexton, K.; Bodnar, W.; Tryndyak, V.; Latendresse, J.R.; Swenberg, J.A.; Beland, F.A.; Pogribny, I.P.; et al. Epigenetic alterations in liver of C57BL/6J mice after short-term inhalational exposure to 1,3-butadiene. Environ. Health Perspect. 2011, 119, 635–640. [Google Scholar] [CrossRef][Green Version]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix effects: The Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef]
- Annesley, T.M. Ion suppression in mass spectrometry. Clin. Chem. 2003, 49, 1041–1044. [Google Scholar] [CrossRef]
- Bylda, C.; Thiele, R.; Kobold, U.; Volmer, D.A. Recent advances in sample preparation techniques to overcome difficulties encountered during quantitative analysis of small molecules from biofluids using LC-MS/MS. Analyst 2014, 139, 2265–2276. [Google Scholar] [CrossRef]
- Zhang, H.; Xin, B.; Caporuscio, C.; Olah, T.V. Bioanalytical strategies for developing highly sensitive liquid chromatography/tandem mass spectrometry based methods for the peptide GLP-1 agonists in support of discovery PK/PD studies. Rapid. Commun. Mass Spectrom. 2011, 25, 3427–3435. [Google Scholar] [CrossRef]
- Gates, K.S.; Nooner, T.; Dutta, S. Biologically relevant chemical reactions of N7-alkylguanine residues in DNA. Chem. Res. Toxicol. 2004, 17, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Lin, W.P. Quantitative analysis of multiple exocyclic DNA adducts in human salivary DNA by stable isotope dilution nanoflow liquid chromatography-nanospray ionization tandem mass spectrometry. Anal. Chem. 2011, 83, 8543–8551. [Google Scholar] [CrossRef] [PubMed]
- Hang, B. Formation and repair of tobacco carcinogen-derived bulky DNA adducts. J. Nucleic. Acids 2010, 2010, 709521. [Google Scholar] [CrossRef] [PubMed]
- Yang, W. Structure and mechanism for DNA lesion recognition. Cell Res. 2008, 18, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Shuck, S.C.; Short, E.A.; Turchi, J.J. Eukaryotic nucleotide excision repair: From understanding mechanisms to influencing biology. Cell Res. 2008, 18, 64–72. [Google Scholar] [CrossRef]
- Fousteri, M.; Mullenders, L.H. Transcription-coupled nucleotide excision repair in mammalian cells: Molecular mechanisms and biological effects. Cell Res. 2008, 18, 73–84. [Google Scholar] [CrossRef]
- Moggs, J.G.; Yarema, K.J.; Essigmann, J.M.; Wood, R.D. Analysis of incision sites produced by human cell extracts and purified proteins during nucleotide excision repair of a 1,3-intrastrand d(GpTpG)-cisplatin adduct. J. Biol. Chem. 1996, 271, 7177–7186. [Google Scholar] [CrossRef] [PubMed]
- Kuraoka, I.; Robins, P.; Masutani, C.; Hanaoka, F.; Gasparutto, D.; Cadet, J.; Wood, R.D.; Lindahl, T. Oxygen free radical damage to DNA. Translesion synthesis by human DNA polymerase eta and resistance to exonuclease action at cyclopurine deoxynucleoside residues. J. Biol. Chem. 2001, 276, 49283–49288. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, N.W.; Yang, L.; Weisenburger, D.D.; Vose, J.; Beseler, C.; Rogan, E.G.; Cavalieri, E.L. Urinary biomarkers suggest that estrogen-DNA adducts may play a role in the aetiology of non-Hodgkin lymphoma. Biomarkers 2009, 14, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.; Nair, U.J.; Sun, X.; Wang, Y.; Arab, K.; Bartsch, H. Quantifying etheno-DNA adducts in human tissues, white blood cells, and urine by ultrasensitive (32)P-postlabeling and immunohistochemistry. Methods Mol. Biol. 2011, 682, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Mikes, P.; Korinek, M.; Linhart, I.; Krouzelka, J.; Dabrowska, L.; Stransky, V.; Mraz, J. Urinary N3 adenine DNA adducts in humans occupationally exposed to styrene. Toxicol. Lett. 2010, 197, 183–187. [Google Scholar] [CrossRef]
- Peters, S.; Talaska, G.; Jonsson, B.A.; Kromhout, H.; Vermeulen, R. Polycyclic aromatic hydrocarbon exposure, urinary mutagenicity, and DNA adducts in rubber manufacturing workers. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1452–1459. [Google Scholar] [CrossRef]
- Degner, A.; Arora, R.; Erber, L.; Chao, C.; Peterson, L.A.; Tretyakova, N.Y. Interindividual Differences in DNA Adduct Formation and Detoxification of 1,3-Butadiene-Derived Epoxide in Human HapMap Cell Lines. Chem. Res. Toxicol. 2020, 33, 1698–1708. [Google Scholar] [CrossRef]
- Koc, H.; Tretyakova, N.Y.; Walker, V.E.; Henderson, R.F.; Swenberg, J.A. Molecular dosimetry of N-7 guanine adduct formation in mice and rats exposed to 1,3-butadiene. Chem. Res. Toxicol. 1999, 12, 566–574. [Google Scholar] [CrossRef]
- Knutson, C.G.; Skipper, P.L.; Liberman, R.G.; Tannenbaum, S.R.; Marnett, L.J. Monitoring in vivo metabolism and elimination of the endogenous DNA adduct, M1dG {3-(2-deoxy-beta-D-erythro-pentofuranosyl)pyrimido[1,2-alpha]purin-10(3H)-one}, by accelerator mass spectrometry. Chem. Res. Toxicol. 2008, 21, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999, 424, 83–95. [Google Scholar] [CrossRef]


| Day | Mean | RSD (%) | Accuracy (%) | N |
|---|---|---|---|---|
| 1 | 1.01 ± 0.12 | 14.7 | 101.8 ± 15.0 | 3 |
| 2 | 1.16 ± 0.09 | 10.0 | 116.9 ± 11.7 | 3 |
| 3 | 1.19 ± 0.07 | 7.8 | 119.2 ± 5.1 | 3 |
| Interday | 1.12 ± 0.07 | 8.3 | 112.6 ± 7.7 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erber, L.; Goodman, S.; Jokipii Krueger, C.C.; Rusyn, I.; Tretyakova, N. Quantitative NanoLC/NSI+-HRMS Method for 1,3-Butadiene Induced bis-N7-guanine DNA-DNA Cross-Links in Urine. Toxics 2021, 9, 247. https://doi.org/10.3390/toxics9100247
Erber L, Goodman S, Jokipii Krueger CC, Rusyn I, Tretyakova N. Quantitative NanoLC/NSI+-HRMS Method for 1,3-Butadiene Induced bis-N7-guanine DNA-DNA Cross-Links in Urine. Toxics. 2021; 9(10):247. https://doi.org/10.3390/toxics9100247
Chicago/Turabian StyleErber, Luke, Samantha Goodman, Caitlin C. Jokipii Krueger, Ivan Rusyn, and Natalia Tretyakova. 2021. "Quantitative NanoLC/NSI+-HRMS Method for 1,3-Butadiene Induced bis-N7-guanine DNA-DNA Cross-Links in Urine" Toxics 9, no. 10: 247. https://doi.org/10.3390/toxics9100247
APA StyleErber, L., Goodman, S., Jokipii Krueger, C. C., Rusyn, I., & Tretyakova, N. (2021). Quantitative NanoLC/NSI+-HRMS Method for 1,3-Butadiene Induced bis-N7-guanine DNA-DNA Cross-Links in Urine. Toxics, 9(10), 247. https://doi.org/10.3390/toxics9100247






